Abstract
Hypertension is the most common medical disorder encountered during pregnancy. A recent report highlighted hypertensive disorders as one of the major causes of pregnancy-related maternal deaths in the United States. Significant advances in our understanding of preeclampsia, a form of hypertension unique to pregnancy, have occurred in recent years. The optimal timing and choice of therapy for hypertensive pregnancy disorders involves carefully weighing the risk-versus-benefit ratio for each individual patient, with an overall goal of improving maternal and fetal outcomes. In this review, we summarize the mechanisms thought to be involved, review the current management guidelines for hypertensive pregnancy disorders as recommended by international guideline groups, and outline some newer perspectives on management.
Keywords: Hypertension, Pregnancy, Preeclampsia, Pre-eclampsia, Management, Therapy, Mechanisms, Target organs, Guidelines
Introduction
Hypertension is the most common medical disorder seen during pregnancy, affecting about 6% to 8% of pregnancies [1]. Hypertension in pregnancy includes a spectrum of conditions (Table 1), most notably preeclampsia, a form of hypertension unique to pregnancy that occurs de novo or may be superimposed on chronic hypertension. The other forms, chronic and gestational hypertension, usually have more benign courses [1].
Table 1.
Classification of blood pressure in pregnancy
| Condition | Definition and management |
|---|---|
| Preeclampsia or eclampsia | A pregnancy-specific disorder that is a multisystem disease characterized by hypertension ≥ 140/±90 mm Hg on at least two occasions at least 6 hours apart, and proteinuria ≥ 300 mg in a 24-hour urine collection, after 20 weeks gestation. Antihypertensive therapy is indicated for sustained blood pressure elevations ≥ 160 mm Hg systolic or 105 mm Hg diastolic. The goal of blood pressure reduction in emergency situations should be a gradual reduction of blood pressure to the normal range. |
| The convulsive form of preeclampsia, eclampsia, affects 0.1% of all pregnancies. | |
| Preeclampsia superimposed on chronic hypertension | ≤ 30% of women with chronic hypertension develop preeclampsia (de novo proteinuria), usually in the third trimester; proteinuria is not seen in uncomplicated chronic hypertension. |
| Chronic hypertension | BP ≥ 140/± 90 mm Hg before pregnancy or before the 20th week of gestation. The NHBPEP Working Group advises that antihypertensive medication can be safely withheld in this group, provided that blood pressure remains < 150–160 mm Hg systolic and 100–110 mm Hg diastolic while the patient is off medications. |
| Gestational hypertension | New onset of hypertension ≥ 140/±90 mm Hg on at least two occasions at least 6 hours apart, after 20 weeks gestation in the absence of proteinuria (< 300 mg in a 24-hour urine collection). |
| If blood pressure returns to normal by 12 weeks postpartum, the diagnosis of transient hypertension of pregnancy can be assigned. If elevated blood pressure persists, the diagnosis of chronic hypertension is made. |
NHBPEP National High Blood Pressure Education Program.
(Adapted from the Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy [1].)
Preeclampsia, a pregnancy-specific disorder characterized by hypertension (≥ 140/90 mm Hg) and proteinuria (≥ 300 mg in a 24-hour urine), affects 3% to 4% of all pregnancies worldwide. Risk factors include primiparity, previous preeclampsia, increased maternal body mass index (BMI) before pregnancy, ethnicity (black women are more at risk), multiple gestations, and underlying medical conditions such as renal disease and diabetes mellitus [2]. In low-income and middle-income countries, preeclampsia and its convulsive form, eclampsia, are associated with 10% to 15% of direct maternal deaths [3]. Risks to the fetus include premature delivery, growth retardation, and death. The only definitive treatment of preeclampsia is delivery. Treatment of severe hypertension is necessary to prevent cerebrovascular, cardiac, and renal complications in the mother.
In this review, we summarize the mechanisms thought to be involved, as well as the current recommendations for management of hypertensive pregnancy disorders, as published by international guideline groups. In addition, we discuss some of the controversies associated with antihypertensive treatment in pregnancy.
Mechanisms of Hypertension in Pregnancy
The normal physiological changes occurring in pregnancy include an increase in cardiac output and blood volume, generalized vasodilatation, and a decrease in blood pressure [4]. Because of gestational physiology, blood pressure decreases during the first trimester, reaches its lowest point by mid-pregnancy, and then usually returns to pre-pregnancy levels during the third trimester. The metabolic changes of normal pregnancy, such as hyperlipidemia and hypercoagulable and inflammatory states, are amplified further in preeclampsia. In recent years, significant advances have occurred in our understanding of the pathophysiology and mechanisms of hypertensive disorders of pregnancy, particularly preeclampsia.
It has been suggested that preeclampsia is a condition that involves numerous and constant interactions among the placental, immunologic, and cardiovascular systems [5••]. It is a syndrome associated with impaired early placentation and dysfunctional trophoblast development, defective placental angiogenesis, and an exaggerated maternal systemic inflammatory response [6••,7•,8••,9••]. Figure 1 highlights some of the implicated factors, the effects on the mother and the fetus, and the long-term consequences.
Figure 1.
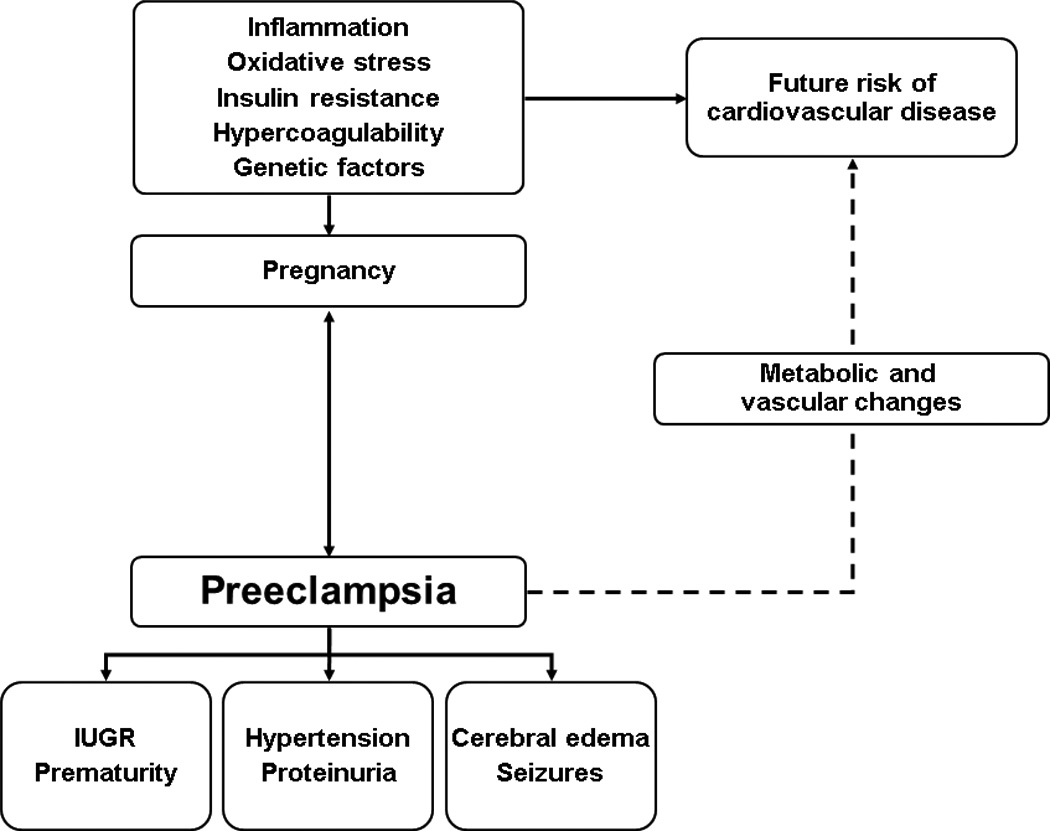
Preeclampsia: implicated factors and short-term and long-term consequences. IUGR intrauterine growth retardation; PRES posterior reversible encephalopathy syndrome.
Placental Abnormalities
A growing body of evidence indicates that endothelial dysfunction plays a crucial role in the pathogenesis of pre-eclampsia. Several different mechanisms may contribute to endothelial cell dysfunction in preeclampsia, including hypoxia, alterations in placental angiogenic factors and the renin-aldosterone-angiotensin II axis, excessive oxidative stress and syncytiotrophoblast debris, immune maladaption, and genetic factors [6••]. Preeclampsia has been described as a “two-stage” disease: stage I refers to abnormal placentation, and stage II refers to the subsequent systemic endothelial activation and its resultant clinical manifestations [10].
Placental development includes trophoblast differentiation in the placenta, invasion of trophoblast into the decidua, and trophoblast-induced remodeling of the spiral arteries, all vital for a normal pregnancy [5••]. However, in preeclampsia, there is abnormal development and differentiation of the villous syncytiotrophoblast and inadequate invasion of the placental extravillous trophoblasts into the myometrium of the uterus, causing insufficient spiral artery remodeling, disruption of the placental barrier, and release of necrotic and aponecrotic trophoblast fragments [9••]. An interesting pathological feature found with impaired vascular remodeling of the spiral arteries is lipid deposition into their arterial walls [11]. This feature has been described as “acute atherosis,” similar to the early stages of atherosclerosis, and appears to improve after delivery [11].
Inadequate placental perfusion as a result of insufficient spiral artery remodeling is believed to result in hypoxia-reperfusion–type injuries to the placenta. Women with preeclampsia have alterations in placental hypoxia-inducible factor (HIF) and its targets [12]. Invasive cytotrophoblasts express several angiogenic factors regulated by HIF, including vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and VEGF-receptor 1 (VEGFR-1); expressions of these proteins are altered in preeclampsia [13]. Placental hypoxia also appears to enhance the formation of syncytial knots and the shedding of syncytiotrophoblast basement membrane fragments into the maternal circulation [14•]. Other factors released at this time include leukocyte and platelet membrane particles, reactive oxygen species, activated neutrophils, cytokines, growth factors, and angiogenic factors, which further affect the maternal endothelium [14•].
Natural killer (NK) cells at the maternal/fetal interface are also thought to play a part in preeclampsia biology [6••]. They have been implicated in immune tolerance needed for placental development, induction of angiogenic factors, and vascular remodelling [15].
Placental ischemia leads to a biologic response by the placenta, which produces and secretes a series of modulators of angiogenesis, some of which cross the maternal placental barrier and adversely affect the mother [7•].
Angiogenic Factors
In recent years, there has been much focus on biomarkers of preeclampsia that may have potential roles in the clinical management of this condition. The soluble forms of VEGFR-1 (sVEGFR-1) and endoglin (sEng), an endothelial receptor for transforming growth factor beta, have been extensively investigated, as they appear to be directly involved in the systemic endothelial dysfunction of the mother [7•].
Work by Maynard and others has led to a greater understanding of the process of defective angiogenesis associated with preeclampsia [16–18]. Regulation of placental angiogenesis is essential for a healthy placenta and a successful pregnancy [9••]. A balance between proangiogenic factors, VEGF and PlGF, and anti-angiogenic factors, sVEGFR-1 and sEng, is important for normal placental development. The level of PlGF is reduced in patients who will subsequently develop preeclampsia, whereas sVEGFR-1 and sEng are increased, particularly in early-onset preeclampsia. In a rat model of preeclampsia, enhanced production of sVEGFR-1 and reactive oxygen species (ROS) has been reported in a placental ischemic model of preeclampsia [19]. In this setting, ROS may be implicated in the hypertension associated with chronic sVEGFR-1 excess during pregnancy.
A recent report from a consensus meeting concerning pre-eclampsia markers concluded that these markers can be used to predict preeclampsia a few weeks before the onset of clinical symptoms, but their application for early prediction varies according to the diagnostic test used [9••]. In addition, dysregulation of angiogenic markers appears not to be specific for preeclampsia; similar changes occur in intrauterine growth retardation (IUGR) not associated with preeclampsia, particularly in the case of sEng [9••].
Placental Steroid and Peptide Hormones
The placenta synthesizes and secretes important steroid and peptide hormones [20]. Myatt demonstrated that the nitration of the protein p38 MAP kinase is increased in preeclamptic placentas and is associated with loss of catalytic activity, and hypothesized that nitration of proteins in the placenta, including receptors, transporters, enzymes and structural proteins, can alter proteins and placental functions, thus influencing fetal growth and development [20].
Placental protein PP13 is found in the brush border of syncytiotrophoblasts, at the maternal-fetal interface (the placenta being the only organ that produces PP13). It is involved in implantation of the embryo and vascular remodeling. PP13 appears to be low during the early stages of pregnancy in women who subsequently develop preeclampsia; it may be a predictive biomarker, but further work is needed [7•, 9••].
With respect to defective placental steroidogenesis, a recent study has demonstrated an association between reduced estrogen levels and activity of placental aromatase with increasing severity of preeclampsia [21].
Oxidative Stress
There is support for the role of oxidative stress in preeclampsia. Evidence for oxidative stress in animal studies of preeclampsia has been demonstrated by the administration of an inhibitor of nitric oxide (NO) synthesis, L-NAME (L-nitroarginine methyl-ester), into pregnant rats; the result mimics clinical features of preeclampsia, such as hypertension, proteinuria, and thrombocytopenia [22].
Agonistic Autoantibodies Against the Angiotensin II Type-1 Receptor
Autoantibodies directed against the angiotensin-II type-1 receptor (AT1-AA) have been detected in women with preeclampsia [23]. AT1 receptor autoantibodies from the circulation of women with preeclampsia can reproduce the features of preeclampsia and increase both sVEGFR-1 and sEng in pregnant mice [24]. However, as discussed in a recent review by Hertig et al. [7•], these autoantibodies also have been found in some normal pregnancies without preeclampsia, so their exact role in the pathogenesis of preeclampsia remains to be determined.
Hypercoagulability
The hypercoagulable state of normal pregnancy is even more pronounced in women with preeclampsia. Levels of procoagulants such as tissue plasminogen activator, plasminogen activator inhibitor, homocysteine, von Willebrand factor, and fibronectin are all upregulated in preeclampsia [25]. One theory is that this maternal hypercoagulable state, along with low-pressure placental blood flow, may cause deposition of fibrin and the formation of thrombi, further worsening endothelial dysfunction and placental ischemia [26].
Anticardiolipin Antibodies
A recent meta-analysis concluded that although elevated levels of anticardiolipin antibodies are associated with preeclampsia, there is insufficient evidence for their use as predictors of preeclampsia in clinical practice [27].
Complement Activation
In a mouse model of preeclampsia, inhibition of placental complement prevents oxidative stress and placental dysfunction, as well as proteinuria and renal pathologic features of preeclampsia [28]. Genetic mutations resulting in abnormal control of the complement alternative pathway have also been demonstrated in patients with HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) [29]. In human studies, genes for the complement regulatory proteins, membrane cofactor protein (MCP), factor I, and factor H, have recently been studied in patients from the PROMISSE Study (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) [30]. They provide evidence for the role of heterozygous mutations in these genes in the development of preeclampsia, and set the stage for future studies that will determine whether elevations of split products, as generated by activation of complement pathways, may serve as predictors of preeclampsia.
Genetics and Proteomics
Genetic mechanisms involved in preeclampsia have been highlighted by Carty et al. [31•]. Women with a family history of preeclampsia are at nearly three times higher risk of developing preeclampsia compared with those without a family history [32]. The systolic blood pressure in the offspring of preeclamptic women may be elevated as early as 12 years of age [33], although lifestyle may be an equally important contributory factor. The GOPEC (Genetics of Pre-eclampsia) consortium studied seven previously reported candidate genes in 657 carefully phenotyped women with preeclampsia, but none of the single-nucleotide polymorphisms examined achieved statistical significance [34].
The use of urinary proteomics in predicting preeclampsia has also been reviewed [35]. Urinary biomarkers, including fibrinogen alpha chain, collagen alpha chain, and uromodulin fragments, may have a role in the diagnosis of late-onset preeclampsia [35].
Insulin Resistance
Insulin resistance and obesity are some of the metabolic abnormalities associated with preeclampsia. These factors not only may be implicated in the pathogenesis of preeclampsia but also may be associated with the future risk of cardiovascular disease [31•].
Target Organs of Preeclampsia
Preeclampsia is characterized by widespread vasoconstriction, increased vascular resistance, and decreased cardiac output [4]. Maternal endothelial damage in the kidney includes glomerular endotheliosis, generalized swelling and vacuolization of the endothelial cells, and loss of the capillary space. In an early animal study, VEGF dysregulation was shown to play a critical role in renal disease in pregnancies affected by preeclampsia [36]. Mice lacking one VEGF allele in renal podocytes developed the typical renal pathology found in preeclampsia, suggesting that glomerular capillary function is under the strict gene-dosage–dependent control of VEGF. In a study of autopsy material from women with severe preeclampsia, the podocyte-specific proteins nephrin and synaptopodin were shown to be downregulated [37]. Clinically, urinary podocytes (podocyturia) were found in increased numbers in patients with preeclampsia [38].
Preeclampsia includes activation of the coagulation system that ranges from mild thrombocytopenia to the more severe HELLP syndrome. This coagulopathy is due to widespread endothelial dysfunction, with increased fibronectin and platelet aggregation, as well as shortened platelet survival and depressed antithrombin III levels [39]. Manifestations of preeclampsia that can be seen within the liver include intracellular fatty changes, areas of infarction, subcapsular hematomas, and, rarely, liver rupture.
Cerebral edema and neurologic sequelae can be seen in patients with preeclampsia or eclampsia. Mechanisms thought to be involved include diminished cerebral autoregulation, effects of endothelial dysfunction on the blood-brain barrier, or both [40]. Reversible posterior leukoencephalopathy syndrome (RPLS), also called posterior reversible encephalopathy syndrome (PRES), has also been observed in patients with preeclampsia. It is characterized by reversible, posterior-predominant white and gray matter lesions on brain MRI [41].
The full clinical presentation of preeclampsia and differential diagnosis has been reviewed by others [8••, 42].
Management of Hypertension in Pregnancy
Table 2 summarizes the recommendations for treatment that are most widely accepted and practiced. It is important to note that BP levels requiring therapy in pregnancy, although somewhat different among various groups and professional societies, have generally been set at higher systolic and diastolic levels than for the general population. There are several reasons for this difference. First, there have been few well-designed clinical trials establishing the benefit of treating mild chronic hypertension during pregnancy (typically defined in the relevant literature as systolic blood pressure [SBP] of 140 to 160 mm Hg and/or diastolic blood pressure [DBP] of 90 to 100 mm Hg). As a result, the contemporary treatment approach is based on the assumption that hypertension lasting 4 or 5 months in a young woman without other risk factors does not increase her risk for cardiovascular disease during the pregnancy or later in life. This approach is further justified by the concern that decreased blood pressure in the mother may compromise perfusion of the uteroplacental unit and fetal circulation. The choice of antihypertensive therapies has been limited to those that have proven to be relatively safe, have a long history of clinical use, and have a side-effect profile acceptable to most obstetricians.
Table 2.
Recommended management options for treating hypertension in pregnancy
| Strategy | Recommendations |
|---|---|
| Pharmacologic agents: First-line | |
| Methyldopa | Drug of choice according to all groups |
| Labetalol | May be associated with fetal growth restriction |
| Oxprenolol | A first-line agent by SOMANZ [46] |
| Pharmacologic agents: Second-line | |
| Nifedipine | May inhibit labor; widely used |
| Hydralazine | Long experience, with few adverse events documented |
| Beta blockers (atenolol not recommended) [43, 45] | Atenolol presents risk of growth restriction when started in first or second trimester |
| Diazoxide | IV bolus for acute BP lowering in severe hypertension [46] |
| Prazosin | Consider as a second-line agent by SOMANZ [46]; not recommended by SOGC [45] |
| Clonidine | Alternative option |
| Hydrochlorothiazide | Can cause volume contraction and electrolyte disorders |
| IV Magnesium sulphate | For prevention and treatment of seizures in severe preeclampsia or eclampsia |
| Other agents | |
| Low-dose aspirin | Advised for women at high risk [60, 61]; used prophylactically in women with a history of preeclampsia at < 28 weeks [47] |
| Fish oil supplementation | Not recommended [1, 60] |
| Calcium supplementation | May have a role in decreasing incidence of preeclampsia; definite role in populations with low calcium intake [1, 61] |
| Vitamin C and E | Not recommended [55, 60, 61] |
| Steroid therapy | Only for fetal lung maturation [45, 46, 60] |
| Contraindicated | |
| ACE inhibitors, angiotensin II receptor blockers, direct renin inhibitors |
ACE angiotensin-converting enzyme; BP blood pressure; IV intravenous; SOGC Society of Obstetricians and Gynaecologists of Canada; SOMANZ Society of Obstetric Medicine of Australia and New Zealand.
Recommendations from the Current Guidelines
The report of the National High Blood Pressure Education Program (NHBPEP) Working Group [1] advises that antihypertensive medication may be safely withheld in women with a history of chronic hypertension and recommends restarting treatment at SBP greater than 150 to 160 mm Hg and/or DBP of 100 to 110 mm Hg, or in the presence of left ventricular hypertrophy or renal insufficiency. According to the Working Group Report of the NHBPEP, methyldopa and hydralazine are recommended as initial oral or intravenous therapy, respectively. In current practice, antihypertensive medications other than methyldopa and hydralazine are being used more often in pregnancy (Table 2), particularly in patients for whom blood pressure control cannot be achieved with these agents, or in the presence of intolerable adverse effects. Methyldopa, labetalol, beta blockers (other than atenolol), slow-release nifedipine, and the use of a diuretic in preexisting hypertension are considered as therapeutic options. Angiotensin-converting enzyme (ACE) inhibitors are not recommended. In preeclampsia, antihypertensive therapy can be withheld unless there is persistent DBP of 105 to 110 mm Hg or higher. For emergency treatment in preeclampsia, intravenous hydralazine or labetalol and oral nifedipine can be used.
The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletins [43, 44] recommend that antihypertensive therapy be used for women with a history of chronic hypertension who develop severe hypertension in pregnancy, for maternal benefit. Treatment of uncomplicated mild hypertension is not beneficial, beta blockers and ACE inhibitors are not recommended, and methyldopa and labetalol are appropriate first-line agents. For the treatment of preeclampsia, the ACOG guidelines are the same as those from the NHBPEP group.
According to the clinical practice guidelines of the Society of Obstetricians and Gynaecologists of Canada [45], for women with severe hypertension (>160/≥110 mm Hg), blood pressure should be lowered to less than 160 mm Hg SBP and less than 110 mm Hg DBP in women with chronic hypertension and gestational hypertension in pregnancy. Initial agents to be used include labetalol, nifedipine, or hydralazine. In women with nonsevere hypertension (140–159/90–109 mm Hg), BP should be lowered to 130 to 155 mm Hg SBP and 80 to 105 mm Hg DBP, when there are no comorbid conditions. For women with comorbidities, SBP should be lowered to 130 to 139 mm Hg, and DBP to 80 to 89 mm Hg. The antihypertensives that can be considered include methyldopa, labetalol, other beta blockers, and calcium channel blockers. Atenolol, prazosin, and ACE inhibitors are not recommended.
The guidelines of the Society of Obstetric Medicine of Australia and New Zealand (SOMANZ) [46] recommend that antihypertensive treatment be commenced in all women with SBP at least 170 mm Hg or DBP at least 110 mm Hg. Several rapidly acting agents are considered suitable: intravenous labetalol, hydralazine, or diazoxide, or oral nifedipine. Treatment for mild to moderate hypertension of 140 to 160/90 to 100 mm Hg is optional and will reflect local practice. They consider methyldopa, labetalol, and oxprenolol to be first-line agents, and hydralazine, nifedipine, and prazosin to be second-line agents. ACE inhibitors are not recommended.
The 2007 European Society of Hypertension (ESH) Guidelines for the Management of Arterial Hypertension [47] include recommendations for hypertension in pregnancy. In the presence of gestational hypertension and preeclampsia, antihypertensives are indicated for blood pressures at least 140/90 mm Hg; SBP of 170 mm Hg or higher or DBP of 110 mm Hg or higher requires emergency treatment. Oral methyldopa, labetalol, calcium antagonists, and (less frequently) beta blockers are the drugs of choice. Nitroglycerin is suggested for preeclampsia with pulmonary edema; diuretics are not advised. Emergency treatment includes intravenous labetalol, oral methyldopa, and oral nifedipine. This group does not recommend intravenous hydralazine because of concerns about perinatal adverse events.
Podymow et al. extensively reviewed the individual antihypertensive agents mentioned above, including their adverse effects [48••].
Controversies and Perspectives in Management
The emergence over the past decade of new evidence with respect to both the pathophysiology of preeclampsia and the benefits of early hypertension treatment in the general population may affect the management of hypertensive pregnant patients. The notion that pregnant women with chronic hypertension are at low risk for cardiovascular complications within the short duration of pregnancy may be in question, given the current trend towards advanced maternal age at first pregnancy. These women may have other cardiovascular risk factors, such as obesity or hyperlipidemia, and/or signs of target organ hypertensive damage. In addition, modern methods of assisted reproduction (such as in vitro fertilization), have enabled women with cardiovascular risk factors that are associated with decreased fertility (such as diabetes mellitus and renal disease) to conceive. In these women, treatment of hypertension of even a short duration may improve their cardiovascular risks, especially in view of recent studies in the general population showing an important correlation between the time taken to achieve goal blood pressure and clinical outcomes—that is, outcome is better with earlier and more effective treatment [49, 50]. Finally, recent studies have indicated that cerebral vascular events in women with severe preeclampsia and eclampsia may occur when SBP exceeds 150 mm Hg; these studies have called for a paradigm shift, recommending antihypertensive therapy when the SBP reaches or exceeds 155 to 160 mm Hg [51]. Indeed, most investigators agree that antihypertensive therapy in the peripartum period should be initiated when DBP approaches 100 mm Hg or blood pressure is greater than or equal to 150/100 mm Hg [52]. As abrupt decreases in BP may adversely affect uteroplacental perfusion, treatment of hypertension mandates close maternal and fetal monitoring as the blood pressure is lowered. The ultimate therapeutic goal is to prevent maternal complications without compromising fetal well-being.
Management Beyond Antihypertensive Therapy
Magnesium Sulphate and Other Anticonvulsants
A Cochrane review of treatment of women with preeclampsia reported that magnesium sulphate more than halves the risk of eclampsia and probably reduces maternal death [53•]. In women with eclampsia, magnesium sulphate reduces the risk ratio of maternal death and of recurrence of seizures, compared with diazepam.
Antiplatelet agents
A review of 59 trials involving 37,560 women found that low doses of aspirin reduced the risk of preeclampsia by 17%, the risk of fetal or neonatal deaths by 14%, and the relative risk of preterm births by 8% [54]. Doses up to 75 mg appear to be safe.
Antioxidants to Prevent Preeclampsia
It has been demonstrated that supplementation with vitamin C (at a dose of 1000 mg daily) and vitamin E (at a dose of 400 IU daily) does not reduce the rates of either serious adverse outcomes of pregnancy-associated hypertension or preeclampsia among low-risk, nulliparous women [55••].
Calcium Supplementation
A review of calcium supplementation during pregnancy for preventing hypertensive disorders concluded that calcium supplementation appears to approximately halve the risk of preeclampsia and reduces the risk of preterm birth and the rare occurrence of the composite outcome “death or serious morbidity” [56]. Of note, most women in these trials had a low-calcium diet.
Other Options
Other management options such as the use of corticosteroids, plasma volume expansion, or interventions such as rest or exercise have not been validated [3]. A suggested management paradigm can be found in the comprehensive review of preeclampsia by Steegers et al [8••].
Novel or Potential Therapies
A number of potential therapeutic targets are under investigation. Among the more recent are aminopeptidases, heme oxygenase 1, and marinobufagenin.
Aminopeptidases such as placental leucine aminopeptidase (P-LAP) and aminopeptidase A (APA) do not cross the placental barrier. In the pregnant, spontaneously hypertensive rat, APA acts as an antihypertensive agent, degrading vasoactive peptides, and as a result, it normalizes blood pressure [57]. The role of aminopeptidases as potential therapeutic agents is being investigated.
A recent study by George et al. examined heme oxygenase 1 (HO-1) induction in a rat model of placental ischemia [58]. These authors suggest two potential pathways through which HO-1 may act: normalization of angiogenic balance in the placenta and reduction in oxidative stress. Both pathways are potential targets for treatment in preeclampsia.
Researchers including Uddin et al. have investigated the role of marinobufagenin (MBG), a cardiotonic steroid, and its antagonist resibufogenin (RBG), in experimental animal models of preeclampsia [59]. They have demonstrated that in a rat model of preeclampsia, MBG inhibits first-trimester cytotrophoblast cell function, and urinary excretion of MBG is elevated prior to the development of hypertension and proteinuria. MBG also causes hypoxia and ischemia, leading to an imbalance of proangiogenic and antiangiogenic factors. RBG, when given early in pregnancy, prevented the development of hypertension, proteinuria, and intrauterine growth restriction.
Conclusions
Our knowledge of the pathophysiology of preeclampsia is rapidly evolving and may lead to potential new therapeutic targets for this condition. The suggested management guidelines from several international expert groups help guide physicians in the management of hypertension in pregnancy. However, their definitions diverge with respect to what constitutes high blood pressure (particularly severe hypertension) in pregnancy, and the thresholds that require treatment. Important questions, such as whether pregnancy outcomes may be improved with earlier and better blood pressure control and what should be the optimal treatment targets, are complicated by the lack of prospective trials specifically addressing these issues. These questions must be evaluated in future studies that are adequately powered to compare the effects of different blood pressure targets on maternal and fetal outcomes. For instance, CHIPS (Control of Hypertension in Pregnancy Study) is a prospective, randomized, multicenter trial evaluating the effects on perinatal and maternal complications of a DBP target of 100 mm Hg versus 85 mm Hg.
While awaiting the results of this trial, the optimal timing and choice of therapy must remain a matter of carefully weighing the risk/benefit ratio for each individual patient, with an overall goal of improving maternal and fetal outcomes.
Footnotes
Disclosure
Conflicts of Interest: C. Brown: None; V. Garovic: As inventor of technology detecting podocyturia in preeclampsia, has rights to receive royalties from licensing.
Contributor Information
Catherine M. Brown, Catherine M. Brown, MD, Division of Nephrology and Hypertension, Mayo Clinic College of Medicine, 200 First Street SW, Rochester, Minnesota 55905, USA., brown.catherine@mayo.edu
Vesna D. Garovic, Vesna D. Garovic, MD, Division of Nephrology and Hypertension, Mayo Clinic College of Medicine, 200 First Street SW, Rochester, Minnesota 55905, USA., garovic.vesna@mayo.edu
References and Recommended Reading
Recently published papers of interest have been highlighted as
• Of importance
•• Of major importance
- 1.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 2.Trogstad L, Magnus P, Stoltenberg C. Pre-eclampsia: Risk factors and causal models. Best Pract Res Clin Obstet Gynaecol. 2011 Feb 22; doi: 10.1016/j.bpobgyn.2011.01.007. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin MK, Roberts JM. Hemodynamic changes. In: Lindheimer MD, Roberts JM, Cunningham FG, editors. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Stamford, Conn: Appleton & Lange; 1999. pp. 69–102. [Google Scholar]
- 5. James JL, Whitley GS, Cartwright JE. Pre-eclampsia: fitting together the placental, immune and cardiovascular pieces. J Pathol. 2010;221(4):363–378. doi: 10.1002/path.2719. This is a comprehensive review of pathological changes in preeclampsia.
- 6. Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–158. doi: 10.1152/physiol.00043.2008. This article provides one of the most up-to-date reviews of angiogenesis in preeclampsia.
- 7. Hertig A, Liere P. New markers in preeclampsia. Clin Chim Acta. 2010;411(21–22):1591–1595. doi: 10.1016/j.cca.2010.07.020. This review provides information on new biomarkers in preeclampsia.
- 8. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. doi: 10.1016/S0140-6736(10)60279-6. This is one of the few reviews to provide both an overview of pathogenesis and clinical aspects of preeclampsia.
- 9. Cetin I, Huppertz B, Burton G, et al. Pregenesys pre-eclampsia markers consensus meeting: What do we require from markers, risk assessment and model systems to tailor preventive strategies? Placenta. 2011;32(Suppl):S4–S16. doi: 10.1016/j.placenta.2010.11.022. This article provides the most comprehensive review of markers of preeclampsia and summarizes the conclusions of a recent consensus meeting on the topic.
- 10.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia—novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–1034. doi: 10.1161/HYPERTENSIONAHA.110.157743. [DOI] [PubMed] [Google Scholar]
- 12.Rajakumar A, Brandon HM, Daftary A, et al. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25(10):763–769. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, McMaster M, Woo K, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7(3):375–384. doi: 10.1111/j.1538-7836.2008.03259.x. This article provides important knowledge of the vascular biology of preeclampsia.
- 15.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 16.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 18.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 19.Tam Tam KB, Lamarca B, Arany M, et al. Role of reactive oxygen species during hypertension in response to chronic antiangiogenic factor (sFlt-1) excess in pregnant rats. Am J Hypertens. 2011;24(1):110–113. doi: 10.1038/ajh.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31(Suppl):S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hertig A, Liere P, Chabbert-Buffet N, et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203(5):477 e471–477 e479. doi: 10.1016/j.ajog.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993;169(5):1316–1320. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]
- 23.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou CC, Zhang Y, Irani RA, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14(8):855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis. 2008;2(4):249–259. doi: 10.1177/1753944708094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111. doi: 10.1186/1477-7827-1-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.do Prado AD, Piovesan DM, Staub HL, Horta BL. Association of anticardiolipin antibodies with preeclampsia: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(6):1433–1443. doi: 10.1097/AOG.0b013e3181fe02ec. [DOI] [PubMed] [Google Scholar]
- 28.Qing X, Redecha PB, Burmeister MA, et al. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011;79(3):331–339. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- 29.Fakhouri F, Jablonski M, Lepercq J, et al. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood. 2008;112(12):4542–4545. doi: 10.1182/blood-2008-03-144691. [DOI] [PubMed] [Google Scholar]
- 30.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8(3):e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carty DM, Delles C, Dominiczak AF. Preeclampsia and future maternal health. J Hypertens. 2010;28(7):1349–1355. doi: 10.1097/HJH.0b013e32833a39d0. This summarizes very well the effects of preeclampsia on women’s cardiovascular health.
- 32.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oglaend B, Forman MR, Romundstad PR, et al. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens. 2009;27(10):2051–2054. doi: 10.1097/HJH.0b013e328330052a. [DOI] [PubMed] [Google Scholar]
- 34.Disentangling fetal and maternal susceptibility for pre-eclampsia: a British multicenter candidate-gene study. Am J Hum Genet. 2005;77(1):127–131. doi: 10.1086/431245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carty DM, Siwy J, Brennand JE, et al. Urinary proteomics for prediction of preeclampsia. Hypertension. 2011;57(3):561–569. doi: 10.1161/HYPERTENSIONAHA.110.164285. [DOI] [PubMed] [Google Scholar]
- 36.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garovic VD, Wagner SJ, Petrovic LM, et al. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant. 2007;22(4):1136–1143. doi: 10.1093/ndt/gfl711. [DOI] [PubMed] [Google Scholar]
- 38.Garovic VD, Wagner SJ, Turner ST, et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007;196(4):320 e321–320 e327. doi: 10.1016/j.ajog.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 40.Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50(1):14–24. doi: 10.1161/HYPERTENSIONAHA.106.079442. [DOI] [PubMed] [Google Scholar]
- 41.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 42.Sibai BM. Imitators of severe pre-eclampsia/eclampsia. Clin Perinatol. 2004;31(4):835–852. doi: 10.1016/j.clp.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.ACOG Practice Bulletin no. 29. Chronic hypertension in pregnancy. ACOG Committee on Practice Bulletins. Obstet Gynecol. 2001;98(1):177–185. doi: 10.1016/s0029-7844(01)01471-5. [DOI] [PubMed] [Google Scholar]
- 44.ACOG Practice Bulletin no. 33. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 45.Magee LA, Helewa M, Moutquin JM, von Dadelszen P Hypertension Guideline Committee, Society of Obstetricians and Gynaecologists of Canada. Treatment of the hypertensive disorders of pregnancy. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3) Suppl 1:S24–S36. [Google Scholar]
- 46.Lowe SA, Brown MA, Dekker GA, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49(3):242–246. doi: 10.1111/j.1479-828X.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 47.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25(6):1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 48. Podymow T, August P. Antihypertensive drugs in pregnancy. Semin Nephrol. 2011;31(1):70–85. doi: 10.1016/j.semnephrol.2010.10.007. This is a comprehensive and detailed review of the antihypertensive drugs used in pregnancy.
- 49.Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363(9426):2049–2051. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 50.Gradman AH, Basile JN, Carter BL, Bakris GL. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4(1):42–50. doi: 10.1016/j.jash.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Martin JN, Jr, Thigpen BD, Moore RC, et al. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105(2):246–254. doi: 10.1097/01.AOG.0000151116.84113.56. [DOI] [PubMed] [Google Scholar]
- 52.August P. Preeclampsia: new thoughts on an ancient problem. J Clin Hypertens (Greenwich) 2000;2(2):115–123. [PubMed] [Google Scholar]
- 53. Duley L, Gulmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;11:CD000025. doi: 10.1002/14651858.CD000025.pub2. This Cochrane review provides a systematic and up-to-date review of magnesium sulphate treatment.
- 54.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 55. Roberts JM, Myatt L, Spong CY, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282–1291. doi: 10.1056/NEJMoa0908056. The findings in this article provide important information on the treatment of preeclampsia.
- 56.Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2010;(8):CD001059. doi: 10.1002/14651858.CD001059.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Mizutani S, Wright JW, Kobayashi H. Placental leucine aminopeptidase- and aminopeptidase A- deficient mice offer insight concerning the mechanisms underlying preterm labor and preeclampsia. J Biomed Biotechnol. 2011;2011:286947. doi: 10.1155/2011/286947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.George EM, Cockrell K, Aranay M, et al. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57(5):941–948. doi: 10.1161/HYPERTENSIONAHA.111.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uddin MN, Horvat D, DeMorrow S, et al. Marinobufagenin is an upstream modulator of Gadd45a stress signaling in preeclampsia. Biochim Biophys Acta. 2011;1812(1):49–58. doi: 10.1016/j.bbadis.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 60.National Collaborating Centre for Women’s and Children’s Health. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. London: National Institute for Health and Clinical Excellence (CG107); 2010. [Accessed May 31, 2011]. http://www.nice.org.uk/nicemedia/live/13098/50475/50475.pdf. [Google Scholar]
- 61.Magee LA, Helewa M, Moutquin JM, von Dadelszen P Hypertension Guideline Committee, Society of Obstetricians and Gynaecologists of Canada. Prediction, prevention, and prognosis of preeclampsia. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3) Suppl 1:S16–S23. [Google Scholar]


