To the Editor
Eosinophilic esophagitis (EoE) is characterized by patchy infiltration into the esophagus by eosinophils, inflammatory leukocytes not found in the healthy esophagus.1-3 The pathologic diagnosis can be difficult because the degree of eosinophil infiltration varies greatly within the esophagus, leading to possible underdiagnosis if the tissue is sampled insufficiently. When clinical suspicion for EoE is high, consensus practice requires sampling at 4 to 5 sites throughout the esophagus.4-7 However, five 2 mm biopsy specimens represent less than 0.7% of the 20- to 25-cm-long esophageal mucosa and might result in under-diagnosis of EoE if mucosal eosinophilia is particularly patchy.4 Here we report the analysis of a unique full circumferential esophagectomy specimen from a patient with known EoE who underwent a partial esophagectomy for a concomitant early-stage esophageal adenocarcinoma. We obtained serial cross-sections of the esophagus and mapped eosinophil density across the entire esophageal epithelium. We performed statistical analysis of the data with a Monte Carlo simulation to predict the number of biopsy specimens required to make a diagnosis of EoE in patients with variable eosinophil densities.
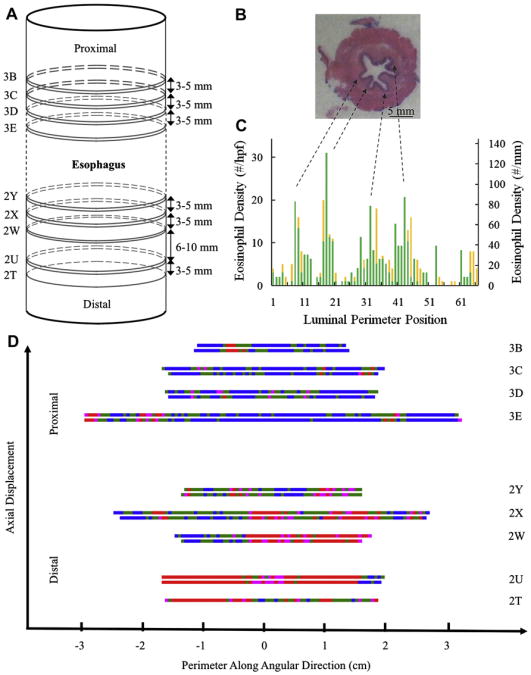
With approval from the University of Utah Institutional Review Board, an esophagectomy specimen from a 68-year-old patient with known EoE was obtained at the time of surgery for early-stage T1N0M0 esophageal adenocarcinoma. Before cancer diagnosis, the patient had solid-food dysphagia (approximately 20 years), seasonal allergies, food impaction (2 years before esophagectomy), both nondysplastic Barrett esophagus and a short distal esophageal stricture (2 years before esophagectomy), and each set of pre-resection biopsy specimens (obtained 3 years, 2 years, and 6 months [>100 eosinophils/high-power field {hpf}] before esophagectomy), indicating persistent eosinophilia and confirming the EoE diagnosis. The patient did not respond to aerosolized steroids, and antigen elimination diets were not attempted. Per guidelines for Barrett esophagus, twice-daily high-dose protein pump inhibitor therapy had been prescribed since diagnosis. Less than 1 year before esophagectomy, adenocarcinoma was discovered, indicating distal esophagectomy. Neither adjuvant therapy nor radiation treatment was performed. After removing routine esophageal segments for cancer diagnosis and staging, 4 additional tissue segments were removed and fixed in formalin. No indication of cancerous cells was found in these marginal segments, but significant eosinophilia consistent with precancerous diagnosis was found. These segments were sectioned horizontally (ie, traverse to the esophageal longitudinal axis) at 3- to 5-mm intervals to yield pairs of tissue slices separated by 3 to 5 μm (Fig 1, A). Each of the 17 sections was designated by surgical pathology with a number and letter representing the segment and section, respectively. The sections examined were 5 cm or more away from the tumor, which was at the gastroesophageal junction.
Fig 1.
Histology, mapping, and data analysis. A, Sequential ordering of sections within the esophagectomy. B, Digital image of representative hematoxylin and eosin–stained esophageal section 3D. C, Representative eosinophil density as a function of luminal perimeter position for section 3D. D, Eosinophil density map as a function of both luminal perimeter and axial location at 3 eosinophils/hpf or less (blue), 4 to 14 eosinophils/hpf (green), 15 to 19 eosinophils/hpf (pink), and 20 eosinophils/hpf or greater (red).
Tissue was imaged with an Olympus microscope (Olympus, Center Valley, Pa) at × 400 magnification. Each microscopic hpf had a diameter of 0.54 mm, representing 0.23 mm2 of tissue. The eosinophil count for each site was performed manually, 1 hpf at a time, around the entire luminal perimeter (Fig 1, C, and Table I). The number of sites containing a diagnostic level of eosinophils (either 15 eosinophils/hpf [65 eosinophils/mm2] or 20 eosinophils/hpf [87 eosinophils/mm2]) was determined for each of the 17 circumferential sections.8
Table I. Section attributes from proximal to distal.
| Segment no. | No. of sites or hpf/section | Perimeter (mm)* | Equivalent diameter (cm)* | Sites ≥15 eosinophils/hpf | Sites ≥15 eosinophils/hpf† | Sites ≥20 eosinophils/hpf | Sites ≥20 eosinophils/hpf† |
|---|---|---|---|---|---|---|---|
| 3B | 46 | 24.8 | 0.79 | 3 | 6.5% | 3 | 6.5% |
| 3B | 48 | 25.9 | 0.83 | 6 | 12.5% | 5 | 10.4% |
| 3C | 69 | 37.3 | 1.19 | 5 | 7.2% | 3 | 4.3% |
| 3C | 65 | 35.1 | 1.12 | 4 | 6.2% | 3 | 4.6% |
| 3D | 66 | 35.6 | 1.13 | 5 | 7.6% | 1 | 1.5% |
| 3D | 64 | 34.6 | 1.10 | 4 | 6.3% | 2 | 3.1% |
| 3E | 116 | 62.6 | 1.99 | 15 | 12.9% | 8 | 6.9% |
| 3E | 117 | 63.2 | 2.01 | 6 | 5.1% | 5 | 4.3% |
| 2Y | 55 | 29.7 | 0.95 | 13 | 23.6% | 7 | 12.7% |
| 2Y | 56 | 30.2 | 0.96 | 17 | 30.4% | 8 | 14.3% |
| 2X | 98 | 52.9 | 1.68 | 37 | 37.8% | 25 | 25.5% |
| 2X | 95 | 51.3 | 1.63 | 40 | 42.1% | 26 | 27.4% |
| 2W | 56 | 30.2 | 0.96 | 33 | 58.9% | 24 | 42.9% |
| 2W | 61 | 32.9 | 1.05 | 38 | 62.3% | 32 | 52.5% |
| 2U | 69 | 37.3 | 1.19 | 60 | 87.0% | 55 | 79.7% |
| 2U | 68 | 36.7 | 1.17 | 59 | 86.8% | 53 | 77.9% |
| 2T | 66 | 35.6 | 1.13 | 38 | 57.6% | 29 | 43.9% |
| Total | 1215 | 383 | 31.5% | 289 | 23.8% |
Perimeter = Number of sites × field of view (0.54 mm); equivalent diameter = perimeter/π.
Fifteen eosinophils/hpf = 65 eosinophils/mm2; 20 eosinophils/hpf = 87 eosinophils/mm2.
A Monte Carlo simulation evaluated the relationship between eosinophil density and the number of biopsy specimens required to make a diagnosis of EoE.9 This simulation mimics the random process of selecting a biopsy site during endoscopy by using a random-number generator to select a site based on the esophagectomy data. A “biopsy” is defined in the simulation as 4 adjacent 0.54-mm fields of view because a typical endoscopic esophageal biopsy specimen measures approximately 2 mm. The biopsy result was considered “positive” if at least 1 of the 4 adjacent fields of view contained at least 15 eosinophils. Statistics (ie, the average, μ, and SD, σ) were compiled from 10,000 simulated endoscopies with biopsy specimens drawn from one of 3 eosinophil densities: low (average of 5.7% of sites ≥15 eosinophils/hpf from the 3 lowest-density sections, one from each of 3C, 3D, and 3E in Table I), average (31.5% of sites ≥15 eosinophils/hpf across all sections), and high (average of 79.3% of sites ≥15 eosinophils/hpf from the 3 highest-density sections, one from 2W and both from 2U). The simulation was repeated 2 to 40 times to represent multiple biopsies per endoscopy. The 95% confidence levels for EoE diagnosis (ie, the number of biopsies required to be sure that 95% of patients with EoE will satisfy the diagnosis criteria) were determined by μ–1.96σ from a single-tailed normal distribution for each eosinophil density to account for significant variability in the outcome of the simulated endoscopies because of the heterogeneity (ie, patchiness) of eosinophil density.
The research protocol was approved by the Huntsman Cancer Institute, University of Utah, Institutional Review Board on April 23, 2010, with the case number IRB_00040035. In the manuscript all identifiers have been removed. The patient agreed to the procurement of the esophagectomy specimen before surgery for research purposes.
The tissue sections show the eosinophil density to be highly variable in this patient with EoE both axially and circumferentially. Fig 1, B, shows a typical 360° hematoxylin and eosin–stained cross-section. By methodically counting the intraepithelial eosinophils in each sequential 0.54-mm field of view, the eosinophil density was mapped for the entire perimeter of each tissue section. Fig 1, C, shows an example of eosinophil density variation per hpf (Fig 1, C, left axis) or per square millimeter (Fig 1, C, right axis). Each section contains 1 to 14 areas or patches comprised of 1 or more sites exceeding the diagnostic threshold of 15 eosinophils/hpf. The greatest peak eosinophil count was 176 eosinophils/hpf (770 eosinophils/mm2), the average eosinophil density was approximately 14.8 eosinophils/hpf (64 eosinophils/mm2), and some fields of view did not contain any eosinophils. Correlation of eosinophil density in Fig 1, C, with the topology of Fig 1, B, indicates that eosinophil peak counts appear to cluster between luminal folds and associate with locally increased vasculature.
Fig 1, D, shows a representation of eosinophil density in each tissue section shaded for infiltration intensity. Pink areas meet the threshold of 15 eosinophils/hpf or greater, and red areas meet the threshold of 20 eosinophils/hpf or greater. The greatest percentage of areas with high eosinophil density was seen in the more distal portions of the esophagectomy. In total, 31.5% of the hpfs contained eosinophil densities of 15 eosinophils/hpf or greater. When using the more stringent threshold of 20 eosinophils/hpf or greater, only 23.8% of hpfs were diagnostic (Table I).8
Remarkably, Fig 1, C, shows that many of the esophageal cross-sections contain eosinophil infiltrates that appear to follow Gaussian distributions. The apparently random location of the infiltrate peaks and their Gaussian-shaped distributions suggest that eosinophil infiltrates are triggered at specific localized points within the esophagus presumably by localized release of chemoattractants at “point sources.”
This study addresses a fundamental gap in our knowledge regarding eosinophil infiltration in patients with EoE by spatially mapping the distribution of eosinophil density derived from a several-centimeters-long esophagectomy specimen of a patient with known EoE. The resulting distributions demonstrate significant variability in eosinophil density. Because endoscopic biopsies typically sample only 2 mm of tissue, eosinophil peak infiltrates can be easily missed.
From this distinct dataset, we used a statistical (Monte Carlo) simulation to predict the number of biopsy specimens required for EoE diagnosis based on the underlying eosinophil density.8 On the basis of the standard of care of 4 to 5 biopsy specimens per endoscopy, the simulations indicate a 62% to approximately 100% probability of detecting EoE (≥1 biopsy specimen with ≥15 eosinophils/hpf). Our analysis suggests that the current standard of care might result in underdiagnosis of EoE in areas of low eosinophil density. Patients with EoE would require 31 or greater, 12 or greater, or 4 or greater random biopsy specimens to detect the disease with 95% confidence from regions of low, average, and high eosinophil density, respectively. This level of confidence accounts for spatial variability in the eosinophil density, which leads to significant variability in the outcome of the simulated endoscopies, providing confidence that 19 of 20 patients with EoE will receive a positive diagnosis.
Finally, because esophagectomies are not routinely available in patients with EoE, this analysis strongly indicates the need for improved imaging of eosinophil infiltration patterns in vivo to accurately diagnose and understand this disease. This study suggests that more intensive biopsy sampling might lead to greater diagnostic accuracy and provide additional insight into the disease, particularly in the early stages when eosinophil infiltrate densities might be more modest but when the patient might also benefit the most from measured interventions.
Acknowledgments
L.F.P. gratefully acknowledges University of Utah startup funds from the Department of Chemical Engineering and support from the University of Utah TCP grant program, the Utah Governor's Office of Economic Development, NIH (1R43AG039929-D1), and the National Science Foundation (CBET-1125490). K.A.P. gratefully acknowledges support from the AGA Castell Esophageal Research Award 2010.
Footnotes
Disclosure of potential conflict of interest: J. C. Fang is founder of Veritract and is a consultant for Merit Medical and Nestlé Nutrition. L. F Pease III has received research support from the University of Utah, the Utah Governor's Office of Economic Development, Novo Contour, and the National Science Foundation. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:22–6. doi: 10.1097/01.mpg.0000188740.38757.d2. [DOI] [PubMed] [Google Scholar]
- 2.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–61. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125:1660–9. doi: 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64:313–9. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Shah A, Kagalwalla AF, Gonsalves N, Melin-Aldana H, Li BU, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol. 2009;104:716–21. doi: 10.1038/ajg.2008.117. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Aderoju A, Woosley JT, Sandler RS, Shaheen NJ. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007;102:2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 8.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood S, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Pease LF. Optimizing the yield and selectivity of high purity nanoparticle clusters. J Nanoparticle Res. 2011;13:2157–72. [Google Scholar]