Abstract
Introduction:
Obesity-hypoventilation syndrome (OHS) is associated with significant morbidity and mortality and requires measurement of arterial pCO2 for diagnosis.
Objective:
To determine diagnostic predictors of OHS among obese patients with suspected obstructive sleep apnea/hypopnea syndrome (OSAHS).
Methods:
Retrospective analysis of data on 525 sleep clinic patients (mean age 51.4 ± 12.7 years; 65.7% males; mean BMI 34.5 ± 8.1). All patients had sleep studies, and arterialized capillary blood gases (CBG) were measured in obese subjects (BMI > 30 kg/m2).
Results:
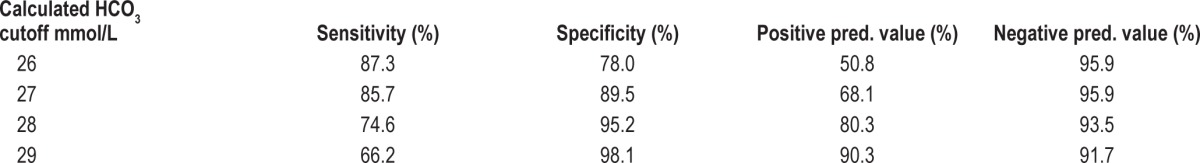
Of 525 patients, 65.5% were obese, 37.2% were morbidly obese (BMI > 40 kg/m2); 52.3% had confirmed OSAHS. Hypercapnia (pCO2 > 6 kPa or 45 mm Hg) was present in 20.6% obese and 22.1% OSAHS patients. Analysis of OHS predictors showed significant correlations between pCO2 and BMI, FEV1, FVC, AHI, mean and minimum nocturnal SpO2, sleep time with SpO2 < 90%, pO2, and calculated HCO3 from the CBG. PO2 and HCO3 were independent predictors of OHS, explaining 27.7% of pCO2 variance (p < 0.0001). A calculated HCO3 cutoff > 27 mmol/L had 85.7% sensitivity and 89.5% specificity for diagnosis of OHS, with 68.1% positive and 95.9% negative predictive value.
Conclusion:
We confirmed a high prevalence of OHS in obese OSAHS patients (22.1%) and high calculated HCO3 level (> 27 mmol/L) to be a sensitive and specific predictor for the diagnosis of OHS.
Citation:
Macavei VM; Spurling KJ; Loft J; Makker HK. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J Clin Sleep Med 2013;9(9):879-884.
Keywords: Obstructive sleep apnea, obesity hypoventilation syndrome
Obesity is now considered a global epidemic, and it is projected that by the year 2025, 36% of females and 47% of males will be obese in the UK.1 A recent US study on obesity epidemic showed a levelling off trend for 2009-2010 compared with 2003-2008, with a prevalence of obesity of 35.5% among adult men and 35.8% among adult women.2 This rise in obesity is likely to lead to an increase in respiratory consequences such as obesity-hypoventilation syndrome (OHS), previously known as Pickwickian syndrome.3 It is defined as a combination of obesity (body mass index [BMI] > 30 kg/m2) and daytime hypercapnia (pCO2 > 45 mm Hg or > 6 kPa) in the absence of other known causes of alveolar hypoventilation.4 Obesity is also a main risk factor for obstructive sleep apnea/hypopnea syndrome (OSAHS). Some studies have found hypercapnia in 10% to 20% of OSAHS patients; however, these studies were limited by small sample size, biased recruitment of patients with COPD or severe obesity, and the exclusive enrollment of one gender group and/or ethnic background.5–7 The exact prevalence of OHS is not known, but it is likely the prevalence is different in unselected OSAHS patients.
Little is known about prevalence of OHS in the obese population, irrespective of OSAHS. Current estimates suggest that around 0.3% to 0.4% of the population may have OHS.8,9 Prevalence of OHS is set to increase with rising obesity; therefore, accurate assessment of prevalence of OHS is critical for planning health services to make provision for this condition. Furthermore, the need for early detection of OHS is clear because delay in diagnosis and treatment is associated with significant morbidity and mortality.10,11 If left untreated, OHS is associated with a mortality of 23% at 18 months following discharge from hospital; adequate treatment of OHS reduces this to 3%. In addition, untreated OHS patients are likely to require invasive mechanical ventilation with longer hospital stay.11
BRIEF SUMMARY
Current Knowledge/Study Rationale: The need for early detection of obesity hypoventilation syndrome (OHS) is clear because delay in the diagnosis and treatment is associated with significant morbidity and mortality. In this study we aimed to determine the prevalence of obesity-hypoventilation syndrome among obese patients with suspected sleep apnea and ascertain the validity of previously reported predictors of OHS.
Study Impact: Our study suggests that a normal calculated HCO3 from the CBG can exclude daytime hypercapnia in obese patients, while an elevated bicarbonate and/or nocturnal hypoxia should prompt further investigation for OHS. Routine measurement of bicarbonate in obese patients with suspected sleep apnea can be a useful screening tool for diagnosis of OHS and/or sleep disordered breathing.
The standard assessment to screen for daytime hypercapnia is measurement of arterial blood gases. Performing this measurement in all obese individuals for diagnosis of OHS has its obvious limitations, in terms of the invasive nature of the test and resources required. Identification of simpler clinical predictors of OHS may help with early diagnosis and management. It has been suggested that obese patients with hypercapnia have higher BMI, more severe OSAHS, and worse restrictive chest wall mechanics than normocapnic obese patients.8 There are no clear thresholds with regard to the above parameters that could guide clinicians in identifying those with hypercapnia. A useful and practical measurement appears to be the bicarbonate level. It is readily available and is less invasive than arterial puncture and therefore an attractive choice as a screening tool for hypercapnia.7,12 However, clinical usefulness of this approach needs further evaluation.
Given the high volume of need for noninvasive ventilation for suspected OHS as a cause of hypercapnic respiratory failure at our institution,13 a quality improvement project was undertaken to systematically identify patients with OHS presenting to the sleep disorders center for evaluation.
In this study we aimed to determine prevalence of OHS among obese patients with suspected OSAHS and to check the validity of previously reported predictors of OHS such as body mass index, sleep apnea, and bicarbonate level.
MATERIAL AND METHODS
The study cohort comprised of 525 consecutive patients referred to the sleep clinic at North Middlesex University Hospital, London, UK, between January 2009 and January 2011. They were evaluated according to a specifically designed protocol which included presenting symptoms, past medical history (respiratory, metabolic and cardiovascular comorbidities, smoking status), and demographic details (age, gender, and ethnicity). Subsequent anthropometric measurements such as height, weight, neck size, waist/hip ratio, Mallampati score, Epworth Sleepiness Scale score, and spirometry values were obtained.
Four hundred twenty-five patients underwent limited polygraphy sleep study (Embletta; Stowood Scientific Instruments, Oxford UK), which measured oronasal flow using pressure transducer, thoraco-abdominal movement using respiratory inductance plethysmography (RIP), and nocturnal oxygen saturation and heart rate using pulse oximetry.
One hundred patients underwent home overnight oximetry (Konica-Minolta Pulsox-300i; Stowood Scientific Instruments, Oxford UK), which measured nocturnal oxygen saturation and heart rate.
Capillary blood gases (CBG) were measured only in obese subjects (BMI > 30 kg/m2) to identify the presence of hypercapnia in both patients with and without OSAHS. Earlobe arterialized blood sampling was performed as per method described in the British Thoracic Society (BTS) guidelines.14 CBG measurements were taken between 10:00 and 12:00 in sitting position, following 20 min rest. The earlobe was warmed with a hot compress, and rubefacient cream was used during the resting period. Hypercapnia was defined as pCO2 > 6 kPa (45 mm Hg), and normocapnia as pCO2 ≤ 6 kPa (45 mm Hg). HCO3 level was calculated using the Henderson-Hasselbalch formula from the CBG.
The analysis and interpretation of the polygraphy and oximetry data was performed using proprietary software (Somnologica for Embletta version 5.1.0 and DS-5 version 2.10, Stowood Scientific Instruments, Oxford UK). Spirometry was obtained from all patients (Medisoft Hyp'Air, Pulmolink, Kent UK), along with reference values calculated from patient height, weight, and age using equations from the European Respiratory Society.15
Apnea was defined as a cessation of airflow at the upper airway lasting > 10 seconds. Hypopnea was defined as a decrease in airflow, chest, or abdominal excursions with > 50% associated with oxygen desaturation ≥ 4% below baseline value. The apnea-hypopnea index (AHI) was calculated as the number of apnea and hypopnea episodes per hour of sleep. Patients with an AHI > 5 were diagnosed with obstructive sleep apnea (OSAHS). Severity of OSAHS was scaled according to AHI: mild (5-15), moderate (15-30), and severe (> 30). The oxygen desaturation index (ODI) was calculated by establishing the total number of episodes of significant oxygen desaturation (> 4% fall from baseline) per hour of sleep. ODI and mean oxygen saturation were recorded on portable polygraph or overnight oximeter from all 525 subjects. Minimum nocturnal SpO2, total recording time with SpO2 < 90% (TRT90), percentage of time spent in supine vs non-supine, supine AHI, and apnea index were calculated from the portable polygraphy sleep studies on 425 cases.
Obesity was classified according to World Health Organization (WHO) criteria at BMI > 30 kg/m2 into 3 classes: class 1 (30-34.9), class 2 (35-39.9), and class 3 or morbid obesity (≥ 40). Patients with BMI > 30 kg/m2 and daytime hypercapnia (pCO2 > 6 kPa or 45 mm Hg) were categorized as OHS.
Ethical approval for this study was obtained from National Research Ethics Service Committee (Reference: 11/LO/1891).
Data Analysis
Patient's clinical details, physiological measurements, and sleep study findings were entered into Stata 10 software for statistical analysis. Demographic, anthropometric, pulmonary function tests, arterial blood gases, and sleep study data of all obese patients were analyzed. The prevalence of OHS was determined by establishing proportion of patients meeting known criteria for the diagnosis of OHS. First, group comparison analysis was performed between normocapnic versus hypercapnic obese patients using Student t-test, χ2, or Mann-Whitney U test when applicable. Second, univariate analysis between daytime pCO2 and other anthropometric variables (neck size, waist hip ratio, BMI), spirometry values (FEV1, FVC, FEV1/FVC ratio), capillary blood gases (pH, pO2, calculated HCO3) and sleep data (AHI, ODI, mean SpO2, minimum nocturnal SpO2, TRT90, % time in supine position) were calculated using Pearson correlation or Kendall tau test when applicable. Finally, multiple regression analysis was completed taking into account pCO2 as dependent variable and the other parameters who correlated with pCO2 after univariate analysis as independent variables. Statistical significance was set at p < 0.05 (two-tailed).
RESULTS
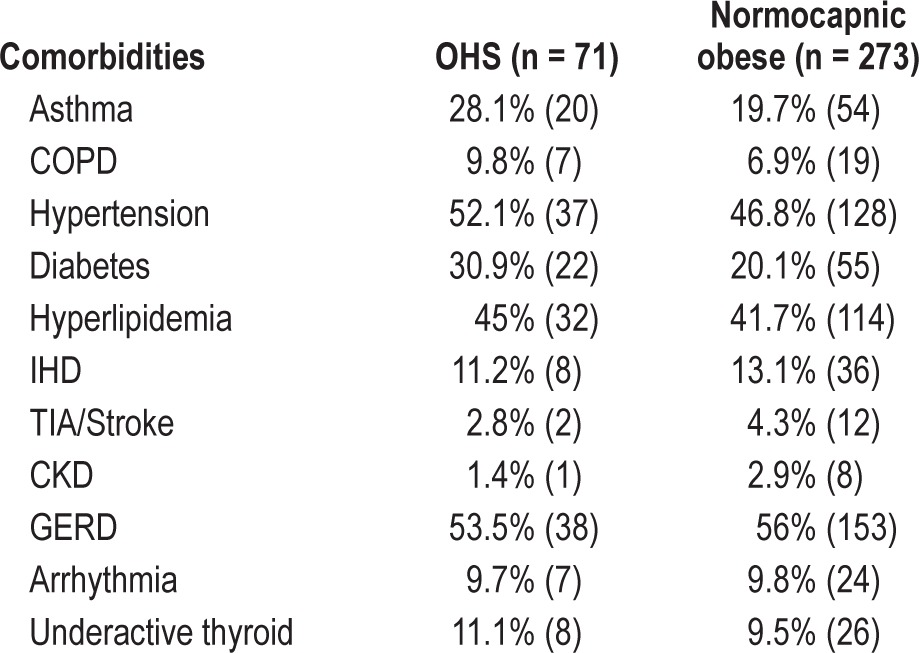
Of 525 consecutive patients included in the retrospective analysis, mean age ± SD was 51.4 ± 12.7 years, and mean BMI 34.5 ± 8.1 kg/m2; 345 were males (65.7%). A total of 344 patients (65.5%) were obese, of which 128 (37.2%) were morbidly obese with a BMI > 40 kg/m2 as presented in Figure 1. Of 525 subjects, 122 (23.2%) were current smokers, 148 (28.2%) ex-smokers and 255 (48.6%) nonsmokers. Prevalence of airflow obstruction was 9.7% (51/525), defined by FEV1/FVC ratio < 70%. Diabetes prevalence was 17.7% (93/525) in our study population and 60.3% (317/525) had a family history of snoring or sleep apnea. Cardiovascular risk factors such as hypertension 39.81% (209/525) or hypercholesterolemia 36.9% (194/525) were common. Obstructive sleep apnea prevalence among obese patients was 79.9% (275/344 patients with AHI > 5 and symptoms), with mean AHI of 32.5 ± 23.9 and ESS 11.8 ± 5.7. OHS prevalence among OSAHS patients was 61/275 (22.1%). Patients with comorbidities that may cause daytime hypercapnia such as severe COPD (FEV1/FVC ratio < 50%; 4 patients) were excluded from the OHS group. Respiratory (asthma, COPD), metabolic (diabetes, hyperlipidemia) and thyroid comorbidities were more common in OHS group when compared to normocapnic obese patients as described in Table 1.
Figure 1. Study population flow diagram.
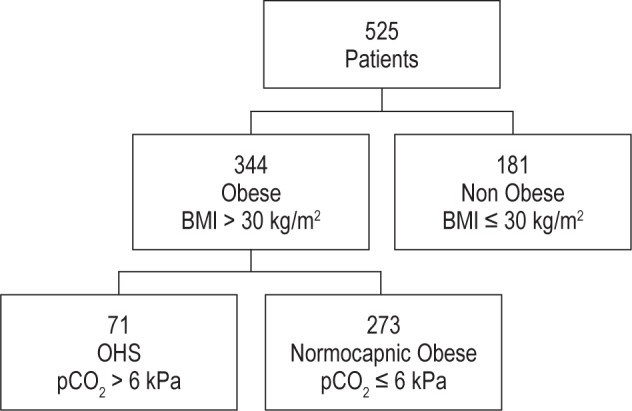
Table 1.
Patients comorbidities in OHS versus normocapnic obese group
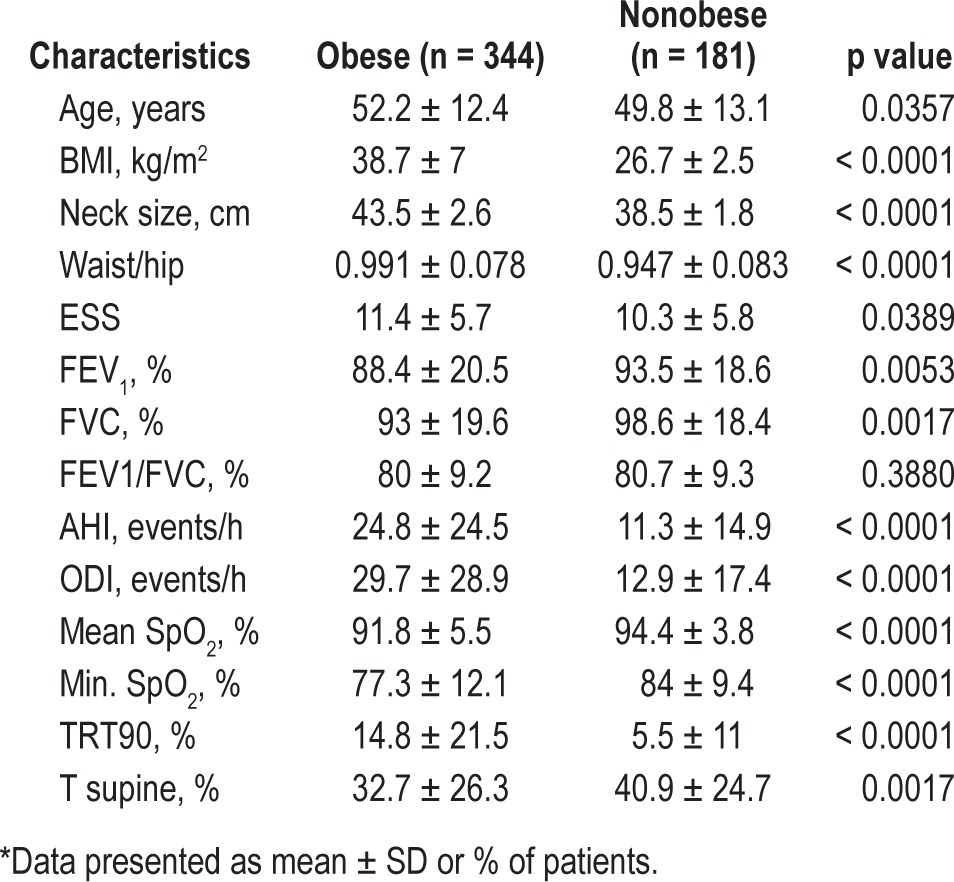
A total of 71/344 patients (20.6%) fulfilled the OHS criteria of concurrent obesity (BMI > 30 kg/m2) and daytime hypercapnia (pCO2 > 6kPa or 45 mm Hg). Table 2 summarizes differences between obese and nonobese patients. Obese patients were older, with increased anthropometric measurements (neck, waist/hip ratio) and lower spirometry values (FEV1, FVC). Obese patients had significantly higher sleep variables (AHI, ODI), lower oxygen saturation during sleep (mean SpO2, minimum nocturnal SpO2), spending more time with SpO2 below 90%, and less time in supine position.
Table 2.
Clinical characteristics of obese versus non obese patients*
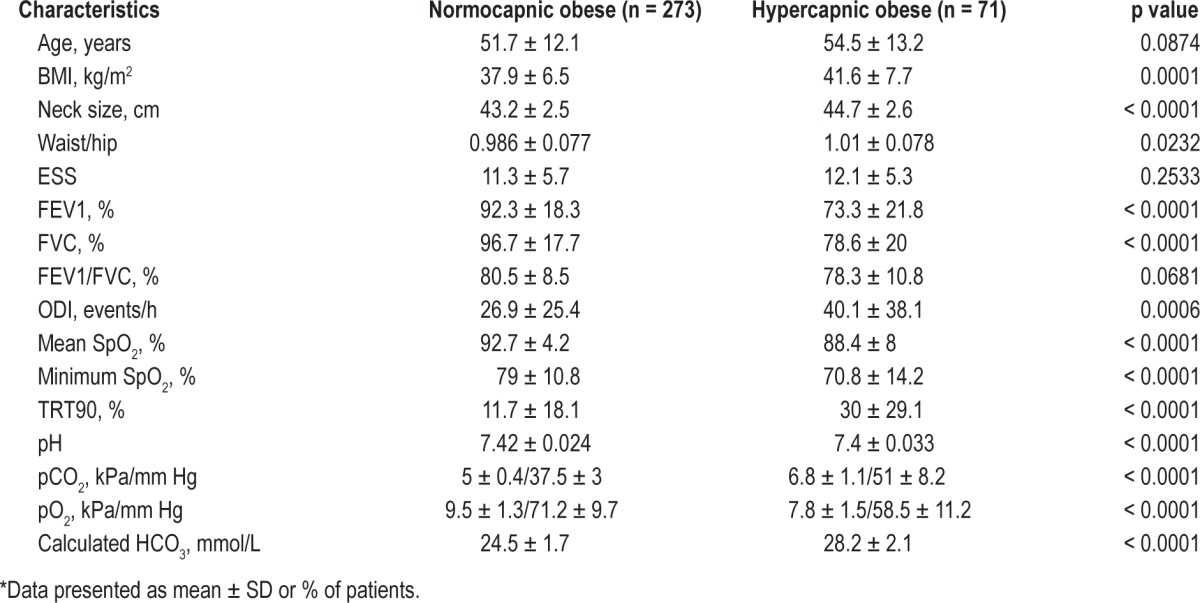
A comparison between obesity hypoventilation patients and normocapnic obese is described in Table 3. Of 344 obese patients, 220 (63.9%) were males. Mean age for obese group ± SD was 52.2 ± 12.4 years. Ethnic distribution showed 257 (74.7%) white, 61 (17.7%) black, 19 (5.5%) Asian, and other 7 (2%). OSAHS (AHI > 5 and symptomatic) was more common in hypercapnic obese patients 85.9 % (61/71) versus normocapnic obese 80.2% (219/273).
Table 3.
Clinical characteristics of normocapnic obese patients versus OHS patients*
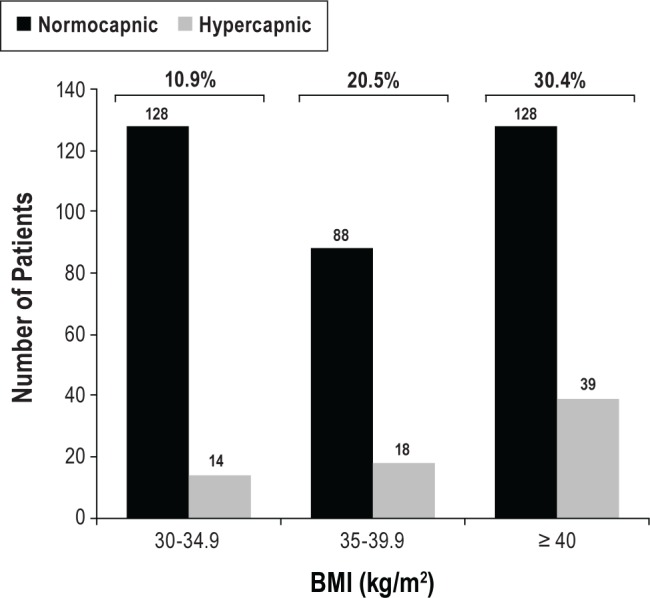
Prevalence of daytime hypercapnia increased with BMI as shown in Figure 2; 10.9% (14/128) in class 1 obesity, 20.5% (18/88) in class 2 obesity, and 30.4% (39/128) in morbidly obese patients.
Figure 2. OHS distribution in obese patients.
Hypercapnic patients were older, heavier, had lower spirometry values, and experienced more sleep events than normocapnic obese patients. Anthropometric measurements (BMI, neck size, waist hip ratio), pulmonary function volumes (FEV1, FVC), sleep data (ODI, mean SpO2, minimum nocturnal SpO2, TRT90), and CBG data (pH, pO2, pCO2, calculated HCO3) were statistically significant between the 2 groups.
Univariate analysis using Pearson correlation or Kendall tau test showed a significant relationship between pCO2 and BMI (r = 0.187; p = 0.0223), FEV1 (r = -0.271; p = 0.0218), FVC (r = -0.253, p = 0.0328), AHI (r = 0.271; p = 0.0083), mean SpO2 (r = -0.214; p = 0.0091), minimum nocturnal SpO2 (r = -0.248; p = 0.0026), time spent with SpO2 below 90% (TRT90, r = 0.328; p = 0.0014), pO2 (r = -0.389; p = 0.0008), and calculated HCO3 (r = 0.306; p = 0.0093).
The relationship between pCO2 and several OHS predictors was estimated using multiple regression allowing for BMI, FEV1, FVC, AHI, mean SpO2, minimum nocturnal SpO2, TRT90, pO2, and calculated HCO3. This model was overall significant (p = 0.0005), and variables taken into account were responsible for 52.2% of pCO2 variance. Following stepwise multiple regression, pO2, and calculated HCO3 were found to be independent predictors of OHS, explaining 27.7% of pCO2 variance (p < 0.0001).
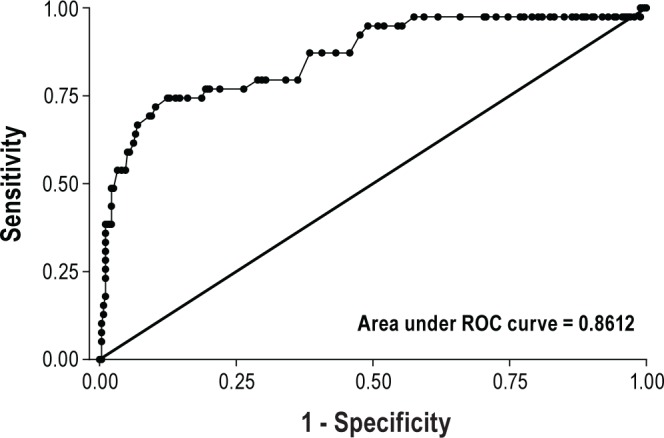
Logistic regression analysis was used to determine various serum bicarbonate thresholds and their statistical value in detecting daytime hypercapnia as presented in Figures 3 and 4.
Figure 3. Bicarbonate performed well as a diagnostic test, with a curve above 45°, rising steeply close to y-axis then flattening out, with the best cut-off giving high sensitivity and specificity.
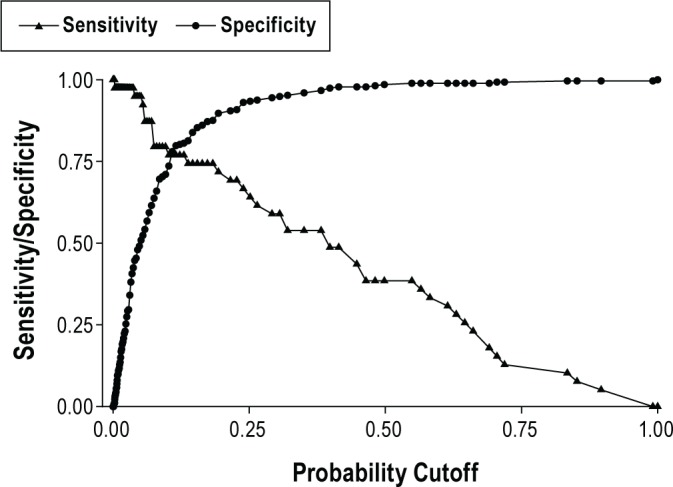
Figure 4. Receiver operating characteristic (ROC) curve analysis for serum bicarbonate levels was performed using logistic regression.
A calculated bicarbonate threshold of 27 mmol/L was the most effective in detecting obesity hypoventilation syndrome, with 85.7% sensitivity and 89.5% specificity as described in Table 4.
Table 4.
Calculated bicarbonate from the CBG cutoff values
DISCUSSION
We found a high prevalence (20.6%) of daytime hypercapnia (OHS) in obese patients attending our sleep clinic. The prevalence increased with an increase in obesity, from 10.9% in mild obesity to 30.4% in morbid obesity. The high prevalence in OHS obese patients in our study was mainly due to high prevalence of obstructive sleep apnea in our obese and hypercapnic obese patients—80% and 86%, respectively. Therefore overall prevalence of OHS in obese sleep apnea patients (22.1%) was similar to prevalence in obese patients (20.6%). Although 51.4% of our patients (obese and non-obese) were either current smokers or ex-smokers, only 9.7% had evidence of airflow obstruction. Patients with moderate to severe airflow obstruction (FEV1/FVC ratio < 50%) as well other causes of daytime hypercapnia were excluded. This study was performed at an altitude of 57 ft, which is just above sea level. Therefore it is unlikely that respiratory alkalosis secondary to altitude hypoxia could lead to a compensatory bicarbonate production.
The exact prevalence of OHS among the general population is unknown; however, the estimated prevalence among the US obese population is thought to be 0.3%.7,8 At the other extreme, the prevalence of OHS among hospitalized adult patients with a BMI > 35 kg/m2 is high, at 31%.9 Several studies have found that as BMI increased, the OHS prevalence increased significantly up to 48% when the BMI was > 50.5–7,10,16,17,19 In the Asian population, OHS is associated with OSAHS at a lower BMI, mainly due to cephalometric differences, such as narrowing of the oropharynx and inferior displacement of the hyoid bone.24–26 In this study, BMI and several other anthropometric measurements (waist/hip ratio, neck size) registered significantly higher values in OHS group versus normocapnic obese patients. We found a prevalence of OHS among obese patients of 20.6%, increasing to 30.4% in those who were morbidly obese. The association between daytime hypercapnia and obesity can be explained by a higher incidence of severe OSAHS among morbidly obese patients.
Most previous studies have examined the prevalence of OHS in patients attending sleep clinic or patients with a confirmed diagnosis of OSAHS. The prevalence among sleep clinic patients has varied from 10% to 20% in different studies, and much higher in patients with confirmed obstructive sleep apnea from 20% to 30%.5,6,17,21,24 A relatively low prevalence of 9% was seen among Japanese OSAHS patients with a mean age of 48 years and BMI of 29.24 Other studies with more selective inclusion criteria such as patients from one gender or of a distinct ethnic group reported higher prevalence up to 38%.16,18 A high prevalence of OHS in some previous studies could also be due to the inclusion of patients with significant airflow limitation. In this study, the prevalence of OHS among patients with OSAHS was 22.1%. This relatively high prevalence of OHS despite a relatively low average BMI of 34.5 kg/m2 compared to other studies may be explained by a high overall prevalence of OSAHS (80%).
A practical tool used for the screening of OHS when interpreting sleep studies, seems to be the amount of time spent with SpO2 < 90%.27 A recent meta-analysis study showed significantly increased time spent with SpO2 < 90% in OHS versus normocapnic OSAHS patients (56% vs 19%).7 Other authors have found this sleep variable to be an independent predictor for OHS, accounting for 24% variance of pCO2.21 In our study, TRT90 was significantly increased in the OHS group—30% compared to 11.7% in normocapnic obese patients. Sleep data variables (mean SpO2, minimum nocturnal SpO2, and TRT90) showed consistent correlation between severity of nocturnal hypoxia and daytime hypercapnia. In our study, AHI correlated with pCO2 after univariate analysis, and unlike other studies was not found to be an independent predictor for OHS.10,19–21
Although gold standard for diagnosis of OHS remains raised arterial pCO2, an increase in the bicarbonate level due to metabolic compensation of respiratory acidosis has been found to be a sensitive screening tool for daytime hypercapnia.7,27
In our study, bicarbonate level was calculated from the CBG data using arterial pH and pCO2 partial pressure in Henderson-Hasselbalch formula. Previous comparison between measured versus calculated bicarbonate levels in 17,621 samples, has found an R2 value of 0.93, indicating a close relationship between serum and calculated HCO3 values.28
In our study using logistic regression with various thresholds for HCO3, a cutoff value of 27 mmol/L had the highest sensitivity (85.7%) and specificity (89.5%) in detecting OHS.
In a previous study using serum bicarbonate sampling, Mokhlesi et al. found after ROC curve analysis an identical HCO3 threshold of 27 mmol/L to have a sensitivity of 92% and a specificity of 50% in identifying OHS. The authors have suggested using serum bicarbonate in conjunction with other markers of OSAHS severity such as AHI when screening for OHS.27 That study revealed a prevalence of OHS of 20% in sleep apnea patients with a mean BMI of 43 and a mean AHI of 62.27 In our study, we found a similar prevalence of OHS in sleep apnea patients of 22.1%, despite a lower severity of sleep apnea with a mean BMI of 34.5 and a mean AHI of 32.5.
In terms of study limitations, we acknowledge that calculated HCO3 from the CBG using Henderson-Hasselbalch formula is not identical with serum bicarbonate measurements, and a 1 mmol/L variation of in HCO3 can have a significant impact on sensitivity and specificity when screening for OHS. Therefore we recommend serum bicarbonate sampling in conjunction with other clinical markers of nocturnal hypoxia.
Previous studies have confirmed high levels of agreement between serum bicarbonate measurements from venous versus arterial samples, suggesting that venous samples can be an acceptable substitute for arterial measurement in clinical setting.22 However, development of commercially available bicarbonate finger strips may further help with noninvasive screening for daytime hypercapnia, allowing an earlier diagnosis of OHS, especially in primary practice.
CONCLUSION
Early identification of daytime hypercapnia using simple clinical predictors can avoid invasive arterial blood gas sampling and reduce morbidity and mortality associated with OHS. A normal HCO3 value may exclude daytime hypercapnia, while an elevated bicarbonate and/or nocturnal hypoxia should guide clinicians to look for OHS.
The outcome of this study has changed our clinical practice so that we routinely measure serum bicarbonate level on all obese patients followed by the measurement of capillary blood gas pCO2 in patients with raised HCO3 > 27 mmol/L.
We conclude that routine measurement of serum bicarbonate in obese patients can be a useful screening tool for early diagnosis of OHS and/or sleep disordered breathing.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Jennifer Quinn, Epidemiologist Consultant at London School of Hygiene and Tropical Medicine for useful suggestions. Research work was performed at Sleep and Ventilation Unit, Department of Respiratory Medicine, North Middlesex University Hospital, London, UK.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- BTS
British Thoracic Society
- CBG
capillary blood gas
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- ESS
Epworth Sleepiness Scale
- FEV1
forced expiratory volume in 1st second
- FVC
forced vital capacity
- HCO3
serum/arterial bicarbonate
- GERD
gastroesophageal reflux disease
- IHD
ischemic heart disease
- ODI
oxygen desaturation index
- OHS
obesity hypoventilation syndrome
- OSAHS
obstructive sleep apnea/hypopnea syndrome
- RIP
respiratory inductance plethysmography
- SpO2
pulse oximeter oxygen saturation
- SD
standard deviation
- TIA
transient ischemic attack
- TRT90
total recording time with SpO2 below 90%
- UK
United Kingdom
- US
United States
- WHO
World Health Organization
REFERENCES
- 1.McPherson K, Marsh T, Brown M. London: Government Office for Sciences; 2007. Foresight: Tackling obesities: Future choices - modelling future trends in obesity and their impact on health. Available from: www.foresight.gov.uk/Obesity/14.pdf. [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Burwell CS, Robin ED, Whaley RD, Bickelmann AG. Extreme obesity associated with alveolar hypoventilation: a Pickwickian Syndrome. Am J Med. 1956;21:811–8. doi: 10.1016/0002-9343(56)90094-8. [DOI] [PubMed] [Google Scholar]
- 4.Al Dabal L, BaHammam AS. Obesity hypoventilation syndrome. Ann Thorac Med. 2009;4:41–9. doi: 10.4103/1817-1737.49411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler R, Chaouat A, Schinkewitch P, et al. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest. 2001;120:369–76. doi: 10.1378/chest.120.2.369. [DOI] [PubMed] [Google Scholar]
- 6.Laaban J-P, Chailleux E Observatory Group of ANTADIR. Daytime hypercapnia in adult patients with obstructive sleep apnea syndrome in France, before initiating nocturnal nasal continuous positive airway pressure therapy. Chest. 2005;127:710–5. doi: 10.1378/chest.127.3.710. [DOI] [PubMed] [Google Scholar]
- 7.Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans A. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11:117–24. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 8.Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and meta analysis of cohort studies. Chest. 2009;136:787–96. doi: 10.1378/chest.09-0615. [DOI] [PubMed] [Google Scholar]
- 9.Mokhlesi B, Saager L, Kaw RQ. Should we routinely screen for hypercapnia in sleep apnea patients before elective non cardiac surgery? Cleve Clin J Med. 2010;77:60–1. doi: 10.3949/ccjm.77a.09105. [DOI] [PubMed] [Google Scholar]
- 10.Quint JK, Ward L, Davison AG. Previously undiagnosed obesity hypoventilation syndrome. Thorax. 2007;62:462–3. doi: 10.1136/thx.2006.075945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowbar S, Burkhart KM, Gonzales R, et al. Obesity associated hypoventilation in hospitalised patients: prevalence, impact and outcome. Am J Med. 2004;116:1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Macavei V, Spurling KJ, Makker H. Validation of raised serum bicarbonate for diagnosis of Obesity Hypoventilation Syndrome. Eur Respir J. 2012;40(Suppl. 56):569s. [Google Scholar]
- 13.Liddicoat H, Zammitt C, Loft J, Moonsie I, Makker H. The effect of body mass index on outcomes of acute non-invasive ventilation (NIV) in a district general hospital. Thorax. 2010;65:A143. [Google Scholar]
- 14.Guideline for emergency oxygen use in adult patients. Thorax. 2008;63(Suppl VI):vi1–vi68. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault J-C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J. 1993;6(Suppl. 16):5–40. [PubMed] [Google Scholar]
- 16.Akashiba T, Kawahara S, Kosaka N, Ito D. Determinants of chronic hypercapnia in Japanese men with obstructive sleep apnea syndrome. Chest. 2002;121:415–21. doi: 10.1378/chest.121.2.415. [DOI] [PubMed] [Google Scholar]
- 17.Golpe R, Jimenez A, Carpizo R. Diurnal hypercapnia in patients with obstructive sleep apnoea syndrome. Chest. 2002;122:1100–1. doi: 10.1378/chest.122.3.1100. [DOI] [PubMed] [Google Scholar]
- 18.Leech JA, Onal E, Baer P, Lopata M. Determinants of hypercapnia in occlusive sleep apnea syndrome. Chest. 1987;92:807–13. doi: 10.1378/chest.92.5.807. [DOI] [PubMed] [Google Scholar]
- 19.Kawata N, Tatsumi K, Terada J, et al. Daytime hypercapnia in obstructive sleep apnoea syndrome. Chest. 2007;132:1832–8. doi: 10.1378/chest.07-0673. [DOI] [PubMed] [Google Scholar]
- 20.Dharmagunawardena R, Zammit C, Makker H. Prevalence and predictors of obesity hypoventilation syndrome. Int J Respir Care. 2011:19–22. [Google Scholar]
- 21.Resta O, Foschino-Barbaro MP, Bonfitto P, et al. Prevalence and mechanisms of diurnal hypercapnia in a sample of morbidly obese subjects with obstructive sleep apnoea. Respir Med. 2000;94:240–6. doi: 10.1053/rmed.1999.0732. [DOI] [PubMed] [Google Scholar]
- 22.Herrington WG, Nye HJ, Hammersley MS, Watkinson PJ. Are arterial and venous samples clinically equivalent for the estimation of pH, serum bicarbonate and potassium concentration in critically ill patients? Diabet Med. 2012;29:32–5. doi: 10.1111/j.1464-5491.2011.03390.x. [DOI] [PubMed] [Google Scholar]
- 23.Verin E, Tardif C, Pasquis P. Prevalence of daytime hypercapnia or hypoxia in patients with OSAS and normal lung function. Respir Med. 2001;95:693–6. doi: 10.1053/rmed.2001.1120. [DOI] [PubMed] [Google Scholar]
- 24.Akashiba T, Akahoshi T, Kawahara S, et al. Clinical characteristics of obesity-hypoventilation syndrome in Japan: a multi-center study. Intern Med. 2006;45:1121–5. doi: 10.2169/internalmedicine.45.1747. [DOI] [PubMed] [Google Scholar]
- 25.Sakakibara H, Tong M, Matsushita K, Hirata M, Konishi Y, Suetsugu S. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J. 1999;13:403–10. doi: 10.1183/09031936.99.13240399. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Fujimoto K, Urushibata K, Matsuzawa Y, Kubo K. Cephalometric analysis in obese and nonobese patients with obstructive sleep apnea syndrome. Chest. 2003;124:212–8. doi: 10.1378/chest.124.1.212. [DOI] [PubMed] [Google Scholar]
- 27.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55, 10:1347–62. [PubMed] [Google Scholar]
- 28.Kumar V, Karon BS. Comparison of measured and calculated bicarbonate values. Clin Chem. 2008;54:1586–7. doi: 10.1373/clinchem.2008.107441. [DOI] [PubMed] [Google Scholar]


