Abstract
Brain glutamate has been shown to play an important role in reinstatement to drug seeking, a behavior considered to be of relevance to relapse to drug taking in humans. Therefore, glutamate receptors, in particular metabotropic glutamate (mGlu) receptors, have become important targets for medication development for the treatment of drug dependence. In this review article, we focus on the mGlu7 receptor subtype, and discuss recent findings with AMN082, a selective mGlu7 receptor allosteric agonist, in animal models with relevance to drug dependence. Systemic or local administration of AMN082 into the nucleus accumbens (NAc), a critical brain region involved in reward and drug dependence processes, inhibited the reinforcing and motivational effects of cocaine, heroin and ethanol, as assessed by the intravenous drug self-administration procedure. In addition, AMN082 inhibited the reward-enhancing effects induced by cocaine, as assessed in the intracranial self-stimulation procedure, and cocaine- or cue-induced reinstatement of drug-seeking behavior. In vivo microdialysis studies indicated that systemic or intra-NAc administration of AMN082 significantly decreased extracellular γ-aminobutyric acid (GABA) and elevated extracellular glutamate, but had no effect on extracellular dopamine in the NAc, suggesting that a non-dopaminergic mechanism underlies the effects of AMN082 on the actions of cocaine. Further, data indicated that AMN082-induced changes in glutamate were the net effect of two actions: one is the direct inhibition of glutamate release by activation of mGlu7 receptors on glutamatergic neurons; another is the indirect increases of glutamate release mediated by decreases in GABA transmission. These increases in extracellular glutamate functionally antagonized cocaine-induced inhibition of NAc-ventral pallidum GABAergic neurotransmission, and therefore, the rewarding effects of cocaine. In addition, elevated extracellular glutamate activated presynaptic mGlu2/3 autoreceptors which in turn inhibited cocaine priming- or cue-induced enhancement of glutamate release and reinstatement of drug-seeking behavior. Taken together, these findings suggest that the mGlu7 receptor is an important target for medication development for the treatment of drug dependence. AMN082 or other mGlu7 receptor allosteric agonists may have potential as novel pharmacotherapies for cocaine addiction.
Keywords: AMN082, glutamate, cocaine, addiction, GABA
1. Introduction
Drug abuse is a serious social and health problem, for which no effective treatments are currently available. A better understanding of the neurophysiological mechanisms involved in drug dependence is important for the development of effective medications for treatment of drug addiction. The majority of previous studies investigating the neuromechanisms of drug dependence have been largely focused on the mesocorticolimbic dopamine system that originates from the midbrain ventral tegmental area (VTA) and projects to the forebrain nucleus accumbens (NAc) and the prefrontal cortex (PFC) (Kalivas and Volkow, 2005; Koob, 1992; Wise, 1996b). Studies suggest that the excitatory glutamate transmission in the NAc also plays an important role in the development and maintenance of drug dependence, particularly in reinstatement to drug-seeking behavior (Kalivas et al., 2009; Kalivas et al., 2005; Tzschentke and Schmidt, 2003; Wise and Morales, 2010). One mechanism via which the glutamatergic system affects drug dependence is direct or indirect modulation of dopaminergic system activity (Tzschentke and Schmidt, 2003; Wise and Morales, 2010). For instance, both the VTA and the NAc receive rich glutamatergic afferent projections from various brain areas such as the PFC, amygdala and hippocampus (Christoph et al., 1986; Floresco et al., 2001; Floresco et al., 1998; Geisler et al., 2007). Direct interaction of glutamate and dopamine has been found in the VTA, NAc, PFC and the amygdala (Omelchenko and Sesack, 2007; Sesack et al., 2003). Glutamate efflux in the VTA increases the bursting activity of dopaminergic neurons and increases dopamine release in projection areas, such as the NAc, the PFC and the amygdala (Tzschentke and Schmidt, 2003; Wise, 1996b; Wise and Morales, 2010). Thus, administration of most drugs of abuse increases both dopamine and glutamate transmission (Kalivas et al., 2005; Wise and Morales, 2010). These actions are hypothesized to be critically involved in different aspects of drug dependence, such as reinforcement, withdrawal and reinstatement to drug use (Kalivas and Volkow, 2005; Wise and Morales, 2010). Therefore, efforts have been undertaken to block the reinforcing effects of drugs of abuse, as well as reinstatement of drug-seeking, by pharmacological manipulation of glutamate transmission (Gass and Olive, 2008; Kalivas et al., 2009). A large body of literature has indicated that decreases of glutamatergic transmission by modulation of glutamate receptors are effective in attenuating the reinforcing effects of drugs of abuse and in prevention of reinstatement to drug-seeking behavior (Gass and Olive, 2008; Olive, 2009). Table 1 provides descriptions of the main behavioral procedures used to study the reinforcing effects of drugs of abuse and drug seeking.
Table 1.
Descriptions of behavioral procedures used in the study of drug dependence
| Procedures | Description | References |
|---|---|---|
| Self-administration | An operant conditioning procedure during which a reinforcer (e.g., drug, food) is administered in a response-contingent manner after the completion of the reinforcement schedule requirement. Two different reinforcement schedules are commonly used (see below). | (Markou et al., 1993; O’Brien and Gardner, 2005) |
| Fixed-ratio schedule of reinforcement | The reinforcer is delivered after the fixed ratio (i.e., the predetermined number of responses) is completed. This schedule provides a measure of drug intake and primary reinforcement efficacy. | (Markou et al., 1993; O’Brien and Gardner, 2005; Richardson and Roberts, 1996) |
| Progressive-ratio schedule of reinforcement | Progressively increased numbers of operant responses are required by the subject in order to receive successive reinforcers. The last ratio value successfully completed, before a predetermined period of time with no reinforcement delivery, is defined as the break-point. This schedule provides a measure of incentive motivation for the reinforcer. | (Hodos, 1961; Markou et al., 1993; Richardson and Roberts, 1996) |
|
| ||
| Intracranial self-stimulation (ICSS) | An operant procedure in which experimental animal learns to deliver brief electrical pulses through an electrode surgically implanted into specific brain reward regions mediating both natural and ICSS reward. The minimal stimulation intensity that maintains ICSS behavior is termed the reward threshold. Drugs of abuse, such as opiates and psychostimulants, lower ICSS thresholds, reflecting an enhancement by these drugs of brain reward function(Markou and Koob, 1992; O’Brien and Gardner, 2005; Wise, 1996a). This procedure provides a measure, ICSS thresholds, that allows the assessment of the reward enhancing properties of drugs of abuse. | (Markou and Koob, 1992; O’Brien and Gardner, 2005; Wise, 1996a) |
|
| ||
| Reinstatement (drug-, cue- or stress-induced reinstatement of drug-seeking behavior) | After the establishment of stable drug self- administration behavior, drug-reinforced lever responding is extinguished (e.g., extinction). Reinstatement of drug-seeking behavior is subsequently triggered by a priming injection of drugs (drug- induced), the contingent and/or non-contingent presentation of cues previously associated with drug delivery (cue-induced), or a stressor (stress-induced). | (Epstein et al., 2006; Katz and Higgins, 2003; O’Brien and Gardner, 2005; Shaham et al., 2003) |
2. Metabotropic glutamate receptors and drug addiction
The actions of glutamate are mediated by activation of ionotropic and metabotropic glutamate (mGlu) receptors (Kew and Kemp, 2005). Numerous studies indicated that blockade of ionotropic receptors, such as N-methyl-D-aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxale propionate (AMPA) receptors, inhibited drug reinforcement and reinstatement of drug-seeking behavior of most drugs of abuse (Bisaga et al., 2000; Gass and Olive, 2008). However, compounds acting at ionotropic glutamate receptors are severely limited in their therapeutic potential because NMDA/AMPA receptors are ubiquitously involved in fast excitatory synaptic transmission throughout the brain. Thus, blockade of ionotropic glutamate receptors may be associated with severe adverse effects, including neurotoxicity and psychotogenicity (Bisaga et al., 2000). Metabotropic glutamate receptors are slow-acting and modulatory of glutamate transmission. These receptors are located pre- and postsynaptically, as well as on glial cells (Schoepp, 2001). Metabotropic glutamate receptors are classified into three groups based on sequence homology, signal transduction mechanisms and pharmacologic properties (Pin and Duvoisin, 1995). Group I receptors (mGlu1 and mGlu5) are coupled via Gq proteins to phospholipase C, and are primarily located postsynaptically where they positively regulate the excitatory effects of glutamate on NMDA receptors (Attucci et al., 2001; Awad et al., 2000; Pisani et al., 2001). Accordingly, blockade of mGlu1 or mGlu5 receptors decreases glutamate neurotransmission (Attucci et al., 2001). Group II receptors (mGlu2 and mGlu3) are Gi protein-coupled receptors. Activation of these receptors inhibits adenylate cyclase activity, attenuates L- and N-type voltage-sensitive Ca2+ channels and activates K+ channels. Both mGlu2 and mGlu3 receptors are mainly located presynaptically, and function as inhibitory autoreceptors regulating glutamate release or heteroreceptors controlling the release of neurotransmitters other than glutamate (Niswender and Conn, 2010; Schoepp, 2001). Among the Group III receptors, mGlu4, mGlu7 and mGlu8 receptors are coupled to Gi/o proteins and are largely located presynaptically. Activation of these receptors inhibits adenylate cyclase activity, attenuates N-type voltage-sensitive Ca2+ channels and activates K+ channels, thereby, decreasing the release of neurotransmitters, including glutamate. The mGlu6 receptors are located postsynaptically in ON-bipolar cells of the retina (Ferraguti and Shigemoto, 2006; Niswender and Conn, 2010).
It is accepted that compounds acting at metabotropic glutamate receptors may be more likely, than compounds acting at ionotropic glutamate receptors to induce the desired therapeutic effects with minimal side-effects because of the modulatory properties of metabotropic glutamate receptors (Liechti and Markou, 2008; Markou, 2007a, b; Olive, 2009). The first compelling evidence supporting this notion came from the findings that mice lacking the mGlu5 receptor gene did not self-administer cocaine, but acquired stable food self-administration behavior (Chiamulera et al., 2001). Moreover, while cocaine did not produce locomotor-stimulant and locomotor sensitization effects in mGlu5 receptor null mutant mice, these mice showed normal basal locomotor activity compared with their wildtype littermates. Importantly, the genetic manipulation findings were mimicked by administration of the mGlu5 receptor selective antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) in wildtype mice (Chiamulera et al., 2001). These findings indicate that the reinforcing and hyperactivity properties of cocaine are absent in mGlu5 receptor null mutant mice, suggesting that the mGlu5 receptor is critically involved in cocaine dependence and that blockade of mGlu5 receptors may be an effective treatment for cocaine dependence with few unwanted side-effects. Using pharmacological manipulation methods, a large body of literature has demonstrated that decreases of glutamatergic neurotransmission by blockade of Group I mGlu5 receptors or by activation of presynaptic Group II mGlu2/3 receptors inhibit the rewarding and motivational effects of abused drugs, such as cocaine, morphine, heroin, nicotine and alcohol, with an acceptable side-effect profile (Heilig and Egli, 2006; Lea and Faden, 2006; Liechti and Markou, 2008; Markou, 2007b; Moussawi and Kalivas, 2010; Olive, 2009). However, up to recently, little was known as to whether decreasing glutamate release by activation of presynaptic Group III mGlu receptors has similar efficacy in reversing the effects of drugs of abuse as actions at Group I and Group II metabotropic glutamate receptors.
The mGlu7 receptor is the most highly conserved receptor subtype among all mGlu receptors across mammalian species (Ferraguti and Shigemoto, 2006), suggesting that this receptor plays an important role in central nervous system function (Conn and Niswender, 2006). Thus, results with an mGlu7 receptor pharmacological agent in experimental animals are highly likely to generalize to humans. This receptor plays a critical role in shaping synaptic responses for glutamatergic neurotransmission because it is located directly in the presynaptic zone of the synaptic cleft of glutamatergic synapses (Bradley et al., 1996; Cartmell and Schoepp, 2000; Dalezios et al., 2002; Kosinski et al., 1999; Shigemoto et al., 1996). Among the Group III metabotropic glutamate receptors, the mGlu7 receptor is the most widely distributed presynaptic subtype, and acts not only as autoreceptor to regulate the glutamate release from the presynaptic terminal, but also as heteroreceptor to regulate the release of inhibitory γ-aminobutyric acid (GABA), and, possibly the release of monoamines (Bradley et al., 1998; Cartmell and Schoepp, 2000).
Previous studies have shown that intracerebral administration of L-(+)-2-Amino-4-phosphonobutyric acid (L-AP4), a non-selective, non-systemically active Group III mGlu receptor agonist (Schoepp et al., 1999), significantly decreased extracellular dopamine and glutamate levels in the NAc (Hu et al., 1999; Xi et al., 2003), and attenuated cocaine- and amphetamine-induced hyperactivity and increases of striatal dopamine levels (David and Abraini, 2003; Mao et al., 2000). These findings suggest the potential usefulness of Group III receptor agonists for the treatment of drug dependence. Although the receptor subtypes responsible for the effects of L-AP4 remains unknown, among Group III mGlu receptors, the mGlu7 receptor has the highest density in reward-related brain regions, such as the striatum, ventral pallidum, amygdala, and olfactory bulb (Bradley et al., 1998; Ferraguti and Shigemoto, 2006; Kosinski et al., 1999), suggesting that the mGlu7 receptor has a particularly crucial role in modulating neurotransmitter release in pathways involved in drug dependence (Xi et al., 2003).
3. AMN082, the first widely available and selective mGlu7 receptor allosteric agonist
The prototypical Group III ligands developed previously, such as the receptor agonist L-AP4 and antagonist (RS)-alpha-methylserine-O-phosphate (MSOP), are orthosteric compounds that bind at the highly conserved glutamate binding site in the N terminal of the receptor (Niswender and Conn, 2010; Thomas et al., 1996). Although these compounds have significant Group selectivity, they do not distinguish between members of a receptor Group. Moreover, both L-AP4 and MSOP cannot penetrate the blood-brain barrier (Yang, 2005). Thus, development and preclinical testing of selective Group III ligands with good brain penetration will significantly contribute to uncovering the role of each subtype in the brain and will lead to the discovery and development of novel therapeutic treatments for psychiatric disorders including drug dependence.
By using a random high-throughput screen of chemical libraries, Mitsukawa and colleagues identified a highly selective mGlu7 receptor allosteric agonist AMN082 (N,N′-dibenzhydrylethane-1,2-diamine dihydrochloride) (Mitsukawa et al., 2005). The chemical structure of AMN082 is entirely different from L-glutamate and L-AP4 (Mitsukawa et al., 2005) (see Figure 1). AMN082 fully activates mGlu7 receptors by binding to a different site from the glutamate-binding pocket utilized by conventional orthosteric ligands; that is, AMN082 binds to the trans-membrane domain of the mGlu7 receptor, while L-glutamate and L-AP4 bind to the extracellular N-terminal domain (Mitsukawa et al., 2005). Thus, AMN082 itself is devoid of intrinsic agonist activity in the absence of glutamate, but potentiates the effectiveness of glutamate at mGlu7 receptors, making it a positive allosteric modulator (PAM) (Conn and Niswender, 2006; Mitsukawa et al., 2006). It should be noted that direct interaction with the receptors by orthosteric agonists may produce some adverse effects, such as excessive activation of the receptors, receptor desensitization or loss of the receptor activity by pulsatile release of neurotransmitter (Marino and Conn, 2006). Therefore, allosteric modulation of mGlu receptor activity, that does not directly alter glutamate activity, constitutes a desirable pharmacological approach for the treatment of neuropsychiatric disorders, such as drug dependence, with few unwanted effects (Jin et al., 2010; Niswender and Conn, 2010) (also see below). This hypothesis was enlightened by evidence that benzodiazepines, that are GABAA receptor positive allosteric modulators, have similar pharmacological effectiveness as GABAA receptor orthosteric agonists for the treatment of anxiety and sleep disorders, while having lower potentially lethal effects than orthosteric agonists that directly activate GABA receptors (Mohler et al., 2002).
Figure 1.

Chemical structures of L-glutamate, L-AP4 and AMN082.
There are several lines of evidence supporting the potency and selectivity of AMN082 at mGlu7 receptors: 1) In vitro phosphoinositol hydrolysis, GTPγS binding and cytoplasmic calcium measurements data indicate that AMN082 has ~30–150-fold selectivity for mGlu7 receptors over other mGlu, NMDA or AMPA receptor subtypes (Mitsukawa et al., 2005); 2) AMN082 potently inhibits forskolin-stimulated cAMP accumulation in mGlu7b receptor-expressed cells with an EC50 value of ~64 nM (Mitsukawa et al., 2005), and in mGlu7a receptor-expressed cells with an EC50 value of ~109 nM (Bradley et al., 2012). However, AMN082 is far more potent than orthosteric ligands, such as L-glutamate (EC50 23.6 μM) and L-AP4 (EC50 213 μM) (Bradley et al., 2012); 3) AMN082 (3 μM) enhances GTPγS binding using membranes from transfected mammalian mGlu7 receptor cells by over 160% relative to the maximal agonist activity of L-glutamate (Mitsukawa et al., 2005); 4) AMN082 increases plasma levels of the stress hormones corticosterone and adrenocorticotropic hormone (ACTH) in wildtype littermates, but not in mGluR7 knockout mice (Mitsukawa et al., 2005); 5) Administration of AMN082 induce anxiolytic-like effects in wildtype mice, but not in mGluR7 knockout mice (Stachowicz et al., 2008); 6) The inhibitory effects of AMN082 on forskolin-stimulated cAMP accumulation can be blocked by the selective mGlu7 receptor allosteric antagonist MMPIP [6-(4-methoxyphenyl)-5-methyl-3-pyridin-4-ylisoxazolo[4,5-c]pyridin-4(5H)-one]), that may share the same binding site with AMN082 in the trans-membrane domain (Bradley et al., 2012; Suzuki et al., 2007); 7). After knowndown of mGlu7 receptors in mGlu7 receptor-expressed cells by small interfering RNA, 1 μM AMN082 inhibits forskolin-stimulated cAMP accumulation to 78% of control level in these cells. In contrast, the same dose of AMN082 inhibits cAMP accumulation up to 37% in mGlu7 receptor control cells (Suzuki et al., 2007). Finally, AMN082 is orally active and readily penetrates the blood-brain barrier (Mitsukawa et al., 2005). All these findings suggest that AMN082 is a valuable pharmacological tool for studying the function of mGlu7 receptors (Conn and Niswender, 2006). Indeed, soon after the introduction of this compound, many studies have unraveled the role of the mGlu7 receptor in glutamate transmission in the central nervous system, and in neuropsychiatric disorders, such as depression, anxiety and pain (see details below). Based on the important role of glutamate in drug dependence, we have explored the potential involvement of mGlu7 receptors in processes relating to drug dependence in animal studies.
4. Activation of mGlu7 receptors inhibits the reinforcing and motivational effects of drugs of abuse
The rewarding effects of drugs of abuse play a key role in driving acquisition and initiation of drug use, as well as in the maintenance of substance use and dependence (Koob, 1992; Wise and Bozarth, 1987). Thus, treatments that block these effects of drugs of abuse could help people maintain abstinence. The fact that addictive drugs serve as reinforcers in maintaining operant drug-taking behavior has led to the development of the intravenous drug self-administration procedure as an animal model that has good face validity for drug-taking behaviors in humans (Markou et al., 1993; O’Brien and Gardner, 2005). In this procedure, animals are surgically prepared with intravenous catheters and allowed to self-administer drugs of abuse by performing an operant response, such as level pressing. Both the fixed-ratio and progressive-ratio (FR and PR, respectively) schedules of reinforcement for intravenous self-administration are commonly used procedures to study the reinforcing and motivational effects of drugs of abuse in experimental animals. FR schedules of reinforcement, in which a specific fixed number of lever presses results in delivery of a drug infusion, assess the primary reinforcing effects of drugs of abuse. PR schedules, in which progressively increased numbers of operant responses are imposed upon animal in order to receive successive drug infusions, evaluate incentive motivation for the drug reinforcers (Hodos, 1961; Markou et al., 1993; O’Brien and Gardner, 2005; Richardson and Roberts, 1996). The last ratio value successfully completed is defined as the break-point and is considered a measure of reinforcing efficacy or strength (Hodos, 1961) or motivation for the drug (Markou et al., 1993; Richardson and Roberts, 1996).
We found that systemic administration of AMN082 (3, 10, 20 mg/kg) dose-dependently decreased the number of cocaine infusions administered under an FR2 schedule of reinforcement and lowered PR break-points (Figure 2A and 2B) for cocaine self-administration in rats (Li et al., 2009). These results suggest that AMN082 not only inhibits the primary reinforcing effects of cocaine and thus cocaine consumption, but also inhibits the incentive motivational effects of cocaine. Consistent with our findings on cocaine self-administration, AMN082 also significantly inhibited heroin self-administration in rats, ethanol self-administration in mice (Salling et al., 2008), and ethanol consumption and preference in rats (Bahi et al., 2011). Further, microinjections of AMN082 into the NAc or ventral pallidum (VP), but not the dorsal striatum, also inhibited cocaine self-administration (Figure 3). Microinjections of MMPIP, a selective mGlu7 receptor antagonist, into the NAc prevented AMN082 from decreasing cocaine self-administration (Li et al., 2009), suggesting that the pharmacological actions of AMN082 are mediated by activation of mGlu7 receptors in the NAc-VP pathway. Importantly, AMN082 neither affects sucrose consumption at doses that inhibit cocaine self-administration in rats (Figure 2C) (Li et al., 2009), nor alters saccharin (sweet) fluid intake (Bahi et al., 2011). The effects of pharmacological manipulation on level responding depends to a large extent on baseline response rates, and drugs affect more high rates of responding than low response rates (Sanger and Blackman, 1976). Therefore, the fact that the compound had no effect on the higher rate responding supported by food reinforcement provides further evidence for the selectivity of the compound for drug reinforcement. AMN082 does not alter either basal or cocaine-enhanced locomotor activities (Bahi et al., 2011; Li et al., 2009). In contrast to these findings, AMN082 (10 mg/kg) was reported to produce a short-lasting (5–10 min) inhibitory effect on basal locomotor activity in mice (Palucha et al., 2007; Salling et al., 2008) and rats (Palucha-Poniewiera et al., 2010). At the highest dose tested (20 mg/kg), AMN082 inhibits fast-running rotarod performance, an effect that lasted for approximately1 hour (Li et al., 2009). These data suggest that although AMN082 may have some mild and short-lasing non-specific effects on locomotor activity, overall there do not appear to be severe unwanted side-effects.
Figure 2.

Systemic administration of AMN082 inhibits cocaine self-administration under FR2 (A) and PR (B) schedules of reinforcement, but has no effect on oral sucrose self-administration (C). * p<0.05, **p<0.01, ***p<0.001, compared to the vehicle control group. Data taken with permission from Neuropsychopharmacology.
Figure 3.

Microinjection of AMN082 into the NAc (A) or VP (B), but not the dorsal striatum (C), inhibits cocaine self-administration, an effect that was blocked by co-administration of MMPIP, a selective mGlu7 receptor antagonist. *p<0.05, **p<0.01, ***p<0.001, compared to the vehicle control group. Data taken with permission from Neuropsychopharmacology.
5. Effects of AMN082 on the reward enhancing effects of drugs of abuse
One of the pharmacological effects of drugs of abuse that is common to drugs from various pharmacological classes and which is hypothesized to contribute to the perpetuation of drug dependence is the enhancement of brain reward. This action may partly account for the intrinsic reinforcing properties of drugs of abuse and also reflect how drugs of abuse potentiate or summate with the reinforcing actions of non-drug rewarding stimuli (Bauco and Wise, 1997; Markou and Koob, 1992; O’Brien and Gardner, 2005; Wise, 1996a). The intracranial self-stimulation (ICSS) procedure is the most commonly used experimental animal procedure to evaluate the rewarding enhancing effects of drugs of abuse. In this procedure, animals are trained to respond for rewarding electrical self-stimulation via a stimulation electrode implanted in specific brain reward loci, such as the medial forebrain bundle or the VTA or the NAc (Kornetsky, 2004; Markou and Koob, 1992; Wise, 1996a). The minimal stimulation intensity that maintains ICSS behavior is termed the reward threshold. Various drugs of abuse, such as opiates and psychostimulants, lower ICSS thresholds, reflecting an enhancement by these drugs of brain reward function (Markou and Koob, 1992; O’Brien and Gardner, 2005; Wise, 1996a). Therefore, the ICSS procedure provides a unique measure to assess the reward enhancing properties of drugs of abuse, and to evaluate compounds that have the therapeutic potential to block the reward enhancing effects of drugs of abuse.
To determine whether the mGlu7 receptor is involved in brain reward function and whether pharmacologic activation of mGlu7 receptors interferes with the reward enhancing effects of drugs of abuse, we assessed the effects of AMN082 on cocaine-induced lowering of ICSS thresholds in rats that reflect a reward enhancing effect (Li et al., 2009). Administration of AMN082 (1, 3, 10, 20 mg/kg) dose-dependently attenuated the ICSS threshold-lowering effects of acute cocaine, indicating blockade of the reward enhancing effects of cocaine (Figure 4A). In contrast, AMN082 by itself, at the same dose range, had no effect on ICSS thresholds (Figure 4B), suggesting that AMN082 produces neither reward-like nor aversive-like effects on brain reward function, and that mGlu7 receptors selectively modulate the actions of cocaine, but not brain reward function itself. Therefore, the results indicate that in health organisms, stimulation of mGlu7 receptors by exogenous agents does not change brain reward function. However, this finding does not necessary suggest that these receptors are not involved in the modulatory actions of glutamatergic neurotransmission during a normal health state. Moreover, it appears that mGlu7 receptors are most sensitive to pharmacologic manipulations during periods of intense synaptic activity, such as increased dopamine or glutamate neurotransmission induced by cocaine administration. This properties of the mGlu7 receptor are different from the effects produced by other mGlu receptor compounds, such as the mGlu2/3 receptor agonist LY379268 or the mGlu5 receptor antagonist MPEP, which themselves elevate reward threshold when administered alone, in addition to blocking the reward enhancing effects of cocaine or nicotine (Harrison et al., 2002; Kenny et al., 2005; Kenny et al., 2003; Liechti and Markou, 2007). Consistent with the inhibition by AMN082 of cocaine-enhanced brain reward, system administration of AMN082 attenuated the threshold-lowering effects of acute heroin administration. Taken together, systemic administration of AMN082, a selective mGlu7 receptor allosteric agonist, not only inhibits the primary rewarding and motivational effects of cocaine and heroin, but also inhibits the reward-enhancing effect of cocaine and heroin with few unwanted side-effects.
Figure 4.

Systemic administration of AMN082 inhibits the reward-enhancing effects produced by cocaine (A), while AMN082 itself has no rewarding or aversive-like effects (B). * p<0.05, **p<0.01, compared to the cocaine-alone treatment group. ### p<0.001, compared to the vehicle treatment group. Data taken with permission from Neuropsychopharmacology.
6. A Nucleus Accumbens – Ventral Pallidum GABAergic mechanism may underlie the actions of AMN082 on cocaine reward
In vivo microdialysis studies indicated that systemic or local administration of AMN082 into the NAc, VP or dorsal striatum did not alter basal or cocaine-induced increases in extracellular dopamine in either drug naïve rats or in cocaine self-administering rats 24 hrs after cocaine self-administration, suggesting that a non-dopaminergic mechanism mediates the antagonism of cocaine reward by this compound (Figure 5A) (Li et al., 2009). This neurochemical finding is consistent with previous anatomical findings showing that the mGlu7 receptors are undetectable on brain dopaminergic neurons (Kosinski et al., 1999; Shigemoto et al., 1997). Nevertheless, we note reports that activation of Group III mGlu receptors by L-AP4 inhibits dopamine release in the NAc (Hu et al., 1999) and the dorsal striatum (Mao et al., 2000). Since L-AP has higher potencies on mGlu4 and mGlu8 receptors than on mGlu7 receptors (Schoepp et al., 1999), it is likely that L-AP4-induced reduction in dopamine is mediated by activation of mGlu4 and/or mGlu8 receptors rather than of mGlu7 receptors. To further study which non-dopaminergic mechanisms underlie the actions of AMN082, we used in vivo microdialysis to measure extracellular dopamine, glutamate and GABA in the same microdialysis samples. AMN082 decreased extracellular GABA and increased extracellular glutamate (see below for explanation of this effect), but had no effect on extracellular dopamine in the NAc (Li et al., 2008). Strikingly, with the continue perfusion of the GABAB receptor antagonist 2-hydroxy-saclofen into the NAc, AMN082 significantly decreased NAc glutamate. This pattern of results suggests that the increases in glutamate may be secondary to the decreases in GABA transmission (Li et al., 2008). Thus, the increases in NAc glutamate induced by AMN082 administration appear to be the net effects of two opposite actions: one is inhibition of glutamate release by activation of mGlu7 receptors on glutamatergic neurons (direct effects); another is inhibition of GABA transmission that would normally inhibit glutamate release, and thus disinhibiting glutamate release (indirect effects). How this increase in NAc glutamate levels and decreases in GABA levels lead to decreases in the motivational and reinforcing effects of cocaine is possibly explained by the mechanism hypothesized in the paragraph below.
Figure 5.

Systemic administration of AMN082 do not alter cocaine-induced increase in extracellular dopamine (DA) in the NAc (A), but blocks cocaine-induced reduction in GABA release in the ventral pallidum (B), one of the NAc GABAergic projection areas. *p<0.05, **p<0.01, ***p<0.001, compared to baseline (before cocaine) of each treatment group. Data taken with permission from Neuropsychopharmacology.
It has been proposed that inhibition of medium spiny GABAergic output neurons in the NAc, that project predominantly to the ventral pallidum (Bennett and Bolam, 1994; Groenewegen et al., 1996), serve as one of the final common pathways mediating psychostimulant reward (Koob and Bloom, 1988; Wise and Bozarth, 1987; Xi and Gardner, 2008). Glutamate and dopamine are proposed to have opposite actions on their target neurons in the NAc. Glutamate is excitatory, while dopamine is inhibitory on the activity of these medium spiny neurons (Carlezon and Wise, 1996). Conceptually congruent with this hypothesis, studies show that acute cocaine, that increases NAc dopamine by inhibiting dopamine reuptake, decreases extracellular GABA levels in the ventral pallidum (Bennett and Bolam, 1994; Li et al., 2009; Torregrossa and Kalivas, 2008; Torregrossa et al., 2008). Thus, we have proposed that AMN082-induced increases in glutamate may functionally antagonize cocaine- or dopamine-induced inhibition of medium spiny GABAergic neurons and the reduction in GABA release in the ventral pallidum (Li et al., 2009; Xi and Gardner, 2008). In addition, AMN082-induced decreases of NAc GABA may enhance the excitability of the postsynaptic GABAergic neurons, thereby antagonizing cocaine-induced inhibition of these neurons as well. Consistent with our hypothesis, treatment with AMN082 attenuated cocaine-induced decreases of ventral pallidum GABA release in both naïve rats and cocaine self-administering rats (Figure 5B) (Li et al., 2009). These data suggest that a NAc-VP GABAergic mechanism may underlie the antagonism by AMN082 of the rewarding and motivational effects of cocaine.
7. Activation of mGlu7 receptors inhibits reinstatement of drug-seeking behavior
A major challenge in the treatment of drug addiction in humans is the high rate of relapse to drug-taking and drug-seeking behavior even after prolonged periods of abstinence. In both humans and experimental animals, drug craving and reinstatement (a putative animal model of relapse; Shaham et al., 2003; however, see Katz and Higgins, 2003) to drug-seeking can be precipitated by re-exposure to drugs of abuse, presentation of environmental stimuli (cues) previously associated with drug taking, or environmental stressors (Bossert et al., 2005; Katz and Higgins, 2003; Le and Shaham, 2002; Shalev et al., 2002). Accordingly, reinstatement of drug-seeking behavior is considered a putative model of relapse in humans (Epstein et al., 2006; Katz and Higgins, 2003; O’Brien and Gardner, 2005; Shaham et al., 2003). In the reinstatement procedure, animals are first trained to self-administer drug of abuse by performing operant responses (e.g., acquisition of drug self-administration). After the establishment of stable drug-taking behavior, the animals are subjected to extinction procedures where the drug is no longer available (e.g., extinction of self-administration). Animals are considered to have met the extinction criteria when they lever-press less than 10 times per extinction session for at least 3 consecutive days. Then, the animals are exposed to drugs of abuse, drug-associated environmental cues, or stressors (foot-shock), in order to reinstate the operant behavior that previously resulted in drug delivery (e.g., reinstatement testing).
This reinstatement procedure was used to assess whether AMN082 has similar efficacy against drug-seeking behaviors as it does against drug-taking behaviors (Li et al., 2010). Administration of AMN082, at the same doses that inhibit cocaine self-administration, blocked cocaine-induced reinstatement of cocaine-seeking behavior in rats (Figure 6A). Similarly, local administration of AMN082 into the NAc or VP also inhibited cocaine-induced reinstatement of cocaine-seeking behavior, an effect that was blocked by co-administration of the mGlu7 receptor antagonist MMPIP (Figure 7), suggesting that the antagonistic effects of AMN082 on cocaine seeking are mediated by activation of mGlu7 receptors in the NAc-VP pathway. To determine whether this action generalizes to reinstatement of drug-seeking behavior for other drugs of abuse, the effects of AMN082 on heroin-triggered reinstatement of heroin-seeking behavior were investigated and the effects were found to be similarly inhibitory. Although both the 10 mg/kg and 20 mg/kg doses of AMN082 exhibited robust inhibitory effects on drug-seeking, only 20 mg/kg AMN082 significantly inhibited sucrose-induced reinstatement of sucrose seeking (Figure 6B), suggesting that AMN082 has limited selectivity in inhibiting drug-seeking over food-seeking behaviors.
Figure 6.
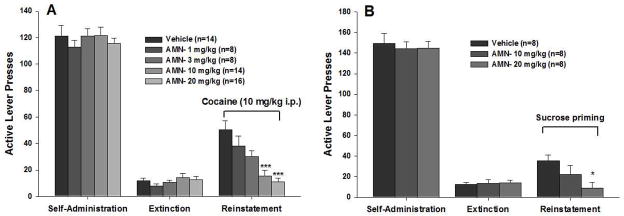
Systemic administration of AMN082 dose-dependently inhibits cocaine (A) and sucrose (B)-induced reinstatement of reward-seeking behavior. *p<0.05, ***p<0.001, compared to the vehicle control group during the reinstatement testing. Data taken with permission from Journal of Neurochemistry.
Figure 7.

Microinjection of AMN082 into the NAc (A) or VP (B), but not the dorsal striatum (C), inhibits cocaine-induced reinstatement of drug-seeking behavior. Co-administration of MMPIP, a selective mGlu7 receptor antagonist, with AMN082 blocks the inhibitory effect of AMN082 on cocaine-seeking behavior. *p<0.05, **p<0.01, compared to the vehicle control group. Data taken with permission from Journal of Neurochemistry.
8. A glutamate mGlu 2/3 receptor mechanism underlies the antagonism of cocaine seeking by AMN082
To determine whether a dopamine-dependent mechanism underlies the antagonism of cocaine seeking, we first measured cocaine- and AMN082-induced changes in extracellular dopamine in the NAc, VP and dorsal striatum (Li et al., 2010). AMN082 did not alter cocaine-induced increases in extracellular dopamine in these brain regions in rats during reinstatement testing (Figure 8A), suggesting again that a non-dopaminergic mechanism underlies the actions of AMN082 on reinstatement of drug-seeking.
Figure 8.
Systemic administration of AMN082 do not alter cocaine-induced increases in extracellular dopamine (DA) (A), but blocks cocaine-induced increases in extracellular glutamate (B) in the NAc in rats during reinstatement testing. Pretreatment with the mGlu2/3 receptor antagonist LY341495 (1 mg/kg, i.p.) blocks the antagonism by AMN082 of cocaine-enhanced extracellular glutamate (C) and cocaine-induced reinstatement of drug-seeking behavior (D). *p<0.05, **p<0.01, ***p<0.001, compared to baseline; # p<0.05, ##p<0.01, ###p<0.001, compared to vehicle group. Data taken with permission from Journal of Neurochemistry.
Emerging evidence suggests that neuroadaptations in glutamate transmission play an important role in reinstatement of drug-seeking (Baker et al., 2003; Kalivas et al., 2009; Thomas et al., 2001). Specifically, there is a reduction in basal levels of glutamate transmission in the NAc in rats during extinction or withdrawal from cocaine self-administration, manifested as a reduction in basal extracellular glutamate, glutamate release or presynaptic glutamate immunoreactivity (Baker et al., 2003; Keys et al., 1998; Kozell and Meshul, 2003; McFarland et al., 2003; Meshul et al., 1998; Pierce et al., 1996). Congruent with these findings, basal levels of extracellular glutamate are ~50% lower in rats 24 hrs after cocaine self-administration (0.35 ± 0.10 μM) or in rats after 2–3 weeks of extinction from cocaine self-administration (0.43 ± 0.10 μM) compared to drug naïve rats (0.77 ± 0.11 μM) (Li et al., 2008; Li et al., 2010; Li et al., 2009). This reduction in basal levels of extracellular glutamate has been shown to be associated with chronic cocaine-induced down-regulation of glial cystine-glutamate exchanger (Baker et al., 2003), that causes a reduction in nonvesicular glutamate release via the cystine-glutamate transporters (Kalivas et al., 2009; Shih and Murphy, 2001). In contrast, re-exposure to drugs of abuse such as cocaine or heroin, or cocaine-associated cues induces a significant increase in extracellular glutamate in the NAc, that has been shown to be related to reinstatement of drug-seeking behavior (Cornish and Kalivas, 2000; Kalivas et al., 2009; Knackstedt and Kalivas, 2009). The increased glutamate response seems to be brain region specific because cocaine priming significantly increased extracellular glutamate only in the NAc (Li et al., 2010; McFarland et al., 2003), but not in the VP or the dorsal striatum (Li et al., 2010). These data are consistent with previous findings that chronic cocaine decreased basal levels of extracellular glutamate only in the NAc, but not in the PFC or the dorsal striatum (Baker et al., 2003; Pierce et al., 1996). Further, mechanistic studies suggest that cocaine-induced increases in NAc glutamate involve glutamate from neuronal sources (McFarland et al., 2003; Xi et al., 2006). Moreover, chronic cocaine administration induced a down-regulation in presynaptic mGlu2/3 receptor phosphorylation and function that has been hypothesized to be related to the increase in glutamate response to cocaine (Xi et al., 2002). Accordingly, increased presynaptic mGlu2/3 receptor function would attenuate cocaine- or cue-induced increase in extracellular glutamate, and therefore, prevent reinstatement to drug-seeking behavior (Xi and Gardner, 2008).
As stated above, systemic administration of AMN082 significantly elevates extracellular glutamate in the NAc. Thus, we further hypothesized that increased extracellular glutamate would increase presynaptic mGlu2/3 autoreceptor function and antagonize cocaine-induced increases in extracellular glutamate and reinstatement to drug-seeking. To test this glutamate mGlu2/3 receptor hypothesis, we used in vivo microdialysis to measure extracellular glutamate levels in rats during reinstatement of drug-seeking. AMN082 administration attenuated both cocaine priming-induced increases in extracellular glutamate in the NAc and reinstatement of cocaine-seeking (Figure 8B) (Li et al., 2010). Strikingly, this antagonism of cocaine-seeking and cocaine-induced increase in glutamate release was blocked by systemic or intra-NAc local administration of LY341495, a selective mGlu2/3 receptor antagonist, or of MMPIP, a selective mGlu7 receptor antagonist (Figure 8C and 8D). Taken together, these results suggest that a glutamate-mGlu2/3 receptor mechanism underlies the antagonism of cocaine seeking after mGlu7 receptor activation. This finding is consistent with previous studies demonstrating that elevation of extracellular glutamate by increasing cystine-glutamate transporter or blockade of presynaptic CB1 receptors attenuates cocaine-induced reinstatement of drug-seeking in rats (Baker et al., 2003; Xi et al., 2006). Similarly, elevation of brain NAAG, an endogenous mGlu3 receptor agonist, by administration of 2-PMPA, a selective NAAG degradation enzyme inhibitor, also inhibits cocaine-induced reinstatement of drug seeking (Xi et al., 2010). Further, administration of the mGlu2/3 receptor agonist LY378268 decreased reinstatement of cocaine (Baptista et al., 2004), heroin (Bossert et al., 2004), alcohol (Zhao et al., 2006) and nicotine (Liechti et al., 2007) seeking in rats. Taken together, a glutamate-mGlu2/3 receptor mechanism appears to play an important role in reinstatement of drug-seeking, and augmentation of presynaptic mGlu2/3 receptor function significantly attenuates cocaine-induced reinstatement (relapse) of drug-seeking.
9. mGlu7 receptor involvement in other psychiatric disorders
Glutamate is the major excitatory neurotransmitter in the central nervous system and is critically involved in many processes in the brain functions, including fast and slow excitatory neurotransmission, synaptic plasticity and control of basal neuronal activity. Abnormal glutamate transmission is associated with a range of psychiatric and neurological disorders. Because of the inherently modulatory role of mGlu7 receptors in glutamate neurotransmission, AMN082 has been shown to have potentially beneficial effects not only for the treatment of drug addiction, but also for other neurological and psychiatric disorders such as depression, anxiety, pain and Parkinson’s disease.
Considering that mGlu7 receptor knockout mice display dysregulation of the hypothalamic-pituitary-adrenal axis, Mitsukawa and colleagues investigated the effects of AMN082 on plasma stress hormones in mice (Mitsukawa et al., 2005). They found that oral administration of AMN082 significantly increased plasma corticosterone and corticotrophin levels in wildtype mice, but not in mGlu7 receptor deficient mice. This finding is consistent with a report that intra-ventricular microinjection of L-AP4 and L-serine-O-phosphate (L-SOP), two Group III mGlu receptor agonists, activated hypothalamic-pituitary-adrenal axis (Johnson et al., 2001), suggesting that mGlu7 receptors regulate stress-related psychiatric disorders, such as depression and anxiety. Consistent with this notion, systemic administration of AMN082 dose-dependently inhibited immobility in the forced swim test and the tail suspension test in wildtype mice (Bradley et al., 2012; Palucha et al., 2007), but not in mGlu7 receptor knockout mice (Palucha et al., 2007). Moreover, AMN082 exhibited antidepressant actions in the differential reinforcement of low rates of responding-30 test, another assay sensitive to antidepressants, in rats (Bradley et al., 2012). These data suggest that AMN082 may have therapeutic antidepressant-like effects.
In addition, administration of AMN082 prevented the acquisition of conditioned fear and enhanced its extinction (Fendt et al., 2008), and facilitated between-session fear extinction in rats (Toth et al., 2011), while down-regulation of mGlu7 receptors using short interfering RNA attenuated the extinction of learned aversion (Fendt et al., 2008). These findings suggest that mGlu7 receptor activation facilitates extinction of aversive memories, and therefore, may be useful for the treatment of anxiety disorders. This suggestion is further supported by the finding that intra-amygdala administration of AMN082 impaired fear acquisition in rats (Siegl et al., 2008), and systemic administration of AMN082 induced anxiolytic-like effects in wildtype mice, but not in mGlu7 receptor knockout mice, as assessed by the modified stress-induced hyperthermia and four-plate tests (Stachowicz et al., 2008). However, the anxiolytic effects of AMN082 seem incompatible with findings in mGlu7 receptor gene knockout mice, which also show anxiolytic-like effects (Callaerts-Vegh et al., 2006; Cryan et al., 2003). The discrepancy between pharmacological and genetic modulation of the mGlu7 receptor may be due to the genetic compensation and development adaptations during early development of gene knockout mice. Studies using gene silencing approaches should be considered to further elucidate the role of mGlu7 receptors in anxiety.
Behavioral studies in animal models of pain are conflicting. Osikowicz and colleagues reported that systemic administration of AMN082 attenuated allodynia and hyperalgesia in mice (Osikowicz et al., 2008). Further, acute or chronic administration of AMN082 enhanced the analgesic effects of morphine (Osikowicz et al., 2008). Moreover, AMN082 inhibited inflammatory pain-induced and incision-induced hypersensitivity in rats (Dolan et al., 2009). These data suggest analgesic effects of AMN082. In contrast to these findings, microinjection of AMN082 into the central nucleus of the amygdala facilitates pain responses in rats (Palazzo et al., 2008), and intra-periaqueductal gray administration of AMN082 decreased tail flick latency and thermoceptive thresholds in rats, suggesting activation of mGlu7 receptors could worsen pain perception (Marabese et al., 2007).
L-AP4, the Group III mGlu receptor agonist, has been shown to reduce motor symptoms in 6-hydroxy-dopamine-lesioned rats (Lopez et al., 2007; Senkowska and Ossowska, 2003), suggesting that Group III mGlu receptors represent promising therapeutic targets for the treatment of Parkinson’s disease. By using the novel mGlu7 allosteric receptor agonist AMN082, Greco and colleagues demonstrated that AMN082 reversed motor dysfunction in an animal model of Parkinson’s disease in rats, suggesting that the therapeutic effects of L-AP4 could be mediated by activation of mGlu7 receptors. Thus, mGlu7 receptors could be a new target for medication development for the treatment of Parkinson’s disease (Greco et al., 2010).
10. Potential “off-target” profile of AMN082
Although most of the studies cited above indicate that AMN082 is a highly selective mGlu7 receptor allosteric agonist, there are findings suggesting that some effects of AMN082 may not be mediated by the mGlu7 receptors. AMN082 neither altered mGlu7 receptor-induced intracellular Ca++ mobilization in mGlu7 receptor-expressing cell lines (Suzuki et al., 2007), nor induced mGlu7 receptor-mediated activation of GIRK potassium channels in HEK cells (Ayala et al., 2008). It also failed to activate mGlu7 receptors at the Shaffer collateral-CA1 synapse (Ayala et al., 2008). An early study indicated that the antidepressant-like effects of AMN082 are mediated by activation of mGlu7 receptor as supported by the evidence that AMN082 induced decreases in the immobility time in the forced swim test and the tail suspension test only in the wildtype, but not the mGlu7 receptor knockout, mice (Palucha et al., 2007), while a follow up study suggests that a serotonin receptor mechanism may be involved also in this effect (Palucha-Poniewiera et al., 2010). Finally, it is reported when incubated with rat liver microsomes, AMN082 was metabolized rapidly into the primary metabolite Met-1, which not only had similar allosteric agonist profile at the mGlu7 receptors, but also had appreciable affinity at multiple monoamine transporters including the serotonin transporter, and the dopamine and norepinephrine transporter (Sukoff Rizzo et al., 2011). These findings suggest that AMN082 could be a prodrug and its antidepressant-like effects could be mediated by Met-1 that activates mGlu7 receptors and alters monoamine reuptake (Sukoff Rizzo et al., 2011). However, a recent study showed that the antidepressant actions of AMN082 in the tail suspension test were blocked by the selective AMPA receptor antagonist NBQX, and the behaviorally efficacious doses of AMN082 modulate phosphorylation of AMPA and NMDA receptor subunits in the hippocampus, suggesting that the antidepressant effects of AMN082 are mediated by modulation of glutamatergic signaling (Bradley et al., 2012). Thus, interpretation of data evaluating the effects of AMN082 administration should be interpreted with caution. Nevertheless, it should be noted that many of the claims made above about the role of mGlu7 receptors in various behaviors with relevance to drug dependence involved the use of antagonists at mGlu7 receptors or the use of mGlu7 receptor knockout mice boosting confidence about the attribution of these effects to mGlu7 receptors. Further, a recent study by Toth and colleagues investigating extinction of conditioned fear, demonstrated that the effects of AMN082 on extinction retention could not be reproduced by the serotonin reuptake inhibitor fluoxetine (Toth et al., 2011). Overall, the review of this literature suggests that the availability of more selective PAMs for the mGlu7 receptor than AMN082 would be proven to be useful tools in the delineation of the role of these receptors in multiple neuropsychiatric disorders, as well as the evaluation of the potential therapeutic effects of such compounds for central nervous system disorders.
11. Summary
Activation of mGlu7 receptors by AMN082 significantly inhibits the rewarding and motivational effects of drugs of abuse, such as cocaine, heroin and ethanol as evaluated by the intravenous self-administration procedure under both FR and PR schedules of reinforcement. AMN082 also inhibits the reward-enhancing effects induced by cocaine and heroin as evaluated by the ICSS procedure, and blocks cocaine- and heroin-induced reinstatement of drug-seeking behavior. These findings suggest an important role of mGlu7 receptors in drug reward and reinstatement of drug-seeking behavior. Further, a series of in vivo microdialysis studies suggest that a non-dopaminergic mechanism underlies the antagonism by AMN082 of drug reward and reinstatement to drug-seeking. An increase in extracellular glutamate in the NAc, that is secondary to a reduction in NAc GABA release, appears to play a central role in these actions. Specifically, AMN082-enhanced NAc glutamate may functionally antagonize cocaine- or dopamine-induced reduction in NAc-VP GABAergic transmission, and therefore, antagonize the primary rewarding, reward-enhancing and motivational effects of cocaine. Such an increase in glutamate may also inhibit cocaine- or cue-induced reinstatement (relapse) of drug-seeking behavior by activating presynaptic mGlu2/3 autoreceptors that inhibit cocaine-induced increase in glutamate release from neuronal terminals. Because AMN082 itself does not significantly inhibit locomotion and oral sucrose-taking behavior, and has no rewarding or aversive effects on brain reward function, it is suggested that mGlu7 receptors are a highly promising therapeutic target for medication development for the treatment of drug dependence, and in particular psychomotor stimulant dependence that has been investigated the most extensively so far. Nevertheless, there are data suggesting the potential role of mGlu7 receptors in heroin and ethanol (Bahi et al., 2011; Salling et al., 2008) dependence also. Thus, AMN082 or other mGlu7 selective receptor agonists may have therapeutic potential for drug dependence with few unwanted side-effects, such as sedation and suppression of seeking of natural rewards.
mGlu7 receptors inhibited the reinforcing and motivational effects of cocaine
mGlu7 receptors inhibited the reward-enhancing effects induced by cocaine
mGlu7 receptors inhibited cocaine-induced reinstatement of drug-seeking behavior
mGlu7 receptors are important target for the treatment of drug dependence
Acknowledgments
XL was supported by a new investigator award by the Tobacco-Related Disease Research Program from the State of California. AM was supported by NIH grant DA011946. Z-XX was supported by Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
FINANCIAL DISCLOSURES
AM has received contract research support from Lundbeck, Bristol-Myers Squibb Co., F. Hoffman-La Roche, Pfizer, and Astra-Zeneca, and honoraria/consulting fees from Abbott GmbH and Company, AstraZeneca, and Pfizer during the past 3 years. AM has a patent application on the use of metabotropic glutamate compounds for the treatment of nicotine dependence. The remaining authors report no financial conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br J Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Niswender CM, Luo Q, Banko JL, Conn PJ. Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated. Neuropharmacology. 2008;54:804–814. doi: 10.1016/j.neuropharm.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Fizia K, Dietz M, Gasparini F, Flor PJ. Pharmacological modulation of mGluR7 with AMN082 and MMPIP exerts specific influences on alcohol consumption and preference in rats. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2010.00310.x. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Synergistic effects of cocaine with lateral hypothalamic brain stimulation reward: lack of tolerance or sensitization. J Pharmacol Exp Ther. 1997;283:1160–1167. [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Popik P, Bespalov AY, Danysz W. Therapeutic potential of NMDA receptor antagonists in the treatment of alcohol and substance use disorders. Expert Opin Investig Drugs. 2000;9:2233–2248. doi: 10.1517/13543784.9.10.2233. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J Neurosci. 1996;16:2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Rees HD, Yi H, Levey AI, Conn PJ. Distribution and developmental regulation of metabotropic glutamate receptor 7a in rat brain. J Neurochem. 1998;71:636–645. doi: 10.1046/j.1471-4159.1998.71020636.x. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Uslaner JM, Flick RB, Lee A, Groover KM, Hutson PH. The mGluR7 allosteric agonist AMN082 produces antidepressant-like effects by modulating glutamatergic signaling. Pharmacol Biochem Behav. 2012;101:35–40. doi: 10.1016/j.pbb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Beckers T, Ball SM, Baeyens F, Callaerts PF, Cryan JF, Molnar E, D’Hooge R. Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J Neurosci. 2006;26:6573–6582. doi: 10.1523/JNEUROSCI.1497-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Niswender CM. mGluR7’s lucky number. Proc Natl Acad Sci U S A. 2006;103:251–252. doi: 10.1073/pnas.0510051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17:2409–2417. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- Dalezios Y, Lujan R, Shigemoto R, Roberts JD, Somogyi P. Enrichment of mGluR7a in the presynaptic active zones of GABAergic and non-GABAergic terminals on interneurons in the rat somatosensory cortex. Cereb Cortex. 2002;12:961–974. doi: 10.1093/cercor/12.9.961. [DOI] [PubMed] [Google Scholar]
- David HN, Abraini JH. Blockade of the locomotor stimulant effects of amphetamine by group I, group II, and group III metabotropic glutamate receptor ligands in the rat nucleus accumbens: possible interactions with dopamine receptors. Neuropharmacology. 2003;44:717–727. doi: 10.1016/s0028-3908(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Dolan S, Gunn MD, Biddlestone L, Nolan AM. The selective metabotropic glutamate receptor 7 allosteric agonist AMN082 inhibits inflammatory pain-induced and incision-induced hypersensitivity in rat. Behav Pharmacol. 2009;20:596–604. doi: 10.1097/FBP.0b013e32832ec5d1. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K, Maier R, Natt F, Husken D, Kelly PH, McAllister KH, Hoyer D, van der Putten H, Cryan JF, Flor PJ. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatry. 2008;13:970–979. doi: 10.1038/sj.mp.4002073. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Lopez S, van der Putten H, Flor PJ, Amalric M. Metabotropic glutamate 7 receptor subtype modulates motor symptoms in rodent models of Parkinson’s disease. J Pharmacol Exp Ther. 2010;332:1064–1071. doi: 10.1124/jpet.109.162115. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Kelly G, Chamberlain M. Changes in rat serum corticosterone after treatment with metabotropic glutamate receptor agonists or antagonists. J Neuroendocrinol. 2001;13:670–677. doi: 10.1046/j.1365-2826.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Keys AS, Mark GP, Emre N, Meshul CK. Reduced glutamate immunolabeling in the nucleus accumbens following extended withdrawal from self-administered cocaine. Synapse. 1998;30:393–401. doi: 10.1002/(SICI)1098-2396(199812)30:4<393::AID-SYN6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: implications for abuse. Neurosci Biobehav Rev. 2004;27:777–786. doi: 10.1016/j.neubiorev.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Kosinski CM, Risso Bradley S, Conn PJ, Levey AI, Landwehrmeyer GB, Penney JB, Jr, Young AB, Standaert DG. Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J Comp Neurol. 1999;415:266–284. [PubMed] [Google Scholar]
- Kozell B, Meshul K. Alterations in nerve terminal glutamate immunoreactivity in the nucleus accumbens and ventral tegmental area following single and repeated doses of cocaine. Psychopharmacology (Berl) 2003;165:337–345. doi: 10.1007/s00213-002-1296-7. [DOI] [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Lea PMt, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gardner EL, Xi ZX. The metabotropic glutamate receptor 7 (mGluR7) allosteric agonist AMN082 modulates nucleus accumbens GABA and glutamate, but not dopamine, in rats. Neuropharmacology. 2008;54:542–551. doi: 10.1016/j.neuropharm.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li J, Gardner EL, Xi ZX. Activation of mGluR7s inhibits cocaine–induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. J Neurochem. 2010;114:1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li J, Peng XQ, Spiller K, Gardner EL, Xi ZX. Metabotropic glutamate receptor 7 modulates the rewarding effects of cocaine in rats: involvement of a ventral pallidal GABAergic mechanism. Neuropsychopharmacology. 2009;34:1783–1796. doi: 10.1038/npp.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol. 2007;74:1299–1307. doi: 10.1016/j.bcp.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Role of the Glutamatergic System in Nicotine Dependence: Implications for the Discovery and Development of New Pharmacological Smoking Cessation Therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J Neurosci. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Lau YS, Wang JQ. Activation of group III metabotropic glutamate receptors inhibits basal and amphetamine-stimulated dopamine release in rat dorsal striatum: an in vivo microdialysis study. Eur J Pharmacol. 2000;404:289–297. doi: 10.1016/s0014-2999(00)00633-6. [DOI] [PubMed] [Google Scholar]
- Marabese I, Rossi F, Palazzo E, de Novellis V, Starowicz K, Cristino L, Vita D, Gatta L, Guida F, Di Marzo V, Maione S. Periaqueductal gray metabotropic glutamate receptor subtype 7 and 8 mediate opposite effects on amino acid release, rostral ventromedial medulla cell activities, and thermal nociception. J Neurophysiol. 2007;98:43–53. doi: 10.1152/jn.00356.2007. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Markou A. Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol Psychiatry. 2007a;61:17–22. doi: 10.1016/j.biopsych.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Markou A. The role of metabotropic glutamate receptors in drug reward, motivation and dependence. Drug News Perspect. 2007b;20:103–108. doi: 10.1358/dnp.2007.20.2.1083435. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, Noguchi K, Emre N, Ellison G. Cocaine-induced changes in glutamate and GABA immunolabeling within rat habenula and nucleus accumbens. Synapse. 1998;30:211–220. doi: 10.1002/(SICI)1098-2396(199810)30:2<211::AID-SYN11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Mombereau C, Lotscher E, Uzunov DP, van der Putten H, Flor PJ, Cryan JF. Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders. Neuropsychopharmacology. 2006;31:1112–1122. doi: 10.1038/sj.npp.1300926. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Yamamoto R, Ofner S, Nozulak J, Pescott O, Lukic S, Stoehr N, Mombereau C, Kuhn R, McAllister KH, van der Putten H, Cryan JF, Flor PJ. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc Natl Acad Sci U S A. 2005;102:18712–18717. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139:117–126. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology. 2008;55:537–545. doi: 10.1016/j.neuropharm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha-Poniewiera A, Branski P, Lenda T, Pilc A. The antidepressant-like action of metabotropic glutamate 7 receptor agonist N,N′-bis(diphenylmethyl)-1,2-ethanediamine (AMN082) is serotonin-dependent. J Pharmacol Exp Ther. 2010;334:1066–1074. doi: 10.1124/jpet.110.169730. [DOI] [PubMed] [Google Scholar]
- Palucha A, Klak K, Branski P, van der Putten H, Flor PJ, Pilc A. Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology (Berl) 2007;194:555–562. doi: 10.1007/s00213-007-0856-2. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Salling MC, Faccidomo S, Hodge CW. Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharmacol Biochem Behav. 2008;91:14–20. doi: 10.1016/j.pbb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Senkowska A, Ossowska K. Role of metabotropic glutamate receptors in animal models of Parkinson’s disease. Pol J Pharmacol. 2003;55:935–950. [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Shih AY, Murphy TH. xCt cystine transporter expression in HEK293 cells: pharmacology and localization. Biochem Biophys Res Commun. 2001;282:1132–1137. doi: 10.1006/bbrc.2001.4703. [DOI] [PubMed] [Google Scholar]
- Siegl S, Flor PJ, Fendt M. Amygdaloid metabotropic glutamate receptor subtype 7 is involved in the acquisition of conditioned fear. Neuroreport. 2008;19:1147–1150. doi: 10.1097/WNR.0b013e328307f295. [DOI] [PubMed] [Google Scholar]
- Stachowicz K, Branski P, Klak K, van der Putten H, Cryan JF, Flor PJ, Andrzej P. Selective activation of metabotropic G-protein-coupled glutamate 7 receptor elicits anxiolytic-like effects in mice by modulating GABAergic neurotransmission. Behav Pharmacol. 2008;19:597–603. doi: 10.1097/FBP.0b013e32830cd839. [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Leonard SK, Gilbert A, Dollings P, Smith DL, Zhang MY, Di L, Platt BJ, Neal S, Dwyer JM, Bender CN, Zhang J, Lock T, Kowal D, Kramer A, Randall A, Huselton C, Vishwanathan K, Tse SY, Butera J, Ring RH, Rosenzweig-Lipson S, Hughes ZA, Dunlop J. The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise? J Pharmacol Exp Ther. 2011;338:345–352. doi: 10.1124/jpet.110.177378. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Tsukamoto N, Fushiki H, Kawagishi A, Nakamura M, Kurihara H, Mitsuya M, Ohkubo M, Ohta H. In vitro pharmacological characterization of novel isoxazolopyridone derivatives as allosteric metabotropic glutamate receptor 7 antagonists. J Pharmacol Exp Ther. 2007;323:147–156. doi: 10.1124/jpet.107.124701. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thomas NK, Jane DE, Tse HW, Watkins JC. alpha-Methyl derivatives of serine-O-phosphate as novel, selective competitive metabotropic glutamate receptor antagonists. Neuropharmacology. 1996;35:637–642. doi: 10.1016/0028-3908(96)84635-1. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW. Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement. J Pharmacol Exp Ther. 2008;325:556–566. doi: 10.1124/jpet.107.130310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Tang XC, Kalivas PW. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett. 2008;438:142–145. doi: 10.1016/j.neulet.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I, Dietz M, Peterlik D, Huber SE, Fendt M, Neumann ID, Flor PJ, Slattery DA. Pharmacological interference with metabotropic glutamate receptor subtype 7 but not subtype 5 differentially affects within- and between-session extinction of Pavlovian conditioned fear. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996a;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996b;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Shen H, Baker DA, Kalivas PW. Inhibition of non-vesicular glutamate release by group III metabotropic glutamate receptors in the nucleus accumbens. J Neurochem. 2003;87:1204–1212. doi: 10.1046/j.1471-4159.2003.02093.x. [DOI] [PubMed] [Google Scholar]
- Yang ZQ. Agonists and antagonists for group III metabotropic glutamate receptors 6, 7 and 8. Curr Top Med Chem. 2005;5:913–918. doi: 10.2174/1568026054750272. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]



