Abstract
Aims: The aim of this study was to estimate the overall impact of alcohol on US race- and sex-specific age-adjusted cirrhosis mortality rates and to consider beverage-specific effects that represent changes in drinking patterns over time, comparing states with large and small African-American/White cirrhosis mortality differentials. Methods: Using series data from 1950 to 2002, the effects of per capita alcohol consumption on cirrhosis mortality for African American and White men and women were estimated using generalized least squares panel models on first-differenced data. Granger causality tests explored geographic patterning of racial differences in cirrhosis mortality. Results: Cirrhosis mortality was significantly positively related to apparent consumption of alcohol, with an overall impact of 8–14%/l of ethanol. This effect was driven by spirits which were more strongly associated with mortality for African-American women and for African-American men in states with larger mortality differentials. This disparity first emerged in New York and spread through the Northeast and into Midwestern states. Conclusion: Differences in the contribution of alcohol to cirrhosis mortality rates suggest variation by race and gender in life-course patterns of heavy consumption, illicit liquor and spirits use, as well as birth cohort effects.
INTRODUCTION
Mortality from liver cirrhosis has long been linked to alcohol consumption (Terris, 1967). This is, particularly, true for the kinds of chronic heavy drinking patterns often observed in alcohol-dependent individuals, but it is also seen for other habitually moderate and heavy drinking patterns. The role of other risk factors for cirrhosis and their interactions with alcohol use are still being explored. Recent research has considered genetic differences in alcohol metabolism, which may result in greater damage for some groups from the same dose of alcohol. For example, Stewart (2002) found evidence for increased liver damage from the same dose of alcohol for African-American and Hispanic drinkers relative to White drinkers, and a much stronger risk for women when compared with men from a given dose of alcohol has long been observed in individual-level studies (Tuyns et al., 1983). The nature of the relationship between alcohol intake and cirrhosis is difficult to study due to the long period of drinking typically required to develop disease and the challenge of assessing alcohol consumption over such a long period of the life-course, given the documented lack of stability in individual drinking patterns over time (Kerr et al., 2002). To understand possible differences by the sex and racial/ethnic group and building on our recent study of panel data methods for the analysis of alcohol consumption and cirrhosis (Ye and Kerr, 2011), the present study estimates the overall impact of alcohol on cirrhosis mortality in the USA using aggregate-level models that consider beverage-specific effects and focus on a subset of 24 states in which the non-White population is predominantly African American.
With our focus on African-American and White cirrhosis mortality, we seek to update and expand analyses presented in Herd's (1985) article documenting extremely high cirrhosis mortality rates among African Americans in certain US geographic regions with predominantly urban African-American populations. She identified the Mid-Atlantic and North Central regions as areas where there was a significant cirrhosis mortality differential between African Americans and Whites. These differentials were not seen in southern states with large rural African-American populations. In these urban African-American populations, Herd also identified birth cohorts between 1901 and 1950 as presenting a dramatic acceleration of cirrhosis mortality at relatively younger ages. A similar but much less dramatic birth cohort effect also was observed in mortality rates for the White population in these regions. Herd explains the racial difference in cirrhosis mortality trends through a detailed historical analysis of African-American migration from the rural South to urban areas in the North over the first half of the twentieth century. These migrants were exposed to a wetter culture both before and after Prohibition, but they were, especially, drawn into the liquor trade during Prohibition, which created a subculture of moonshine drinking that continued up until recently in some areas of the country. Differences in life-course drinking patterns between African Americans and Whites also may have contributed to the cirrhosis mortality rate differential. Relative to Whites, African Americans tend to start drinking and, thus, to experience problems at older ages, and they also continue drinking later into adulthood (Caetano, 1984, 1989). This propensity for continued heavy drinking at older ages is a key hypothesis regarding the cirrhosis mortality differential given the etiology of the disease (Lelbach, 1975; Skog, 1984).
The present study builds on Herd's analyses by including 27 additional years of data for each state. Using data on sex-specific, age-standardized cirrhosis mortality rates for Whites and African Americans from 1950 to 2002, we are able to look at patterns of cirrhosis mortality across gender- and race-defined groups and to model relationships with an alcohol consumption series spanning the same time period. The availability of this 53-year series is, especially, important in the case of cirrhosis, because during the 1990s, African-American cirrhosis mortality rates declined to levels equal the rates for Whites of the same gender. Thus, our data allow observation of the entire process of divergence and convergence ∼40 years later. We describe mortality rate trends and racial differentials in a subset of US states with significant African-American populations in conjunction with per capita alcohol consumption trends, and model time-series relationships between alcohol consumption and cirrhosis mortality further stratifying by the size of the mortality differential between African Americans and Whites. Finally, we use Granger causality tests to explore differences in the temporal pattern of African-American male cirrhosis mortality trends across states to determine how these detrimental drinking patterns may have spread geographically.
METHODS
This study was approved by the Public Health Institute's Institutional Review Board as an exempt study of non-identifiable secondary data (IRB #I04–015).
Data
Yearly liver cirrhosis mortality rates were taken from the Vital Statistics of the USA (National Office of Vital Statistics, 1954–1960; National Center for Health Statistics, 1961–1969) from 1950 to 1967 and from the National Center for Health Statistics Compressed Mortality File series (National Center for Health Statistics, 2000, 2003, 2004) for the years 1968–2002. These sources report deaths according to underlying cause, rather than using multiple cause coding, so that each death is attributed to only one cause. The rates were age-standardized to the 2000 US population using the number of deaths in each specific age group along with population estimates for that group. State- and age-specific population estimates came from the same sources as the mortality data from 1968 to 2002. The 1968 population estimates were used with data from the 1950 and 1960 US Decennial Census to calculate population estimates for all remaining years, with interpolations assuming a constant rate of increase (or decrease).
Natural logs of yearly age-standardized cirrhosis mortality rates were calculated for each state. Liver cirrhosis was defined based on several revisions of the International Classification of Diseases (ICD) using code 581 for ICD-6 and ICD-7, code 571 for ICD-8 and ICD-9 and codes K70, K73 and K74 for ICD-10. The data include White and non-White male and female mortality from 1950 to 1967, because more specific race/ethnicity categories were not available; the age-standardized mortality rates for White and African-American men and women are used from 1968 to 2002. The limited classification of race/ethnicity in the first 17 years of the series should not pose a serious problem for these analyses, as the states included in this study had total populations that were overwhelmingly classified as either White or Black by the US Decennial Census (U.S. Census Bureau, 2011), as shown in Table 1. For example, in 1950, from 99.2 to 100% of the total population was included in these two groups; in 1970, the range was from 98.7 to 99.8%.
Table 1.
Race by state: 1950, 1970 and 1990
| State | 1990 |
1970 |
1950 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total population | % White | % Black | Total population | % White | % Black | Total population | % White | % Black | |
| Arkansas | 2 ,350,725 | 82.7 | 15.9 | 1,923,295 | 81.4 | 18.3 | 1,909,511 | 77.6 | 22.3 |
| Alabama | 4,040,587 | 73.6 | 25.3 | 3,444,165 | 73.6 | 26.2 | 3,061,743 | 67.9 | 32.0 |
| Georgia | 6,478,216 | 71.0 | 27.0 | 4,589,575 | 73.9 | 25.9 | 3,444,578 | 69.1 | 30.9 |
| Kentucky | 3,685,296 | 92.0 | 7.1 | 3,218,706 | 92.6 | 7.2 | 2,944,806 | 93.1 | 6.9 |
| Louisiana | 4,219,973 | 67.3 | 30.8 | 3,641,306 | 69.8 | 29.8 | 2,683,516 | 67.0 | 32.9 |
| Mississippi | 2,573,216 | 63.5 | 35.6 | 2,216,912 | 62.8 | 36.8 | 2,178,914 | 54.6 | 45.3 |
| Massachusetts | 6,016,425 | 89.8 | 5.0 | 5,689,170 | 96.3 | 3.1 | 4,690,514 | 98.3 | 1.6 |
| South Carolina | 3,486,703 | 69.0 | 29.8 | 2,590,516 | 69.3 | 30.5 | 2,117,027 | 61.1 | 38.8 |
| Tennessee | 4,877,185 | 83.0 | 16.0 | 3,923,687 | 83.9 | 15.8 | 3,291,718 | 83.9 | 16.1 |
| Texas | 16,986,510 | 75.2 | 11.9 | 11,196,730 | 86.8 | 12.5 | 7,711,194 | 87.2 | 12.7 |
| Virginia | 6,187,358 | 77.4 | 18.8 | 4,648,494 | 80.9 | 18.5 | 3,318,680 | 77.8 | 22.1 |
| Connecticut | 3,287,116 | 87.0 | 8.3 | 3,031,709 | 93.5 | 6.0 | 2,007,280 | 97.3 | 2.7 |
| Delaware | 666,168 | 80.3 | 16.9 | 548,104 | 85.1 | 14.3 | 318,085 | 86.1 | 13.7 |
| DC | 606,900 | 29.6 | 65.8 | 756,510 | 27.7 | 71.1 | 802,178 | 64.6 | 35.0 |
| Illinois | 11,430,602 | 78.3 | 14.8 | 11,113,976 | 86.4 | 12.8 | 8,712,176 | 92.4 | 7.4 |
| Maryland | 4,781,468 | 71.0 | 24.9 | 3,922,399 | 81.5 | 17.8 | 2,343,001 | 83.4 | 16.5 |
| Michigan | 9,295,297 | 83.4 | 13.9 | 8,875,083 | 88.3 | 11.2 | 6,371,766 | 92.9 | 6.9 |
| Missouri | 5,117,073 | 87.7 | 10.7 | 4,676,501 | 89.3 | 10.3 | 3,954,653 | 92.4 | 7.5 |
| New Jersey | 7,730,188 | 79.3 | 13.4 | 7,168,164 | 88.6 | 10.7 | 4,835,329 | 93.3 | 6.6 |
| New York | 17,990,455 | 74.4 | 15.9 | 18,236,967 | 86.8 | 11.9 | 14,830,192 | 93.5 | 6.2 |
| North Carolina | 6,628,637 | 75.6 | 22.0 | 5,082,059 | 76.8 | 22.2 | 4,061,929 | 73.4 | 25.8 |
| Ohio | 10,847,115 | 87.8 | 10.6 | 10,652,017 | 90.6 | 9.1 | 7,946,627 | 93.5 | 6.5 |
| Pennsylvania | 11,881,643 | 88.5 | 9.2 | 11,793,909 | 91.0 | 8.6 | 10,498,012 | 93.9 | 6.1 |
| West Virginia | 1,793,477 | 96.2 | 3.1 | 1,744,237 | 95.9 | 3.9 | 2,005,552 | 94.3 | 5.7 |
Data for per capita apparent consumption of ethanol (in liters) from beer, wine, spirits and combined for total alcohol are derived from volume sales by beverage type using state- and year-specific estimates of the mean alcohol content. Alcohol sales figures came from US beer, wine and spirits industry statistics for the years 1950–1969 and from the Alcohol Epidemiologic Data System (Nephew et al., 2004) for 1970–2002. Estimates of alcohol concentration for each beverage type and year were developed from a variety of industry and government alcohol monopoly sources (Kerr et al., 2004; Kerr et al., 2006a, b). We used a 0.7 geometric distributed lag truncated after 5 years for the alcohol variables, because previous consumption is theoretically relevant to cirrhosis risk (Ye and Kerr, 2011) and because the lag relationship fit the data better than current year data. As several years of data are lost in the creation of distributed lags, models start in 1955.
Analysis strategy
States with large numbers and percentages of African-American residents were the focus of the analyses; we included those states where 90–100% of the population was either African American or White. As noted above, figures from the US Decennial Census were used to determine the number of African-American residents and the proportion of non-White residents in each state. States with large Hispanic and Native American non-White populations (i.e. those where >10% of the total population was of a race/ethnicity other than White or African American), particularly in the west, were not included. Graphical analyses of White and African-American cirrhosis mortality trends for males identified two groups of states corresponding to the regions identified by Herd (1985). The 13 states with a large differential between race groups were Connecticut, Delaware, District of Columbia, Illinois, Maryland, Michigan, Missouri, New Jersey, New York, North Carolina, Ohio, Pennsylvania and West Virginia. The 11 states where such a difference is not seen for men were mainly southern states (Alabama, Arkansas, Georgia, Kentucky, Louisiana, Mississippi, South Carolina, Tennessee, Texas and Virginia) and Massachusetts. States with a large cirrhosis mortality rate difference for men also showed a similar difference for women. In the group of states with a small men's mortality rate difference, some states showed a similar pattern for women, but others had a large differential in women's mortality rates, with substantially higher cirrhosis mortality for African-American women compared with White women.
Panel models were estimated using generalized least squares (GLS). GLS is a generalized method specifying the variance-covariance matrix of the error structure, allowing the modeling of differences in variances across panels (heteroscedasticity) as well as panel-specific, first-order AR error terms. Panel models were fit using STATA version 10 (Stata Corp, 2007). The first difference of both the log mortality rate and alcohol consumption series was used to remove unit roots present in the original series, which is a conservative approach to address state-specific time trends. The panel models estimated the effects of total alcohol consumption volume on cirrhosis mortality rates and then separate models estimated the effects of beer, wine and spirits consumption volumes (Kerr and Ye, 2011; Ye and Kerr, 2011). Each model was estimated separately for each sex- and race-defined group, first using data from all 24 states in the subsample of interest, and then for the two subgroups of states with large and small African-American/White mortality rate differentials. A final set of models for African Americans adjust for the White cirrhosis mortality rate. These models examine the contribution of alcohol to the mortality differential by documenting alcohol's association with mortality rate changes for African Americans beyond those experienced by Whites. All panel models controlled for the years corresponding to an ICD version change. Coefficients represent the percentage change in cirrhosis mortality in response to a 1 l change in ethanol consumption.
The geographic patterning of mortality rate changes over time among African-American males gives an indication of the spread of detrimental drinking patterns across states. Granger causality tests (Granger, 1989) used lagged values of cirrhosis mortality rates from one state to predict the current value of cirrhosis mortality rates in another state. All pair-wise comparisons of states were tested. These tests incorporated two-lagged values and a 0.05 significance level was used for rejecting the null hypothesis of no relationship. Granger causality is determined when a significant relationship is found in one direction but not in the other, in this case, indicating a mortality rise (or decline) in one state was followed by a rise (or decline) in another.
RESULTS
Trends
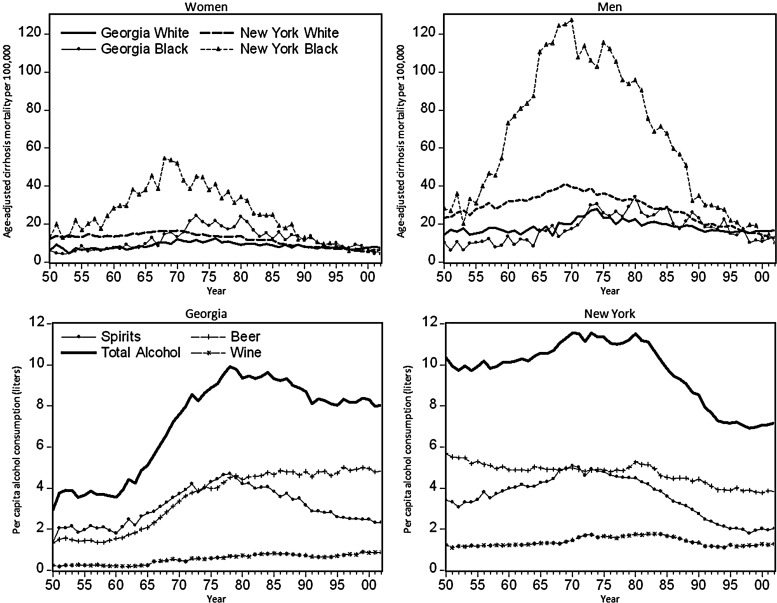
Cirrhosis mortality and per capita alcohol consumption trends are shown for all states in selected years in Table 2 and in Fig. 1 for two states that illustrate the large and small African-American/White mortality differentials. Overall, both male and female cirrhosis mortality rates increased by ∼50% over the 1950–1975 period, with most of the increase coming after 1965. Rates then decline through the mid-1990s to less than half of their peak. The largest racial/ethnic differences are seen in New York, where the African-American male mortality rate reaches 127 per 100,000, which is about triple the White male peak rate (41 per 100,000). Similarly, African-American female mortality rates peak at ∼55 per 100,000 and appear to follow a trend similar to African-American males. An important feature of these rate comparisons is the similarity of rates in the 1950s and again in the 1990s. Whatever the process involved in the mortality differential, it can be seen to begin and end in during the period of study. Another important feature is the similarity of trends in mortality with trends in per capita consumption of spirits. The trends in total alcohol consumption were similar but proportionally smaller, as would be expected given the non-linear risk relationship between alcohol consumption and cirrhosis (Skog, 1984).
Table 2.
Total alcohol sales and cirrhosis mortality rates for 1960, 1975 and 1990, stratified by Black–White mortality differential
| States with historically high-Black–White cirrhosis mortality differential |
States with historically low-Black–White cirrhosis mortality differential |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | Year | Total alcohol sales | Cirrhosis mortality rates |
State | Year | Total alcohol sales | Cirrhosis Mortality Rates |
||||||
| White males | Black males | White females | Black females | White males | Black males | White females | Black females | ||||||
| Connecticut | 1960 | 10.3764 | 27.78 | 11.82 | 13.48 | 31.97 | Alabama | 1960 | 3.1992 | 14.72 | 14.67 | 5.91 | 5.62 |
| 1975 | 10.6334 | 28.49 | 45.63 | 11.41 | 21.36 | 1975 | 7.4424 | 17.80 | 17.22 | 8.69 | 10.55 | ||
| 1990 | 8.9372 | 17.51 | 24.64 | 8.11 | 12.14 | 1990 | 7.3134 | 17.60 | 27.40 | 9.39 | 14.22 | ||
| Delaware | 1960 | 9.9347 | 33.98 | 36.50 | 9.18 | 10.42 | Arkansas | 1960 | 3.6487 | 13.46 | 9.46 | 5.77 | 6.50 |
| 1975 | 11.5765 | 26.32 | 75.48 | 19.96 | 31.37 | 1975 | 6.5266 | 17.54 | 20.94 | 6.00 | 9.41 | ||
| 1990 | 10.7819 | 19.99 | 17.33 | 9.37 | 12.48 | 1990 | 6.9881 | 15.90 | 22.63 | 4.86 | 10.58 | ||
| District of Columbia | 1960 | 20.4727 | 41.05 | 35.44 | 22.00 | 36.85 | Georgia | 1960 | 3.5659 | 15.63 | 13.50 | 7.65 | 7.45 |
| 1975 | 22.5130 | 64.30 | 139.35 | 26.22 | 68.58 | 1975 | 8.8746 | 23.40 | 25.78 | 11.33 | 19.15 | ||
| 1990 | 15.3720 | 22.26 | 87.23 | 12.02 | 32.91 | 1990 | 8.7022 | 17.10 | 23.14 | 8.02 | 11.29 | ||
| Illinois | 1960 | 9.3919 | 28.54 | 21.91 | 10.99 | 16.95 | Kentucky | 1960 | 5.8027 | 16.61 | 13.82 | 6.19 | 9.98 |
| 1975 | 11.6435 | 28.24 | 56.52 | 10.80 | 22.63 | 1975 | 7.8718 | 22.44 | 36.56 | 8.85 | 11.90 | ||
| 1990 | 9.6870 | 20.11 | 45.29 | 8.09 | 18.93 | 1990 | 6.9081 | 16.69 | 17.42 | 6.72 | 10.10 | ||
| Maryland | 1960 | 9.7761 | 24.94 | 18.78 | 10.03 | 13.46 | Louisiana | 1960 | 8.0980 | 23.20 | 14.60 | 6.91 | 7.26 |
| 1975 | 12.3336 | 30.06 | 44.03 | 12.03 | 19.91 | 1975 | 10.0151 | 23.10 | 30.08 | 9.74 | 10.79 | ||
| 1990 | 9.2463 | 15.12 | 25.66 | 6.18 | 9.76 | 1990 | 9.6503 | 15.74 | 22.40 | 8.10 | 9.02 | ||
| Michigan | 1960 | 9.1248 | 23.75 | 14.67 | 10.19 | 12.90 | Massachusetts | 1960 | 8.9646 | 31.60 | 32.88 | 14.18 | 16.09 |
| 1975 | 10.9562 | 31.80 | 79.63 | 14.14 | 37.66 | 1975 | 12.0614 | 35.74 | 25.68 | 14.24 | 18.93 | ||
| 1990 | 8.9931 | 19.50 | 51.06 | 9.42 | 20.03 | 1990 | 9.4819 | 21.45 | 18.01 | 9.00 | 12.40 | ||
| Missouri | 1960 | 7.5867 | 20.58 | 24.94 | 7.25 | 16.58 | Mississippi | 1960 | 1.5024 | 10.93 | 5.64 | 5.42 | 5.22 |
| 1975 | 9.2292 | 19.92 | 36.23 | 7.72 | 17.86 | 1975 | 7.8068 | 14.98 | 14.54 | 6.52 | 3.53 | ||
| 1990 | 8.5868 | 13.36 | 22.05 | 6.11 | 15.70 | 1990 | 8.0428 | 15.49 | 21.50 | 6.92 | 5.79 | ||
| New Jersey | 1960 | 10.5188 | 27.64 | 22.75 | 12.84 | 15.01 | South Carolina | 1960 | 4.2323 | 13.32 | 5.83 | 4.40 | 3.52 |
| 1975 | 10.9974 | 29.41 | 47.85 | 13.45 | 24.46 | 1975 | 9.2181 | 26.75 | 22.30 | 8.61 | 14.81 | ||
| 1990 | 9.5024 | 19.91 | 33.02 | 8.83 | 17.41 | 1990 | 9.8558 | 17.63 | 32.09 | 8.03 | 11.79 | ||
| New York | 1960 | 10.1244 | 32.09 | 73.12 | 13.39 | 28.32 | Tennessee | 1960 | 3.7977 | 12.63 | 6.73 | 5.78 | 4.37 |
| 1975 | 11.3481 | 36.18 | 115.43 | 13.75 | 37.95 | 1975 | 7.5976 | 18.36 | 29.10 | 7.44 | 13.22 | ||
| 1990 | 8.5368 | 21.13 | 34.80 | 8.20 | 12.78 | 1990 | 7.4197 | 17.89 | 31.84 | 7.76 | 12.56 | ||
| North Carolina | 1960 | 4.0044 | 15.36 | 7.84 | 6.37 | 5.65 | Texas | 1960 | 6.5513 | 16.23 | 14.01 | 9.23 | 7.96 |
| 1975 | 8.1125 | 25.86 | 37.92 | 11.12 | 25.50 | 1975 | 10.1785 | 23.83 | 26.98 | 10.84 | 10.93 | ||
| 1990 | 7.8878 | 18.08 | 35.44 | 7.58 | 14.46 | 1990 | 9.3666 | 20.84 | 21.08 | 9.05 | 9.01 | ||
| Ohio | 1960 | 8.1339 | 24.98 | 26.55 | 10.45 | 16.05 | Virginia | 1960 | 6.9861 | 18.29 | 15.30 | 7.36 | 7.06 |
| 1975 | 8.8914 | 25.54 | 51.43 | 10.03 | 25.63 | 1975 | 9.2235 | 26.96 | 26.33 | 12.32 | 15.87 | ||
| 1990 | 7.7575 | 16.20 | 29.23 | 6.90 | 13.33 | 1990 | 8.1068 | 15.44 | 22.47 | 6.37 | 12.96 | ||
| Pennsylvania | 1960 | 8.1361 | 23.57 | 16.78 | 8.91 | 10.60 | |||||||
| 1975 | 9.4076 | 26.95 | 43.83 | 9.57 | 24.68 | ||||||||
| 1990 | 8.1661 | 17.09 | 30.50 | 6.97 | 15.06 | ||||||||
| West Virginia | 1960 | 5.0265 | 18.09 | 3.53 | 5.86 | 15.90 | |||||||
| 1975 | 7.2741 | 27.30 | 33.70 | 9.35 | 34.74 | ||||||||
| 1990 | 6.5721 | 17.79 | 61.46 | 5.98 | 11.35 | ||||||||
Fig. 1.
Graphs illustrating cirrhosis mortality differentials between White and African-American women (top left) and men (top right) and alcohol consumption trends for Georgia (bottom left) and New York (bottom right) from 1950 to 2002.
In contrast, the rates seen in Georgia illustrate the patterns found in most southern states (and Massachusetts): alcohol consumption and cirrhosis mortality increased for both Whites and African Americans, but peak rates generally were lower and did not differ as widely between the race groups. Mortality for African-American males in Georgia started at a lower rate than for White men, but it increased more rapidly as alcohol consumption rose during the 1960s. African-American women in Georgia also show a steeper increase in mortality than White women, but cirrhosis mortality for African-American women increased to twice the White women's rate during the 1970s, which was a pattern similar to that seen in the states with a bigger mortality differential for men.
Panel estimates
Overall, the panel models (Table 3) indicate that apparent consumption of total alcohol and of spirits (while adjusting for beer and wine consumption) are each strongly associated with cirrhosis mortality for both White and African-American men and women, with the effects magnified for African Americans. When analyzing the data stratifying by the size of the states' differential in White and African-American cirrhosis mortality, the overall pattern remains, with significant effects of total alcohol on cirrhosis and larger effect sizes in the 13 states with large mortality differentials and smaller effect sizes for total alcohol in the 11 states without large differentials. This pattern also is seen for the effect of spirits on African-American men's mortality, with stronger associations in the 13 states with large mortality differentials than in the other 11 states. This is in stark contrast to the relatively large effects for spirits on cirrhosis mortality for African-American women in both groups of states. No significant associations between wine or beer and cirrhosis mortality were seen other than a significant negative association between wine consumption and cirrhosis for African-American women. However, this ‘protective’ effect of wine consumption was not seen in models testing the association separately (not shown) indicating that this result was due to correlations between the beverage type trends.
Table 3.
Associations between alcohol sales and US cirrhosis mortality rates, 1950–2002
| White men |
African-American men |
White women |
African-American women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | B | SE | B | SE | |
| All 24 states with predominantly African-American non-White populations | ||||||||||||
| Model 1 | ||||||||||||
| Total alcohol | 0.086 | 0.012** | 0.135 | 0.024** | 0.119 | 0.024** | 0.076 | 0.013** | 0.138 | 0.027** | 0.132 | 0.027** |
| White cirrhosis mortalitya | — | — | — | — | 0.212 | 0.055** | — | — | — | — | 0.075 | 0.054 |
| Model 2 | ||||||||||||
| Beer | 0.022 | 0.026 | −0.021 | 0.057 | −0.028 | 0.056 | −0.008 | 0.03 | −0.029 | 0.062 | −0.028 | 0.062 |
| Wine | −0.11 | 0.069 | 0.128 | 0.148 | 0.125 | 0.148 | −0.054 | 0.081 | −0.213 | 0.168 | −0.209 | 0.168 |
| Spirits | 0.179 | 0.023** | 0.234 | 0.043** | 0.212 | 0.043** | 0.173 | 0.027** | 0.351 | 0.054** | 0.337 | 0.054** |
| White cirrhosis mortality | — | — | — | — | 0.198 | 0.056** | — | — | — | — | 0.073 | 0.055 |
| 13 states with large African-American/White cirrhosis mortality differential | ||||||||||||
| Model 1 | ||||||||||||
| Total alcohol | 0.101 | 0.017** | 0.151 | 0.031** | 0.136 | 0.032** | 0.091 | 0.018** | 0.166 | 0.035** | 0.151 | 0.035** |
| White cirrhosis mortality | — | — | — | — | 0.173 | 0.068** | — | — | — | — | 0.117 | 0.071+ |
| Model 2 | ||||||||||||
| Beer | −0.004 | 0.039 | −0.12 | 0.092 | −0.118 | 0.092 | 0.003 | 0.043 | −0.029 | 0.096 | −0.026 | 0.097 |
| Wine | −0.088 | 0.084 | 0.19 | 0.178 | 0.182 | 0.178 | −0.066 | 0.099 | 0.011 | 0.205 | 0.032 | 0.203 |
| Spirits | 0.207 | 0.028** | 0.251 | 0.048** | 0.23 | 0.05** | 0.179 | 0.031** | 0.307 | 0.061** | 0.273 | 0.061** |
| White cirrhosis mortality | — | — | — | — | 0.17 | 0.069** | — | — | — | — | 0.108 | 0.073 |
| 11 states with small African-American/White cirrhosis mortality differential | ||||||||||||
| Model 1 | ||||||||||||
| Total alcohol | 0.059 | 0.018** | 0.113 | 0.039** | 0.095 | 0.039* | 0.049 | 0.02* | 0.089 | 0.044* | 0.086 | 0.044* |
| White cirrhosis mortality | — | — | — | — | 0.256 | 0.092** | — | — | — | — | 0.01 | 0.083 |
| Model 2 | ||||||||||||
| Beer | 0.033 | 0.042 | 0.034 | 0.089 | 0.026 | 0.089 | −0.05 | 0.05 | −0.141 | 0.101 | −0.144 | 0.101 |
| Wine | −0.163 | 0.128 | 0.071 | 0.285 | 0.094 | 0.283 | −0.042 | 0.147 | −0.68 | 0.305* | −0.698 | 0.306* |
| Spirits | 0.118 | 0.045** | 0.181 | 0.095+ | 0.149 | 0.095 | 0.167 | 0.052** | 0.462 | 0.108** | 0.462 | 0.11** |
| White cirrhosis mortality | — | — | — | — | 0.236 | 0.095** | — | — | — | — | 0.023 | 0.083 |
Results from semi-logged, differenced GLS models allowing for panel heteroscedasticity and panel-specific, first-order autoregressive error structure. Models use distributed lags for alcohol and adjust for changes to ICD coding in 1958, 1968, 1979 and 1999. SE, standard error. States with a large African-American/White differential: CT, DE, DC, IL, MD, MI, MO, NJ, NY, NC, OH, PA and WV. States without a large African-American/White differential: AL, AR, GA, KY, LA, MS, SC, TN, TX, VA and MA.
aWhite cirrhosis mortality rate is sex-specific.
*P < 0.05, **P < 0.01, +P < 0.10.
Differential mortality models (Table 3) also emphasized varying contributions of alcohol, and specifically spirits consumption, to cirrhosis mortality for the two groups of states. After adjusting for White men's cirrhosis mortality (which was significant in all models), there was a significant and positive association of total alcohol and of spirits with African-American men's cirrhosis mortality overall and in the group of states with a large African-American/White mortality differential. The results for women diverged from those for men, in that White women's cirrhosis mortality was not a significant predictor of African-American women's mortality, and the coefficient for spirits actually was much larger in the states with a small African-American/White mortality differential than in the states with a large differential.
Analyses of the timing of mortality changes
Granger causality tests assessed the timing of mortality rate changes for African-American men in the states where the large mortality differential was observed. Results were consistent with graphical inspection of cirrhosis mortality trends. New York was the first state where African-American mortality rates began to rise. Next were the nearby states of New Jersey and Maryland, followed by Connecticut, Delaware, Washington DC and Illinois. The last group of states was North Carolina, Pennsylvania, West Virginia, Ohio, Michigan and Missouri. These patterns make sense geographically and may give some insight into the underlying process through which the spread of risky drinking patterns and cultures emerged.
DISCUSSION
Findings for men clearly support the hypothesis put forth by Herd (Herd, 1985). In the northern, urban states, spirits consumption was responsible for a large increase in cirrhosis mortality among African-American men (25%/l of ethanol, compared with 18%/l in the southern states). The effect of spirits on cirrhosis mortality of White men in the northern states also was stronger than in the southern states (21 vs. 12%/l of ethanol). The data for African-American women do not conform as closely to this hypothesis, however. Spirits consumption was associated with an increase in African-American women's cirrhosis mortality of 31–46%/l of ethanol, depending on region. The differential mortality models suggest that African-American men's cirrhosis mortality in the southern states (those without a large differential) is less directly linked to spirits than in the northern states, but that African-American women's cirrhosis mortality is strongly related to spirits consumption in both regions of the country. These patterns are very similar to those observed with a smaller grouping of states based on wetness (data not shown), which suggests that drinking norms in these areas may be a factor.
Over the period from 1950 to 1975, there was a dramatic increase in age-standardized cirrhosis mortality rates in the USA, with a disproportionate increase for African-American men and women in the Mid-Atlantic and North Central states. White rates increased by 50–100% and African-American rates showed double or triple this increase in these regions. This mortality trend in these states paralleled a 50% increase in per capita spirits consumption and a 30–40% increase in per capita total alcohol intake. The age-standardized mortality increase, particularly for African Americans, was composed of two components. First, as demonstrated by Herd (1985), there was a substantial reduction in the average age of death from cirrhosis starting with the 1901 birth cohort with continued decline through the 1930–1950 birth cohorts. As cirrhosis death is more common at older ages, increases in mortality in younger groups will disproportionately increase the age-standardized rate and present a significant public health concern. Second, more cirrhosis deaths occurred in both the White and African-American populations over time, indicating that a larger proportion of each group engaged in long-term heavy drinking. Both of these changes were likely larger in the African-American population.
Herd (1985) presents several hypotheses as potential explanations for the observed mortality pattern. Given the size of the mortality increase, it is likely that more than one factor contributed. First, heavier volume and more extreme drinking patterns likely occurred in the 20–40 age group, given the large increases in mortality at relatively younger ages. A greater propensity for continued heavy drinking into the 30s and 40s among African Americans (Caetano, 1984; Caetano and Kaskutas, 1996; Johnson et al., 1998) was likely an important factor in the mortality differential. Second, the drinking patterns associated with spirits consumption, such as drinking higher concentration beverages and achieving higher blood alcohol levels also may have played a role. African Americans drink proportionately more spirits than Whites even today, (Kerr and Greenfield, 2007) and they also may have been more likely to drink moonshine (illicitly produced liquor) (Herd, 1985). Moonshine drinking is likely to be associated with drinking to intoxication, and the liquor often contains contaminants such as lead and methanol (Holstege et al., 2004), which could result in greater liver damage. Third, differences in diet and nutritional status between heavy drinkers by race may result in more harm to the liver from a given amount of drinking. For example, pork consumption has been found to be related to higher cirrhosis mortality risk, (Bode et al., 1998), and pork consumption is higher among US African Americans than Whites (Gans et al., 2003; McCabe-Sellers et al., 2007). Fourth, there may be differences in genetic susceptibility, such that relative to Whites, African Americans are at an increased risk of liver damage from a given amount of alcohol (Stewart, 2002). Finally, chronic hepatitis B infection can amplify the negative effects of alcohol on the liver, and infection rates may have been higher in US African Americans (Herd, 1985; Kim, 2009).
The present study cannot identify whether any or all of these potential explanations are true or to what extent they may account for the differential effects of spirits consumption on cirrhosis mortality by race. It does, however, suggest avenues for future research. The rise in the number of individuals dying from cirrhosis indicates an expansion of heavy drinking patterns or an increase in longevity of such patterns among both race groups, but to a greater extent among African Americans. The rapidity of mortality rate rise ∼1965 corresponds with rising per capita spirits consumption, but it also may be related to a lagged effect of a post-World War II drinking boom in the general US population. In populations without a pool of long-term heavy drinkers, cirrhosis mortality will not increase directly with consumption and heavy drinking at first, as liver damage builds over time. The timing of the 1965 rise at ∼20 years after the war suggests the possibility of such an effect. The increase in per capita consumption (especially spirits) also was important and may have been associated with a steep rise in the prevalence of detrimental drinking patterns, such as daily heavy drinking or all-day drinking. Examination of survey data from this period could potentially shed some light on this phenomenon, but little such data are available.
The other side of the rise and divergence in cirrhosis rates is the decline and convergence that took place from ∼1980 to the mid-1990s. During this period, total alcohol and spirits consumption declined, and the cirrhosis mortality rates of African Americans declined at a steeper pace than those of Whites. By 1970, moonshine consumption had declined to a lower level (Licensed Beverage Industries, 1971) and hepatitis B prevalence may have also declined over time (Kim, 2009). The nutritional status of heavy drinkers also may have improved over this period, although diets in the USA generally are considered to have worsened over this period. Since 1980 obesity, average body mass index and related diseases such as diabetes have also increased substantially in the USA (Ogden et al., 2006; Reither et al., 2009). Recent studies have established these as risk factors for liver cirrhosis (Marchesini et al., 2008; Garcia-Compean et al., 2009; Hart et al., 2010; Liu et al., 2010); however, declining cirrhosis mortality rates suggest that these causes did not strongly influence US cirrhosis mortality trends through 2002. Further the evaluation of birth cohort patterns of mortality is warranted to establish the cohorts in which mortality rates first began to decline. The 53-year view on alcohol consumption and mortality presented here shows a surprisingly symmetric pattern, suggesting a 50-year wave of consumption change, similar to waves of consumption of varying lengths observed in other countries (Skog, 1986). While African American and White drinking cultures may have differed in some respects, it does appear that the timing of trends was closely related, possibly due to the influence of similar market and cultural factors.
Funding
This work was supported by the NIH (R01 AA014362 and P50 AA005595).
Conflict of interest statement. None declared.
REFERENCES
- Bode C, Bode JC, Erhardt JG, et al. Effect of the type of beverage and meat consumed by alcoholics with alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:1803–5. [PubMed] [Google Scholar]
- Caetano R. Ethnicity and drinking in Northern California: a comparison among whites, blacks and Hispanics. Alcohol Alcohol. 1984;19:31–44. [PubMed] [Google Scholar]
- Caetano R. Madrid, Spain: 1989. Drinking patterns and problems among white, black and Hispanic men in the U.S. general population. 1989 Paper presented at the V Congreso Iberoamericano sobre Drogodependencias y Alcoholismo. [Google Scholar]
- Caetano R, Kaskutas LA. Changes in drinking problems among whites, blacks and Hispanics: 1984–1992. Subst Use Misuse. 1996;31:1547–71. doi: 10.3109/10826089609063991. [DOI] [PubMed] [Google Scholar]
- Gans KM, Burkholder GJ, Risica PM, et al. Baseline fat-related dietary behaviors of white, Hispanic, and black participants in a cholesterol screening and educational project in New England. J Am Diet Assoc. 2003;103:699–706. doi: 10.1053/jada.2003.50135. [DOI] [PubMed] [Google Scholar]
- Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280–88. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CWJ. Forecasting in Business and Economics. Boston: Academic Press; 1989. [Google Scholar]
- Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd D. Migration, cultural transformation and the rise of black liver cirrhosis mortality. Br J Addict. 1985;80:397–410. doi: 10.1111/j.1360-0443.1985.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Holstege CP, Ferguson JD, Wolf CE, et al. Analysis of moonshine for contaminants. Clin Toxicol. 2004;42:597–601. doi: 10.1081/clt-200026976. [DOI] [PubMed] [Google Scholar]
- Johnson FW, Gruenewald PJ, Treno AJ, et al. Drinking over the life course within gender and ethnic groups: a hyperparametric analysis. J Stud Alcohol. 1998;59:568–80. doi: 10.15288/jsa.1998.59.568. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK. Distribution of alcohol consumption and expenditures and the impact of improved measurement on coverage of alcohol sales in the 2000 National Alcohol Survey. Alcohol Clin Exp Res. 2007;31:1714–22. doi: 10.1111/j.1530-0277.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Ye Y. Beverage-specific mortality relationships in US population data. Contemp Drug Probl. 2011;38:561–78. doi: 10.1177/009145091103800406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr WC, Fillmore KM, Bostrom A. Stability of alcohol consumption over time: evidence from three longitudinal surveys from the United States. J Stud Alcohol. 2002;63:325–33. doi: 10.15288/jsa.2002.63.325. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Brown S, Greenfield TK. National and state estimates of the mean ethanol content of beer sold in the U.S. and their impact on per capita consumption estimates: 1988 to 2001. Alcohol Clin Exp Res. 2004;28:1524–32. doi: 10.1097/01.alc.0000141641.72726.c1. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Tujague J. Estimates of the mean alcohol concentration of the spirits, wine, and beer sold in the U.S. and per capita consumption: 1950–2002. Alcohol Clin Exp Res. 2006a;30:1583–91. doi: 10.1111/j.1530-0277.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Tujague J, et al. The alcohol content of wine consumed in the US and per capita consumption: new estimates reveal different trends. Alcohol Clin Exp Res. 2006b;30:516–22. doi: 10.1111/j.1530-0277.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49:S28–34. doi: 10.1002/hep.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelbach WK. Quantitative aspects of drinking in alocholic lever cirrhosis. In: Khanna JM, Israel Y, Kalant Hs, editors. Alcoholic Liver Pathology. Toronto, Canada: Addiction Research Foundation of Ontario; 1975. pp. 1–18. [Google Scholar]
- Licensed Beverage Industries, Inc. Moonshine. The Poison Business. New York, NY: Licensed Beverage Industries; 1971. [Google Scholar]
- Liu B, Balkwill A, Reeves G, et al. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ. 2010;340:c912. doi: 10.1136/bmj.c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini G, Moscatiello S, Di Domizio S, et al. Obesity-associated liver disease. J Clin Endocrinol Metab. 2008;93:S74–80. doi: 10.1210/jc.2008-1399. [DOI] [PubMed] [Google Scholar]
- McCabe-Sellers BJ, Bowman S, Stuff JE, et al. Assessment of the diet quality of the US adults in the lower Mississippi delta. Am J Clin Nutr. 2007;86:697–706. doi: 10.1093/ajcn/86.3.697. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Vital Statistics of the United States, 1959–1967. Washington, DC: U.S. Public Health Service; 1961–1969. [Google Scholar]
- National Center for Health Statistics. Compressed Mortality File 1968–1988. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- National Center for Health Statistics. Compressed Mortality File 1989–1998. Hyattsville, MD: National Center for Health Statistics; 2003. [Google Scholar]
- National Center for Health Statistics. Compressed Mortality File 1999–2002. Hyattsville, MD: National Center for Health Statistics; 2004. [Google Scholar]
- National Office of Vital Statistics. Vital Statistics of the United States, 1950–1958. Washington, DC: U.S. Public Health Service; 1954–1960. [Google Scholar]
- Nephew TM, Yi H-y, Williams GD, et al. U.S. Apparent Consumption of Alcoholic Beverages Based on State Sales, Taxation, or Receipt Data [NIH Publication No. 04–5563] Vol. 1. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Alcohol Epidemiologic Data System; 2004. [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Reither EN, Hauser RM, Yang Y. Do birth cohorts matter? Age-period-cohort analyses of the obesity epidemic in the United States. Soc Sci Med. 2009;69:1439–48. doi: 10.1016/j.socscimed.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog O-J. The risk function for liver cirrhosis from lifetime alcohol consumption. J Stud Alcohol. 1984;45:199–208. doi: 10.15288/jsa.1984.45.199. [DOI] [PubMed] [Google Scholar]
- Skog O-J. The long waves of alcohol consumption: a social network perspective on cultural change. Social Networks. 1986;8:1–32. [Google Scholar]
- Stata Corp. Stata Statistical Software: Release 10.0. College Station, TX: Stata Corporation; 2007. [Google Scholar]
- Stewart SH. Racial and ethnic differences in alcohol-associated aspartate aminotransferase and γ-glutamyltransferase elevation. Arch Intern Med. 2002;162:2236–9. doi: 10.1001/archinte.162.19.2236. [DOI] [PubMed] [Google Scholar]
- Terris M. Epidemiology of cirrhosis of the liver: national mortality data. Am J Public Health. 1967;57:2076–88. doi: 10.2105/ajph.57.12.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyns A, Péquignot G, Esteve J. Greater risk of ascitic cirrhosis in females in relation to alcohol consumption. Int J Epidemiol. 1983;13:53–7. doi: 10.1093/ije/13.1.53. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Washington, DC: U.S. Census Bureau; 2011. United States Census: American FactFinder. Archived by WebCite® at http://www.webcitation.org/6CAljhzJd. (14 November 2012, date last accessed) [Google Scholar]
- Ye Y, Kerr WC. Alcohol and liver cirrhosis mortality: comparison of methods for the analyses of time-series panel data models in the US. Alcohol Clin Exp Res. 2011;35:108–115. doi: 10.1111/j.1530-0277.2010.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]