Abstract
Recent studies have demonstrated the therapeutic potential of cannabinoids to treat pain, yet none have compared the analgesic effectiveness of smoked marijuana to orally administered Δ9-tetrahydrocannabinol (THC; dronabinol). This randomized, placebo-controlled, double-dummy, double-blind study compared the magnitude and duration of analgesic effects of smoked marijuana and dronabinol under well-controlled conditions using a validated experimental model of pain. Healthy male (N=15) and female (N=15) daily marijuana smokers participated in this outpatient study comparing the analgesic, subjective, and physiological effects of marijuana (0.00, 1.98, or 3.56% THC) to dronabinol (0, 10, or 20 mg). Pain response was assessed using the cold-pressor test (CPT): participants immersed their left hand in cold water (4 °C), and the time to report pain (pain sensitivity) and withdraw the hand from the water (pain tolerance) were recorded. Subjective pain and drug effect ratings were also measured as well as cardiovascular effects. Compared with placebo, marijuana and dronabinol decreased pain sensitivity (3.56% 20 mg), increased pain tolerance (1.98% 20 mg), and decreased subjective ratings of pain intensity (1.98, 3.56% 20 mg). The magnitude of peak change in pain sensitivity and tolerance did not differ between marijuana and dronabinol, although dronabinol produced analgesia that was of a longer duration. Marijuana (1.98, 3.56%) and dronabinol (20 mg) also increased abuse-related subjective ratings relative to placebo; these ratings were greater with marijuana. These data indicate that under controlled conditions, marijuana and dronabinol decreased pain, with dronabinol producing longer-lasting decreases in pain sensitivity and lower ratings of abuse-related subjective effects than marijuana.
Keywords: abuse liability, analgesia, cannabinoids, pain, sex differences
INTRODUCTION
Cannabinoids have long been thought to be effective in reducing pain (Russo, 2008) and findings from double-blind, placebo-controlled studies have substantiated such hypotheses demonstrating that smoked and vaporized marijuana (Abrams et al, 2007; Ellis et al, 2009; Wilsey et al, 2008, 2013), oral Δ9-tetrahydrocannabinol (THC; dronabinol), the primary psychoactive component of marijuana (Mechoulam et al, 1970; Svendsen et al, 2004), and Sativex (Nurmikko et al, 2007), an oromucuosal preparation of THC and cannabidiol, decrease subjective ratings of pain in neuropathic pain populations. Because of its recent legalization for medical use in a number of US states (Hoffmann and Weber, 2010), marijuana is now being prescribed therapeutically to manage a wide variety of pain conditions (Reinarman et al, 2011). Although marijuana is not regulated for purity or potency, and its smoked route of administration presents health risks that may limit marijuana's potential as a viable therapeutic option (Moir et al, 2008), it is argued to be preferable to dronabinol because of its faster onset and shorter duration of action allowing patients to titrate dose to the desired effect (Gowing et al, 1998). However, dronabinol lacks the health risks associated with smoked marijuana, and has been shown to retain the therapeutic effects of marijuana for treatment of nausea (Musty and Rossi, 2001) and anorexia in HIV-positive patients, while producing somewhat reduced intoxication compared to marijuana (Haney et al, 2005, 2007). The purpose of this study was to extend the comparison of the therapeutic effects of marijuana and dronabinol to measures of analgesia.
As discussed above, clinical studies have demonstrated the analgesic effects of cannabinoids in pain patients (Svendsen et al, 2004; Abrams et al, 2007; Nurmikko et al, 2007; Wilsey et al, 2008; Ellis et al, 2009; Ware et al, 2010), but none have compared the effects of marijuana to dronabinol. Therefore, this within-subject, randomized, placebo-controlled, double-dummy, double-blind study directly compared the therapeutic analgesic potential of marijuana to dronabinol in response to a laboratory measure of acute pain in healthy, daily marijuana smokers. Although assessing the therapeutic effects of an analgesic in a target population has clinical relevance, comparing these effects in a population without pain has the advantage of isolating the drug effects to the experimental nociceptive stimulus in the absence of chronic pain, which may fluctuate in intensity between laboratory sessions. The analgesic effects of two strengths of marijuana (1.98 and 3.56% THC) were compared with two doses of dronabinol (10 and 20 mg), which produce comparable effects on food intake and subjective-ratings in +HIV patients (Haney et al, 2005, 2007), using a laboratory model of pain that has predictive validity for clinical efficacy of analgesics in non-pain populations (Zacny et al, 1996; Conley et al, 1997; Kowalczyk et al, 2006).
In addition to their potential analgesic effects, marijuana and dronabinol produce additional effects, some of which may be therapeutically advantageous, for eample, increased appetite, decreased nausea, improved sleep (Musty and Rossi, 2001; Haney et al, 2007), whereas others are disadvantageous, such as intoxication and dependence with daily use (Cooper and Haney, 2009). How these effects correspond to analgesia is largely unknown but is an important mitigating factor in defining therapeutic utility. Therefore, this study compared the analgesic effects of marijuana and dronabinol with their cardiovascular and subjective effects related to intoxication and abuse potential. Further, because pain perception and efficacy of analgesic medication vary as a function of sex (Zacny and Beckman, 2004), this study enrolled equal numbers of men and women to control for and conduct exploratory analyses of potential sex-dependent differences in pain response and drug effects.
MATERIALS AND METHODS
Participants
Volunteers aged 21–45 years were recruited through newspaper advertisements, and those who met inclusion/exclusion criteria after an initial telephone screen were invited to the laboratory for further screening. Before enrollment, participants gave written informed consent, received a psychiatric and medical evaluation, and provided a detailed drug use and medical history. Participants were accepted into the study if they were healthy, as determined by a physical examination, electrocardiogram, and urine and blood chemistries. All eligible participants currently smoked ⩾3 marijuana cigarettes at least four times a week for the previous 4 weeks before screening, as determined by urine toxicology and self-report. Volunteers were excluded if they repeatedly used other illicit drugs, as determined by urine toxicology and self-report, or met the criteria for alcohol dependence. Those reporting pain were excluded, as were those who currently used over-the-counter or prescription medications each day, with the exception of oral contraceptives. Meeting Diagnostic and Statistical Manual (of Mental Disorders), fourth edition revised criteria for current or past Axis I psychopathology was also exclusionary. Female subjects were excluded if they were pregnant or nursing. Volunteers were told that the study objective was to determine the effects of smoked marijuana and dronabinol on pain response, mood, and physiology, and that during each session they would take a capsule and smoke a portion of a marijuana cigarette, but that the strength of the capsules and marijuana would vary. Participants were admitted into the study only after written informed consent to participate was given and eligibility criteria were verified. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accord with the Declaration of Helsinki.
Design and Procedures
The study included five outpatient sessions over the course of 2–4 weeks at the New York State Psychiatric Institute. Sessions, which were separated by at least 48 h to prevent medication carryover effects, began around 0900 hours, and were 6–7 h in duration. Before study onset, participants were familiarized with computerized tasks, the cold-pressor test (CPT), and study procedures with 1–2 training sessions, during which dronabinol and marijuana were not administered. During each session, one capsule containing placebo or dronabinol (10 or 20 mg) was administered to the participant 45 min before marijuana was smoked (0, 1.98, or 3.56% THC). Only one active dose of marijuana or dronabinol was administered within a session. A within-subject design was used in which all participants received all strengths of dronabinol and marijuana. The order of dosing was randomized.
Experimental session
Participants were instructed to not eat breakfast before each session or smoke marijuana or cigarettes after midnight the night before each session. Upon arrival to the laboratory, carbon monoxide levels were measured to confirm no recent smoking, breath alcohol levels were assessed, and use of illicit drugs other than marijuana was determined by a urine toxicology screen. If carbon monoxide levels indicated that the participant had smoked marijuana or a cigarette before arrival (⩾8 p.p.m.), the session was rescheduled. Pregnancy tests were also carried out before the first and fifth session. A standardized breakfast was provided to all participants before session onset.
Before capsule administration, participants completed baseline subjective-effects questionnaires and CPT; heart rate and blood pressure were measured using a Sentry II vital signs monitor (Model 6100; NBS Medical Services, Costa Mesa, CA). At 45 min after capsule administration, participants smoked a marijuana cigarette according to a cued-smoking procedure shown to produce reliable increases in heart rate and plasma THC levels (Foltin et al, 1987): Investigators instructed participants to ‘inhale' (5 s), ‘hold smoke in lungs' (10 s), and ‘exhale'. Participants smoked according to this procedure until 70% of the cigarette was pyrolized (3–7 puffs), with a 40-s interval between puffs. Heart rate, blood pressure, pain ratings derived from the CPT, and subjective pain ratings were assessed at set timepoints throughout the session (60, 90, 120, 150, 180, 270, and 360 min after capsule administration; 15, 45, 75, 105, 135, 225, and 315 min after initiation of marijuana smoking). Subjective ratings of mood and drug effect were also completed at these timepoints. Cigarette smokers were permitted to smoke at predetermined intervals throughout the session to minimize nicotine withdrawal symptoms. At the end of each session (about 5 h after smoking), participants were free to leave the laboratory after passing field sobriety tasks.
Cold pressor test
The cold pressor apparatus consisted of two water coolers, fitted with a wire cradle and an aquarium pump for water circulation. One cooler was filled with warm water (37 °C) and the other was filled with cold water (4 °C). Participants removed jewelry from the hand and forearm at the beginning of the session; during the test, participants were instructed to rest his/her hand with fingers spread apart on the wire cradle. Each CPT began with an immersion of the left hand into the warm water bath for 3 min. During this time, blood pressure and heart rate were measured. After removal of the hand from the warm water, skin temperature of the thumbpad was recorded and participants listened to a standardized script describing the procedures. Participants then immersed the left hand into the cold water bath, and were instructed to report the first painful sensation after immersion. They were asked to tolerate the stimulus as long as possible, but were permitted to withdraw their hand from the cold water at any point. Maximum immersion time was 2 min. Latency to first feel pain (pain sensitivity) and latency to withdraw the hand from the water (pain tolerance) were recorded. Blood pressure and heart rate were measured before and after each immersion using the arm that was not immersed in the water bath. The experimenter administering the CPT was of the same sex as the volunteer.
Subjective effects
Ratings of pain and drug effect were measured throughout the session.
Pain intensity and bothersomeness scales
Immediately after removing the hand from the cold water, participants rated pain intensity and bothersomeness of the cold water stimulus on a scale from 0 to 10, 0 being ‘not painful/bothersome at all' and 10 being ‘most painful/bothersome feeling imaginable.'
McGill Pain Questionnaire
A 15-item shortened form of the McGill Pain Questionnaire (MPQ) (Melzack, 1987) was used to assess the sensory and affective dimensions of the pain experience immediately following the CPT. Participants were asked to describe the pain by choosing among a series of possible answers (None (score=1) to Severe (score=4)) when prompted by a descriptor (‘Throbbing,' ‘Shooting,' ‘Stabbing,' etc). Scores were added across all 15 items to generate a sum score, which ranged between 15 and 60. This questionnaire was completed immediately after participants withdrew their hand from the cold water.
Subjective drug-effect questionnaires
Subjective drug effects were measured using visual analog scales (VASs), a series of 100-mm lines anchored with ‘not at all' (0 mm) and ‘extremely' (100 mm). Participants were instructed to indicate on the line how they felt at that moment.
Subjective effect-VAS
Participants were asked to rate their mood and physical symptoms on a modified 44-item visual analog scale (VAS) intended to measure affective and physical subjective drug effects (see Haney et al (1999) for description of the original 50-question version).
Marijuana rating form
Subjective marijuana-related drug effects were assessed using a 5-item VAS asking participants to rate the strength of the drug effect, good effect, bad effect, drug liking, and willingness to take the drug again. Participants were also asked to indicate whether they thought the marijuana was ‘placebo' or ‘active.'
Capsule rating form
Subjective drug effects associated with capsules were assessed using a 5-item VAS similar to the MRF, asking participants to rate the strength of the capsule drug effect, good effect, bad effect, drug liking, and willingness to take the drug again. Participants were also asked to indicate whether they thought the capsule contained placebo or active medication.
Drugs
Capsules (size 00 opaque capsules with lactose filler) containing placebo or dronabinol (10 or 20 mg) were prepared by the New York State Psychiatric Institute Research Pharmacy. Marijuana cigarettes (0.00, 1.98, or 3.56% THC; ca. 800 mg) were provided by the National Institute on Drug Abuse. Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 h before the session.
Data Analysis
Repeated-measures analysis of variance with planned comparisons were used to assess dronabinol and marijuana's analgesic, subjective, and cardiovascular effects. There were two within-group factors, drug condition (marijuana strength and dronabinol dose) and time point. Dependent variables included pain sensitivity and tolerance, subjective pain and drug effects as assessed by the pain intensity and bothersomeness scales, the MPQ, subjective effect-VAS (SE-VAS), MRF, and CRF scales, and heart rate. For each drug condition, pain sensitivity and tolerance were calculated for each participant as the percent of the baseline pre-dosing CPT response; differences in the baseline CPT effects were analyzed according to sex and dose condition. For each of the above dependent measures, six planned comparisons between dosing conditions were completed. Both marijuana strengths and dronabinol doses were compared with the placebo condition (four comparisons), marijuana and dronabinol effects were compared according to dose/strength (two comparisons: 1.98% THC marijuana compared with 10 mg dronabinol and 3.56% THC marijuana compared with 20 mg dronabinol). Results were considered statistically significant when p-values were equal to or less than 0.05 using Huynh–Feldt corrections. Statistical power was not sufficient to assess sex-dependent effects; however, an exploratory between-groups analysis was performed to assess for trends suggesting sex differences for baseline pain measures and drug effects.
RESULTS
Demographic Characteristics
Table 1 portrays the demographic characteristics of the participants who completed the study; there were no significant demographic differences between male (n=15) and female subjects (n=15). An additional four volunteers enrolled, but did not complete the study. Of the four that discontinued, one did so for personal reasons and three were unreliable.
Table 1. Demographic Characteristics of Study Participants.
| Males (N=15) | Females (N=15) | |
|---|---|---|
| Age (years old) | 27±5 | 27±7 |
| Race (B/W/M) | 10/4/1 | 10/2/3 |
| Marijuana use | ||
| Years regular use | 8.0±5.3 | 9.1±6.3 |
| Days per week | 6.6±0.9 | 6.1±1.4 |
| Dollars per week | 70.0±59.8 | 41.1±20.0 |
| MJ cigarettes per day | 6.0±4.3 | 8.0±8.4 |
Note: Data are presented as means (± SD) or as frequency.
Race is indicated as Black (B), White (W), and Mixed (M).
Analgesic Effects
CPT: pain sensitivity and tolerance
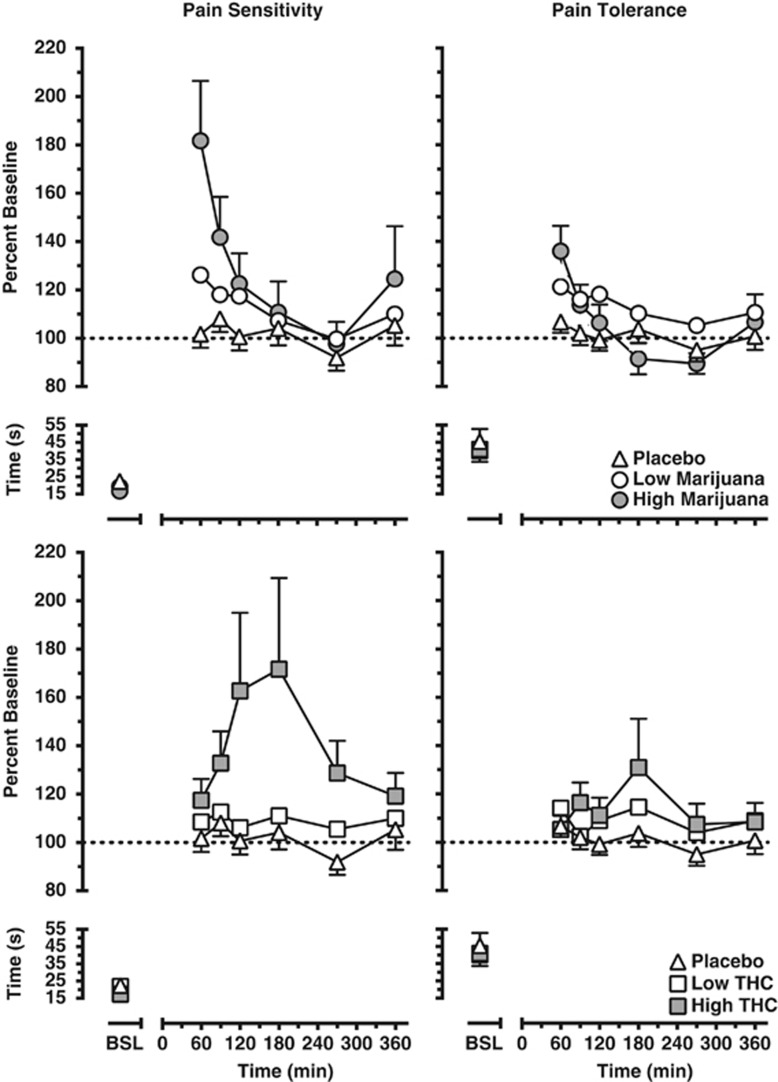
Figure 1 portrays the time course of pain sensitivity (latency to first report pain, left panels) and pain tolerance (latency to withdraw the hand from the cold water, right panels) as a function of marijuana strength (top panels) and dronabinol dose (bottom panels). Baseline pain sensitivity and tolerance did not differ across dosing conditions. Drug effects on pain sensitivity and tolerance peaked 15 min after marijuana was smoked and 180 min after dronabinol was administered. In terms of pain sensitivity, the latency to first report pain was significantly increased by the high marijuana strength (3.56% 13.1±3.9 s difference from baseline) and dronabinol dose (20 mg; 12.1±5.6 s difference from baseline) conditions relative to the placebo (0.3±1.0 s difference from baseline) (p⩽0.01). Comparisons of pain sensitivity according to dronabinol dose and marijuana strength did not reveal differences between the two drugs. In terms of pain tolerance, low marijuana strength cigarettes (1.98% 4.9±3.4 s difference from baseline) and both dronabinol doses (10 and 20 mg; 2.8±2.9 s difference from baseline and 6.1±4.4 s difference from baseline, respectively) increased the latency to report pain (p⩽0.05) relative to placebo (1.5±1.4 s difference from baseline). The high marijuana strength cigarettes (3.56%) did not significantly alter pain tolerance relative to placebo across the session; although this strength increased pain tolerance up to an hour after smoking (9.0±3.0 s difference from baseline), a decrease in pain tolerance was observed at later time points. Marijuana and dronabinol did not produce significantly different effects on pain latency.
Figure 1.
Pain sensitivity (left panels) and tolerance (right panels) as calculated by percent baseline latency (seconds) to report pain and withdraw the hand from the cold water. Data are presented as mean values for the group according to marijuana strength (top panels) or dronabinol dose (bottom panels) and time point. Refer to the Results section for explanation of significant differences between drug conditions.
Subjective pain ratings
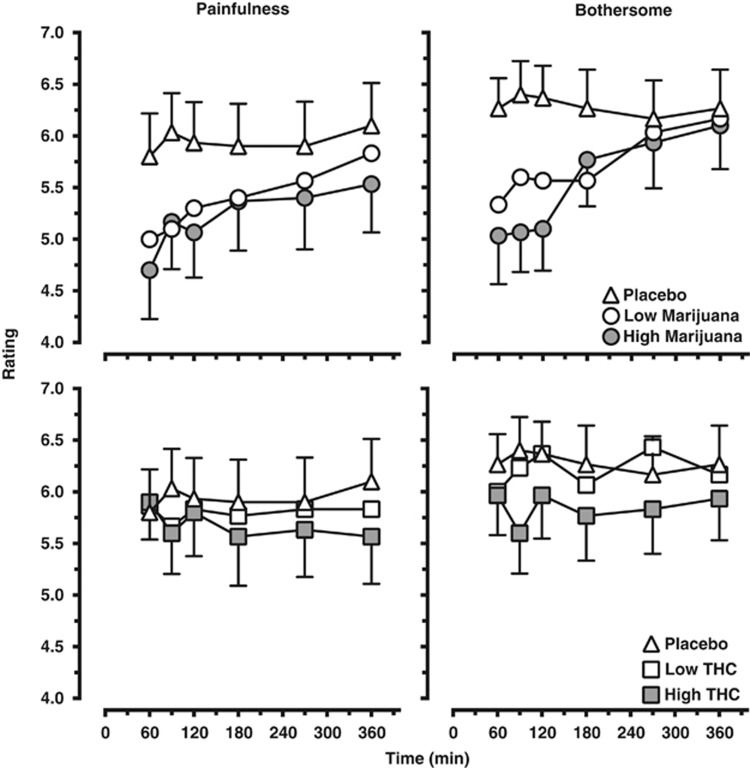
Effects of marijuana and dronabinol on subjective pain ratings measured using the Pain Intensity and Bothersomeness scales are shown in Figure 2 (top panels, marijuana; bottom panels, dronabinol). Baseline subjective pain ratings did not differ across dosing conditions. Both marijuana strengths (p⩽0.001) and the high dronabinol dose (p⩽0.05) decreased subjective ratings of pain intensity and bothersomeness of the CPT relative to placebo, whereas the low dronabinol dose did not affect either of these ratings. Comparisons of subjective pain ratings as a function of marijuana strength and dronabinol dose revealed that both strengths of marijuana produced greater decreases in subjective pain ratings relative to the respective dronabinol doses (p⩽0.01).
Figure 2.
Subjective ratings of the painfulness (left panel) and bothersomeness (right panel) as assessed immediately after the hand was withdrawn from the cold water. Data are presented as mean ratings for the group as a function of marijuana strength (top panels) or dronabinol dose (bottom panels) and time point. Refer to the Results section for explanation of significant differences between drug conditions.
Subjective drug effects
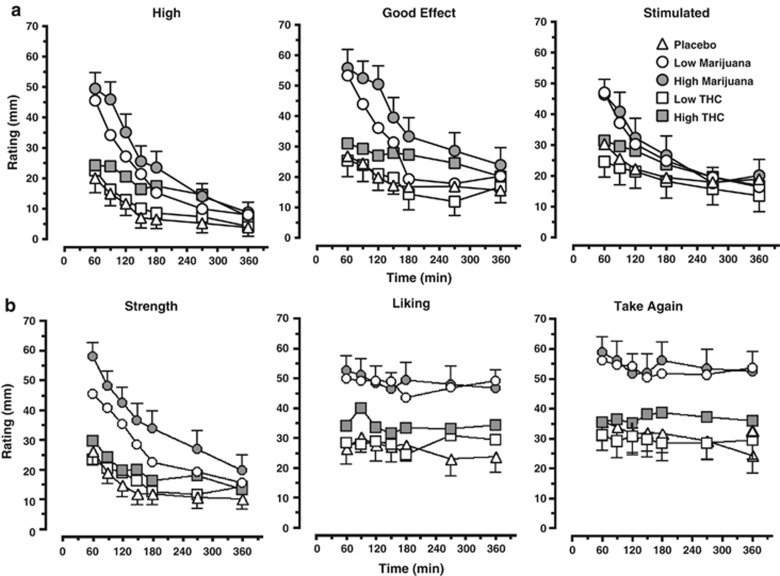
Figure 3 illustrates ratings of subjective drug effects according to marijuana strength and dronabinol dose as measured by the SE-VAS (Figure 3a) and MRF (Figure 3b). Subjective ratings of drug effects on the SE-VAS peaked 15 min after active marijuana was smoked and 60–90 min after active dronabinol was administered. Both marijuana strengths and the high dronabinol dose increased ratings of ‘High' and ‘Good drug effect' relative to placebo (p⩽0.001), and both marijuana strengths increased ratings of ‘Stimulated' (p⩽0.001); the low dronabinol dose decreased ratings of ‘Stimulated' relative to placebo (p⩽0.05) but did not alter ratings of ‘High' and ‘Good drug effect' relative to placebo. For these three effects, both marijuana strengths produced higher ratings relative to the respective dronabinol doses (p⩽0.001).
Figure 3.
Participant ratings of subjective drug effects ‘High,' ‘Good drug effect,' and ‘Stimulated' as measured by the subjective effect-visual analog scale (SE-VAS) (a) and ratings of marijuana strength, liking, and willingness to take again as measured by the Marijuana Rating Form (MRF) (b). Data are presented as mean group ratings as a function of drug condition and time point. Refer to the Results section for explanation of significant differences between drug conditions.
Subjective ratings of marijuana quality and effects as measured by the MRF demonstrated that relative to placebo, both marijuana strengths and the high dronabinol dose increased ratings of marijuana strength, liking, and willingness to take again (p⩽0.01); the low dronabinol dose did not alter these ratings. For these three effects, both marijuana strengths produced higher ratings relative to the respective dronabinol doses (p⩽0.001).
Subjective ratings of capsule effects as measured by the CRF averaged across each session revealed that both marijuana strengths and the high dronabinol dose produced small (<5 mm increase) but significant increases in subjective ratings of willingness to take the capsule again relative to placebo (p⩽0.01), and the high dronabinol dose and marijuana strength increased ratings of capsule liking (<6 mm; p⩽0.01) (data not shown). The low marijuana strength produced higher ratings for these three effects relative to the low dronabinol dose (p⩽0.01) (data not shown). Ratings did not differ between the high marijuana strength and dronabinol dose. For both the MRF and CRF, subjective ratings of drug effects peaked 15 min after active marijuana was smoked and 60–90 min after active dronabinol was administered.
Marijuana and dronabinol did not increase negative subjective drug effect ratings on the SE-VAS, MRF, or CRF.
Cardiovascular Effects
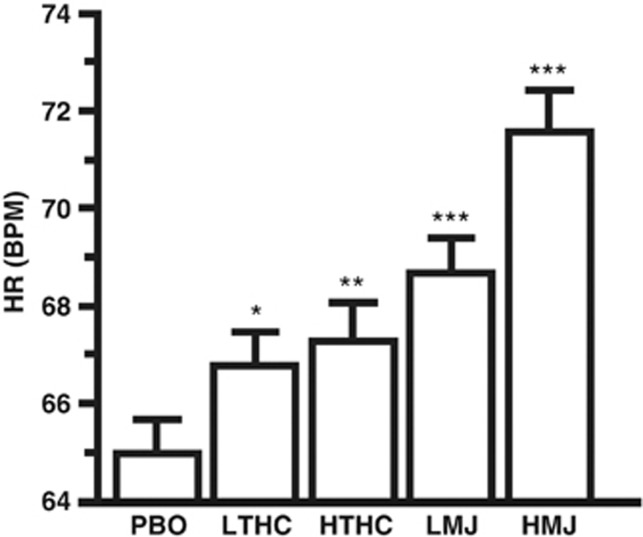
Figure 4 portrays the average change in heart rate over the session as a function of marijuana strength and dronabinol dose. Both marijuana strengths and dronabinol doses increased heart rate relative to placebo (p⩽0.05). Both marijuana strengths produced greater increases in heart rate relative to the respective dronabinol doses (p⩽0.01).
Figure 4.
Cardiovascular effects of placebo, dronabinol, and marijuana presented as the average (±SEM) heart rate (beats per minute (b.p.m.)) for post-smoking time points for each drug condition. Significant differences between placebo and dronabinol or marijuana are indicated by *p⩽0.05, **p⩽0.01, and ***p⩽0.001.
Sex Differences
There was no evidence of sex differences for baseline pain measures, or for the analgesic, subjective, or physiological effects of marijuana or dronabinol.
DISCUSSION
This study demonstrates dose- and time-dependent cannabinoid-induced analgesia in response to an acute pain response to the cold pressor test. The magnitude and duration of analgesic effects varied according to drug condition: Dronabinol administration decreased pain sensitivity and increased pain tolerance that peaked later and lasted longer relative to smoked marijuana, whereas marijuana produced a greater attenuation of subjective ratings of pain intensity relative to dronabinol. In addition to analgesia, marijuana and dronabinol increased subjective drug effect ratings associated with abuse liability, with marijuana eliciting greater increases in these ratings relative to dronabinol. Although the time course for marijuana-induced increases in subjective drug ratings paralleled its analgesic effects, this was not the case for dronabinol. Peak subjective ratings of dronabinol's drug effects occurred 60 min after administration, whereas dronabinol-induced decreases in pain sensitivity and increases in pain tolerance were highest about 4 h after drug administration, suggesting that the magnitude and time course of these subjective drug effects do not necessarily predict analgesia in the CPT.
Clinical studies have demonstrated the analgesic effectiveness of cannabinoids including marijuana and dronabinol in neuropathic pain populations (Svendsen et al, 2004; Abrams et al, 2007; Nurmikko et al, 2007; Wilsey et al, 2008; Ellis et al, 2009; Ware et al, 2010). However, the cannabinoids proved to be less effective in attenuating other pain modalities; dronabinol (5 mg) failed to decrease postoperative pain (Buggy et al, 2003) and sensitivity to a thermal stimulus (Roberts et al, 2006), and a higher dronabinol dose (20 mg) failed to reduce acute inflammatory pain, hyperalgesia (Kraft et al, 2008), or increase pain threshold in a pressure test (Naef et al, 2003). Although dronabinol did not produce analgesia in these experimental pain models, a single study successfully demonstrated the effectiveness of smoked marijuana in reducing sensitivity to radiant heat stimulation (Greenwald and Stitzer, 2000). In this study utilizing healthy volunteers, the analgesic effects of both marijuana and dronabinol were evident using the CPT, producing a robust increase in pain sensitivity and a significant, but smaller in magnitude, increase in pain tolerance. These findings demonstrate that under controlled conditions, both dronabinol and marijuana reliably elicit dose- and time-dependent analgesia in response to the CPT for most measures and are in concordance with the preclinical studies demonstrating the antinociceptive effectiveness of cannabinoid subtype 1 (CB1) receptor agonists (eg, Hama and Sagen, 2011).
For one measure, the low but not the high marijuana strength cigarettes significantly increased pain tolerance relative to placebo. Although the high marijuana strength increased pain tolerance at the earlier timepoints, the effect dissipated and decreased at the later timepoints below baseline values. A similar effect has been identified in an earlier study assessing marijuana-induced analgesia in a model of postinjury pain, where the responses to nociceptive stimuli were determined after a transdermal injection of capsaicin (Wallace et al, 2007). Under this facilitated pain state, the moderate marijuana strength decreased pain, whereas the higher marijuana strength increased pain at a single timepoint. The same study also failed to demonstrate marijuana-induced analgesia in response to warm and cold stimuli under a non-facilitated pain state. These findings indicate that marijuana's effects on the pain response seem to be contingent upon dose, time, and the experimental pain model. As such, unlike the preclinical findings that have identified the antinociceptive effects of CB1 agonists across a variety of pain modalities, the analgesic effects of dronabinol and marijuana in humans are dependent upon the nociceptive stimulus.
The potential use of cannabinoids to treat pain and their limitations have been widely discussed in review and opinion publications (eg, Rahn and Hohmann, 2009). Adverse effects of cannabinoids that can limit their therapeutic use are negative subjective effects and impaired cognition (as discussed in Haney, 2007). In this study, dronabinol and marijuana did not produce negative subjective effects. Marijuana increased heart rate, as did dronabinol to a lesser extent, but the magnitude of these changes were not clinically significant. Although the cognitive effects of dronabinol and marijuana were not assessed in this study, previous reports from our laboratory have demonstrated that these dronabinol doses and marijuana strengths minimally affect performance on tasks designed to measure memory, attention, and psychomotor skills in ongoing marijuana smokers (Haney et al, 2005). By contrast, in non-marijuana smokers, lower doses of dronabinol have been shown to elicit negative subjective effects in healthy volunteers (Haney, 2007) and in patient populations (Tramer et al, 2001). Although these effects are dose-dependent and can be mitigated by careful dose titration, it is still unknown if analgesia can be achieved at doses that do not produce negative effects in a non-marijuana smoking population. Another potential limitation to the use of cannabinoids for pain is the development of tolerance and dependence, an issue that impacts the therapeutic potential of opioids. Although tolerance to the analgesic effects of cannabinoids may require escalating doses over time, CB1 agonists have a favorable safety profile (Burns and Ineck, 2006) relative to opioids, which can have respiratory depressant effects at high doses (Smith and Bruckenthal, 2010). A primary caveat of these findings is that the study population consisted of daily marijuana smokers; this study limitation should be considered when interpreting the findings and placing them within the context of the potential therapeutic feasibility of cannabinoids. The study inclusion criteria were specifically designed to control for marijuana use in effort to decrease the variability in cannabinoid-elicited effects that may have arisen because of tolerance. However, selecting for this population limits the generalizability of the findings. As discussed above, the analgesic effects of marijuana and dronabinol are hypothesized to be greater in lighter or non-marijuana users, but the probability for negative effects (ie, negative subjective effects and decrements in cognitive performance) would similarly increase.
The exploratory analysis for sex-dependent effects did not detect differences between male and female participants in baseline pain response or to dronabinol or marijuana's analgesic effects. The lack of sex-dependent differences in baseline (non-drug) pain response was unexpected given that the majority of investigations exploring sex differences in response to an experimental cold pain stimulus report male participants to exhibit lower pain sensitivity and higher tolerance relative to female participants (for a review see Fillingim et al, 2009). Sex-dependent differences in dronabinol and marijuana's analgesic effects were also expected given that the synthetic cannabinoid nabilone decreases pain in an experimental heat pain in female participants but not male participants (Redmond et al, 2008). One possibility for the lack of sex-dependent effects in this study is because the experimenter administering the CPT was the same sex as the volunteer. Manipulating the sex of the experimenter has been shown to alter pain sensitivity and tolerance in the CPT, thus potentially contributing to sex-dependent differences in pain response when an experimenter of the opposite sex performs the CPT (Gijsbers and Nicholson, 2005; Fillingim et al, 2009). Another factor that significantly contributes to sex-dependent differences in pain sensitivity is fluctuations of reproductive hormones that occur as a function of menstrual-cycle phase in laboratory animals (for a review see Craft et al, 2004), which was not controlled for in this study. Although recent findings suggest that menstrual cycle phase has only a subtle effect on pain responsivity in humans (Kowalczyk et al, 2006; Kowalczyk et al, 2010), the evidence for sex-dependent differences in nabilone's analgesic effects (Redmond et al, 2008) suggests that changes in cannabinoid-induced analgesia as a function of sex and menstrual-cycle phase is an area that warrants further research.
CONCLUSION
Cannabinoids have been shown to alleviate neuropathic pain associated with disease (multiple sclerosis (Svendsen et al, 2004; Rog et al, 2007; Zajicek and Apostu, 2011), HIV (Ellis et al, 2009) and injury (Berman et al, 2004). This study is the first to demonstrate the dose- and route-dependent analgesic effectiveness of cannabinoids for acute experimentally-induced pain in a pain-free population, evidence that supports the role of cannabinoids for the management of pain.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This research was supported by US National Institute on Drug Abuse Grant DA19239, DA09236, and DA02775. We acknowledge and appreciate the exceptional assistance of Divya Lakhaney, Elyssa Berg, and Michael Harakas in data collection.
References
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106:169–172. doi: 10.1016/s0304-3959(03)00331-2. [DOI] [PubMed] [Google Scholar]
- Burns TL, Ineck JR. Cannabinoid analgesia as a potential new therapeutic option in the treatment of chronic pain. Ann Pharmacother. 2006;40:251–260. doi: 10.1345/aph.1G217. [DOI] [PubMed] [Google Scholar]
- Conley KM, Toledano AY, Apfelbaum JL, Zacny JP. Modulating effects of a cold water stimulus on opioid effects in volunteers. Psychopharmacology. 1997;131:313–320. doi: 10.1007/s002130050298. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatry. 2009;21:104–112. doi: 10.1080/09540260902782752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 1987;20:87–93. doi: 10.1016/0376-8716(87)90079-2. [DOI] [PubMed] [Google Scholar]
- Gijsbers K, Nicholson F. Experimental pain thresholds influenced by sex of experimenter. Percept Mot Skills. 2005;101:803–807. doi: 10.2466/pms.101.3.803-807. [DOI] [PubMed] [Google Scholar]
- Gowing LR, Ali RL, Christie P, White JM. Therapeutic use of cannabis: clarifying the debate. Drug Alcohol Rev. 1998;17:445–452. doi: 10.1080/09595239800187281. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- Hama A, Sagen J. Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain. Brain Res. 2011;1412:44–54. doi: 10.1016/j.brainres.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, et al. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007;45:545–554. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology. 2005;181:170–178. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362:1453–1457. doi: 10.1056/NEJMp1000695. [DOI] [PubMed] [Google Scholar]
- Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7:151–160. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, Vosburg SK, Comer SD. Sex differences and hormonal influences on response to mechanical pressure pain in humans. J Pain. 2010;11:330–342. doi: 10.1016/j.jpain.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, et al. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology. 2008;109:101–110. doi: 10.1097/ALN.0b013e31817881e1. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Shani A, Edery H, Grunfeld Y. Chemical basis of hashish activity. Science. 1970;169:611–612. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21:494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- Musty R, Rossi R. Effects of Smoked Cannabis and Oral Δ9-Tetrahydrocannabinol on Nausea and Emesis After Cancer Chemotherapy: A Review of State Clinical Trials. Journal of Cannabis Therapeutics. 2001;1:43–56. [Google Scholar]
- Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88. doi: 10.1016/s0304-3959(03)00163-5. [DOI] [PubMed] [Google Scholar]
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133:210–220. doi: 10.1016/j.pain.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond WJ, Goffaux P, Potvin S, Marchand S. Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin. 2008;24:1017–1024. doi: 10.1185/030079908x280635. [DOI] [PubMed] [Google Scholar]
- Reinarman C, Nunberg H, Lanthier F, Heddleston T. Who are medical marijuana patients? Population characteristics from nine California assessment clinics. J Psychoactive Drugs. 2011;43:128–135. doi: 10.1080/02791072.2011.587700. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530:54–58. doi: 10.1016/j.ejphar.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007;29:2068–2079. doi: 10.1016/j.clinthera.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4:245–259. doi: 10.2147/tcrm.s1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Bruckenthal P. Implications of opioid analgesia for medically complicated patients. Drugs Aging. 2010;27:417–433. doi: 10.2165/11536540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329:253. doi: 10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323:16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Schulteis G, Atkinoson JH, Wolfson T, Lazzaretto D, Bentley H, et al. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology. 2007;107:785–796. doi: 10.1097/01.anes.0000286986.92475.b7. [DOI] [PubMed] [Google Scholar]
- Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182:E694–E701. doi: 10.1503/cmaj.091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136–148. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506–521. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Beckman NJ. The effects of a cold-water stimulus on butorphanol effects in males and females. Pharmacol Biochem Behav. 2004;78:653–659. doi: 10.1016/j.pbb.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Coalson DW, Young CJ, Klafta JM, Lichtor JL, Rupani G, et al. Propofol at conscious sedation doses produces mild analgesia to cold pressor-induced pain in healthy volunteers. J Clin Anesth. 1996;8:469–474. doi: 10.1016/0952-8180(96)00126-2. [DOI] [PubMed] [Google Scholar]
- Zajicek JP, Apostu VI. Role of cannabinoids in multiple sclerosis. CNS Drugs. 2011;25:187–201. doi: 10.2165/11539000-000000000-00000. [DOI] [PubMed] [Google Scholar]