Abstract
Background
Behavioral studies suggest that alcohol intoxication impairs speed and accuracy of word recognition and categorization, but alcohol’s effects on the brain during verbal cognitive processing have not been adequately understood. Using event-related potentials (ERP) and a word recognition paradigm, this study investigated the effects of alcohol intoxication on prelexical, semantic, and mnemonic aspects of verbal processing.
Methods
Concurrent measures of ERPs and skin conductance responses (SCRs) were obtained in a word repetition priming task and permitted a comparison of the effects of alcohol on the central and autonomic physiological systems. Social drinkers participated in all four cells of the within-subjects balanced placebo design in which effects of alcohol and instructions as to the beverage content (expectancy) were manipulated. The average peak blood alcohol level was raised to 0.045%.
Results
None of the manipulations affected behavioral performance and expectancy had no effect on any of the measures. In contrast, alcohol ingestion attenuated the temporo-parietal N180 suggesting an impairment in prelexical pattern recognition processes. Alcohol significantly increased the amplitude of N450 and the latency of P580, particularly on trials evoking sympathetic arousal as measured with SCRs.
Conclusions
Although behavioral measures were unaffected, ERPs showed that a moderately low alcohol dose affected verbal processing during both early, prelexical and late, semantic stages. Alcohol significantly increased the difficulty of semantic access and integration as reflected in larger N450 amplitude and longer P580 latency. This effect was particularly prominent on arousal-related trials, suggesting that alcohol impairs processes that modulate cognitive functioning. The lack of an interaction between the factors of repetition and beverage suggests that a moderately low alcohol dose exerts these effects via the semantic and integration systems rather than via memory processes.
Keywords: Alcohol, Verbal processing, Event-related potentials, N400, Electrodermal activity
INTRODUCTION
It has been established that alcohol intoxication affects multiple cognitive and psycho-motor functions including verbal processing and memory. Intoxication impairs reaction speed and accuracy in word categorization, recall and recognition tasks (Erblich and Earleywine, 1995; Haut et al., 1989; Maylor et al., 1987; Miller et al., 1978; Mungas et al., 1994; Williams and Rundell, 1984). Furthermore, paired-associates learning and verbal fluency are decreased by acute alcohol intake (Hartcollis and Johnson, 1956; Peterson et al., 1990). However, in spite of this behavioral evidence, the effects of alcohol on the brain during verbal processing and memory have not been adequately studied. Based on their capacity to reflect synaptic current flows that carry out cortical information processing with millisecond accuracy, event-related potentials (ERPs) could offer important insights into the effects of alcohol on neurophysiological stages of verbal processing.
Most of the studies investigating changes in the ERPs after acute intoxication or a chronic abuse have used different versions of an “oddball” paradigm requiring detection of a rarely presented target among the standard stimuli in auditory, visual and somatosensory modalities. Chronic alcohol use affects late endogenous potentials (primarily reflected in a smaller P3 amplitude) across sensory modalities and under different experimental conditions, possibly indicating abnormalities in multiple functional systems in the brain. Irreversibility of these effects even after long abstinence (Porjesz and Begleiter, 1987), in conjunction with evidence obtained from high-risk individuals (Rodriguez Holguin et al., 1999), has given rise to a vulnerability marker hypothesis whereby attenuated P3 amplitude may suggest a genetic susceptibility to alcohol dependence (Begleiter and Porjesz, 1999; Monteiro and Schuckit, 1988; Pfefferbaum et al., 1991). The most common finding of the studies investigating effects of alcohol intoxication on healthy social drinkers, is also attenuated P3 deflection (Jääskeläinen et al., 1996; Marinkovic et al., 2001; Porjesz and Begleiter, 1981, 1985, 1996). This effect is inversely related to the alcohol dose (Rohrbaugh et al., 1987; Teo and Ferguson, 1986) and is modulated by task difficulty (Campbell et al., 1984). However, such simple, nonsemantic paradigms may engage different brain processes than the cognitively more challenging tasks involving verbal material that rely on semantic and memory networks (Halgren, 1990a; Halgren et al., 1998). Consequently, although the effects of acute alcohol intoxication on ERPs during simple discrimination tasks under various conditions have been described, evidence on the effects of alcohol on stages of verbal memory processing is still lacking. Of particular interest is a negativity peaking at about 400 ms (N400) which has been studied extensively in language studies. The N400 is evoked by potentially meaningful material such as words (written, spoken or signed) and pictures and is thought to reflect access to a semantic network and the ease of semantic contextual integration (Brown and Hagoort, 1993; Halgren, 1990b; Holcomb, 1993; Kutas and Van Petten, 1988).
In addition to its deleterious effects on ERPs, a central measure of stimulus processing, alcohol affects autonomic functioning as well (Lyvers and Maltzman, 1991; Richter et al., 1977). Skin conductance responses (SCRs) are a good indicator of orienting response (OR) and sympathetic arousal (Boucsein, 1992) and can offer a good complement to the ERP indices of central activity. Indeed, concurrent measures of ERPs and SCRs revealed that alcohol selectively attenuated the P3a to novel sounds only on trials that also evoked SCR (Marinkovic et al., 2001). Thus, utilization of ERP measures in conjunction with autonomic measures may help discern the selective effects of alcohol on central word processing and sympathetic arousal as well as the interplay between the two physiological systems. It may provide insight into modulatory brain processes (e.g. arousal circuits) that aid and moderate cognition.
A repetition priming paradigm that probes verbal recognition memory was employed in this study since it yields a robust effect on the ERPs (Rugg, 1985; Smith and Halgren, 1987). Furthermore, neural generators of the elicited ERP deflections have been studied extensively (Halgren et al., 1994a; Halgren et al., 1994b; Nobre et al., 1994). This approach allowed an investigation of selective effects of a moderately low alcohol dose on early prelexical and late semantic verbal processing stages, as well as its effects on memory processes. A within-subject placebo design permitted an examination of the pharmacological effects of alcohol versus the instructions as to the beverage content (expectancy), with the same subjects serving as their own controls.
METHODS AND MATERIALS
Subjects
Participants in this study were screened for their past and present alcohol, tobacco and drug use habits, alcohol or drug-related treatment, medical problems, handedness and age. The subjects were recruited from an advertisement in the campus newspaper and from another study. They were all normal, healthy, non-smoking right-handed males, native English speakers with no alcohol or drug-related problems. Their answers on the adapted Alcohol Use Questionnaire (Mills et al., 1983) indicated that they drank alcohol occasionally (two times per week on average) and in low to moderate amounts (3.4 drinks per occasion). MAST (Selzer, 1971) indicated no alcoholism-related symptoms and the subjects reported no family history of alcoholism or drug abuse. In addition, their responses on the Childhood Hyperactivity Questionnaire (HK/MBD) (Tarter et al., 1977), Eysenck Personality Questionnaire (EPQ) (Eysenck and Eysenck, 1975) and Socialization Scale (SSQ) of the California Psychological Inventory (Gough, 1994) were within normal range.
Out of fifteen participants, twelve subjects (mean age = 23.6, SD = 2.6 yrs) completed all four experimental sessions, yielding a total of 48 recording sessions. Prior to the experiment, they all participated in an introductory recording session in order to become familiarized with the laboratory setting and experimental procedures. No drinks were administered at this time but the subjects underwent a brief recording and filled out questionnaires. All individuals gave their written consent approved by the human subject protection review board to participate in the study and were monetarily reimbursed.
Experimental Design and Procedure
A within subject design was employed in this study so that subjects could serve as their own controls in physiological measurements and intoxication manipulations. Balanced placebo design was used in an attempt to assess the effects of beverage content and the effects of instructions as to the beverage content (expectancy). The factors of beverage and expectancy were fully crossed and the participants were tested under all four experimental conditions. The consumed beverage and information concerning the alcohol content varied across sessions but otherwise the same procedure was utilized in all four randomly ordered sessions. In order to maximize credibility of deception conditions (e.g., subjects were sometimes told that they will receive placebo when in fact they consumed alcohol), a low-to-moderate alcohol dose significantly below the legal intoxication level was utilized.
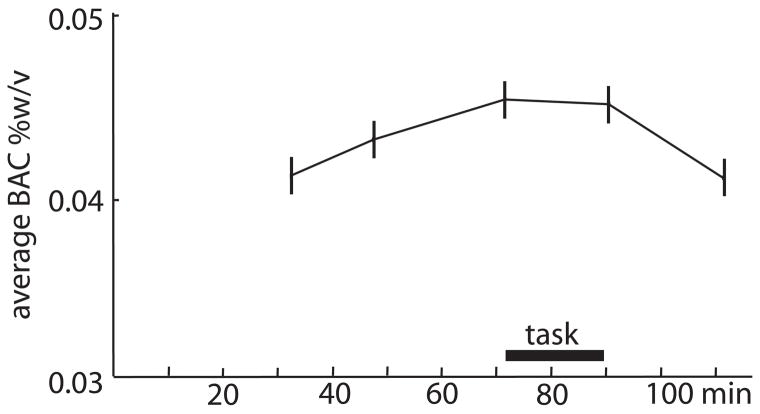
The recording sessions were scheduled at least 48 hours apart and started between 3 and 4 pm in order to minimize potential variability in alcohol metabolism and circadian rhythms. Participants were asked to abstain from alcohol for 24 hours, and from food for 3 hours prior to the experiment. After taking a breathalyzer reading, the experimenter informed the participants about the designated drink for that session and brought in a tray with the appropriate cues (e.g. a vodka bottle). The subjects finished drinking in 10–15 minutes. Following the drink consumption the participants were fitted with the recording electrodes. During the task, they reclined comfortably in an armchair in an electrically shielded room and indicated their responses with a hand-held microswitch. In addition to this task, the protocol included a mood-rating questionnaire and a simple tone discrimination task (Marinkovic et al., 2001). The blood alcohol concentration (BAC) was monitored throughout the experiment with an Alco-sensor III breathalyzer (Intoximeters, Inc.). The task was administered between 72 and 90 min after the subjects were presented with their drinks. The average BAC reached a peak (0.0453%) immediately before the task and remained almost the same (0.045%) when measured immediately after the task (Fig 1). However, because of the interindividual variability in metabolic rate (Reed, 1985), some of the subjects were already on the descending limb of the BAC when others were reaching the peak BAC. Even though the average peak BAC was reached quite late in this study, Fig 1 illustrates its “protracted” nature, suggesting that near-peak BAC was maintained for extended period of time on average. Subjects filled out a short version of the Profile of Mood States (POMS) (McNair et al., 1981) that contained questions about their feelings of “high”. At the end of each experimental session the participants filled out a detailed questionnaire consisting of Likert scales (1–5) querying them about the type and content of the beverage they were given, about their feeling of intoxication, about the task difficulty etc. They were offered warm sandwiches and were invited to read or play computer games. They remained in the laboratory until their BAC diminished to negligible (i.e. 0.01%) levels.
Figure 1.
Average blood alcohol concentration (BAC) ± standard errors of the mean are presented for each measurement point in minutes after the start of drinking. The task was performed at near-peak levels on average, as the BAC levels were 0.04527 %w/v immediately before and 0.045 %w/v immediately after the task.
Beverage Administration
Smirnoff 80 proof vodka (40% alcohol by volume) was administered at a dosage of 0.4 g of ethanol per kg of body weight mixed with chilled grapefruit fruit juice and pineapple-orange-guava frozen concentrate in a 1:5.5 ratio based on a previously conducted pilot study. The beverage administration included the cues (e.g. vodka bottle) appropriate to the instructional “expectancy” condition procedure (Rohsenow and Marlatt, 1981). In the Given Juice/Told Alcohol condition strong olfactory cues were provided by a small piece of vodka-saturated gauze placed in the cap of the bottle unbeknown to participants.
Task
Subjects were instructed to memorize a list of twenty words presented individually on a computer screen for 300 ms every 4 sec, subtending a visual angle of 3–50. At all other times, a fixation target consisting of five star characters was shown at the same screen location. Immediately afterwards, a series of 200 words was presented within the recognition task. Ten words from the initially memorized list were randomly chosen as “target” words requiring a button-press and were shown on half of the trials mixed in among the new, unlearned words. A feedback tone, 100 ms duration, occurred 1500 ms after word onset informing the participants whether their response was correct, high pitch - 500 Hz, or not, low pitch - 100 Hz. All the words were 4–6 letters long, equated across lists on their imagery, concreteness and frequency of occurrence on the basis of published norms (Francis and Kucera, 1982; Paivio et al., 1968). The words had low frequency of occurrence (one to seven per million) and were presented in a random order. A different set of words was used in each session in a randomized manner.
Data Recording
ERPs
The electroencephalogram (EEG) was recorded with a lycra fitted electrode cap (Electro-Cap International, Inc.) from 13 scalp sites: Fz, Cz, Pz, F3, F4, F7, F8, C3, C4, P3, P4, T5, T6 of the 10–20 International system with the electrode on the tip of the nose serving as a reference and the right earlobe as ground. The electrooculogram (EOG) was recorded with bipolarly referred electrodes placed at the outer canthus of the right eye and just above the nasion. The electrode impedance was kept below 5 kOhms. The EEG and EOG were recorded with a Grass 16-channel polygraph with a bandpass of 0.05 to 75 Hz (1/2 amplitude) and were digitized at a rate of 200 Hz (12-bit accuracy).
Only the trials with correct responses and without eyeblinks or other artifacts were included in the analyses, yielding 90.8 (SD = 5.1) and 91.2 (SD = 6.9) trials in averages of new and repeated words respectively. Average voltages were quantified with an automatic algorithm within the following latency windows (corresponding peak latencies): 135–265ms (P180); 270–370ms (N310); 420–480ms (N450); 510–610ms (P580). In addition, peak amplitude and latency of the late positivity were measured within 450–700 ms window. All measures were expressed in microvolts (amplitudes) and milliseconds (latencies) with respect to a baseline period of 100 ms before stimulus onset.
SCRs were recorded from Beckman Ag-AgCl electrodes filled with K-Y jelly placed with adhesive collars (1 cm in diameter) on the volar surface of the first and third fingertips of one hand. Participants were pressing the microswitch with the other hand. The left hand/right hand order was counterbalanced across the four sessions. The SCRs were recorded with Coulbourn Instruments Skin Conductance amplifier through a constant 0.5 volt bridge circuit. The SCR signal was digitized at 20 Hz for 100 ms before each word onset and continued for 3600 ms.
SCRs were measured on each trial with a semi-automatic computer algorithm and were defined as a maximum response within a latency window from 0.5 to 3.5 s post-stimulus onset. In order to ameliorate their skewed distribution, the SCRs were transformed with a square root function. Only the SCRs measured on trials with correct responses were accepted in the analyses that included the following measures: average SCR - response magnitudes averaged across all trials for new and repeated words; and block SCR - response magnitude averages of five consecutive trials for each stimulus type resulting in 20 blocks across the course of the experiment, allowing an insight into response dynamics across the task. Due to equipment failure, SCR data of two participants (each during one experimental session) were not recorded.
Data Analysis
Repeated measures ANOVAs with factors: beverage (given alcohol or placebo), instructions (told alcohol or placebo) and repetition (new vs. repeated) were performed on all measures. Additionally, a factor of electrode sites was included in the ERP analyses. Each of the five ERP time windows was analyzed separately with a repeated measures ANOVA. In order to provide a conservative protection against sphericity assumption violations in the repeated measures ANOVA, the p-values were adjusted with the Huynh-Feldt procedure (Huynh and Feldt, 1980). When simple main effects were investigated, Tukey post hoc procedure (Woodward et al., 1990) was utilized as a protection against inflated probability values. These methods were applied to all analyses and for all response systems.
RESULTS
A nearly perfect recognition performance (mean = 98.3%, SD = 1.8) and reaction times (mean = 641.45 ms, SD = 68.5) were unaffected by any of the experimental factors. The subjects reported to be more “high” when given alcohol than when given juice regardless of the instruction they were given, F(1,11) = 11.56, p < 0.01. Similarly, the subjects’ self-ratings of intoxication indicate that they felt moderately intoxicated, mean = 2.67 (SD = 0.9) on a Likert 1–5 scale. They felt equally inebriated regardless of whether they were instructed that the drink contains alcohol or placebo.
Event-related potentials
Fig. 2 depicts superimposed waveforms obtained in alcohol and placebo conditions averaged across all participants, sessions and repetition conditions for all electrode sites. Visual inspection of the waveforms reveals a large, sharp-onset deflection about 180 ms after stimulus onset which is negative at posterior temporal sites (T5 and T6) and inverts in polarity over central and frontal sites. It is followed by a series of superimposed broad negative deflections that could be observed at most sites, especially fronto-centrally, between about 230 to 450 ms.
Figure 2.
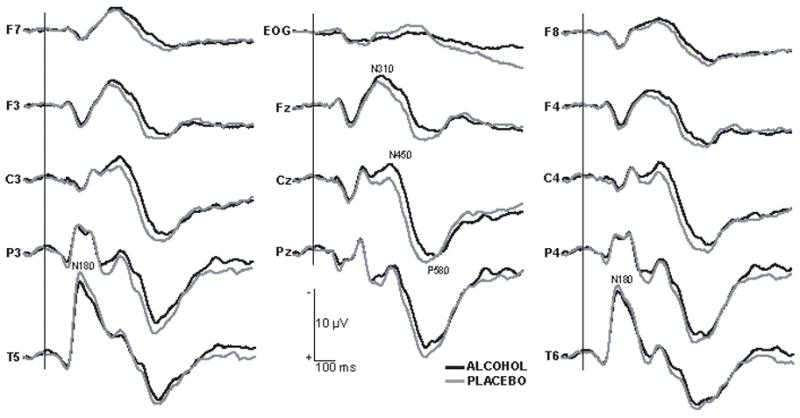
Grand average waveforms obtained in alcohol and placebo conditions. The waveforms were averaged for both new and repeated words, across all participants and sessions. Alcohol intoxication resulted in a selective attenuation of N180 at lateral temporal sites, a widespread increase of N450 amplitude, and an increased latency of P580. Word duration was 300 ms. Negative is up.
Effects of beverage
None of the measures were affected by instructions as to the beverage content (expectancy). A significant beverage x sites interaction, [F(12,132) = 3.26, p < .01] was observed for the earliest measured time window, 135–265 ms post stimulus onset. The only scalp sites where ERPs were differentially affected by alcohol and placebo were T5 and T6, [F(1,11) = 12.21, p < .05]. At this latency, the overall event-related activity recorded over the posterior temporal sites (T5, T6) was by far the largest, as compared to other sites within the present montage. A closer look at the main effect of sites, [F(12,132) = 15.2, p < .001] revealed a laterality difference with larger potentials recorded over left parieto-temporal sites, [F(1,11) = 7.74, p < .05].
Whereas there was no significant effect of beverage on N310 (270–370 ms latency window), the overall laterality effect persisted with waveforms recorded on the left side being more negative than those on the right, [F(1,11) = 11.11, p < .05]. This effect was particularly prominent at temporal sites as potentials recorded at T5 were more negative than at T6, [F(1,11) = 16.23, p < .05].
The waveforms obtained in alcohol and placebo conditions clearly begin to diverge in most sites at about 330 ms after word onset. A significant beverage x sites interaction was obtained for the N450 area (420–480 ms), [F(12,132) = 2.59, p < .05], with alcohol increasing the amplitude of N450. As can be observed in Fig. 2, by this latency the waveform difference is quite pronounced over most scalp regions although it is negligible over posterior temporal sites.
Effects of repetition
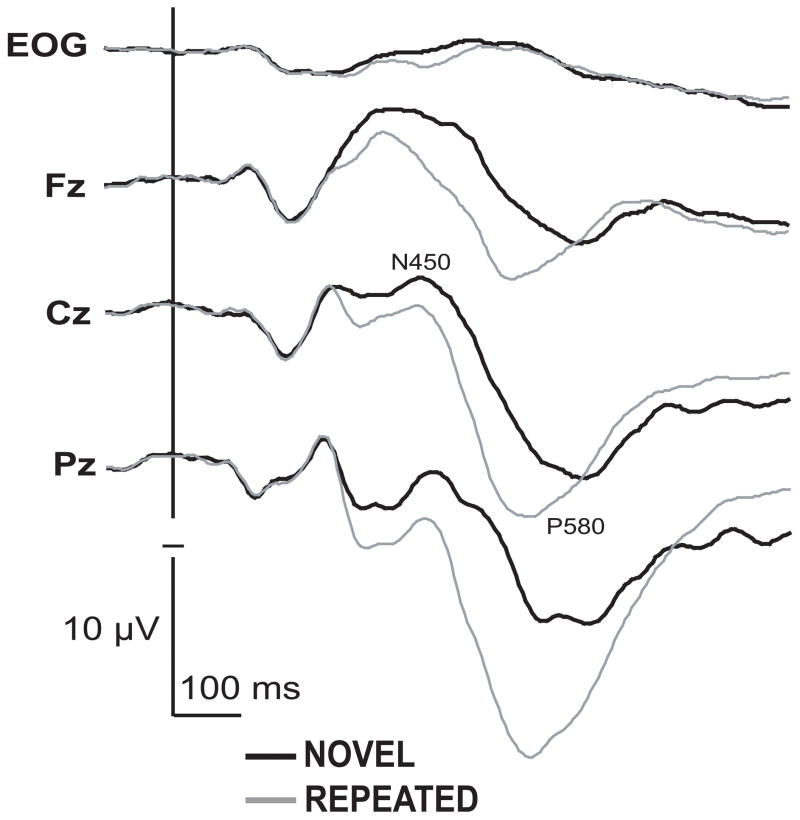
As illustrated in Fig. 3, the main effect of repetition, with novel words evoking a more negative amplitude, was significant within all three measured time windows between 270 and 610 ms post-stimulus onset: N310 [F(1,11) = 10.4, p < .01], N450 [F(1,11) = 37.8, p < .0001], and P580 [F(1,11) = 19.3, p < .001]. Similarly, the repetition x sites interaction was very robust for these three latency windows (270 – 610 ms) as well, with the new-repeated difference increasing in the posterior sites: N310 [F(12,132) = 6.9, p < .001], N450 [F(12,132) = 13.9, p < .0001], and P580 [F(12,132) = 12.0, p < .0001]. Finally, the P580 peak latency was shorter for the repeated target (549 ms) than the novel words (605 ms), [F(1,11) = 53.4, p < .0001].
Figure 3.
Grand average ERP waveforms to novel and repeated words at midline electrode sites, averaged for both beverage and instructions conditions and across all participants. Repetition resulted in a sustained attenuated negativity and increased positivity after 270 ms. Word duration was 300 ms. Negative is up.
Waveforms recorded from the three midline sites in alcohol and placebo conditions are presented separately for the novel and repeated target words in Fig. 4. There was no interaction between the factors of beverage and repetition indicating that although novel words evoked a larger negativity overall, alcohol intoxication affected the novel and target words in a comparable manner. Alcohol affected the series of superimposed negative components without exerting a significant influence on the peak amplitude of the late positivity P580. However, it has significantly prolonged its peak latency, F(1,11) = 8.84, p < .05, (alcohol: 583 ms and placebo: 571 ms).
Figure 4.
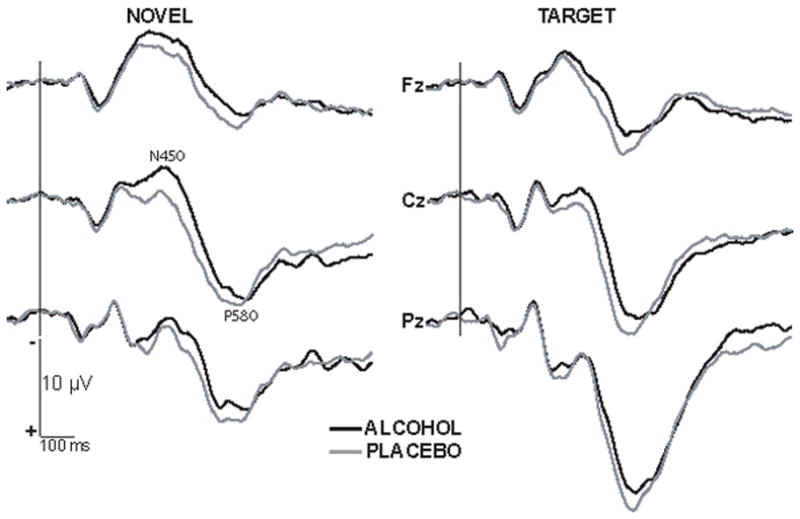
Grand average waveforms obtained in alcohol and placebo conditions for novel and repeated target words separately for midline electrode sites. Alcohol increased the amplitude of N450 and the latency of P580 on both novel and target words in a comparable manner. Negative is up.
Electrodermal Activity
The main effect of stimulus type, [F(1,9) = 56.3, p < .0001] reflected larger average SCRs evoked by target than nontarget words, with the means of 0.22 √μS and 0.16 √μS respectively. Investigation of the dynamics across trials (Fig. 5) revealed that the SCRs habituated markedly within the first ten trials resulting in the main effect of block, [F(19,171) = 7.2, p < .0001].
Figure 5.
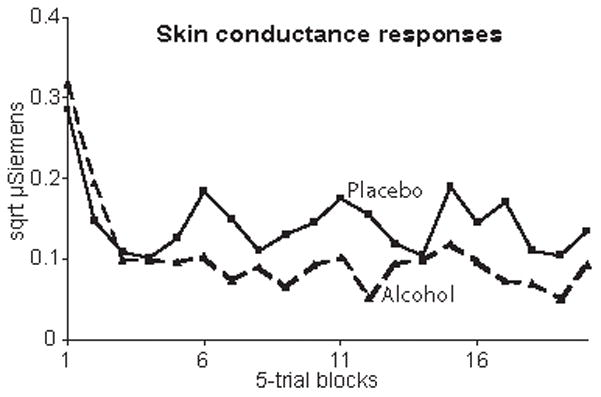
Average skin conductance responses (in √μ Siemens) measured across 5-trial blocks in alcohol and placebo conditions for the whole duration of the task. Alcohol tended to depress SCR amplitude in the last 16 blocks of the task.
Even though the dose used in this study may not have been sufficient to significantly alter skin conductance, alcohol exhibited a trend to depress SCR amplitude in the last 16 blocks of the task [F (1,9) = 4.6, p = 06], in agreement with other studies using higher doses (Carpenter, 1957; Kilpatrick et al., 1980).
ERPs as a Function of Electrodermal Responsivity
In addition to the analyses performed on event-related potentials and peripheral autonomic (i.e. electrodermal) responses separately, their concurrent recording during this task afforded an opportunity to compare directly the activity of the two physiological systems (Lyytinen et al., 1992; Marinkovic et al., 2001). The SCRs were used as a grouping criterion during ERP averaging for each participant and for the repeated and novel words separately. The SCR+ ERP averages contained only those trials on which a measurable phasic SCR was elicited. Conversely, no phasic SCRs were recorded on trials forming the SCR- average waveform. Since SCRs were evoked on fewer trials than not, care was taken to balance the averages with respect to the total number of trials and the ordinal trial number. Averaged ERPs were based on 17.6 and 19 trials for new and repeated words on average. Data of four participants were eliminated from the analyses due to an insufficient number of trials. The waveforms were quantified by obtaining an average voltage within 300–650 ms latency window as well as the peak amplitude and latency for the P580 component.
As shown in Fig. 6, the SCR+ and SCR- waveforms begin to diverge significantly at about 300 ms after word onset especially in the alcohol condition, with a large long-lasting negativity evoked on SCR+ trials. Interaction between the factors of SCR and sites [F(12,84) = 4.3, p < .001] was significant for the entire measured time window, 300–650 ms.
Figure 6.
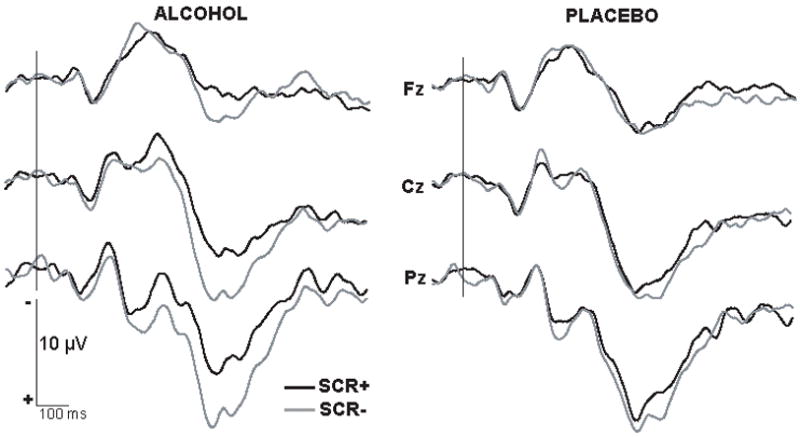
Grand average waveforms obtained on trials with (SCR+) and without (SCR-) accompanying electrodermal reactions recorded from midline electrode sites in alcohol (left column) and placebo (right column) conditions. Alcohol increased the negativity on trials with sympathetic arousal 300–650 ms after stimulus onset. Negative is up.
Analysis of the peak amplitude and latency of the late positivity revealed that a smaller P580 was evoked on SCR+ trials, [F(1,7) = 5.69, p < .05]. This effect was primarily observed in alcohol condition at centro-posterior sites, [F(1,7) = 16.1, p < .05]. Finally, the P580 peak latency was longer on SCR+ as compared to SCR- trials under the alcohol condition only, as suggested by a beverage and SCR interaction [F(1,7) = 5.61, p < .05].
DISCUSSION
The present study investigated the effects of alcohol on central measures of brain activity and on peripheral autonomic measures during a task of verbal recognition. Factors of beverage and repetition affected the overall ERPs independently from each other. Lack of an interaction between these two factors indicates that a moderately low alcohol dose does not impair recent verbal memory in normal participants. Instead, these results suggest that low level alcohol intoxication exerts its influence on an early, (pre-lexical pattern analysis) and late (semantic and contextual integration) processing stages, but not on mnemonic aspects of word processing. Furthermore, the low-to-moderate alcohol dose was found to affect ERP deflections without impairing behavioral accuracy or speed on this verbal recognition task, suggesting superiority of the physiological measures in assessing the adverse effects of alcohol intoxication on verbal processing.
Alcohol effects on pre-lexical pattern analysis
A series of processes that are unrelated to the semantic significance of a word need to take place prior to the actual understanding of a word within its context. Some of those earlier stages (before 220 ms) embody a prelexical pattern recognition process (Gros et al., 2002; Ritter et al., 1982), as they are unaffected by contextual constraints or word processing task demands (Lovrich et al., 1986; Smith and Halgren, 1987). Neuronal ensembles in the ventral visual stream are selectively activated by word-like visual stimuli and subserve processing of their morphological characteristics (Cohen et al., 2002; Dehaene et al., 2002; Nobre et al., 1994). The activation then proceeds anteriorly, encompassing middle and superior temporal areas and inferior prefrontal cortex predominantly on the left (Dale and Halgren, 2001; Fiez and Petersen, 1998; Halgren et al., 1994a).
In the present study a large, left-dominant N180 was recorded over posterior temporal areas. This corresponds to word-specific focal peaks observed in the left inferotemporal cortex at a similar latency with intracranial recordings (Halgren et al., 1994a), magnetoencephalography (Dhond et al., 2001; Marinkovic et al., 2003) and current source estimated ERPs (Curran et al., 1993) during word processing tasks. Alcohol significantly attenuated this large negative deflection at posterior temporal sites only. Thus, effects of alcohol on this relatively early stage of verbal processing may reflect its influences on feature identification and pre-lexical pattern analysis subserved by the ventrotemporal area.
Alcohol effects on semantic and contextual integration
A negative deflection peaking at around 400 ms latency (“N400”) is commonly evoked by potentially meaningful stimuli including spoken, written, or signed words and pictures (Kutas and Federmeier, 2000). The N400 is modulated by priming and is commonly viewed as reflecting attempts to access and integrate a semantic representation into a current context (Brown and Hagoort, 1993; Halgren, 1990b; Holcomb, 1993; Rugg and Doyle, 1994). The N400 amplitude is attenuated by sentence-terminal words that are congruent with the overall meaning of a sentence (Halgren et al., 2002; Kutas and Hillyard, 1980), as well as individually presented words that are repeated, semantically primed, or have higher frequency (Osterhout and Holcomb, 1995; Otten et al., 1993) Smith, 1987 #156]. Multimodal imaging studies sensitive to both spatial and temporal aspects suggest that the N400 to words in the sentential or single-word paradigm may be generated in the same or highly overlapping brain regions (Halgren et al., 2002; Marinkovic et al., 2003).
In the present study, the amplitude of N450 and the latency of P580 were significantly increased by alcohol. At this latency, it is likely that different informational aspects (e.g. semantic, mnestic, contextual, emotional) contribute modulatory influences to the amplitude of the N450. Understanding of a word then results from the process of their integration. A robust effect of repetition with repeated target words evoking a smaller negativity and larger positivity after about 270 ms has been reported in numerous studies and it may reflect an easier access to the lexicon and the semantic store due to priming (Forster and Davis, 1984; Joyce et al., 1999; Nagy and Rugg, 1989; Otten et al., 1993; Rugg, 1985, 1990; Rugg and Doyle, 1994; Rugg and Nieto-Vegas, 1999; Smith and Halgren, 1987; Van Petten et al., 1991). Rather low alcohol dose employed in this study did not affect priming per se, as there was no significant interaction between alcohol and word repetition. However, this result has to be viewed cautiously in light of other evidence suggesting that alcohol affects memory (Acheson et al., 1998; Mungas et al., 1994). Subsequent studies are needed to assess dose-related effects of alcohol on priming under conditions of immediate and delayed repetition priming and with less well-learned stimuli than was the case in this study.
A comparable increase in the N450 negativity to both repeated and novel words indicates that a sensitivity to repetition was preserved. This suggests that alcohol exerts its effect on at least partially different neural processes than those that underlie the effect of repetition itself. It is possible that mild intoxication raised the level of difficulty in semantic integration and prolonged the duration of word evaluation. This may have resulted from lowering the inhibiting effects of the semantic context, increasing the difficulty of semantic and contextual integration. These results are in accord with other evidence (Zhang et al., 1997), showing that chronic alcoholics preserved normal priming to words but exhibited increased negativity at this latency. Increased negativity of the N400 to sentence-terminal words has also been observed in schizophrenic patients suggesting weakening of the constraints provided by the semantic context (Nestor et al., 1997; Salisbury et al., 2000). Future work should determine whether the alcohol-induced N450 abnormality derives from the semantic or contextual aspects of verbal processing.
Recordings obtained with intracerebral electrodes in human subjects indicate multiple N400 generators, including medial and ventral temporal and ventrolateral prefrontal areas (Halgren et al., 1994a; Halgren et al., 1994b; Nobre and McCarthy, 1995). A corroborating evidence comes from recent fMRI and MEG studies suggesting that semantic processing is mediated by ventrolateral prefrontal and ventral temporal regions especially on the left (Buckner et al., 2000; Halgren et al., 2002; Marinkovic et al., 2003). Thus, a low dose of alcohol may affect semantic word processing stage via its influence on the distributed circuits primarily encompassing the left prefrontal and temporal regions.
Alcohol, ERPs and Arousal
A possibility that the effects of alcohol on cognitive ERPs were related to its effects on a more global sympathetic activation was explored using concurrent electrodermal measures of the orienting response (OR). An OR is usually evoked by a stimulus whose characteristics set it apart from other stimuli in the current context, such as its importance for the task performance, emotional significance (all the words used in this task had a neutral emotional valence), difficulty in processing, incertitude about stimulus categorization or any other parameter that makes it different from the stream of other stimuli (Maltzman, 1979; Sokolov, 1963). OR may have an adaptive value in pooling of activation across broad cortical, limbic and subcortical circuits, increasing the likelihood of an appropriate and comprehensive stimulus evaluation and response selection (Halgren and Marinkovic, 1995). Alcohol may affect higher cognitive processes through its effects on the association cortices, but also through its effects on the autonomic functioning and the relevant modulatory processes such as sympathetic arousal. In this study, intoxication tended to decrease SCRs, although this change did not reach significance. Concurrent recording of the ERPs and SCRs during this task indicated that only under alcohol condition a larger ERP negativity in the 300–650 ms latency range was recorded on those trials that also evoked an arousal response. Consequently, these alcohol effects on the N450 and P580 were mainly due to an increased negativity confined to those trials that also evoked autonomic arousal. It is possible that this reflects increased difficulty in semantic integration, as the sympathetic arousal system may have been activated on trials that were somewhat more difficult to process due to alcohol intoxication, or on which participants felt uncertain about their classification, resulting in a larger late negativity (Stuss et al., 1988). Furthermore, it is possible that engagement of the arousal system facilitated performance in order to counteract the effects of alcohol administration. Differences in temporal resolution between the measures of central (ERPs) and peripheral autonomic (SCR) activity obscure the nature of their interaction. That is, it is not clear whether alcohol primarily affected verbal processing, or it primarily resulted in aberrant SCR elicitiation.. Nevertheless, even moderately low alcohol dose seems to alter central activation processes and may selectively impair modulatory brain processes such as arousal, that moderate cognition and behavior. In a related study (Marinkovic et al., 2001) employing an auditory “oddball” task, a large P3a was evoked by novel, distracting tones on the SCR+ trials. Alcohol intoxication abolished the P3a indicating its interference with orienting to novel, unexpected, potentially important events.
Conclusion
In sum, without impairing behavioral measures of verbal recognition, mild alcohol intoxication resulted in an attenuated P180, indexing the prelexical stage of verbal processing. Alcohol also affected the late widespread N450 and P580 potentials, potentially indicating and increased difficulty in semantic and contextual integration. The effects of alcohol on the late potentials mainly occurred on those relatively rare trials that also evoked sympathetic arousal, suggesting that alcohol may selectively impair processes that modulate cognitive processing.
Acknowledgments
This study was conducted at the Dept. of Psychology, University of California, Los Angeles Supported by Sigma-Xi Society, UCLA, AA13402 (KM) and NS18741 (EH).
We thank Kerin Asher for help in data acquisition. We dedicate this work to the memory of Helen George.
Contributor Information
Ksenija Marinkovic, MGH-NMR Center, Harvard Medical School, 149 13th Street, Rm 2301, Charlestown, MA 02129
Eric Halgren, MGH-NMR Center, Harvard Medical School, Radiology, University of Utah, INSERM E9926, Marseilles, France
Irving Maltzman, Psychology Dept., Franz Hall, Univ. of California, Los Angeles, Los Angeles, CA 90095-1563
References
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age- dependent effects. Alcohol Clin Exp Res. 1998;22:1437–42. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model [see comments] Alcohol Clin Exp Res. 1999;23:1125–35. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. Plenum Press; New York: 1992. [Google Scholar]
- Brown C, Hagoort P. The processing nature of the N400: Evidence from masked priming. Journal of Cognitive Neuroscience. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123(Pt 3):620–40. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Campbell K, Marois R, Arcand L. Ethanol and the event-related evoked potentials. Effects of rate of stimulus presentation and task difficulty. Ann N Y Acad Sci. 1984;425:551–5. doi: 10.1111/j.1749-6632.1984.tb23576.x. [DOI] [PubMed] [Google Scholar]
- Carpenter JA. Effects of alcohol beverages on skin conductance. Quarterly Journal of Studies on Alcohol. 1957;18:1–18. [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Curran T, Tucker DM, Kutas M, Posner MI. Topography of the N400: brain electrical activity reflecting semantic expectancy. Electroencephalogr Clin Neurophysiol. 1993;88:188–209. doi: 10.1016/0168-5597(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr Opin Neurobiol. 2001;11:202–8. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13:321–5. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. Sequence of brain activity underlying word-stem completion. Journal of Neuroscience. 2001;21:3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Earleywine M. Distraction does not impair memory during intoxication: support for the attention-allocation model. J Stud Alcohol. 1995;56:444–8. doi: 10.15288/jsa.1995.56.444. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. Hodder & Staughton; London: 1975. [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci U S A. 1998;95:914–21. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster KI, Davis C. Repetition priming and frequency attenuation in lexical access. Journal of Experimental Psychology: Learning, Memory and Cognition. 1984;10:680–698. [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Houghton Mifflin; Boston: 1982. [Google Scholar]
- Gough HG. Theory, development, and interpretation of the CPI socialization scale. Psychol Rep. 1994;75:651–700. doi: 10.2466/pr0.1994.75.1.651. [DOI] [PubMed] [Google Scholar]
- Gros H, Doyon B, Rioual K, Celsis P. Automatic grapheme processing in the left occipitotemporal cortex. Neuroreport. 2002;13:1021–4. doi: 10.1097/00001756-200206120-00008. [DOI] [PubMed] [Google Scholar]
- Halgren E. Human evoked potential. In: Boulton AA, Baker GB, Vanderwolf C, editors. Neuropsychological techniques: Applications to neural systems. Vol. 15. Humana; Clifton, NJ: 1990a. pp. 147–275. [Google Scholar]
- Halgren E. Insights from evoked potentials into the neuropsychological mechanisms of reading. In: Scheibel AB, Wechsler AF, editors. Neurobiology of higher cognitive function. Guilford; New York: 1990b. pp. 103–150. [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K. Spatio-temporal stages in face and word processing. I. Depth-recorded potentials in the human occipital, temporal and parietal lobes [corrected] [published erratum appears in J Physiol Paris 1994;88(2):following 151] J Physiol Paris. 1994a;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices [published erratum appears in J Physiol Paris 1994;88(2):following 151] J Physiol Paris. 1994b;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17:1101–16. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K. Neurophysiological networks integrating human emotions. In: Gazzaniga M, editor. The cognitive neurosciences. MIT Press; Cambridge, MA: 1995. pp. 1137–1151. [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–64. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Hartcollis P, Johnson DM. Differential effects of alcohol on verbal fluency. Quarterly Journal of Studies on Alcohol. 1956;17:183–189. [PubMed] [Google Scholar]
- Haut JS, Beckwith BE, Petros TV, Russell S. Gender differences in retrieval from long-term memory following acute intoxication with ethanol. Physiol Behav. 1989;45:1161–5. doi: 10.1016/0031-9384(89)90103-0. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ. Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology. 1993;30:47–61. doi: 10.1111/j.1469-8986.1993.tb03204.x. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Performance of traditional F tests in repeated measures designs under covariance heterogeneity. Communications on Statistical and Theoretical Mathematics. 1980;A9:61–74. [Google Scholar]
- Jääskeläinen IP, Näätänen R, Sillanaukee P. Effect of acute ethanol on auditory and visual event-related potentials: a review and reinterpretation. Biol Psychiatry. 1996;40:284–91. doi: 10.1016/0006-3223(95)00385-1. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Paller KA, Schwartz TJ, Kutas M. An electrophysiological analysis of modality-specific aspects of word repetition. Psychophysiology. 1999;36:655–65. [PubMed] [Google Scholar]
- Kilpatrick DG, Sutker PB, Best CL, Allain AN. Acute alcohol intoxication and vicarious emotional responsiveness. Addict Behav. 1980;5:191–8. doi: 10.1016/0306-4603(80)90039-8. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–5. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C. Event-related brain potential studies of language. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in psychophysiology. Vol. 3. JAI Press; Greenwich, CT: 1988. pp. 139–187. [Google Scholar]
- Lovrich D, Simson R, Vaughan HG, Jr, Ritter W. Topography of visual event-related potentials during geometric and phonetic discriminations. Electroencephalogr Clin Neurophysiol. 1986;65:1–12. doi: 10.1016/0168-5597(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Maltzman I. Selective effects of alcohol on electrodermal indices of orienting reflexes to signal and nonsignal stimuli. Psychophysiology. 1991;28:559–69. doi: 10.1111/j.1469-8986.1991.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Lyytinen H, Blomberg AP, Naatanen R. Event-related potentials and autonomic responses to a change in unattended auditory stimuli. Psychophysiology. 1992;29:523–34. doi: 10.1111/j.1469-8986.1992.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Maltzman I. Orienting reflexes and classical conditioning in humans. In: Kimmel HD, van Olst EH, Orlebeke JF, editors. Orienting Reflex in Humans. Lawrence Erlbaum; Hillsdale: 1979. pp. 323–351. [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–97. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I. Arousal-related P3a to novel auditory stimuli is abolished by moderately low alcohol dose. Alcohol and Alcoholism. 2001;36:529–539. doi: 10.1093/alcalc/36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylor EA, Rabbitt PM, Kingstone A. Effects of alcohol on word categorization and recognition memory. Br J Psychol. 1987;78:233–9. doi: 10.1111/j.2044-8295.1987.tb02242.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States manual. Educational Testing Service; San Diego, CA: 1981. [Google Scholar]
- Miller ME, Adesso VJ, Fleming JP, Gino A, Lauerman R. Effects of alcohol on the storage and retrieval processes of heavy social drinkers. J Exp Psychol [Hum Learn] 1978;4:246–55. [PubMed] [Google Scholar]
- Mills KC, Neal EM, Peed-Neal I. Handbook for alcohol education: The community approach. Ballinger; Cambridge, MA: 1983. [Google Scholar]
- Monteiro MG, Schuckit MA. Populations at high alcoholism risk: recent findings. J Clin Psychiatry. 1988;49(Suppl):3–7. [PubMed] [Google Scholar]
- Mungas D, Ehlers CL, Wall TL. Effects of acute alcohol administration on verbal and spatial learning. Alcohol Alcohol. 1994;29:163–9. [PubMed] [Google Scholar]
- Nagy ME, Rugg MD. Modulation of event-related potentials by word repetition: the effects of inter-item lag. Psychophysiology. 1989;26:431–6. doi: 10.1111/j.1469-8986.1989.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kimble MO, O’Donnell BF, Smith L, Niznikiewicz M, Shenton ME, McCarley RW. Aberrant semantic activation in schizophrenia: a neurophysiological study. Am J Psychiatry. 1997;154:640–6. doi: 10.1176/ajp.154.5.640. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–3. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. J Neurosci. 1995;15:1090–8. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout L, Holcomb P. Event-related potentials and language comprehension. In: Rugg MD, Coles MGH, editors. Electrophysiology of mind: Event-related brain potentials and cognition. Oxford University Press; Oxford: 1995. [Google Scholar]
- Otten LJ, Rugg MD, Doyle MC. Modulation of event-related potentials by word repetition: the role of visual selective attention. Psychophysiology. 1993;30:559–71. doi: 10.1111/j.1469-8986.1993.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol. 1968;76(Suppl):1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD, Pihl RO. Acute alcohol intoxication and cognitive functioning. J Stud Alcohol. 1990;51:114–22. doi: 10.15288/jsa.1990.51.114. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–50. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human evoked brain potentials and alcohol. Alcohol Clin Exp Res. 1981;5:304–17. doi: 10.1111/j.1530-0277.1981.tb04904.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RE, Van Thiel DH, editors. Alcohol and the brain. Plenum Press; New York: 1985. pp. 139–182. [Google Scholar]
- Porjesz B, Begleiter H. Evoked brain potentials and alcoholism. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of alcoholism: Implications for diagnosis and treatment. Guilford; New York: 1987. pp. 45–63. [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. The pharmacology of alcohol and alcohol dependence. Oxford University Press; New York: 1996. pp. 207–247. [Google Scholar]
- Reed TE. The myth of “the average alcohol response”. Alcohol. 1985;2:515–9. doi: 10.1016/0741-8329(85)90126-0. [DOI] [PubMed] [Google Scholar]
- Richter R, Kielholz P, Hobi V, Ladewig D, Miest P-C, Reggiani G, Schwarz E. Biphasic time-course of alcohol-induced changes in electrodermal activation parameters. Blutalkohol. 1977;14:279–291. [Google Scholar]
- Ritter W, Simson R, Vaughan HG, Jr, Macht M. Manipulation of event-related potential manifestations of information processing stages. Science. 1982;218:909–11. doi: 10.1126/science.7134983. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male subjects at high risk for alcoholism. Biol Psychiatry. 1999;46:281–91. doi: 10.1016/s0006-3223(98)00247-9. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic EA, Frowein HW, Varner JL, Adinoff B, Lane EA, Eckardt MJ, Linnoila M. Dose-related effects of ethanol on visual sustained attention and event-related potentials. Alcohol. 1987;4:293–300. doi: 10.1016/0741-8329(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Marlatt GA. The balanced placebo design: methodological considerations. Addict Behav. 1981;6:107–22. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Rugg MD. The effects of semantic priming and word repetition on event-related potentials. Psychophysiology. 1985;22:642–7. doi: 10.1111/j.1469-8986.1985.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Rugg MD. Event-related brain potentials dissociate repetition effects of high- and low-frequency words. Memory and Cognition. 1990;18:367–379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Doyle MC. Event-related potentials and stimulus repetition in direct and indirect tests of memory. In: Heinze H, Munte T, Mangun GR, editors. Cognitive Electrophysiology. Birkhauser; Boston: 1994. [Google Scholar]
- Rugg MD, Nieto-Vegas M. Modality-specific effects of immediate word repetition: electrophysiological evidence. Neuroreport. 1999;10:2661–4. doi: 10.1097/00001756-199908200-00041. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, O’Donnell BF, McCarley RW, Nestor PG, Shenton ME. Event-related potentials elicited during a context-free homograph task in normal versus schizophrenic subjects. Psychophysiology. 2000;37:456–63. [PMC free article] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–8. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E. Event-related potentials during lexical decision: effects of repetition, word frequency, pronounceability, and concreteness. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:417–21. [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Pergamon Press; New York: 1963. [Google Scholar]
- Stuss DT, Picton TW, Cerri AM. Electrophysiological manifestations of typicality judgment. Brain Lang. 1988;33:260–72. doi: 10.1016/0093-934x(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Tarter RE, McBride H, Buonpane N, Schneider DU. Differentiation of alcoholics. Childhood history of minimal brain dysfunction, family history, and drinking pattern. Arch Gen Psychiatry. 1977;34:761–8. doi: 10.1001/archpsyc.1977.01770190023002. [DOI] [PubMed] [Google Scholar]
- Teo RK, Ferguson DA. The acute effects of ethanol on auditory event-related potentials. Psychopharmacology. 1986;90:179–84. doi: 10.1007/BF00181237. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Kutas M, Kluender R, Mitchiner M, McIsaac H. Fractionating the word repetition effect with event-related potentials. Journal of Cognitive Neuroscience. 1991;3:131–150. doi: 10.1162/jocn.1991.3.2.131. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH. Effect of alcohol on recall and recognition as functions of processing levels. J Stud Alcohol. 1984;45:10–5. doi: 10.15288/jsa.1984.45.10. [DOI] [PubMed] [Google Scholar]
- Woodward JA, Bonett DG, Brecht ML. Introduction to linear models and experimental design. Harcourt Brace Jovanovich; San Diego: 1990. [Google Scholar]
- Zhang XL, Begleiter H, Porjesz B. Do chronic alcoholics have intact implicit memory? An ERP study. Electroencephalogr Clin Neurophysiol. 1997;103:457–73. doi: 10.1016/s0013-4694(97)00044-8. [DOI] [PubMed] [Google Scholar]