Abstract
Objective
To determine the effect of inhibiting aromatase activity on endometrial lesion growth and aromatase expression in a baboon model of induced endometriosis.
Design
Prospective Study
Setting
Institute of Primate Research, Nairobi, Kenya
Animals: 16 olive baboons
Intervention
16 olive baboons with induced endometriosis were examined with laparoscopy 10 months after disease inoculation. Animals in Group 1 (n=10) were treated with 1.25 mg/d of the aromatase inhibitor (AI) letrozole and animals in Group 2 (n=6) were given a placebo for a total of 6 months
Main Outcome Measure
Total number of endometriotic lesions, morphology and volume of leisions as well as semi-quantitative RT-PCR and quantitative PCR for levels of aromatase cytochrome mRNA were measured. Ovarian volumes were evaluated prior to treatment initiation and every 2 months during the study.
Results
Treatment of Group 1 animals with an AI significantly decreased lesion volume from baseline measurements whereas the placebo-treated animals showed an increase in lesion volume. Aromatase mRNA levels in lesions in the AI-treated animals were significantly lower compared with the placebo-treated animals. Ovarian volumes were significantly increased at six months of AI treatment compared to pre-treatment volumes.
Conclusions
These findings suggest that suppression of aromatase cytochrome P450 may inhibit the in vivo growth of endometriotic lesions in baboons.
Keywords: Endometriosis, aromatase inhibitors, baboon model, laparoscopy
INTRODUCTION
Endometriosis is a chronic, heterogeneous condition characterized by the growth of endometrial implants outside of the uterus, and affects 5–10% of reproductive-age women (1–3). Symptoms include dysmenorrhea, dyspareunia, pelvic pain, and infertility. We and others have demonstrated that estradiol (E2), the biologically active form of estrogen, is a potent mitogen that supports growth and inflammation processes in endometriotic lesions (1–7).
The local estrogen content of endometriosis lesions is highly correlated with the levels of steroidogenic enzyme aromatase cytochrome P450 (1, 8, 9). High levels of aromatase mRNA have been found in extraovarian endometriotic lesions and endometriomas when compared to normal endometrium (10). Androstenedione of adrenal and ovarian origin serves as the primary substrate for aromatase activity in endometriotic tissue, catalyzing the reaction to give rise to estrone, which is further converted to the more active estradiol (8, 11–16). Moreover, peritoneal and ovarian endometriotic tissues express the complete set of genes necessary for converting cholesterol to estradiol.
Estrogen production plays a key role in endometriosis, and is a key target for treatment. Its inhibition by GnRH analogues, oral contraceptives, progestins, and aromatase inhibitors (AIs) reduces pelvic disease and pain.(1, 17–19) GnRH analogues act by suppressing ovarian steroidogenesis, resulting in a hypo-estrogenic state; however, GnRH agonists do not block extra-ovarian sources of estrogen including the local production of estrogen by endometriotic lesions and peripheral tissue sites (such as adipose tissue and skin) (1, 11, 18–25). These tissue sources may give rise to significant circulating levels of estrogen despite the inhibitory action of GnRH agonists on ovarian estrogen production.
Aromatase is regulated at the levels of transcriptional expression, protein expression, and enzyme activity in endometriosis. (7, 26, 27). It is involved in a positive feedback loop that favors expression of key steroidogenic genes (1). Estrogen stimulates expression of the COX-2 enzyme, resulting in elevated levels of prostaglandin E2 (PGE2), which is a potent stimulator of aromatase activity in endometriosis (1). This leads to continuous local production of E2 and PGE2 in endometriotic tissue (1). In endometriosis, estrogens promote the growth and invasion of endometriotic tissue, while prostaglandins mediate pain, inflammation and infertility. Because of the integral role of aromatase and estrogens in endometriosis, AIs have been investigated as a potential treatment option for women with this disease (16, 18–20, 28).
A number of human studies have demonstrated that treatment with an AI reduces endometriosis-associated pain; however, only one study has demonstrated that AI treatment reduces laparoscopically visible pelvic endometriosis (17). Moreover, all previous studies in premenopausal women employed a combination of an AI and a second agent due to concerns that the AI would indirectly cause ovarian follicular development.(17, 18, 28–35) Thus, it remains unclear whether AI single-agent therapy can directly reduce pelvic endometriotic implant numbers or lesion size. We therefore conducted the current study in a non-human primate model to help answer this question.
For this study, we used baboons with experimentally induced endometriosis. The use of non-human primates is advantageous for the study of endometriosis because they represent a phylogenetically similar animal model to the human that allows for the evaluation of disease pathogenesis as well as the identification of therapeutic targets (36–42). We previously demonstrated significantly elevated aromatase mRNA in endometriotic lesions from baboons 10 months after experimentally induced endometriosis (13). Here, we used the identical animal model to determine whether the inhibition of aromatase enzyme activity can reduce pelvic endometriosis and decrease aromatase mRNA levels in endometriotic tissues.
MATERIALS AND METHODS
Animals
Sixteen female olive baboons (Papio anubis) of reproductive age and average weight of 10 ± 3 kg were housed in single cages at the Institute of Primate Research, Nairobi, Kenya. The animals were captured from the wild and underwent a mandatory three month quarantine period prior to inclusion in the study. All baboons had normal menstrual cycles, proven fertility, and a normal pelvis with no evidence of spontaneous endometriosis as demonstrated by laparoscopy before the beginning of the study. These animals were only used for this study. Baboon weights, menstrual cycles start date and duration were recorded. The baboons also had daily health checks to monitor for any signs, including distress, that would require veterinary intervention. The study protocol was approved by the Institutional Scientific Review and Animal Use and Care committees of the Institute of Primate Research, Nairobi, Kenya. All animals were humanely euthanized at the end of the study period with intravenous administration of an overdose of sodium pentobarbitone.
Induction of endometriosis
Endometriosis was experimentally induced using intra-peritoneal inoculation of eutopic endometrium as previously described (13, 40, 49). Menstrual tissue was obtained using the Pipel™ biopsy catheter (Cooper Surgical, Trumbull, CT) and the tissue was deposited into the pouch of Douglas, ovarian fossae, and the broad ligaments adjacent to the fallopian tubes by laparoscopic guidance.
Pre-treatment laparoscopic evaluation
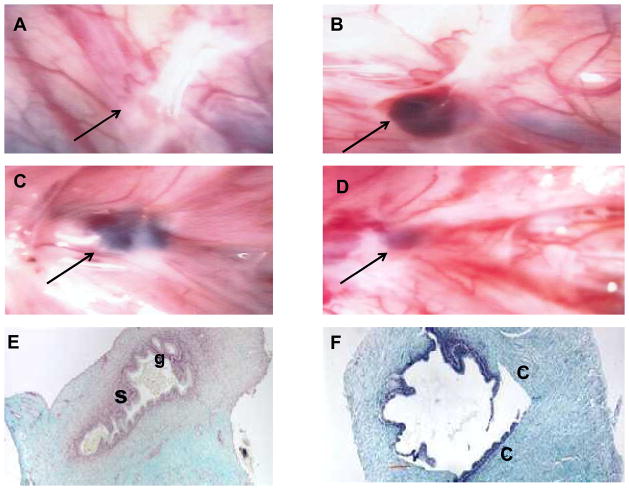
Endometriotic lesion development was documented laparoscopically 10 months after disease induction (Figure 1). Endometriotic lesions were characterized as having typical or suspicious appearance, similar to those seen in human females. Typical lesions were those noted to be black and blue cysts, white plaques with pigmentation, active red hemorrhagic vesicles, or nodules. Suspicious lesions were non-raised areas with orange-brown discoloration or irregular blood vessel patterns. Endometriosis was staged using the revised American Society for Reproductive Medicine classification system and adapted for use in baboons as previously described (44, 50–52).
Figure 1.
Representative laparoscopic photographs with endometriotic lesions (arrows) and histologic samples are shown. (A, B) Placebo group before (A) and after (B) treatment, showing progression of lesion size and change of morphology from subtle to typical blue-black lesion over time. (C,D) Letrozole group before (C) and after (D) treatment, showing lesion diminishing in size post-treatment. (E,F) Histology sections of a lesion treated with placebo (E). Active glandular epithelium [g] and stroma [s] are seen. The letrozole-treated lesion (F) shows inactive glands, absence of stroma, sloughing epithelia, and loose connective tissue [C] invasion.
All typical lesions were counted in each animal and categorized according to lesion volume using the formula Vlesion = a x b x c where a, b, and c represent lesion width, length, and height, respectively. The gross appearance and lesion volumes were also documented on pelvic maps, photographed, and recorded using video-laparoscopy equipment (Sony Corporation, Tokyo, Japan).
AI treatment
Treatment was started 10 months after disease induction immediately following the pre-treatment laparoscopy. The baboons were divided into 2 groups: Group 1 (n=10) was treated with the AI letrozole (Novartis, Basel, Switzerland), a potent inhibitor of aromatase P450 enzyme activity; Group 2 (n=6) was given a placebo and served as the control group. Both groups were administered a single 1.25-mg tablet (either a letrozole or a placebo tablet buried in a banana) at the same time daily for 6 months. During the treatment period, diagnostic laparoscopy was repeated every two months to visualize and measure the ovary size (since follicular cyst formation is a known side effect of AI use). At each laparoscopy, the pelvis and ovaries were examined systematically and the lesions were grossly visualized and counted. Exact measurements of lesions were only done at the pre-randomization and post-treatment laparoscopies.
Post-treatment endometriosis screening
Within a week following the sixth month of treatment, a post-treatment video-laparoscopy was carried out in all animals (n=16) to document changes in individual lesions by calculating the mean number and lesion volume per group. Additionally, we documented lesion regression as well as the gross presence of fibrosis on individual lesions, which indicated healing. The lesion number means were calculated by dividing the total number or volumes of lesions in each group by the number of animals in the group. During the final laparoscopy, excisional biopsies of endometriotic lesions in all 16 animals were taken. Fresh tissue samples were divided into two, with one sample frozen immediately in liquid nitrogen and transferred to −80°C for total RNA extraction at a later time, and a second sample fixed in 10% formalin for histologic analysis. Schematic representation of the treatment strategy is provided in Supplemental Figure 1.
Ovarian Volume assessment
To determine the effect of orally administered aromatase inhibitor on ovarian volume in baboons, we performed a video-laparoscopy one month before treatment, every two months during treatment and one month after treatment in both AI and placebo treated animals. Briefly the right and left ovary in every animal was measured on day 2 of their monthly estrus cycles with a graduated cannula. The length of each ovary was measured in an oblique sagittal section and the width and the height of the ovaries were measured in the frontal section after a 90° rotation of the measuring cannnular. The ovarian volume was calculated by using the formula for a prolate ellipsoid: (V = 4/3 × 3.14 × (d1 /2 × d2 /2 × d3/2) where d1 is length, d2 width and d3 the height of every ovary. An average of the volume of left and right ovary was calculated for every animal. Group means were then compared based on the averaged ovary volume from every individual baboon. All measurements were carried out by one observer to reduce inter-observer differences.
Histologic study
Formalin-fixed endometriotic lesions from letrozole or placebo-treated animals were embedded in paraffin and 4-μm sections were prepared and stained with gomori trichrome for morphologic evaluation. Lesions were observed under light microscopy for cellular effects of letrozole and placebo treatments. Endometriosis was diagnosed by the presence of both endometrial stromal cells and glands. Lesions containing only stroma were excluded from the study.
Semi-quantitative and quantitative RT-PCR
Total RNA from endometriotic tissue was isolated as previously described (53). Equal amounts of total RNA from both AI-treated (Group 1) and placebo-treated (Group 2) animals were analyzed for levels of aromatase mRNA using both semi-quantitative RT-PCR and real-time quantitative PCR. Briefly, cDNA was synthesized from 1 μg of total RNA using Superscript II RT (Invitrogen, Carlsbad, CA).
PCR was carried out using 2μl of cDNA, 2μl of 50 mM MgCl, 1 μl 10m M dNTP, 10 μM sense and antisense primers, and radiolabelled 32P-dCTP (Amersham Biosciences, Piscataway, NJ). Semi-quantitative RT-PCR was carried out using specific primers for the aromatase gene coding region. Expression of GAPDH was used as an internal control to normalize the amount of RNA used for each sample. The specific aromatase primers used to amplify cDNA fragments are shown in Supplemental Table 1. The amplification procedure consisted of initial incubation at 94°C for 2 min to inactivate the RT followed by incubation at 93°C for 1 min, 45°C for 1 min, and 72°C for 1 min for 38 cycles. An aliquot of the PCR product was fractionated on a 2% agarose gel. The gel with the separated cDNA fragments was dried, exposed to Xomat film, and developed. A scanning densitometer was used to determine the ratio of intensity of each DNA band relative to GAPDH. Densitometric analysis of the gel bands was performed using a Phosphor-Imager analyzer (Bio-Rad, Hercules, CA).
Real-time quantitative PCR analysis was performed using the same RNA samples and the Taqman analysis employing the ABI PRISM 7000 Detection system (ABI Biosystems, Carlsbad, CA).. cDNA was produced from 0.5 μg total RNA. Each sample was run in triplicate. The quantification relative to the housekeeping gene GAPDH was used to determine the level of the target gene as previously described (10).
Statistical analysis
Statistical analysis was performed using the analysis of variance (ANOVA) and paired and unpaired Student’s t test. Paired analyses were used when comparing pre-and post-treatment results in the AI treated group. Unpaired analyses were used when comparing AI treatment to controls. The data for ovarian volumes was log transformed prior to analysis. Significance was defined as P<0.05.
RESULTS
Successful experimental induction of endometriosis in baboons
To assess whether endometriosis was successfully induced in these animals, video-laparoscopy with a minimum of 5 biopsies in each animal was performed in each group 10 months after disease induction. Histologic analysis demonstrated that 80% of the lesions biopsied in each animal were consistent with endometriosis (Figure 1). The location and number of the lesions were not significantly different at this time between the treatment and control groups (Table 1, Supplemental Figure 2A;).
Table 1.
Comparison of Endometriotic Lesion Number and Volume Between Aromatase Inhibitor (AI) and Placebo (PLA) Treated baboons Pre and Post Treatment
| AI (n=10) | PLA (n=6) | p-value | ||
|---|---|---|---|---|
| # of total lesions (mean, median, range) | Pre | 83 (10±3, 11, 6–13) | 39 (8±2, 7, 7–10) | NS |
| # of total lesions (mean, median, range) | Post | 42#.* (5±1, 6, 3–7) | 65* (13±4, 13, 9–19) | P<0.05*,# |
| # Fibrotic post- treatment lesions (mean, median, range) | Post | 21 (3±1, 3, 1–4) | 0 | P<0.05* |
| Total Volumes (mm3) (Mean, median, range) | Pre | 81# (4±4, 2, 1–16) | 78 (3±1, 3, 1–8) | NS |
| Total Volumes (mm3) (Mean, median, range) | Post | 22#,* (1±1, 1, 1–13) | 52* (6±5, 5, 1–20) | P<0.05 *,# |
Significantly different from pre-treatment as calculated by an unpaired Student t-test between
Significantly different from pre-treatment AI group as calculated by a paired Student t-test
Letrozole decreases endometriotic lesion volume
In order to assess the in vivo role of aromatase suppression on the progression of endometriosis lesions, the volume of individual lesions were laparoscopically recorded before and after six months treatment in both groups. A total of 80% of the lesions were histologically proven endometriosis. The gross appearance of individual endometriotic lesions in the letrozole treated animals regressed in size, while an increase in size and morphology was noted in the placebo treated animals (Figure 1, Table 1, Supplemental Figure 2A;). A significant decrease from baseline in mean peritoneal endometriotic lesion volume as well as the total number of lesions was noted in the letrozole-treated group. Specifically, 49% of the lesions noted at the pre-treatment laparoscopy disappeared after 6 months of letrozole treatment. With regards to the lesions that did not disappear, AI treatment caused a 54% decrease in lesion size, with fibrotic scar appearing at the site of the lesion. Similar findings were seen when comparing the letrozole- and placebo-treated groups (Supplemental Figure 2A, Table 1, Supplemental Figure 2A). A significant 40% increase from baseline in total lesion number and the volume of the lesions was noted in the placebo-treated group (Table 1; Supplemental Figure 2A).
Aromatase cytochrome mRNA expression levels are decreased in endometriotic tissue from animals treated with letrozole
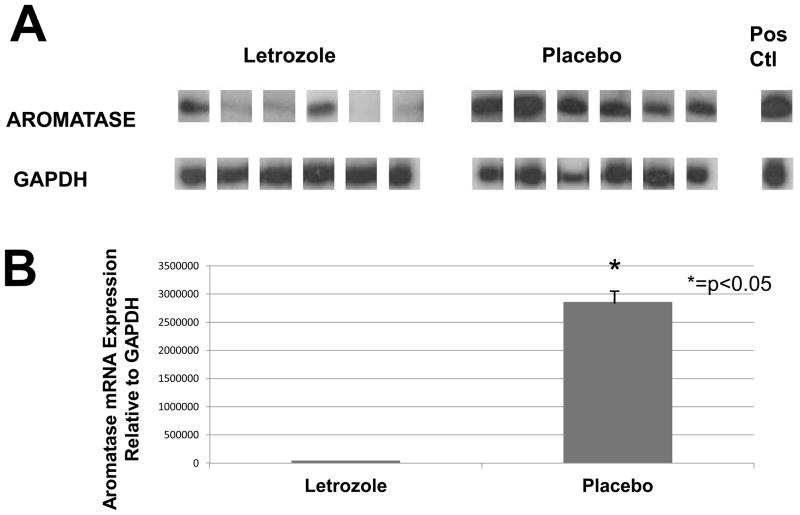
Semi-quantitative RT-PCR (Figure 2A) was used to detect and compare aromatase mRNA levels in endometriotic lesions obtained from baboons after 6 months of letrozole or placebo treatment. A significantly lower aromatase mRNA level was observed in the endometriotic tissue of letrozole-treated animals compared with placebo-treated animals (P<0.05). The results of quantitative PCR are shown in Figure 2B. The observed aromatase cDNA levels were significantly higher in the control animals as compared to the AI-treated animals (P<0.05), consistent with the results of the semiquantitative RT-PCR analysis.
Figure 2.
Aromatase expression in endometriotic tissue. (A) Representative semi-quantitative RT-PCR for aromatase cytochrome P450 expression in endometriotic tissue from 6 letrozole-treated and 6 placebo-treated treated animals. Adipose tissue was used as positive control. Post-treatment expression of aromatase was significantly lower with letrozole compared with placebo (P<0.05). (B) Quantitative PCR shows a significantly lower level of lesion aromatase expression with letrozole treatment vs. placebo treatment (p<0.05) (units of measure= fluorescence units).
Changes in weight, menstrual cyclicity and ovarian volumes
The changes in group mean baboon weight and their monthly menstrual cycle duration were compared within groups during pre-treatment, treatment and after treatment periods of the study. These parameters did not differ (Table 2, p >.05).
Table 2.
Weight and Menstrual Cycle Length Endometriosis-Induced Baboons Treated with Aromatase Inhibitor (AI) or Placebo (PLA)
| WEIGHT (kg) | MENSTRUAL CYCLE LENGTH (DAYS) | |||
|---|---|---|---|---|
| AI | PLA | AI | PLA | |
| Pre -Treatment (mean, SD) | 11.9 ± 2.1 | 13.4 ± 2.6 | 34 ± 3 | 34 ± 3 |
| Treatment (mean, SD) | 12.7 ± 2.1 | 13.5 ± 2.5 | 34 ± 3 | 34 ± 2 |
| Post- Treatment (mean, SD) | 12.7 ± 1.5 | 13.7 ± 0.9 | 32 ± 3 | 33 ± 3 |
Values are means ± Standard deviation (SD). There were no statistically significant differences in weight or menstrual cycle length noted pre-treatment, during treatment of after treatment in either group.
The mean ovarian volume significantly increased with AI treatment (Supplemental Figure 2B, p <.05). A similar increase in ovarian size was not noted in placebo-treated animals.
DISCUSSION
There are many uncontrollable and unknown factors involved with the induction and progression of endometriosis, making it a particularly difficult disorder to study. In addition, the time from onset of disease to diagnosis can range between 8–11 years in women (54). Therefore, an animal model with experimentally induced disease allows investigators to characterize factors involved with the initial onset of disease and throughout disease progression. This type of model is also conducive to investigating the efficacy of various treatments. Baboons remain one of the best animal models in which to study endometriosis (36, 48). The female reproductive anatomy and physiology are similar in baboons and humans. Retrograde menses has been reported as a frequent natural phenomenon in baboons (44). In addition, spontaneous endometriosis can be seen in these animals (45). The body size of the baboon also allows for multiple surgical procedures and repeated collection of samples including blood, tissue, and peritoneal fluid. Therefore, the baboon has emerged a particularly useful model to study the pathophysiology and progression of endometriosis (36, 48).
The present study describes important experimental evidence suggesting that aromatase cytochrome P450 may play an important role in the growth of peritoneal endometriotic lesions, and demonstrates the therapeutic potential of using an AI for slowing the progression of endometriosis. This study supports clinical data showing that women with endometriosis respond to therapies directed at inhibiting aromatase action, and provides visual, histologic, and molecular evidence of the AI mechanism of action in endometriosis (17–21, 28, 29). In addition, similar experiments in a mouse model have shown a resolution of ectopic endometriotic lesions when aromatase is suppressed with inhibitors (12).
We also found that treatment with an AI caused a regression of endometriotic lesions (Figure 1, Table 1, Supplemental Figure 2). This observation underscores the importance of aromatase cytochrome P450 for maintenance of local estrogen concentrations needed for the perpetuation and progression of endometriotic disease (1, 16, 19). In placebo-treated animals, endometriosis disease progression was consistent with what has been previously described (45, 49). Our results are also consistent with the study by Ailawadi et al., which showed lesion regression in women treated with a combination of AI and a progestin (17). Our study, however, used an AI as a single agent, something that has not been done in humans because of the potential effects on the pre-menopausal ovary.
AIs are known to inhibit estrogen production in different body sites, including the brain, ovary, and peripheral tissues (including adipose tissue and skin) (16, 20). In humans, local estrogen production by the brain is, in part, responsible for the suppression of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion. The amount of aromatase in the brain or periphery is small compared with the overwhelming levels of aromatase in granulosa cells of the human antral follicles. Thus, it is likely that while AIs completely inhibit aromatase activity in the brain and periphery, only a fraction of the aromatase activity in the ovary is blocked. In women, there is a compensatory response to E2 depletion, in which the hypothalamus stimulates higher serum FSH secretion and ovarian stimulation. Therefore, when given to women of reproductive age, AIs increase follicular recruitment and may lead to ovarian stimulation and cyst formation (55). Because of this, when AIs are given in premenopausal women, additional drugs are used to effectively down-regulate gonadal estrogen biosynthesis and prevent ovarian cyst formation. In this study, AI treatment did give rise to an increase in ovarian volume in the baboon (Supplemental Figure 2B). However, baboon menstrual cycles were not altered because of this treatment, indicating that this increase in ovarian volume did not affect menstrual regularity (Table 2).
Studies by our laboratory and others have demonstrated an intracrine effect of estrogen in endometriosis (12, 13, 16, 19, 21–25, 27, 56–59). Estrogen produced by aromatase activity in the cytoplasm of endometriotic stromal cells can exert its effects by binding to its own nuclear receptor. Our findings are consistent with those of many human studies reporting high aromatase expression in endometriosis (Figures 2A, 2B). Endometrium obtained from women without endometriosis, however, has been found to lack aromatase expression (22, 57–59).
There are several limitations to our study. We did not measure systemic hormonal levels or collect peritoneal fluid for assessment of inflammatory cytokines. Many of the animals did not have any peritoneal fluid at the time of surgery. Systemic hormone measurements were not done because of cost. Bone density measurements were also not done because of cost.
In conclusion, this study suggests that aromatase cytochrome P450 plays an important role in the pathophysiology of endometriosis in baboons, and that altering the activity of aromatase with AIs may decrease disease burden.
Supplementary Material
Schematic diagram of treatment regiments.
A: Lesion volume before and after treatment with letrozole or placebo (A) Mean surface volume(square millimeters) of peritoneal endometriosis lesions before and after treatment shows a significant decrease in lesion volume with letrozole (*=p<0.05). A significant increase in lesion volume from baseline was noted in the placebo-treated group (*=p<0.05). In addition, letrozole significantly decreased lesion volume compared with placebo (*=p<0.05)(see also Table 3) B: Mean Ovarian volume before (PRE) and duirng 6 months (MO) Aromatase inhibitor (AI) treatment in female baboons (N = 10). The mean of the left and right ovarian volume in individual baboons was used to calculate the group mean. There was a statistically significant difference in post-treatment ovarian volume compared to pre-treatment ovarian volume. (*= p = .05).
Acknowledgments
Funding: These studies were supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD 40093 to ATF as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and a NIH Fogarty Award TW 01339 to ATF and SB; MEP is funded by K12HD050121
The authors would like to acknowledge the contributions of Dr. Idle Farah and Dr. Hastings Ozwara for editorial assistance and Mr. Habib Saibulu and Abdul Majid Maritim for surgical assistance. All of these individuals are based at Institute of Primate Research in Kenya.
REEFERENCES
- 1.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC. Clinical practice. Endometriosis The New England journal of medicine. 2010;362:2389–98. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Seminars in reproductive medicine. 2010;28:36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SF, Roberts NJ, Partridge MR. Comparison of a web-based package with tutor-based methods of teaching respiratory medicine: subjective and objective evaluations. BMC Med Educ. 2007;7:41. doi: 10.1186/1472-6920-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, et al. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002;8:1103–10. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- 7.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal VR, Ashanullah CI, Simpson ER, Bulun SE. Alternatively spliced transcripts of the aromatase cytochrome P450 (CYP19) gene in adipose tissue of women. The Journal of clinical endocrinology and metabolism. 1997;82:70–4. doi: 10.1210/jcem.82.1.3655. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. The Journal of clinical endocrinology and metabolism. 1996;81:3843–9. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 10.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. The Journal of clinical endocrinology and metabolism. 2009;94:623–31. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulun SE. Aromatase deficiency and estrogen resistance: from molecular genetics to clinic. Seminars in reproductive medicine. 2000;18:31–9. doi: 10.1055/s-2000-13481. [DOI] [PubMed] [Google Scholar]
- 12.Fang Z, Yang S, Gurates B, Tamura M, Simpson E, Evans D, et al. Genetic or enzymatic disruption of aromatase inhibits the growth of ectopic uterine tissue. The Journal of clinical endocrinology and metabolism. 2002;87:3460–6. doi: 10.1210/jcem.87.7.8683. [DOI] [PubMed] [Google Scholar]
- 13.Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertility and sterility. 2003;80 (Suppl 2):820–7. doi: 10.1016/s0015-0282(03)00982-8. [DOI] [PubMed] [Google Scholar]
- 14.Simpson ER, Mahendroo MS, Nichols JE, Bulun SE. Aromatase gene expression in adipose tissue: relationship to breast cancer. Int J Fertil Menopausal Stud. 1994;39 (Suppl 2):75–83. [PubMed] [Google Scholar]
- 15.Simpson ER, Zhao Y, Agarwal VR, Michael MD, Bulun SE, Hinshelwood MM, et al. Aromatase expression in health and disease. Recent Prog Horm Res. 1997;52:185–213. discussion -4. [PubMed] [Google Scholar]
- 16.Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49–56. doi: 10.1093/humupd/dmi034. [DOI] [PubMed] [Google Scholar]
- 17.Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertility and sterility. 2004;81:290–6. doi: 10.1016/j.fertnstert.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Amsterdam LL, Gentry W, Jobanputra S, Wolf M, Rubin SD, Bulun SE. Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertility and sterility. 2005;84:300–4. doi: 10.1016/j.fertnstert.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Bulun SE, Fang Z, Imir G, Gurates B, Tamura M, Yilmaz B, et al. Aromatase and endometriosis. Seminars in reproductive medicine. 2004;22:45–50. doi: 10.1055/s-2004-823026. [DOI] [PubMed] [Google Scholar]
- 20.Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertility and sterility. 2006;85:1307–18. doi: 10.1016/j.fertnstert.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 21.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Zhou J, et al. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79:19–25. doi: 10.1016/s0960-0760(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 22.Bulun SE, Zeitoun K, Takayama K, Noble L, Michael D, Simpson E, et al. Estrogen production in endometriosis and use of aromatase inhibitors to treat endometriosis. Endocr Relat Cancer. 1999;6:293–301. doi: 10.1677/erc.0.0060293. [DOI] [PubMed] [Google Scholar]
- 23.Bulun SE, Zeitoun KM, Takayama K, Sasano H. Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance. J Mol Endocrinol. 2000;25:35–42. doi: 10.1677/jme.0.0250035. [DOI] [PubMed] [Google Scholar]
- 24.Bulun SE, Zeitoun KM, Takayama K, Sasano H. Molecular basis for treating endometriosis with aromatase inhibitors. Hum Reprod Update. 2000;6:413–8. doi: 10.1093/humupd/6.5.413. [DOI] [PubMed] [Google Scholar]
- 25.Bulun SE, Zeitoun KM, Takayama K, Simpson E, Sasano H. Aromatase as a therapeutic target in endometriosis. Trends Endocrinol Metab. 2000;11:22–7. doi: 10.1016/s1043-2760(99)00216-7. [DOI] [PubMed] [Google Scholar]
- 26.Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biology of reproduction. 1997;57:514–9. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- 27.Bulun SE, Imir G, Utsunomiya H, Thung S, Gurates B, Tamura M, et al. Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol. 2005;95:57–62. doi: 10.1016/j.jsbmb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Ferrero S, Venturini PL, Gillott DJ, Remorgida V. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: a randomized controlled trial. Reprod Biol Endocrinol. 2011;9:88. doi: 10.1186/1477-7827-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soysal S, Soysal ME, Ozer S, Gul N, Gezgin T. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Hum Reprod. 2004;19:160–7. doi: 10.1093/humrep/deh035. [DOI] [PubMed] [Google Scholar]
- 30.Remorgida V, Abbamonte LH, Ragni N, Fulcheri E, Ferrero S. Letrozole and desogestrel-only contraceptive pill for the treatment of stage IV endometriosis. Aust N Z J Obstet Gynaecol. 2007;47:222–5. doi: 10.1111/j.1479-828X.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 31.Remorgida V, Abbamonte HL, Ragni N, Fulcheri E, Ferrero S. Letrozole and norethisterone acetate in rectovaginal endometriosis. Fertility and sterility. 2007;88:724–6. doi: 10.1016/j.fertnstert.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Lall Seal S, Kamilya G, Mukherji J, De A, Ghosh D, Majhi AK. Aromatase inhibitors in recurrent ovarian endometriomas: report of five cases with literature review. Fertility and sterility. 2011;95:291, e15–8. doi: 10.1016/j.fertnstert.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Ferrero S, Venturini PL, Ragni N, Camerini G, Remorgida V. Pharmacological treatment of endometriosis: experience with aromatase inhibitors. Drugs. 2009;69:943–52. doi: 10.2165/00003495-200969080-00001. [DOI] [PubMed] [Google Scholar]
- 34.Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24:3033–41. doi: 10.1093/humrep/dep302. [DOI] [PubMed] [Google Scholar]
- 35.Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Seracchioli R, et al. Letrozole and norethisterone acetate in colorectal endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;150:199–202. doi: 10.1016/j.ejogrb.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod. 2009;15:577–86. doi: 10.1093/molehr/gap057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazleabas AT. A baboon model for simulating pregnancy. Methods Mol Med. 2006;121:101–10. doi: 10.1385/1-59259-983-4:099. [DOI] [PubMed] [Google Scholar]
- 38.Fazleabas AT. A baboon model for inducing endometriosis. Methods Mol Med. 2006;121:95–9. doi: 10.1385/1-59259-983-4:093. [DOI] [PubMed] [Google Scholar]
- 39.Fazleabas AT, Brudney A, Chai D, Mwenda J. Endometriosis in the baboon. Gynecol Obstet Invest. 2004;57:46–7. [PubMed] [Google Scholar]
- 40.Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann N Y Acad Sci. 2002;955:308–17. doi: 10.1111/j.1749-6632.2002.tb02791.x. discussion 40–2, 96–406. [DOI] [PubMed] [Google Scholar]
- 41.Fazleabas AT, Kim JJ, Srinivasan S, Donnelly KM, Brudney A, Jaffe RC. Implantation in the baboon: endometrial responses. Semin Reprod Endocrinol. 1999;17:257–65. doi: 10.1055/s-2007-1016233. [DOI] [PubMed] [Google Scholar]
- 42.Fazleabas AT, Strakova Z, Kim JJ. Implantation in the baboon. Gynecol Obstet Invest. 2004;57:30–1. [PubMed] [Google Scholar]
- 43.Banaszak S, Brudney A, Donnelly K, Chai D, Chwalisz K, Fazleabas AT. Modulation of the action of chorionic gonadotropin in the baboon (Papio anubis) uterus by a progesterone receptor antagonist (ZK 137. 316) Biology of reproduction. 2000;63:820–5. doi: 10.1095/biolreprod63.3.820. [DOI] [PubMed] [Google Scholar]
- 44.D’Hooghe TM, Bambra CS, Cornillie FJ, Isahakia M, Koninckx PR. Prevalence and laparoscopic appearance of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) Biology of reproduction. 1991;45:411–6. doi: 10.1095/biolreprod45.3.411. [DOI] [PubMed] [Google Scholar]
- 45.D’Hooghe TM, Bambra CS, De Jonge I, Lauweryns JM, Koninckx PR. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75:98–101. doi: 10.3109/00016349609033298. [DOI] [PubMed] [Google Scholar]
- 46.D’Hooghe TM, Bambra CS, Isahakia M, Koninckx PR. Evolution of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) over a 12-month period. Fertility and sterility. 1992;58:409–12. [PubMed] [Google Scholar]
- 47.D’Hooghe TM, Bambra CS, Xiao L, Peixe K, Hill JA. Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus) American journal of obstetrics and gynecology. 2001;184:917–25. doi: 10.1067/mob.2001.111715. [DOI] [PubMed] [Google Scholar]
- 48.Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;4 (Suppl 1):S7. doi: 10.1186/1477-7827-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis) American journal of obstetrics and gynecology. 1995;173:125–34. doi: 10.1016/0002-9378(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 50.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility and sterility. 1997;67:817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 51.Revised American Fertility Society classification of endometriosis: 1985. Fertility and sterility. 1985;43:351–2. doi: 10.1016/s0015-0282(16)48430-x. [DOI] [PubMed] [Google Scholar]
- 52.Classification of endometriosis. The American Fertility Society. Fertility and sterility. 1979;32:633–4. [PubMed] [Google Scholar]
- 53.Glisin V, Crkvenjakov R, Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974;13:2633–7. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- 54.Stratton P, Winkel CA, Sinaii N, Merino MJ, Zimmer C, Nieman LK. Location, color, size, depth, and volume may predict endometriosis in lesions resected at surgery. Fertility and sterility. 2002;78:743–9. doi: 10.1016/s0015-0282(02)03337-x. [DOI] [PubMed] [Google Scholar]
- 55.Schoolcraft WB, Surrey ES, Minjarez DA, Stevens JM, Gardner DK. Management of poor responders: can outcomes be improved with a novel gonadotropin-releasing hormone antagonist/letrozole protocol? Fertility and sterility. 2008;89:151–6. doi: 10.1016/j.fertnstert.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–83. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 57.Bulun SE, Noble LS, Takayama K, Michael MD, Agarwal V, Fisher C, et al. Endocrine disorders associated with inappropriately high aromatase expression. J Steroid Biochem Mol Biol. 1997;61:133–9. [PubMed] [Google Scholar]
- 58.Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. The Journal of clinical endocrinology and metabolism. 1996;81:174–9. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- 59.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–6. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic diagram of treatment regiments.
A: Lesion volume before and after treatment with letrozole or placebo (A) Mean surface volume(square millimeters) of peritoneal endometriosis lesions before and after treatment shows a significant decrease in lesion volume with letrozole (*=p<0.05). A significant increase in lesion volume from baseline was noted in the placebo-treated group (*=p<0.05). In addition, letrozole significantly decreased lesion volume compared with placebo (*=p<0.05)(see also Table 3) B: Mean Ovarian volume before (PRE) and duirng 6 months (MO) Aromatase inhibitor (AI) treatment in female baboons (N = 10). The mean of the left and right ovarian volume in individual baboons was used to calculate the group mean. There was a statistically significant difference in post-treatment ovarian volume compared to pre-treatment ovarian volume. (*= p = .05).