Abstract
Study Objectives:
Characterize disrupted nighttime sleep (DNS) in narcolepsy, an important symptom of narcolepsy.
Methods:
A panel of international narcolepsy experts was convened in 2011 to build a consensus characterization of DNS in patients with narcolepsy. A literature search of the Medline (1965 to date), Medline In-Process (latest weeks), Embase (1974 to date), Embase Alert (latest 8 weeks), and Biosis (1965 to date) databases was conducted using the following search terms: narcolepsy and disrupted nighttime sleep, disturbed nighttime sleep, fragmented sleep, consolidated sleep, sleep disruption, and narcolepsy questionnaire. The purpose of the literature search was to identify publications characterizing the nighttime sleep of patients with narcolepsy. The panel reviewed the literature. Nocturnal sleep can also be disturbed by REM sleep abnormalities such as vivid dreaming and REM sleep behavior disorder; however, these were not reviewed in the current paper, as we were evaluating for idiopathic sleep disturbances.
Results:
The literature reviewed provide a consistent characterization of nighttime sleep in patients with narcolepsy as fragmented, with reports of frequent, brief nightly awakenings with difficulties returning to sleep and associated reports of poor sleep quality. Polysomnographic studies consistently report frequent awakenings/arousals after sleep onset, more stage 1 (S1) sleep, and more frequent shifts to S1 sleep or wake from deeper stages of sleep. The consensus of the International Experts' Panel on Narcolepsy was that DNS can be distressing for patients with narcolepsy and that treatment of DNS warrants consideration.
Conclusions:
Clinicians involved in the management of patients with narcolepsy should investigate patients' quality of nighttime sleep, give weight and consideration to patient reports of nighttime sleep experience, and consider DNS a target for treatment.
Citation:
Roth T; Dauvilliers Y; Mignot E; Montplaisir J; Paul J; Swick T; Zee P. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med 2013;9(9):955-965.
Keywords: Narcolepsy, disrupted nighttime sleep, sleep fragmentation, fragmented nighttime sleep, consolidated sleep
Narcolepsy is a chronic neurologic disorder defined by a tetrad of symptoms: excessive daytime sleepiness (EDS), cataplexy, hypnagogic hallucinations, and sleep paralysis.1 It affects approximately 0.05% of the general population, with variation according to geography and ethnicity.2 Narcolepsy is associated with substantial morbidity and an impaired health-related quality of life (QOL).3–7
EDS is the most common presenting symptom and is necessary for a diagnosis (Table 1),8 although EDS is not specific to narcolepsy.9–11 Cataplexy is the most specific symptom of narcolepsy. It is defined as a sudden, transient partial or total loss of muscle tone triggered by strong emotions, most typically laughing or joking. Although specific, it is not universal, occurring in approximately 75% of patients with narcolepsy as defined by the ICSD-2.2,10,11 Estimates of the prevalence of hypnagogic hallucinations range from one-third to 80% of patients, whereas sleep paralysis affects 25% to 50% of patients.2 Only a minority of patients (10% to 25%) suffer from the complete tetrad of symptoms.2,12
Table 1.
ICSD-2 criteria for the diagnosis of narcolepsy with and without cataplexy.8

Among patients with narcolepsy, disrupted nighttime sleep (DNS) is a common complaint and frequent finding on polysomnographic (PSG) testing, and it is referred to in the contemporary literature as a disease-related symptom. Patients typically report that DNS is more of a problem than are sleep paralysis and hypnagogic hallucinations, and it appears to be more common, with prevalence estimates ranging from approximately 30% to 95%, depending on the definition and sleep assay used.13–15 Thus, DNS may represent an additional characteristic of narcolepsy to be considered as part of a pentad of symptoms rather than EDS and the triad of auxiliary symptoms.
Despite the widespread reference to DNS in narcolepsy, there are few systematic patient-report and PSG data describing specific patterns of DNS or its clinical or QOL impact. Finally, there is no consensus characterization of DNS in narcolepsy that distinguishes it from disturbed sleep in other sleep disorder populations, especially primary insomnia, or that differentiates DNS associated with other sleep disorders commonly seen in patients with narcolepsy (e.g., vivid dreaming, periodic leg movements, rapid eye movement [REM] behavior disorder, and sleep apnea) from DNS as a stand-alone symptom. This is an important distinction: DNS may be secondary to a comorbid sleep disorder including insomnia or sleep apnea, or it may affect patients with narcolepsy independently from such conditions. Many patients with narcolepsy appear to experience DNS as a stand-alone and specific symptom that is not associated with other sleep disorders.1,15–18
To better characterize the most commonly patient-reported and PSG-determined disruptions to nighttime sleep and to distinguish DNS as a consistent and frequently observed feature of narcolepsy—one that is distinct from insomnia and other sleep-related comorbidities—as well as a possible target for treatment, this article reviews the available literature on DNS in narcolepsy. In this review, we focused on sleep disruptions and PSG findings, not ancillary symptoms.
Another approach to characterizing DNS in narcolepsy is to build a consensus among experts that incorporates both clinical experience as well as the published literature. Toward this end, the International Experts' Panel on Narcolepsy was held on June 9-10, 2011, in Minneapolis, Minnesota, to review and discuss the literature on DNS in patients with narcolepsy and their own extensive clinical expertise to clarify the characterization of sleep disturbance in narcolepsy.
The meeting was sponsored by Jazz Pharmaceuticals, Inc., Palo Alto, California. Participants attending the International Experts' Panel on Narcolepsy are listed in the appendix, and portions of the panel's proceedings have been incorporated into this characterization of DNS.
METHODS
We reviewed and included studies that provided objectively and subjectively collected data on nighttime sleep in patients with narcolepsy. These studies were of variable size, design, and scientific rigor, but each provided data on characteristics of night sleep in patients with narcolepsy such as the number and frequency of PSG-recorded and patient-reported arousals and awakenings, the duration of sleep stages and wake time after sleep onset (WASO), patient-reported sleep quality, and other parameters. The literature included comparisons between patients with narcolepsy and normal controls, patients with and without sleep-related comorbidities, and patients receiving and not receiving narcolepsy-specific medications. Overall, the patient report and PSG studies indicate a nighttime sleep pattern characterized by frequent arousals and brief awakenings that occur independent of other sleep disorders and treatments.
Literature Search Methodology
A search of the Medline (1965 to date), Medline In-Process (latest weeks), Embase (1974 to date), Embase Alert (latest 8 weeks), and Biosis (1965 to date) databases was conducted using the following search terms: narcolepsy and disrupted nighttime sleep, disturbed nighttime sleep, fragmented sleep, consolidated sleep, sleep disruption, and narcolepsy questionnaire. There were no date limits on the search.
The purpose of the literature search was to identify publications characterizing the nighttime sleep of patients with narcolepsy. Search results were reviewed by the lead author, with other authors consulted as needed to provide consensus on questions or issues arising during the review process. Studies providing specific PSG and/or patient-reported characterizations of nighttime sleep were selected for inclusion in the current article. Review articles and articles describing sleep-related comorbidities, such as periodic leg movements, obstructive sleep apnea, REM behavior disorder, and nightmares, were excluded so as to be able to identify the nature of disturbed sleep in narcolepsy per se without the contamination of other sleep disorders.
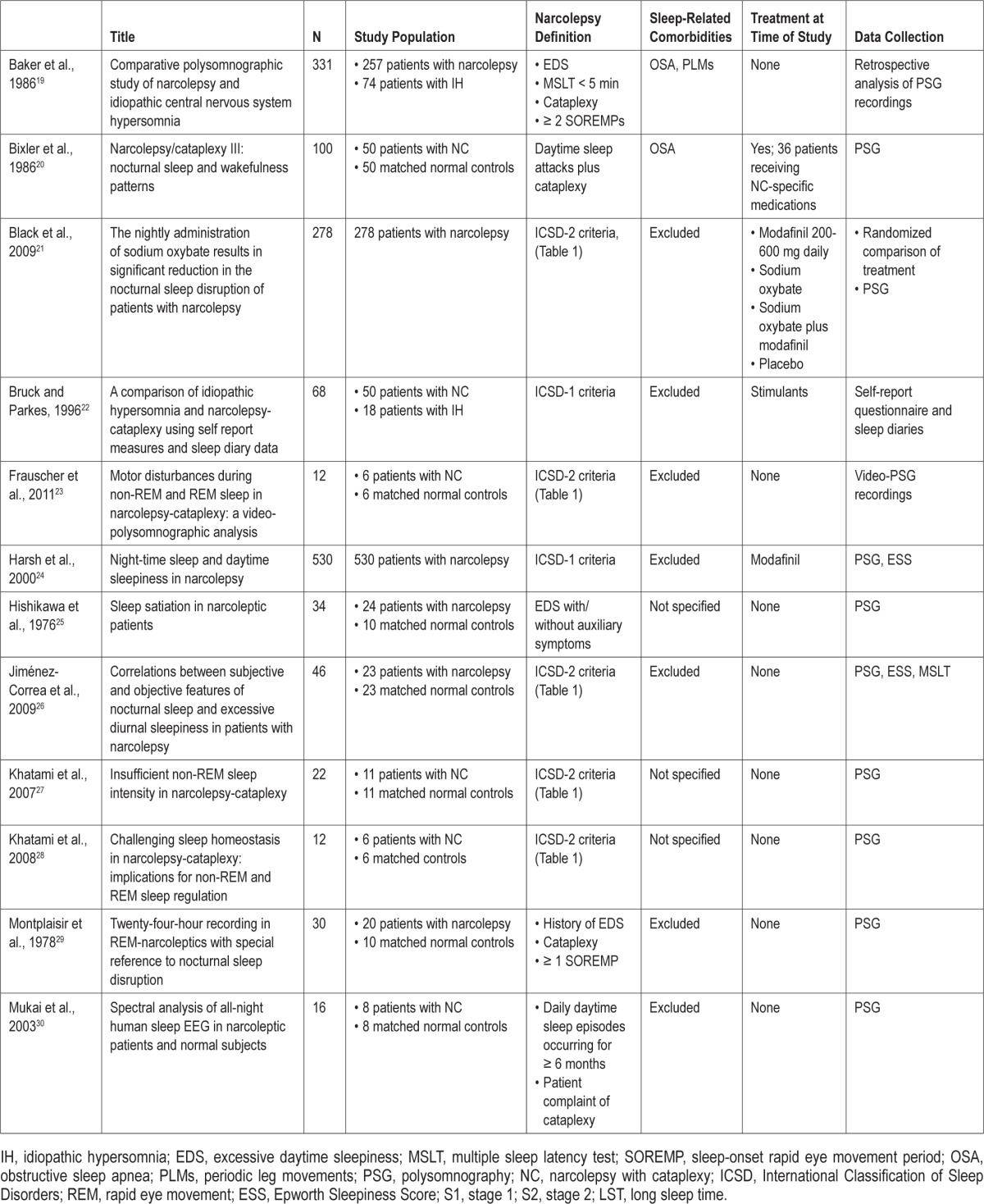
A total of 642 English-language citations were returned and reviewed for characterizations of nighttime sleep in patients with narcolepsy. When inclusion criteria could not be identified through an abstract, full-text articles were reviewed. As shown in the flow of citations through the review process (Figure 1), of the 642 citations, 20 relevant studies were identified: 12 reported PSG characteristics of nighttime sleep in patients with narcolepsy, 4 reported patient-reported outcomes, and 4 reported both PSG and patient-reported characterizations of sleeping patterns. Baseline data from a randomized clinical trial evaluating the effect of sodium oxybate on nighttime sleep in patients with narcolepsy was also included (another trial was excluded because it did not provide baseline PSG data on patients' nocturnal sleep). Thus, a total of 21 studies met the inclusion criteria (Table 2).14,19–38
Figure 1. Flow of citations during the review process.
Table 2.
Publications characterizing the nighttime sleep of patients with narcolepsy
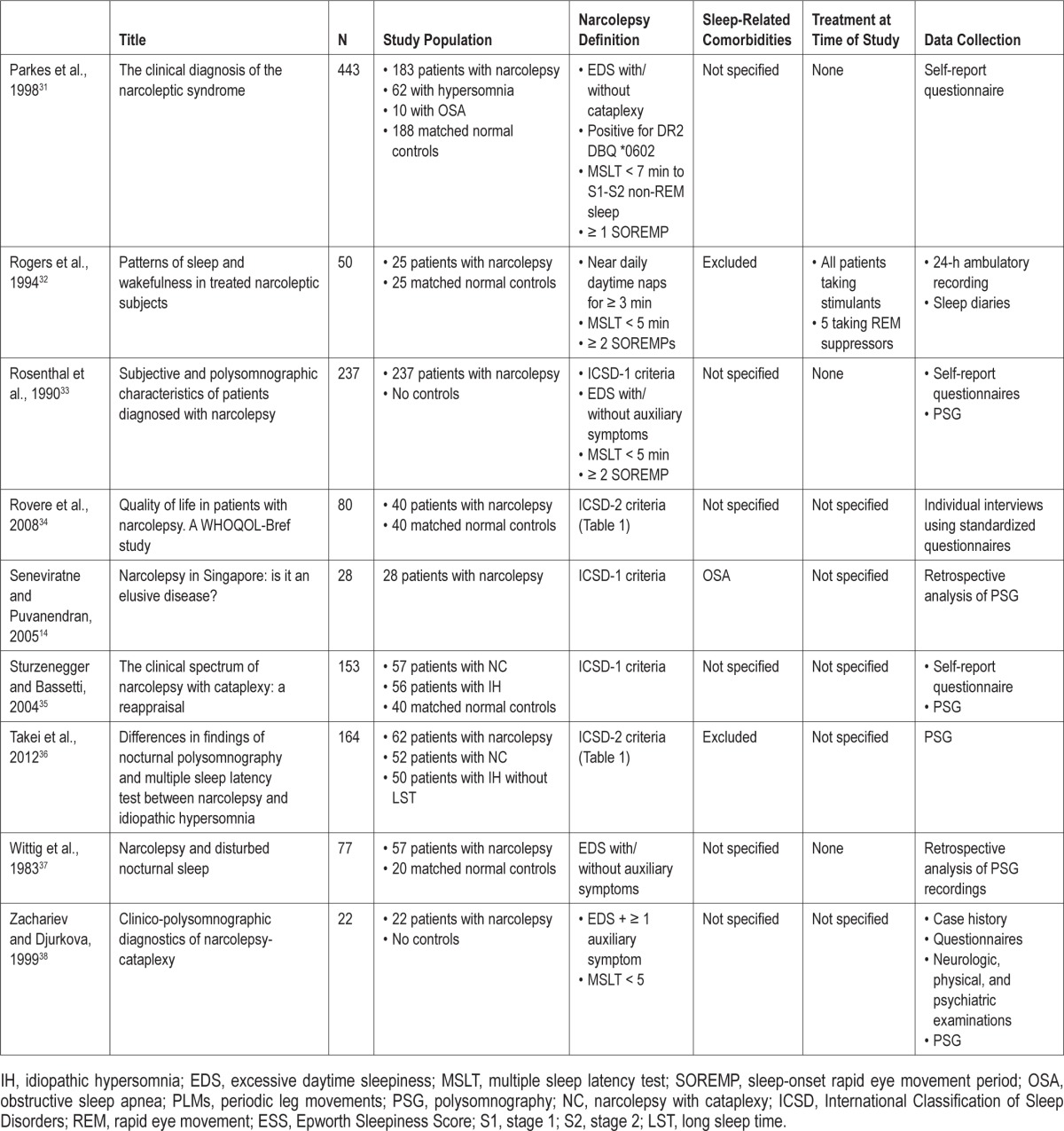
RESULTS
DNS in Narcolepsy
Nighttime Sleep in Narcolepsy
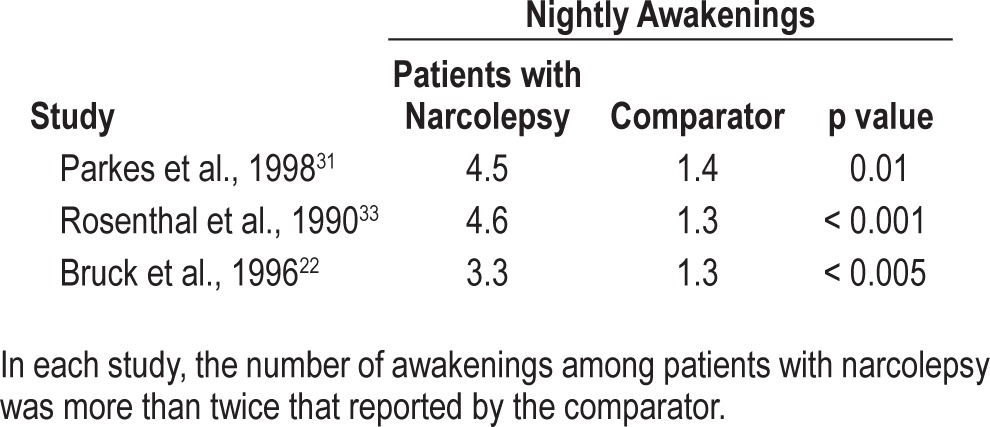
Nighttime sleep in patients with narcolepsy is characterized by a short sleep latency and a sleep-onset REM period in approximately 50% of cases.10,13,39 Aside from the short sleep latency, it is also characterized by an inability to stay asleep.13,22,23,26,31,33,35 Patients with narcolepsy experience frequent awakenings during sleep. The database characterizing such sleep patterns is limited but consistent, with patients reporting frequent awakenings after sleep onset and poor sleep quality,22,31,33–35,38 although only 3 studies provided a quantitative comparison of nocturnal awakenings (Table 3).22,31,33
Table 3.
The number of nightly awakenings reported by patients with narcolepsy and by comparator groups
Patient-Reported DNS
The literature search identified 4 studies that reported on DNS from the patient perspective and 4 studies that included the patient perspective in addition to PSG. Patient reports and sleep diaries from these studies indicate that between 33% and 81% of patients with narcolepsy awaken multiple times throughout the night.23,38 Zachariev and colleagues reported that 16 of 22 (72.7%) consecutive patients with narcolepsy reported DNS, and 36.4% of these patients described the disturbances as frequent awakenings.38 Rosenthal and colleagues, using a 22-item questionnaire, surveyed 237 consecutive adult patients diagnosed with narcolepsy and found that 81.1% of the 119 responders experienced nocturnal sleep disturbances, also characterized as frequent brief awakenings.33 The prevalence of DNS was comparable to the prevalence of patient-reported cataplexy (79.3%), but significantly higher than the prevalence of hypnagogic hallucinations, sleep paralysis, and memory problems (67%, 64.2%, and 46.3%, respectively; p < 0.001 vs. DNS).
In the Rosenthal study, patients with narcolepsy and disturbed sleep had significantly less total sleep time (6 vs. 7 h, respectively; p < 0.001) and reported more frequent nocturnal awakenings (4.6 vs. 1.3 per night, respectively; p < 0.001) than did patients who reported no trouble sleeping. PSG data, recorded 7 days after discontinuation of stimulant medications, confirmed these patient reports. Patients were stratified according to whether they reported the presence or absence of difficulty maintaining nocturnal sleep. According to the PSG studies, patients who reported suffering DNS experienced 13.0 awakenings per night vs. 10.5 among those who did not self-report DNS. PSG also demonstrated that patients with DNS had significantly more stage 1 (S1) sleep (23.0% vs. 17.4%, respectively; p < 0.05), significantly less stage 2 (S2) sleep (49.1% vs. 53.5%, respectively; p < 0.05), and significantly more wake time during sleep (72.5 vs. 45.4 min, respectively; p < 0.01), all characteristics of DNS independent of other sleep-related comorbidities.33
Parkes et al. reported data on the sleep-wake habits of 183 patients with narcolepsy, 62 patients with idiopathic hypersomnia (IH), and 10 patients with obstructive sleep apnea.31 Responses were compared with 188 controls with normal sleep-wake habits and no complaints of EDS or insomnia. Patients with narcolepsy reported a mean 4.5 nightly awakenings versus 1.4 among controls (p < 0.01) and poor sleep quality, measured using a visual analog insomnia score (43.1 vs. 27.6, respectively; p < 0.01).
Sturzenegger and Bassetti compared 40 normal controls with no history of sleep disorders, EDS, or use of sleeping pills with 57 patients with narcolepsy with cataplexy and 56 patients with “nonnarcoleptic hypersomnia,” defined by the presence of subjective EDS and an Epworth Sleepiness Score (ESS) ≥ 10 and other sleep disorders.35 Almost 95% of patients with narcolepsy reported falling asleep rapidly, with sleep latency < 20 minutes. Importantly, patient-reported inability to sleep without awakening was significantly more common among patients with narcolepsy than among normal controls (71% vs. 25%, p value not reported), as were early morning awakenings (83% vs. 43%, respectively; p < 0.001). Among patients with nonnarcoleptic hypersomnia, frequent nocturnal awakenings and early morning awakenings were reported by 67% and 71%, respectively. Among normal controls, these rates were 25% and 43%, respectively (p < 0.001 vs. normal controls for both comparisons).35
As compared to normal control groups, patients with narcolepsy consistently report disturbed and fragmented nocturnal sleep. This experience is distinct from the nocturnal sleep patterns reported by patients with other sleep disorders. For example, Bruck and Parkes compared the sleep-wake behaviors of 18 patients with IH and 50 patients with narcolepsy with cataplexy using questionnaires and sleep diaries.22 Patients with narcolepsy reported a mean 3.3 awakenings per night, for an average total wake time of 34.4 minutes. By comparison, patients with IH reported only 1.3 nocturnal awakenings (p < 0.005 vs. narcolepsy), with an average total wake time of 7.7 minutes (p < 0.05 vs. narcolepsy).
These patient-report studies present a consistent characterization of fragmented, disrupted, and unsatisfactory sleep in patients with narcolepsy. It is clear that nighttime sleep in patients with narcolepsy has its own patterns distinct from those in normal controls and from those with insomnia. Although DNS is important and may be potentially troublesome for patients, there is a paucity of patient-reported data regarding the consequences of DNS. The fact that narcolepsy is associated with a diminished QOL is well established, but how DNS in patients with narcolepsy affects QOL is not well understood.3,5–7,10 In one study using the World Health Organization QOL (WHOQOLBREF) instrument, Rovere et al. compared QOL among 40 patients with narcolepsy and 40 normal controls with fixed daytime work schedules and no history of chronic diseases or sleep disorders.34 Dissatisfaction with sleep was common among patients with narcolepsy, with 22.5% being very unsatisfied and 45.0% unsatisfied. Among normal controls, the rates were 2.5% and 10.0%, respectively (p < 0.001). The rates of regular, satisfied, and very satisfied sleep among patients with narcolepsy were 17.5%, 15.0%, and 0.0%, respectively. Among normal controls, these were 22.5%, 50.0%, and 15.0%, respectively (p < 0.001 for comparisons). The results of this study do not address the QOL impact or other consequences of DNS in narcolepsy. The study does, however, confirm the PSG and sleep questionnaire data indicating high rates of overall dissatisfaction with sleep among patients with narcolepsy.
PSG Findings of DNS in Narcolepsy
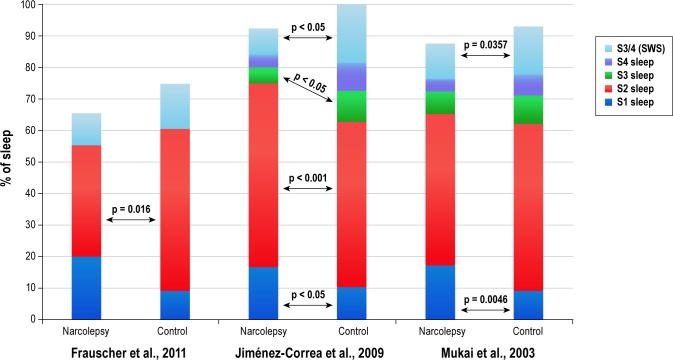
Objective data from the 12 PSG-specific studies,14,19,20,23,25–30,36,37 the 4 with PSG in addition to patient reports,32,33,35,38 and the 1 randomized clinical trial21 confirm the patient-reported data that nighttime sleep in patients with narcolepsy is generally disrupted and fragmented. PSG comparisons between patients with narcolepsy and normal controls with no history of sleep disorders consistently find that patients with narcolepsy experience frequent brief awakenings and arousals (e.g., excessive shifts to S1 sleep or wake from deeper sleep stages), therefore having more time spent in S1 sleep, an elevated WASO, and reduced sleep efficiency relative to normal controls (Table 4 and Figure 2).20,23,24,26–30,32
Table 4.
Polysomnographically identified arousals and wake time after sleep onset (WASO) among untreated patients with narcolepsy and normal controls
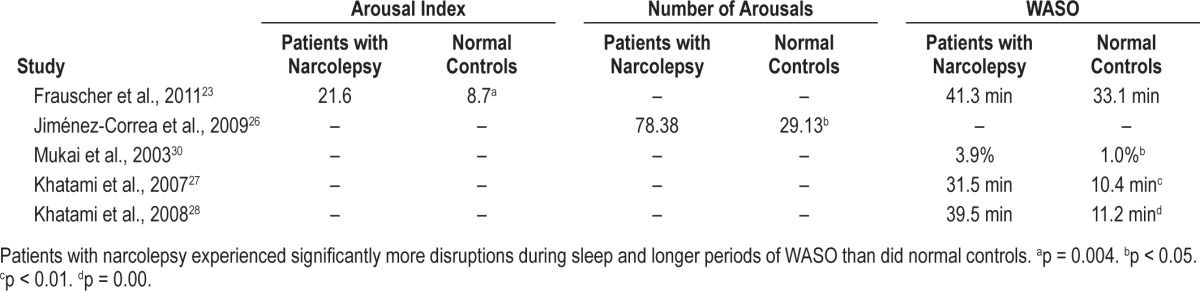
Figure 2. Comparison of the stages of sleep between patients with narcolepsy and normal controls.
S1, stage 1; S2, stage 2; S3, stage 3; S4, stage 4; SWS, slow wave sleep. Frauscher et al.,23 Jiménez-Correa et al.,26 Mukai et al.30
These nighttime sleep patterns are present whether or not patients are receiving stimulant medications,20,22,32 including in drug-naïve patients with narcolepsy,30 albeit in a small sample (N = 8), as well as when compared with other sleep disorders.32,36 These observations provide further support for an association between narcolepsy and DNS that is also independent of stimulant use, and although the studies do not identify underlying causes of DNS, several suggest an abnormality in non-REM (NREM) sleep as a possible mediator. However, no studies have directly compared DNS in patients with narcolepsy who are on and off stimulants. This lack of objective data on the effects of stimulants on DNS suggests the need for studies addressing this issue.
The largest study to characterize PSG-assayed sleep in patients with narcolepsy is a 530-patient database created for a clinical trial of modafinil (US Modafinil in Narcolepsy Multicenter Study Group, 1998).24 All prescription and over-the-counter stimulant- and sedative-containing medications were discontinued 2 weeks prior to patients undergoing 2 consecutive baseline PSG measurements. On both nights, patients displayed evidence of nocturnal sleep disruption, characterized by an arousal index of 12 and an awakening index of 3.8. Analyses of smaller patient groups with narcolepsy in Asia and Eastern Europe have reported similar DNS arousal rates.14,38
Comparisons between patients and normal controls confirm the conclusion that these sleep parameters are deviations from normal sleep. Mukai and colleagues compared PSG data between 8 drug-naïve patients with narcolepsy and 8 healthy normal controls with no history of sleep disorders.30 Patients with narcolepsy had significantly more S1 sleep (17.2% vs. 8.9%, respectively; p < 0.01), more stage shifts (mean, 352.6 vs. 251.8, respectively; p < 0.05), and a higher percentage of WASO (3.9% vs. 1.0%, respectively; p < 0.05) than did normal controls. These investigators suggest that DNS is linked to the higher REM densities seen in patients with narcolepsy.
In several studies, DNS in patients with narcolepsy has been differentially associated with NREM sleep. Frauscher and colleagues, using nocturnal video-PSG studies, compared motor events during REM and NREM sleep in 6 stimulant-free patients with narcolepsy/cataplexy with 6 sex- and age-matched normal controls with no history of sleep disorders, ESS < 10, and no central nervous system-active medication at the time of investigation.23 PSG was performed on 2 consecutive nights and video monitoring for 1 night. All visible movements were defined as a motor event and classified according to topography, number of involved body parts, duration, and its association with arousal.
Patients with narcolepsy with cataplexy had more fragmented sleep with more S1 sleep (20.0 vs. 9.1 min, respectively; p = 0.071) and less S2 sleep (35.5 vs. 51.5 min, respectively; p = 0.022) than did normal controls. The mean motor activity index, defined as movement episodes per hour of sleep excluding electromyographic-related muscle activity in the chin or tibialis anterior channels, was 59.9/h of sleep in patients with narcolepsy with cataplexy versus 15.4/h of sleep among controls (p = 0.004). Motor event indices were similar between REM and NREM sleep periods in the narcolepsy with cataplexy group, whereas motor event indices were higher during REM sleep periods in controls (p = 0.028). Motor events affected significantly more body parts in patients with narcolepsy with cataplexy than controls, and events lasting > 1 second were significantly more common (p = 0.011 and p < 0.001, respectively). The total arousal index among patients and controls was 21.6 versus 8.7, respectively (p = 0.004), and the motor activity-related index was 17.6/h of sleep versus 5.9/h, respectively (p = 0.002). The investigators hypothesized that motor disturbances during NREM sleep in patients with narcolepsy with cataplexy exacerbate DNS in patients with narcolepsy with cataplexy and may be an underlying cause.23
Khatami et al. reported that patients with narcolepsy with cataplexy have decreased NREM sleep intensity and suggested that this may mediate DNS.27 Their analysis compared PSG findings between 11 treatment-naïve patients with narcolepsy with cataplexy with 11 matched controls. As in other studies, patients with narcolepsy with cataplexy exhibited more S1 sleep, lower sleep efficiency, and more frequent awakenings/ arousals than did controls. Patients with narcolepsy exhibited longer NREM-REM cycles than did controls and a more dramatic decline in slow wave sleep across successive NREMREM cycles. This was evidenced by less slow wave activity during the second NREM sleep episode among patients. In parallel, during this second NREM sleep episode, patients with narcolepsy with cataplexy experienced more wake episodes than did controls (p = 0.01).27
Based on the hypothesis that insufficient NREM intensity is associated with sleep disruptions, Khatami et al. compared 6 drug-free patients with narcolepsy with cataplexy and 6 matched controls after 40 hours of wakefulness.28 Prior to sleep deprivation, slow wave activity decreased in both groups across successive NREM-REM sleep cycles, but, as in the previous study, slow wave activity was smaller among patients with narcolepsy during the second NREM-REM cycle (p = 0.02). During recovery sleep, initial slow wave activity increased in both groups, and there were no sustained differences between groups across sequential NREM episodes. That is, during recovery sleep, there were fewer awakenings during the first and second NREM sleep episodes (p = 0.01 and p = 0.02, respectively) than at baseline, suggesting that increased NREM intensity associated with sleep deprivation reduces disruptions to nocturnal sleep among patients with narcolepsy with cataplexy.
These studies provide a consistent characterization of fragmented nighttime sleep in drug-free patients with narcolepsy. Similar results have also been observed in patients receiving active treatments. One study compared normal controls (n = 50) with patients with narcolepsy with cataplexy (n = 50), 36 of whom were being treated for narcolepsy at the time of the study.20 Patients with narcolepsy had significantly greater WASO (92.7 vs. 51.1 min, respectively; p < 0.01), more total wake time (104.9 vs. 77.3 min, respectively; p < 0.01), and a greater percentage of S1 sleep (12.4% vs. 7.8%, respectively; p < 0.01).
Rogers et al. compared PSG and sleep diary data from 25 treated patients with narcolepsy with gender-matched controls.32 All patients were receiving stimulants, and 5 patients were also taking REM-suppressant medication. Treatment response was considered satisfactory by both the patient and the patient's physician. The controls had no history of sleep, neurologic, or psychiatric disorders, alcoholism, use of drugs affecting the central nervous system, or shift work. Patients recorded sleep-wake patterns, activities, and medication use in the diaries and underwent PSG assessment. Total sleep time was comparable between patients and controls, but patients with narcolepsy had more WASO (57.5 vs. 34.5 min, respectively; p ≤ 0.05), S1 sleep (15.3% vs. 10.1%, respectively; p ≤ 0.05), and more frequent awakenings, although this parameter failed to reach statistical significance.
Finally, studies have compared the nighttime sleep of patients with narcolepsy with the nighttime sleep of patients with other sleep disorders.19,36 Baker et al. found that patients with narcolepsy had more DNS than did patients with IH, characterized by more S1 sleep, greater WASO, and lower sleep efficiency.19 More recently, Takei and colleagues reported PSG findings among patients with narcolepsy both with and without cataplexy (n = 52 and n = 62, respectively) and patients with IH without long sleep times (n = 50).36 Patients with narcolepsy with cataplexy experienced more arousals per hour than did those without cataplexy and those with IH (17.1 vs. 11.7 vs. 10.1, respectively; p < 0.01). There was no statistically significant difference in the number of arousals between patients with narcolepsy without cataplexy and those with IH. Patients with cataplexy also had a greater percentage of S1 sleep than did those without cataplexy and those with IH (15.6% vs. 9.2% vs. 10.8%, respectively; p < 0.01). Sleep efficiency was lowest (p < 0.05) and WASO highest (p < 0.01) among patients with cataplexy. The data suggest that DNS occurs more frequently among patients with narcolepsy with cataplexy than without.36
PSG Findings and EDS
Nighttime sleep in narcolepsy, as defined by PSG, parallels that of patient reports of fragmented sleep, with the preponderance and consistency of data suggesting that DNS is a characteristic of narcolepsy per se, not a comorbid condition. Interestingly, DNS may be related to lower drive for sleep as evidenced by a greater decline across the night in slow wave sleep among patients with narcolepsy. This is especially true for patients with narcolepsy with cataplexy. However, this is not a consistent finding.27,28,40,41
Despite the consistency of reports of DNS in narcolepsy, few studies have attempted to correlate DNS with narcolepsy's daytime symptoms.24,26 Those that have, produced inconsistent results or found suggestive, but not completely persuasive, correlations between DNS and EDS.
In the US Modafinil in Narcolepsy Multicenter Study, there was a significant, albeit modest, relation between DNS and daytime sleepiness evaluated with the multiple sleep latency test (MSLT), maintenance of wakefulness test, and ESS. Thus they concluded that whereas DNS may exacerbate EDS, DNS is not the primary cause of EDS in narcolepsy.24
Jiménez-Correa and colleagues compared patients with narcolepsy and healthy matched controls with no history of sleep disorders (n = 23 in each group) and found that patients with narcolepsy had a greater percent of S1 sleep (16.60% vs. 10.57%, respectively; p < 0.05) and S2 sleep (58.36% vs. 52.09%, respectively; p < 0.05), a significantly greater number of arousals (78.38 vs. 29.13, respectively; p < 0.05), and more awakenings lasting < 3 minutes (mean, 35.15 vs. 3.77, respectively; p < 0.05).26 Using measures of daytime sleepiness (ESS, MSLT, subjective sleep quality) and other tools, investigators reported moderate correlations between EDS and DNS. Daytime sleepiness was associated with WASO and frequency of awakenings, and lower total sleep time, sleep efficacy, and percent of slow wave sleep.26
Although fragmented sleep has generally been associated with EDS, the limited number of studies on the role of DNS in producing daytime sleepiness in patients with narcolepsy, and their contrasting results, do not present a clear picture of this relationship. Consequently, further studies are warranted for clarifying this relationship, and addressing the question of whether directly treating DNS has an impact on EDS. Furthermore, since there are interrelationships among disturbed sleep, inflammation, and chronic conditions such as cardiovascular disease and diabetes,42,43 the potential impact of DNS on other health outcomes would also be of interest.
DNS in Narcolepsy versus Insomnia
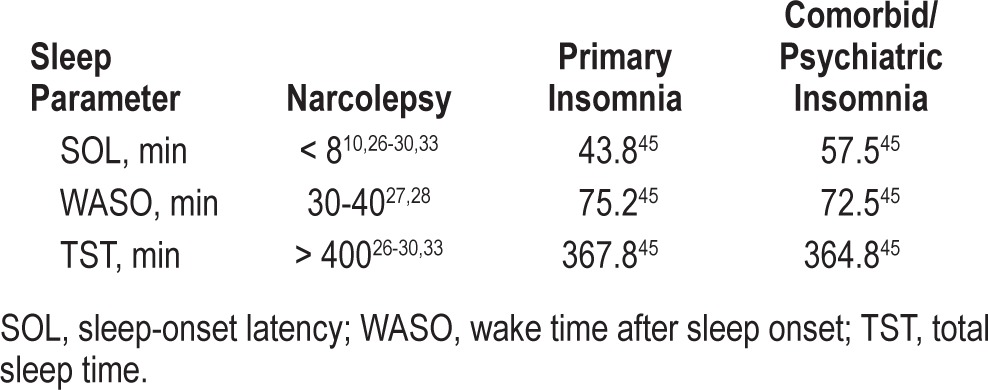
It is important to recognize that patient reports and PSG characterizations of DNS in narcolepsy are part of narcolepsy per se and distinguishable from the sleep disturbances seen in insomnia. Insomnia is defined by a patient report of difficulty falling asleep or staying asleep, or the presence of nonrestorative sleep.44–46 There is obviously overlap in descriptions of insomnia and DNS with narcolepsy, but patients with narcolepsy have no difficulty falling asleep, and the difficulties in sleep maintenance experienced by individuals with insomnia are different from those experienced by patients with narcolepsy. Insomnia is characterized by prolonged periods of wakefulness such as long sleep latency and difficulty falling back to sleep after nocturnal awakenings.43,44 In contrast, patients with narcolepsy fall asleep rapidly and experience frequent arousals but not prolonged wakefulness (Table 5).10,26–30,33,47
Table 5.
Differences in sleep parameters between patients with narcolepsy with and without cataplexy and insomnia
Consensus Characterization of Nighttime Sleep in Narcolepsy
Based on patient-reported and PSG data reviewed and discussed by the panel, the following consensus characterization of DNS in patients with narcolepsy was formulated:
Patients report poor sleep quality and frequent nocturnal awakenings
PSG findings document sleep fragmentation as evidenced by frequent transitions to lighter stages of sleep (S1 and wake)
To evaluate patients with narcolepsy for DNS, the panel recommended consideration of patient reports of the number of awakenings, duration of awake time in bed, and sleep quality. Patient reports should be considered alongside PSG data, including the frequency of shifts from deeper stages of sleep to waking or S1 sleep, frequency of brief awakenings/arousals, sleep efficiency, WASO, and sleep stage distribution, especially times spent in S1 and slow wave sleep and latency to REM sleep.
Treatment of DNS in Narcolepsy
Contemporary guidelines for the treatment of narcolepsy are available from the American Academy of Sleep Medicine (AASM) and European Federation of Neurological Society (EFNS), and numerous reviews have been published in the last decade describing available therapeutic options.7,9,11,48–50 An in-depth review of available therapies is beyond the purview of this article. However, it is worthwhile to note that some available pharmacologic treatments have been associated with improvements in nighttime sleep in narcolepsy.21,52,53 In 2 randomized clinical trials, treatment with sodium oxybate increased slow wave sleep, decreased S1 sleep, and produced dose-dependent reductions in the number of nocturnal awakenings experienced by patients with narcolepsy.21,52 A randomized controlled trial by Mayer and colleagues of ritanserin in patients with narcolepsy found that ritanserin increased nocturnal slow wave sleep and improved patients' perception of feeling refreshed after awakening, although investigators did not see a significant effect in the primary efficacy parameters.53 These findings support the hypotheses that insufficient slow wave activity mediates DNS of narcolepsy. Nevertheless, DNS remains a symptom that typically goes untreated.7,9,11,13
Current guidelines from the AASM endorse “control[ling] nocturnal symptoms of disrupted sleep…when present and troublesome in patients with narcolepsy,”7 whereas guidelines from the EFNS note that improvements in DNS are achievable, but the guidelines do not provide a specific treatment recommendation.9 Therefore, studies are needed that evaluate objective and patient-reported effects of various therapies on the treatment of DNS.
CONCLUSIONS
DNS is a common aspect of narcolepsy that appears to differ from DNS in other sleep disorders including insomnia. DNS in narcolepsy is characterized by patient reports of frequent brief awakenings and by PSG findings of frequent arousals, higher WASO, frequent shifts to wake or increased S1 sleep with reduction in stage 3/stage 4, and overall decreased sleep efficiency. Several studies using PSG suggest that decreased NREM and slow wave activity are possible mediators of the fragmented sleep. Some pharmacologic therapies are associated with improvements in nighttime sleep in narcolepsy. These distinctions further endorse DNS as part of a narcolepsy symptom pentad.
The AASM recommends controlling troublesome nocturnal symptoms of disrupted sleep in patients with narcolepsy but makes no specific treatment recommendations. It is unclear that improving nocturnal sleep leads to improvement in daytime functioning. However, this symptom is potentially troublesome for patients, and treatment may be warranted when patients report its presence and impact on their daily lives.
DISCLOSURE STATEMENT
The International Experts' Panel on Narcolepsy meeting and the development of this article were supported by funding from Jazz Pharmaceuticals PLC, Palo Alto, California. Dr. Tom Roth has served as a consultant for Abbot, Accadia, Acogolix, Acorda, Actelion, Addrenex, Alchemers, Alza, Ancel, Arena, AstraZeneca, Aventis, AVER, Bayer, BMS, BTG, Cephalon, Cypress, Dove, Eisai, Elan, Eli Lilly, Evotec, Forest, Glaxo Smith Kline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson and Johnson, King, Lundbeck, McNeil, MedicNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Otsuka, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Yanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. He has served on speakers bureau for Purdue and Sepracor. He has received research support from Apnex, Aventis, Cephalon, Glaxo Smith Kline, Merck, Neurocrine, Pfizer, Sanofi, Schering Plough, Sepracor, Somaxon, Syrex, Takeda, Transcept, Wyeth, and Xenoport. Prof. Y Dauvilliers has received speaker's honoraria and funding for travel to conferences from UCB Pharma, JAZZ, Cephalon, Novartis, and Bioprojet. Prof. Dauvilliers has participated in advisory boards of UCB pharma, JAZZ and Bioprojet. Dr. Emmanuel Mignot has received research support from Glaxo Smith Kline, Jazz Pharmaceuticals, and Novo Nordisk. He has received an educational gift from Jazz Pharmaceuticals to the Stanford Center for Sleep Sciences. Dr. Jacques Montplaisir received honoraria for speaking engagements from Valeant Pharmaceuticals and Otsuka Pharmaceuticals. He has served as an advisor for Sanofi, Servier, Merck, Jazz, Valeant Pharmaceuticals, and Impax. He has received research grants/support from Merck and Glaxo Smith Kline. Josh Paul has no conflicts of interest to disclose. Dr. Todd Swick has received funding as a principle investigator for: Jazz Pharmaceuticals/Orphan Medical (SXB-6, SXB-7, SXB-15, SXB-19, SXB-22, SXB-26, H-16368, Jazz 06-008, Jazz 06-009, and Jazz 06-010), Takeda Pharmaceuticals, Merck, Cephalon, Sanofi-Aventis, Pfizer, Somaxon, GlaxoSmithKline, and Epix Pharmaceuticals. He has received funding as a consultant to Jazz Pharmaceuticals, Aerial Pharmaceuticals and Concert Pharmaceuticals and Boehringer Ingelheim. He is on the Speakers' Bureau of Glaxo Smith Kline, Sepracor, Jazz Pharmaceuticals, Sanofi-Aventis, and Boehringer-Ingelheim. Dr. Phyllis Zee has received a research educational gift from Philips Respironics to Northwestern University. She is a consultant for Takeda Pharmaceutical, UCB, Merck, Jazz, Purdue, and Ferring. She has stock options from Zeo.
ACKNOWLEDGMENTS
The authors thank Josh Paul, M.A. for his editorial and writing assistance.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- DNS
disrupted nighttime sleep
- EDS
excessive daytime sleepiness
- EFNS
European Federation of Neurological Society
- ESS
Epworth Sleepiness Score
- ICSD
International Classification of Sleep Disorders
- IH
idiopathic hypersomnia
- LST
long sleep time
- MLST
multiple sleep latency test
- NC
narcolepsy with cataplexy
- NREM
non-REM
- OSA
obstructive sleep apnea
- PLMs
periodic leg movements
- PSG
polysomnography
- QOL
quality of life
- REM
rapid eye movement
- S1
stage 1
- S2
stage 2
- SOREMP
sleep-onset rapid eye movement period
- WASO
wake time after sleep onset
APPENDIX
Disrupted Nighttime Sleep in Narcolepsy Scientific Advisory Board
Co-chairs
Emmanuel Mignot, M.D., Ph.D.
Director
Stanford Center for Sleep Sciences
Professor of Psychiatry and Behavioral Sciences
Stanford University
Palo Alto, California
Thomas Roth, Ph.D.
Director
Sleep Disorders and Research Center
Henry Ford Hospital
University of Michigan College of Medicine
Ann Arbor, Michigan
Advisors
Claudio Bassetti, M.D.
Director
Neurocenter of Southern Switzerland
Chairman of the Neurology Department
Ospedale Civico
Lugano, Switzerland
Professor of Neurology
University Hospital
Zürich, Switzerland
Jacques Montplaisir, M.D., Ph.D.
Director
Center for Advanced Studies in Sleep Medicine
Hôpital de Sacré-Cœur de Montréal
Centre d'étude du sommeil
Montréal, Québec, Canada
Jed Black, M.D.
Consulting Professor
Stanford Sleep Medicine Center
Stanford University
Palo Alto, California
Todd Swick, M.D.
Medical Director
The Houston Sleep Center
Assistant Clinical Professor of Neurology
University of Texas-Houston School of Medicine
Houston, Texas
Yves Dauvilliers, M.D.
Professor of Neurology
Director of Sleep Unit
Montpellier, France
University Hospital, Gui de Chauliac
Montpellier, France
Phyllis Zee, M.D., Ph.D.
Professor and Director
Sleep Disorders Center
Northwestern University
Chicago, Illinois
Cynthia McCormick, M.D.
Principal
McCormick Consultation, LLC
Bethesda, Maryland
REFERENCES
- 1.Benca RM. Narcolepsy and excessive daytime sleepiness: diagnostic considerations, epidemiology, and comorbidities. J Clin Psychiatry. 2007;68(suppl 13):5–8. [PubMed] [Google Scholar]
- 2.Mamelak M. Narcolepsy and depression and the neurobiology of gamma hydroxybutyrate. Prog Neurobiol. 2009;89:193–219. doi: 10.1016/j.pneurobio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Dauvilliers Y, Bayard S, Shneerson JM, Plazzi G, Myers AJ, Garcia-Borreguero D. High pain frequency in narcolepsy with cataplexy. Sleep Med. 2011;12:572–6. doi: 10.1016/j.sleep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Vignatelli L, Plazzi G, Peschechera F, Delaj L, D'Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12:19–23. doi: 10.1016/j.sleep.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Jennum P, Knudsen S, Kjellberg J. The economic consequences of narcolepsy. J Clin Sleep Med. 2009;5:240–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Dodel R, Peter H, Spottke A, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007;8:733–41. doi: 10.1016/j.sleep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Morganthaler TI, Kapur VK, Brown TM, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diagnostic and Coding Manual. 2nd ed. Westchester, Illinois: American Academy of Sleep Medicine; 2005. Diagnostic criteria: International Classification of Sleep Disorders. [Google Scholar]
- 9.Billiard M, Dauvilliers Y, Dolenc-Grošelj L, Lammers GJ, Mayer G, Sonka K. Management of narcolepsy in adults. In: Gilhus NE, Barnes MP, Brainin M, editors. European handbook of neurological management. 2nd ed. Oxford, UK: Blackwell Publishing Ltd; 2011. pp. 513–28. [Google Scholar]
- 10.Billiard M. Diagnosis of narcolepsy and idiopathic hypersomnia. An update based on the International Classification of Sleep Disorders, 2nd ed. Sleep Med Rev. 2007;11:377–88. doi: 10.1016/j.smrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Didato G, Nobili L. Treatment of narcolepsy. Expert Rev Neurother. 2009;9:897–910. doi: 10.1586/ern.09.29. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Neurological Disorders and Stroke. Narcolepsy fact sheet. [Accessed January 9, 2012]. http://www.ninds.nih.gov/disorders/narcolepsy/detail_narcolepsy.htm.
- 13.Plazzi G, Serra L, Ferri R. Nocturnal aspects of narcolepsy with cataplexy. Sleep Med Rev. 2008;12:109–28. doi: 10.1016/j.smrv.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Seneviratne U, Puvanendran K. Narcolepsy in Singapore: is it an elusive disease? Ann Acad Med Singapore. 2005;34:90–3. [PubMed] [Google Scholar]
- 15.Bassetti C, Aldrich MS. Narcolepsy. Neurol Clin. 1996;14:545–71. doi: 10.1016/s0733-8619(05)70273-5. [DOI] [PubMed] [Google Scholar]
- 16.Nishino S, Okuro M. Emerging treatments for narcolepsy and its related disorders. Expert Opin Emerg Drugs. 2010;15:139–58. doi: 10.1517/14728210903559852. [DOI] [PubMed] [Google Scholar]
- 17.Zarowski M, Ali-Dinar T, Kothare SV. Narcolepsy. Minerva Pneumol. 2009;48:345–75. [Google Scholar]
- 18.Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8:373–99. doi: 10.1016/j.sleep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker TL, Guilleminault C, Nino-Murcia G, Dement WC. Comparative polysomnographic study of narcolepsy and idiopathic central nervous system hypersomnia. Sleep. 1986;9:232–42. doi: 10.1093/sleep/9.1.232. [DOI] [PubMed] [Google Scholar]
- 20.Bixler EO, Kales A, Vela-Bueno A, Drozdiak RA, Jacoby JA, Manfredi RL. Narcolepsy/cataplexy III: nocturnal sleep and wakefulness patterns. Int J Neurosci. 1986;29:305–16. doi: 10.3109/00207458608986159. [DOI] [PubMed] [Google Scholar]
- 21.Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly administration of sodium oxybate results in significant reduction in the nocturnal sleep disruption of patients with narcolepsy. Sleep Med. 2009;10:829–35. doi: 10.1016/j.sleep.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Bruck D, Parkes JD. A comparison of idiopathic hypersomnia and narcolepsycataplexy using self report measures and sleep diary data. J Neurol Neurosurg Psychiatry. 1996;60:576–8. doi: 10.1136/jnnp.60.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frauscher B, Gschliesser V, Brandauer E, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res. 2011;20:514–21. doi: 10.1111/j.1365-2869.2011.00906.x. [DOI] [PubMed] [Google Scholar]
- 24.Harsh J, Peszka J, Hartwig G, Mitler M. Night-time sleep and daytime sleepiness in narcolepsy. J Sleep Res. 2000;9:309–16. doi: 10.1046/j.1365-2869.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 25.Hishikawa Y, Wakamatsu H, Furuya E, et al. Sleep satiation in narcoleptic patients. Electroencephalogr Clin Neurophysiol. 1976;41:1–18. doi: 10.1016/0013-4694(76)90210-8. [DOI] [PubMed] [Google Scholar]
- 26.Jiménez-Correa U, Haro R, González RO, Velázquez-Moctezuma J. Correlations between subjective and objective features of nocturnal sleep and excessive diurnal sleepiness in patients with narcolepsy. Arq Neuropsiquiatr. 2009;67:995–1000. doi: 10.1590/s0004-282x2009000600006. [DOI] [PubMed] [Google Scholar]
- 27.Khatami R, Landolt HP, Achermann P, et al. Insufficient non-REM sleep intensity in narcolepsy-cataplexy. Sleep. 2007;30:980–9. doi: 10.1093/sleep/30.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatami R, Landolt HP, Achermann P, et al. Challenging sleep homeostasis in narcolepsy-cataplexy: implications for non-REM and REM sleep regulation. Sleep. 2008;31:859–67. doi: 10.1093/sleep/31.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montplaisir J, Billiard M, Takahashi S, Bell IF, Guilleminault C, Dement WC. Twenty-four-hour recording in REM-narcoleptics with special reference to nocturnal sleep disruption. Biol Psychiatry. 1978;13:73–89. [PubMed] [Google Scholar]
- 30.Mukai J, Uchida S, Miyazaki S, Nishihara K, Honda Y. Spectral analysis of all-night human sleep EEG in narcoleptic patients and normal subjects. J Sleep Res. 2003;12:63–71. doi: 10.1046/j.1365-2869.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 31.Parkes JD, Chen SY, Clift SJ, Dahlitz MJ, Dunn G. The clinical diagnosis of the narcoleptic syndrome. J Sleep Res. 1998;7:41–52. doi: 10.1046/j.1365-2869.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 32.Rogers AE, Aldrich MS, Caruso CC. Patterns of sleep and wakefulness in treated narcoleptic subjects. Sleep. 1994;17:590–7. doi: 10.1093/sleep/17.7.590. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal LD, Merlotti L, Young DK, et al. Subjective and polysomnographic characteristics of patients diagnosed with narcolepsy. Gen Hosp Psychiatry. 1990;12:191–7. doi: 10.1016/0163-8343(90)90078-q. [DOI] [PubMed] [Google Scholar]
- 34.Rovere H, Rossini S, Reimão R. Quality of life in patients with narcolepsy. A WHOQOL-Bref study. Arq Neuropsiquiatr. 2008;66:163–7. doi: 10.1590/s0004-282x2008000200004. [DOI] [PubMed] [Google Scholar]
- 35.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 36.Takei Y, Komada Y, Namba K, et al. Differences in findings of nocturnal polysomnography and multiple sleep latency test between narcolepsy and idiopathic hypersomnia. Clin Neurophysiol. 2012;123:137–41. doi: 10.1016/j.clinph.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Wittig R, Zorick F, Piccione P, Sicklesteel J, Roth T. Narcolepsy and disturbed nocturnal sleep. Clin Electroencephalogr. 1983;14:130–4. doi: 10.1177/155005948301400306. [DOI] [PubMed] [Google Scholar]
- 38.Zachariev Z, Djurkova A. Clinico-polysomnographic diagnostics of narcolepsycataplexy. Folia Med (Plovdiv) 1999;41:5–12. [PubMed] [Google Scholar]
- 39.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 40.Besset A, Tafti M, Nobile L, Billiard M. Homeostasis and narcolepsy. Sleep. 1994;17:S29–34. [PubMed] [Google Scholar]
- 41.Tafti M, Rondouin G, Besset A, Billiard M. Sleep deprivation in narcoleptic subjects: effect on sleep stages and EEG power density. Electroencephalogr Clin Neurophysiol. 1992;83:339–49. doi: 10.1016/0013-4694(92)90069-t. [DOI] [PubMed] [Google Scholar]
- 42.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller MA. Association of inflammatory markers with cardiovascular risk and sleepiness. J Clin Sleep Med. 2011;7(5 Suppl):S31–3. doi: 10.5664/JCSM.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3:167–74. doi: 10.1016/j.jsmc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 46.Lineberger MD, Carney CE, Edinger JD, Means MK. Defining insomnia: quantitative criteria for insomnia severity and frequency. Sleep. 2006;29:479–85. doi: 10.1093/sleep/29.4.479. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Ortuño MM, Carney CE, Edinger JD, Wyatt JK, Harris A. Moving beyond average values: assessing the night-to-night instability of sleep and arousal in DSM-IV-TR insomnia subtypes. Sleep. 2011;34:531–9. doi: 10.1093/sleep/34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishino S, Mignot E. Narcolepsy and cataplexy. In: Montagna P, Chokroverty S, editors. Handbook of clinical neurology, vol 99. Sleep disorders, part 2. St. Louis, MO: Elsevier; 2011. pp. 783–814. [DOI] [PubMed] [Google Scholar]
- 49.Mohsenin V. Narcolepsy—master of disguise: evidence-based recommendations for management. Postgrad Med. 2009;121:99–104. doi: 10.3810/pgm.2009.05.2008. [DOI] [PubMed] [Google Scholar]
- 50.Mahmood M, Black J. Narcolepsy-cataplexy: how does recent understanding help in evaluation and treatment. Curr Treat Options Neurol. 2005;7:363–71. doi: 10.1007/s11940-005-0029-8. [DOI] [PubMed] [Google Scholar]
- 51.Chakravorty SS, Rye DB. Narcolepsy in the older adult. Epidemiology, diagnosis, and management. Drugs Aging. 2003;20:361–76. doi: 10.2165/00002512-200320050-00005. [DOI] [PubMed] [Google Scholar]
- 52.Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly use of sodium oxybate is associated with a reduction in nocturnal sleep disruption: a double-blind, placebo-controlled study in patients with narcolepsy. J Clin Sleep Med. 2010;6:596–602. [PMC free article] [PubMed] [Google Scholar]
- 53.Mayer G. Ritanserin improves sleep quality in narcolepsy. Pharmacopsychiatry. 2003;36:150–5. doi: 10.1055/s-2003-41200. [DOI] [PubMed] [Google Scholar]