Abstract
Vascular patterning is critical for organ function. In the eye, there is simultaneous regression of embryonic hyaloid vasculature1 (important to clear the optical path) and formation of the retinal vasculature2 (important for the high metabolic demands of retinal neurons). These events occur postnatally in the mouse. Here we have identified a light-response pathway that regulates both processes. We show that when mice are mutated in the gene (Opn4) for the atypical opsin melanopsin3–5, or are dark-reared from late gestation, the hyaloid vessels are persistent at 8 days post-partum and the retinal vasculature overgrows. We provide evidence that these vascular anomalies are explained by a light-response pathway that suppresses retinal neuron number, limits hypoxia and, as a consequence, holds local expression of vascular endothelial growth factor (VEGFA) in check. We also show that the light response for this pathway occurs in late gestation at about embryonic day 16 and requires the photopigment in the fetus and not the mother. Measurements show that visceral cavity photon flux is probably sufficient to activate melanopsin-expressing retinal ganglion cells in the mouse fetus. These data thus show that light—the stimulus for function of the mature eye—is also critical in preparing the eye for vision by regulating retinal neuron number and initiating a series of events that ultimately pattern the ocular blood vessels.
Prompted by the recognition that newborn mice are light-responsive6 and show light-dependent neuronal connectivity changes7, we proposed the existence of a pathway in which light responsiveness in the early retina might trigger hyaloid regression and thus clearance of the optic axis. To test this, we placed pregnant dams in the dark at late gestation (embryonic (E) day 16–17): pups raised in the dark until 8 days post partum (P8) showed persistent hyaloid vessels (Fig. 1a) and this was confirmed by quantification over a P1–P8 time course (Fig. 1b). Assessment of hyaloid vessel numbers at P15 showed that by this stage they had regressed. This indicated that dark-rearing resulted in a regression delay. Quantification of apoptosis at P5 showed that regardless of whether we quantified the isolated events that predominate early in hyaloid regression or the segmental pattern of apoptosis that follows8, there was a reduction (Fig. 1c) similar quantitatively to previously characterized hyaloid persistence mutants9. These data suggest that a light-response pathway promotes hyaloid regression.
Figure 1. | Hyaloid regression is regulated by light.
a, Hyaloid vessel preparations at the indicated postnatal (P) days from pups reared under normal light conditions (LD) or under constant darkness (DD) from E16–17. Original magnification, ×50. b, As in a but a quantification of vessel number from P1 to P8. P values obtained by analysis of variance (ANOVA). c, P5 apoptotic index in hyaloid vascular cells (isolated apoptosis) or vessels undergoing a segmental pattern of apoptosis. P values obtained by Student's t-test. Sample size (n) as labelled. NS, not significant. Error bars are s.e.m.
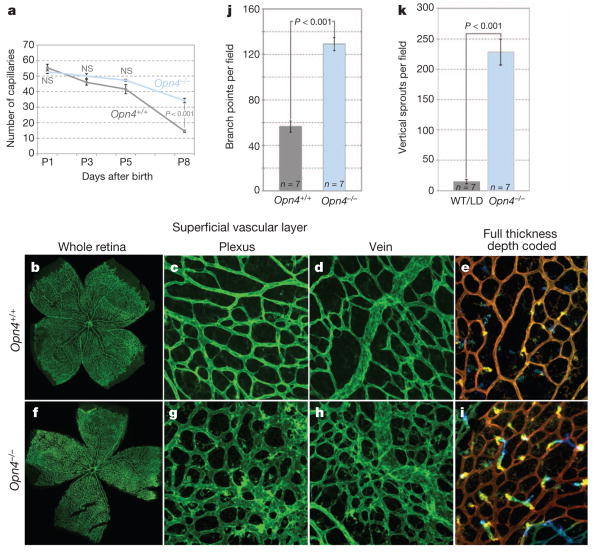
Hyaloid vessel regression and superficial layer retinal angiogenesis occur at the same time in the mouse1,10, and this indicated that dark-rearing might affect both processes. Retinal angiogenesis in mice begins at the day of birth with the extension of vessel precursors from the head of the optic nerve. A superficial layer of vasculature within the retinal ganglion cell (RGC) layer extends to the retinal periphery by P7 (Supplementary Fig. 1a–c). Starting at about P8, angiogenic sprouts extend vertically downwards into the deeper layers of the retina (Supplementary Fig. 1d) and ultimately form the deep vasculature at the outer edge of the inner nuclear layer and the intermediate plexus within the inner plexiform layer2. In mice dark-reared from E16 to E17, the superficial vascular plexus (Supplementary Fig. 1e) showed an increase in density regardless of whether the region was simple plexus (Supplementary Fig. 1f) or at a vein (Supplementary Fig. 1g). Depth-coded P8 image stacks (Supplementary Fig. 1h) showed that there were many more descending vessels (Supplementary Fig. 1h, yellow, blue) than in the wild type (Supplementary Fig. 1h, yellow) and many of these were abnormally located. These changes were confirmed by quantification (Supplementary Fig. 1i, j). Thus, the retinal vasculature is a second vascular structure in the eye where normal development is disrupted by the absence of light.
Melanopsin is expressed from an early stage of both mouse and human gestation and unlike photoreceptor opsins, is known to function in the mouse eye before P10 (ref. 11). Melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) are a subset of RGCs that function in circadian entrainment and the pupillary reflex12. ipRGCs are located in the superficial layers of the retina adjacent to both the retinal and hyaloid vasculatures (Supplementary Fig. 2a–c). This location, the pre-photoreceptor functions of melanopsin6 and the vascular anomalies present in mice that are missing RGCs13, suggested that it was a good candidate to mediate light-dependent vascular development in the eye. To test this possibility, we assessed hyaloid vessel regression and retinal vascular development in mice mutated in Opn4, the melanopsin-encoding gene4,5. Opn4−/− mice showed normal hyaloid vessel numbers at P1 but persistence at P8 (Fig. 2a and Supplementary Fig. 2d). Examination of P15 eyes showed that hyaloid regression was complete in the Opn4−/− mice, indicating that, as with dark-reared mice, hyaloid persistence was not long term. Opn4−/− mice also showed a retinal vascular overgrowth phenotype that qualitatively and quantitatively (Fig. 2b–k) mimicked the changes resulting from dark rearing. To determine whether changes in retinal vascular density endured, we performed a quantitative assessment at P15, P25 and P180 (Supplementary Fig. 3) that showed that elevated vascular density was regionally sustained until at least P180. More generally, the vascular phenotype of the Opn4−/− mice phenocopies that observed in dark-reared mice. This provides an independent means of implicating a light-response pathway in vascular development of the eye and identifies melanopsin as the opsin required.
Figure 2. |Hyaloid regression and retinal angiogenesis are regulated by melanopsin.
a, Quantification of hyaloid vessels in Opn4+/+ and Opn4−/− mice over a P1 to P8 time course. P values obtained by ANOVA. b–i, Low (×100; b, f) and high (×200; c–e, g–i) magnification images of isolectin-labelled P8 retina from wild-type (b–e) and Opn4−/− (f–i) pups raised in normal lighting.e, i, Depth-coded z stack images for wild type (e) and Opn4−/− (i) indicate the appearance of vertical angiogenic sprouts. j, k, Graphs show quantification of branch points (j) and vertical sprouts (k) in animals of the indicated genotypes. WT, wild type. P values obtained by Student's t-test. Errors bars are s.e.m. Sample sizes (n) as labelled.
VEGFA is a potent signal for vascular endothelial cell survival14 that is required for retinal angiogenesis15 and is also present in the vitreous of the rodent16 and human17 eye where the hyaloid vessels reside. We proposed that light-dependent vascular development might be explained by modulation of VEGFA. Consistent with this, homozygous and heterozygous deletion of Vegfafl with the Chx10-cre retinal driver18 gave, respectively, either a hyaloid development failure (Supplementary Fig. 4a) or diminished hyaloid regression (Fig. 3a and Supplementary Fig. 4b). Animmunoblot for vitreous VEGFA over the P1–P8 time course revealed that in control mice, VEGFA164 levels were reduced at P5 but rose again by P8 (Fig. 3b). When three different time courses of VEGFA immunoblots were quantified, the P5 VEGFA signal was about fivefold reduced compared with P1 (Fig. 3b). A low level of VEGFA at P5 is consistent with the idea that it is a key regulator of hyaloid regression because P5 is the time when there are peak levels of vascular endothelial cell apoptosis9.
Figure 3. | Light and melanopsin-dependent regulation of VEGFA expression and hypoxia in the retina.
a, Hyaloid vessel number from P1 to P8 in mice of labelled genotypes. P values obtained by ANOVA. b, Vitreous VEGFA immunoblot (IB) for wild-type mice at P1, P5 and P8 with quantification histogram. c, Immunoblot for P1 or P5 vitreous VEGFA in wild-type and Opn4−/− mice or in mice reared in LD or DD light conditions as labelled. d, ELISA quantification of VEGFA levels in the P5 vitreous of control/LD mouse pups (grey bar) from Opn4−/− mice (pale blue bar)or those raised in constant darkness from E16–17 (DD, blue bar). e, qPCR detection of Vegfa mRNA in P5 retina from control/LD (grey bar), Opn4−/− mice (light blue bar) and dark-reared mice (DD, dark blue bar). P values in b, d, e were obtained by Student's t-test. Sample sizes (n) as labelled. Error bars are s.e.m. f, g, Labelling of flat-mount P5 retinas from wild-type (f) and Opn4−/− (g) mice for blood vessels (isolectin, green) and for hypoxia (red). Retinal myeloid cells label faintly with isolectin. Original magnification, ×100. h, i, Quantification of the relative levels of hypoxyprobe labelling in the retinas of LD and DD mice (c) and wild-type versus Opn4−/− (d) retinas.
Using dark rearing and the Opn4−/− mice, we determined whether actual or functional darkness resulted in a modulation of vitreous VEGFA. In four independent experiments we consistently observed that vitreous VEGFA levels were increased regardless of how light responsiveness was compromised (Fig. 3c). Furthermore, an enzyme-linked immunosorbent assay (ELISA)-based assessment of VEGFA in the P5 vitreous showed that whether pups were dark-reared or mutated in Opn4, the levels of VEGFA were about sevenfold higher than in the control (Fig. 3d). A sevenfold increase in VEGFA in the vitreous of dark-reared and Opn4−/− mice was reflected in similar fold increases in the level of retinal Vegfa messenger RNA as indicated by quantitative polymerase chain reaction (qPCR) (Fig. 3e). Flow sorting/qPCR further showed that Thy1.1+ RGCs and Thy1.1−Vc1.1+ amacrine/horizontal cells exhibited an increase in Vegfa mRNA, although Thy1.1−Vc1.1−PDGFR+ astrocytes did not (Supplementary Fig. 5a). Given the VEGFA dependence of hyaloid vessels (Supplementary Fig. 4) and of retinal angiogenesis15, elevated retinal VEGFA expression is an explanation for the vascular anomalies observed in dark-reared and Opn4−/− mice.
Quantification of BRN3B+ RGCs and calretinin+ amacrine cells in P5 Opn4−/− mice revealed modest increases in the numbers of both cell types (Supplementary Fig. 5d). It has been shown that retinal angiogenesis in the mouse is driven by a hypoxia-response pathway that upregulates VEGFA expression19,20. Because increased cell number can increase oxygen demand, we tested whether the Opn4 mutation and dark rearing resulted in retinal hypoxia (Fig. 3f, g). Quantification of labelling with hypoxyprobe at P5 (Fig. 3h, i) revealed that increased signal was a consequence of both Opn4 mutation and dark rearing. This is consistent with the hypothesis that elevated VEGFA expression in the retina is a consequence of increased oxygen demand due to higher numbers of retinal neurons (Supplementary Fig. 7).
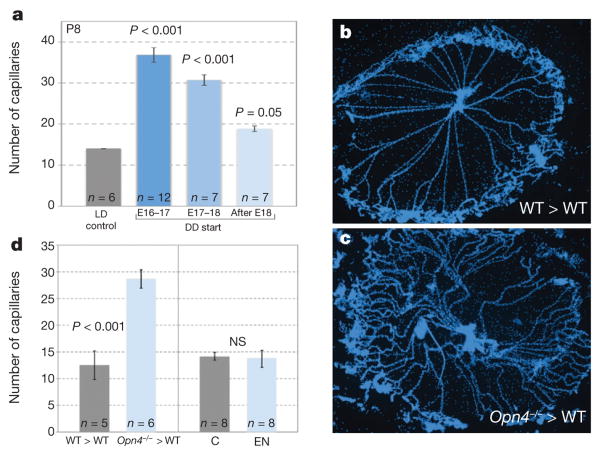
In assessing the role of light responses in vascular development of the eye, our presumption had been that birth was probably a triggering event because vascular patterning events occur after birth and because light levels to the eye of the newborn would increase. To test this, we dark-reared pups from different points of late gestation (E16–17, E17–E18 or after E18) and assessed hyaloid persistence. We observed a dose response where the hyaloid vessels were progressively more persistent with an earlier dark-rearing start (Fig. 4a). In particular, if dark rearing was started after E18 (the day of birth is usually E19) there was almost no effect (Fig. 4a, light blue bar). These data indicate the surprising outcome that the critical light-response period stimulating hyaloid regression is gestational, at or before E16–17. This further raised the possibility that this developmental pathway required a direct fetal light response. To test this assertion directly, we transferred embryos from an Opn4+/−×Opn4+/− cross into a pseudopregnant wild-type female and assessed hyaloid regression. Control, wild-type pups in the transferred litter showed normal hyaloid regression (Fig. 4d, b, grey bar), whereas the Opn4−/− mice showed persistence at P8 (Fig. 4d, c, blue bar). In addition, to test the reciprocal possibility—that the light response of the mother might influence vascular development of the fetal eye—we enucleated female mice, and allowed them to gestate and raise litters under normal lighting conditions. This did not produce hyaloid persistence (Fig. 4d). Combined, these experiments show that melanopsin in the fetus, not the mother, is critical to regulate vascular development of the eye.
Figure 4. | Gestational light controls vascular development in the eye.
a, Quantification of hyaloid vessels in mice raised in normal lighting (LD, grey bar) and those dark-reared from E16–17 (dark-blue bar), E17–18 (medium-blue bar), or after E18 (light-blue bar). b, c, P8 hyaloid vessel preparations from a wild-type embryo transferred into a wild-type pseudopregnant female (WT>WT) and an Opn4−/− embryo transferred into a wild-type pseudopregnant female (Opn4−/− > WT). Original magnification, ×50. d, Left panel: quantification of hyaloid vessels in P8 WT>WT (n = 5) and Opn4−/−>WT (n = 6) pups. Right panel: quantification of hyaloid vessels in normal control pups at P8 (C; n = 8)and P8 pups (n = 8) born to an enucleated female (EN). Sample sizes (n) as labelled. P values in a by ANOVA; P values in d were obtained by Student's t-test. Errors bars are s.e.m.
Measurements of the light level in the visceral cavity of adult mice living under mouse room fluorescent lights (Supplementary Fig. 6) revealed flux densities of 1.4×1013 (for BALB/c) and 1.1×1012 (for C57BL/6) photons cm−2s−1. Published response thresholds for rodent ipRGCs range upwards from approximately 1.2×1010 photons cm−2 s−1 (refs 21–23). Furthermore, it has been shown that ipRGCs have the ability to respond continuously to light stimulation, via melanopsin, for up to 10 h (ref. 23). Although it has been suggested that ipRGCs in newborn mouse pups are less sensitive than in adults22, the reduced sensitivity is about 1.5 log quanta and so the visceral light level in a pigmented animal of 1.1×1012 photons cm−2s−1 may still be above the threshold. These data are consistent with the hypothesis that the mouse fetus can respond directly to light via melanopsin.
These experimental studies identify light as a trigger for hyaloid vessel regression and for suppression of promiscuous angiogenesis in the retina. The observation that dark rearing from late gestation or Opn4 mutation produces essentially identical perturbations of vascular development provides corroborating evidence for involvement of a melanopsin-dependent, light-response pathway (Supplementary Fig. 7). Our data also indicate that the origin of the hyaloid persistence and de-regulated retinal angiogenesis is increased levels of VEGFA originating in retinal neurons. These findings are surprising because, with the exception of neuronal connectivity, it has not been shown previously that light can trigger changes in developmental programs. Our data indicate that the primary light-dependent change is an increase in the number of retinal neurons and that the vascular changes occur in response to increased oxygen demand (Supplementary Fig. 7) considerably later in developmental time. This pathway is an interesting example of one where events unfold slowly over the course of nearly 2 weeks. It will be interesting to determine whether this pathway might influence susceptibility to retinopathy of pre-maturity24, the retinal vasculopathy of pre-term infants in which promiscuous angiogenesis can cause blindness.
2. Methods
a. Mice
Genotyping of Vegfafl (ref. 26), Chx10-cre (ref. 18), Opn4cre (ref. 27), Ai4 (ref. 28) and Opn4−/− (ref. 5) was performed as described. All animal experimentation was carried out using protocols approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Medical Center and at the University of California San Francisco.
b. Hyaloid and retinal labelling and quantification
Hyaloid vessels were collected and stained with Hoechst as well as for TdT-mediated dUTP nick end labelling (TUNEL) as described9. Retinal flat-mounts were prepared and labelled with isolectin29 or for melanopsin4. Hyaloid vessel quantification has been described previously1. Retinal vessel density was quantified by counting vessel junctions using ImageJ for many ×200 microscope fields. Depth-coded three-dimensional image reconstructions were generated using a Zeiss Apotome-equipped microscope in conjunction with Axiovision software. Antibodies for labelling of retinal flat-mounts included anti-Brn3b (Abcam), anti-calretinin (Millipore) and anti-melanopsin (ATS).
c. Assessment of hypoxia
P5 mouse pups were injected with 60 mg kg−1 (about 180 μg per pup) pimonidazole hydrochloride (Hypoxyprobe), and after 45 min were killed and retinas collected. The rabbit primary antibody to pimonidazole hydro-chloride was then used in conjunction with an anti-rabbit Alexa594 secondary to label retinal tissue. We quantified labelling by generating intensity values along line intervals extending from the retinal centre to the periphery. Pixel intensity values from 20 to 25 line intervals per retina and 5 to 6 retinas per genotype were averaged. Significance values were calculated using the MatLab ANOVA test.
d. Isolation and analysis of vitreous
Vitreous was collected from dark-reared pups in a room using red illumination. Eyes from P1 and P5 pups were washed twice in sterile ice cold PBS. Excess PBS was blotted using a kimwipe, a small slit was made through the retina and vitreous collected. ELISA was performed on the vitreous using the Vegfa Quantikine kits (R&D) that include recombinant protein standards. Immunoblots were probed with a unique carboxy-terminal antibody for VEGFA30 from Santa Cruz. Quantification was performed using ImageJ.
e. Cell sorting
For flow sorting using markers for retinal neurons31, retina was dissociated as described25 except 16 mg ml−1 of liberase CI (Roche) and 20μg ml−1 of DNase I (Sigma) was used. Cells were then labelled on ice for 30 min with goat PDGFR-α, washed with PBS and labelled with PerCP-conjugated anti-CD90 (clone OX-7), FITC-conjugated anti-CD57 (clone VC1.1), Alexa fluor 350 and 7-AAD. Cells were sorted with FACSAria II running DiVa software.
f. RNA isolation and qPCR
RNA was isolated using RNeasy (Qiagen). qPCR was performed with QuantiTect SYBR green (Qiagen) using amplification of actin for normalization. In analysing qPCR data, the P values refer to a comparison of the ΔΔCT values. Primers were as follows: Vegfa 5′-GACAGAACAAAGCCAGA-3′, 5′-CACCGCCTTGGCTTGTCAC-3′.
g. Light measurements
To estimate the radiant flux density impinging a mouse housed in a cage in the UCSF animal care facilities, we determined the spectral distribution of fluorescent lights, S(λrelative), illuminating the room and the absolute power (watts cm−2s−1)of these lights. Both measurements were done at floor level. S(λrelative) was measured (Photo Research, PR670 spectraradiometer) as light reflected from a spectrally flat reflective surface (Spectralon Target, Labsphere). The radiant power was measured using a calibrated radiometric detector (UDT Instruments; model S471). S(λrelative) was converted to S(λabsolute) by scaling the area under S(λrelative) to match the radiant power and then converting these values to photons cm−2s−1 for each wavelength. The melanopsin spectral absorbance curve was then convolved with S(λabsolute). The area under this curve was used as a measure of the radiant flux density capable of stimulating the melanopsin pigment (λmax = 479 nm). We calculate that the equivalent of at least 5×1013 photons cm−2 s−1 was available to stimulate melanopsin. Similar measurements and calculations for sunlight (12:00, 20 December 2011) revealed a radiant flux density of 2.6×1016 photons cm−2s−1.
To estimate attenuation of light that could stimulate melanopsin in mouse fetuses in utero, we directed a blue LED (Philips Lumileds Lighting Company; model: Luxeon III star, LXHL-LB3C, peak wavelength = 470 nm) that was positioned 1 inch from the skin to a miniature silicon photodiode light detector placed inside the abdominal cavity. Light penetrated both the skin and sub-dermalmuscle layer. The measurements were done on live, anaesthetized adult mice (intramuscular (IM) injections of ketamine/xylazine).
Supplementary Material
Acknowledgments
We thank D. Bredl, P. Speeg and M. Sarangdhar for technical assistance, A. Delwig and N. Brown for advice. We acknowledge the assistance of the Research Flow Cytometry Core in the Division of Rheumatology at CCHMC, supported in part by NIH AR-47363. This work was supported by the NIH (R.A.L., D.R.C., J.M.K. and R.S.H.) with additional funding from the Abrahamson Pediatric Eye Institute of CCHMC, That Man May See at UCSF, Research to Prevent Blindness (D.R.C.) and March of Dimes (D.R.C.).
Footnotes
Supplementary Information is available in the online version of the paper
Author Contributions R.A.L. and D.R.C. provided project leadership. R.A.L., D.R.C. and S.R. wrote the manuscript. R.A.L., D.R.C. and R.S.H. supervised experimental work. N.F. developed critical reagents and M.B.Y. provided important clinical insights. S.R., C.C., J.F., J.M.K. and D.R.C. performed experimentation and analysis.
References
- 1.Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillarymembrane of the mouse. Anat Embryol (Berl) 1999;200:403–411. doi: 10.1007/s004290050289. [DOI] [PubMed] [Google Scholar]
- 2.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 3.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J, et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci USA. 2010;107:17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 8.Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–3638. doi: 10.1242/dev.124.18.3633. [DOI] [PubMed] [Google Scholar]
- 9.Lobov IB, et al. WNT7b mediates macrophage-inducedprogrammedcell deathin patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 11.Tarttelin EE, et al. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res. 2003;76:393–396. doi: 10.1016/s0014-4835(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 12.Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards MM, et al. The deletion of Math5 disrupts retinal blood vessel and glial development in mice. Exp Eye Res. 2012;96:147–156. doi: 10.1016/j.exer.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 15.Haigh JJ, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 16.Meeson AP, Argilla M, Ko K, Witte L, Lang RA. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development. 1999;126:1407–1415. doi: 10.1242/dev.126.7.1407. [DOI] [PubMed] [Google Scholar]
- 17.Adamis AP, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 18.Rowan S, Cepko CL. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev Biol. 2004;271:388–402. doi: 10.1016/j.ydbio.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Kurihara T, et al. von Hippel-Lindau protein regulates transition from the fetal to the adult circulatory system in retina. Development. 2010;137:1563–1571. doi: 10.1242/dev.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange C, et al. Retina-specific activation of a sustained hypoxia-like response leads to severe retinal degeneration and loss of vision. Neurobiol Dis. 2010;41:119–130. doi: 10.1016/j.nbd.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Sekaran S, et al. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu DC, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci. 2012;32:11478–11485. doi: 10.1523/JNEUROSCI.1423-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connor KM, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nature Protocols. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefater JA, et al. Regulation of angiogenesis by a non-canonical Wnt–Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtenberger BM, et al. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 27.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockmann C, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.