Abstract
Tendon mechanical properties are thought to degrade during aging but improve with exercise. A remaining question is whether exercise in aged animals provides sufficient regenerative, systemic stimulus to restore younger mechanical behaviors. Herein we address that question with tail tendons from aged and exercised rats, which would be subject to systemic effects but not direct loading from the exercise regimen. Twenty-four month old rats underwent one of three treadmill exercise training protocols for 12 months: sedentary (walking at 0° incline for 5 min/day), moderate (running at 0° incline for 30 min/day), or high (running at 4° incline for 30 min/day). A group of 9 month old rats were used to provide an adult control, while a group of 3 month old rats provided a young control. Tendons were harvested at sacrifice and mechanically tested. Results show significant age-dependent differences in modulus, ultimate stress, relaxation rate, and percent relaxation. Relaxation rate was strain-dependent, consistent with nonlinear superposition or Schapery models but not with quasilinear viscoelasticity (QLV). Trends in exercise data suggest that with exercise, tendons assume the elastic character of younger rats (lower elastic modulus and ultimate stress).
Keywords: Viscoelasticity, Systemic exercise effects, Aging
INTRODUCTION
The mechanical behavior of tendon is nonlinear and viscoelastic. While strength and stiffness are predominantly used as distinguishing features for this structural tissue, viscoelastic behaviors are important at physiological strains. Viscoelasticity affects a tissue’s ability to store, translate, and dissipate energy, and adapt to loading conditions over time. Study of the strain dependence of viscoelastic response over the physiologically relevant range is consequently pertinent. The effects of maturation and aging on the mechanical behavior of tendons require further elucidation.
Current literature generally agrees that maturing tendons lose viscoelasticity but increase in stiffness.26,34,35,38 While there is general agreement about the effects of maturation, there is disagreement over the effects of further aging on tendon mechanics. A number of groups suggest that tendon strength and stiffness increase along with a decrease in ultimate strain, making tendons appear stronger, but more brittle and less tough.6,18,19,30,37 Others suggest there is either no effect16,24 or that aging tendons decrease in strength and stiffness.12,25,31,38 Likewise, no investigations lend conclusive evidence of a link between old age and changes in viscoelasticity in tendon;1,23,25,34,38 some studies have reported that aging decreases viscoelasticity,1,38 while others report no significant effect.25
Another debated question is whether exercise can lessen the biomechanical effects of aging on structural soft tissues, including tendon. Investigations on the positive effects of exercise yielded conflicting results. Early animal studies by Nielsen et al.30 saw a lower ultimate stress and modulus in old exercised tendons, which they associated with “younger” mechanical properties even though those properties were mechanically inferior to the stronger and stiffer unexercised old tendons. Others who have reported decreases in mechanical properties in exercised tendons have attributed it to overuse and resulting injury. More recently, in a human in vivo study, a 65% increase in modulus was measured as a result of a weekly strength training protocol.8 This modulus increase suggests that exercise may be mechanically regenerative in older tendons; whether this is due to local or systemic effects is unclear. One study investigating the effect of exercise on tendon mechanics obtained equivocal results; low strength trained rat Achilles tendons exhibited lower viscoelasticity than sedentary controls, while those subjected to a high strength training protocol exhibited more viscoelastic behavior than controls. This unanticipated result was attributed to overuse or injury. It is therefore of interest to examine whether exercise provides a beneficial systemic effect in a tendon which would not be subject to overuse or injury during the exercise protocol.
Previously reported effects in weight-bearing tendons have not defined whether effects are systemic or directly related to mechanical load (or both). Similarly, previous studies have explained unanticipated results by stating that the loaded tendon was subject to overuse or injury. Thus, a study of non-weight bearing tendons would avoid overuse issues and be better able to elucidate systemic vs. mechanical load effects. The goal of this study was to investigate the effects of age on the mechanical and viscoelastic (including relaxation studies at different strain levels) properties of tendon, and to determine systemic effects of exercise in aged tendons in a non-weight bearing tendon.
MATERIALS AND METHODS
Animal Groups and Exercise Training
A total of 33 rats were used in this study. Twenty-one male Fischer 344 × Brown Norway F1 rats (F344BN), aged 24 months, were obtained from the National Institute on Aging colony maintained by Harlan Sprague–Dawley (Indianapolis, IN, USA) for the exercise study. The rats were housed in pairs in a temperature (21° C) controlled animal facility maintained on a 12:12-h light/dark reverse cycle under the care of a full-time veterinarian. Animals had access to food and water ad libitum. Body weight, food intake, and survival were monitored weekly. The rats were randomly assigned to one of three groups: a sedentary control (S) group (n = 7), a moderate intensity (M) exercise group (n = 7), or a high intensity (H) exercise group (n = 7) and subjected to a previously established exercise protocol.5,11,17 Briefly, animals were exercised on a motor-driven treadmill starting at age 24 months and continuing 5 days/week until they were aged 36 months. M animals started with a speed and duration of 3 m/min for 5 min, with slow progression of intensity to 13 m/min for 30 min/ day by week 7 (we included a longer “ramp up” to the final training intensity to lessen the stress of exercise training and improve exercise compliance),5 and this intensity was then maintained for the duration of the 12-month exercise training program. H animals started with a speed and duration of 3 m/min for 5 min, with slow progression of intensity to 13 m/min at 4% incline for 30 min/day by week 75 and this intensity was then maintained for the duration of the 12-month exercise training program. S animals walked on the treadmill 2 days/week at 3 m/min for 5 min to control for handling and treadmill exposure.
A group of 9 month old F344BN rats (n = 6) were used to provide an adult, but not elderly control. A group of 3 month old F344BN rats (n = 6) provided a young control group. These rats were housed and handled in the same fashion as the Sedentary group rats (n = 7), to which they were compared in age-related analyses.
Animal handling and euthanasia were carried out under the guidelines of University of Wisconsin–Madison Animal Use and Care Committee. Rats were anesthetized by inhalation of isoflurane. After sacrifice, tails were collected, placed in airtight plastic bags, and frozen at −20 °C. After thawing in a water bath, dorsal rat tail tendons were carefully dissected from the proximal end of the tail to a location 2/3 down the length of the tail. During dissection, special care was taken to leave the tendon slack while separating it from the bony attachments. The main securing force was placed on the tail backbone itself, not on the tendon being dissected, to avoid damaging the tendons. Dissected tendons were then placed in PBS-soaked gauze, wrapped in aluminum foil, and again stored at −20 °C.
Mechanical Testing
Tendons were thawed prior to testing. Width and thickness were measured at three equidistant locations along the length of the tendon using a 100 mm digital caliper. Using the averages of width and thickness, tendon cross sectional area was calculated assuming an elliptical cross section.
All testing was performed using a servohydraulic mechanical test system (Bionix 858; MTS, Minneapolis, MN, USA) in combination with a computer for data collection.13,14 Identical grips were custom designed with textured gripping surfaces and set screws to grip the proximal and distal portions of the tendons. The bottom grip was secured to a stationary block. The top grip was secured to a load cell (3 month low-strain specimens: 50 lb Stellar Technology, Amherst, NY, USA; all other testing: 1000 lb Honeywell, Morristown, NJ, USA), with displacement controlled and measured by the mechanical testing system. Once loaded, tendons were kept moist by the application of strips of PBS-soaked gauze to each side of the tendon, and the gauze remained saturated by dripping with PBS throughout testing.
Before each test, a 0.1 N preload was applied to remove any slack in the tendon. At this point the grip-to-grip distance was measured using a 100 mm digital caliper and recorded. Strain was calculated as the grip-to-grip displacement divided by the tendon length at the 0.1 N preload. Next, tendons were preconditioned by stretching to 2% strain in a sinusoidal wave for 20 s with a period of 2 s. Tendons were allowed to recover for 10 min before relaxation testing started. They were then stretched to 1, 2, 3, 4, 5, and 6% strain and held for 100 s at each level. Between each increase in strain, tendons were allowed to recover for 1000 s between each test (10 times the length of each relaxation test). To investigate the low-strain relaxation behavior of 3 month old samples, these tendons were stretched to 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 5, and 6% strain for each relaxation test. In order to better extract the true behavior of tendon at strains as low as 0.5 and 0.75%, a moving average smoothing was performed on those data sets.
After each set of relaxation tests, tendons were pulled to failure at a rate of 20 mm/min (~0.5%/s for 60 mm gage length).
Parameter Calculation
Stress was calculated using the following equation:
| (1) |
where σ is engineering stress, F is force, and A is cross sectional area of the unloaded tissue.
Relaxation rate was calculated as the exponent in a power-law curve fit (of the form Atn). Percent stress loss was calculated over the 100 s relaxation test as
| (2) |
where σ 0.1s and σ 100s are the stress at 0.1 and 100 s, respectively.
Parameter Comparison
In order to examine the effects of age on tendon mechanical behavior, elastic modulus, ultimate stress, ultimate strain, relaxation rate, and percent stress loss during relaxation (“% relaxation”) for rats aged 3, 9, and 36 (sedentary groups) months were compared. Exercise effects were likewise examined by comparing the same parameters in rats from the sedentary, moderate exercise, and high exercise groups. To elucidate whether observed effects were due to size changes in the animals (e.g., older animals were larger, or exercised animals weighed less), parameters were compared in rats from three weight groups: ~400 (334–427 g), ~470 (451–494 g), and ~540 (519–570 g).
Statistical Analysis
An ANOVA analysis was performed to determine significance between groups. Post hoc pairwise analysis was performed using Tukey’s method. Significance was set at p ≤ 0.05, trends at p ≤ 0.15. Statistical analyses were performed using Kaleidagraph (Synergy Software, Reading, PA, USA) software.
RESULTS
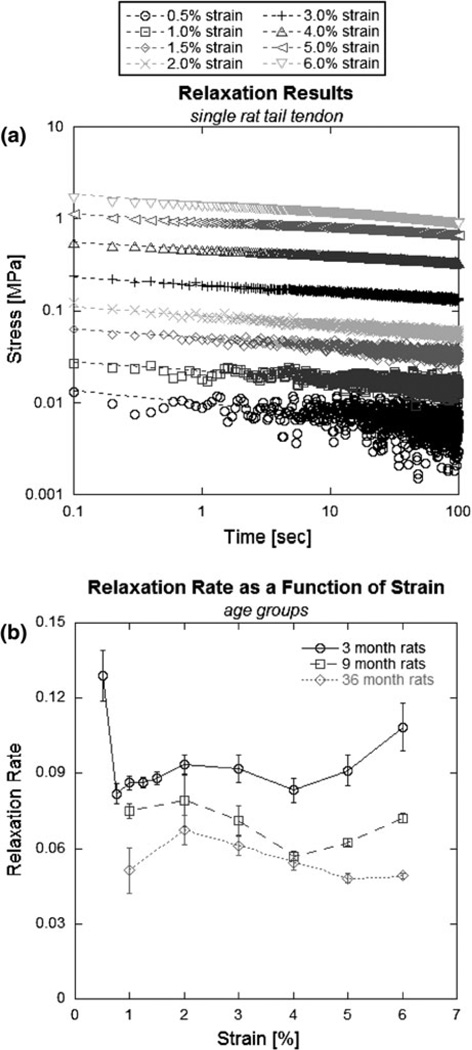
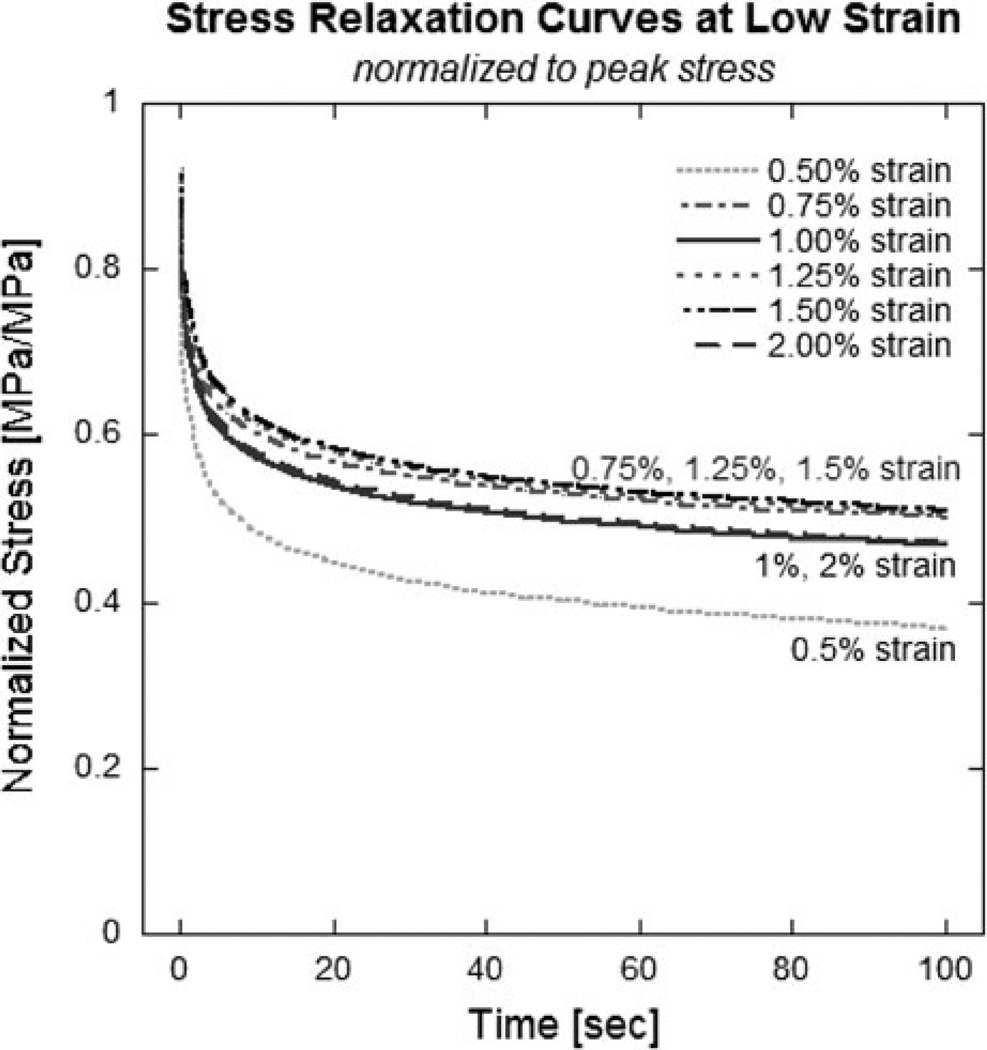
Relaxation rate is represented graphically as the slope of the relaxation curve plotted on a log–log scale (Fig. 1a). Tendon relaxation rate showed strain-dependence, with higher rates at the low and high strains, and relatively constant rates over intermediate strains (Fig. 1a, 1b). Relaxation rate decreased significantly with age (p < 0.001; Table 1), with significant decreases found during maturity (3–9 month, p < 0.001; Table 1) and further decrease during aging of matured tendons (9–36 month, p < 0.001; Table 1), as seen in Fig. 1b. Further examination of low-strain relaxation rate in the young rat group is shown in Fig. 2, in which raw relaxation data were averaged and normalized to peak stress to better demonstrate the rapid stress loss seen at the lowest strain (0.5%).
FIGURE 1.
Relaxation as a function of strain. Relaxation data were plotted on a log–log scale and fitted with power law curves (results from a single 3 month old rat tendon without smoothing shown) (a); the slope of which is the relaxation rate (b). In post hoc analysis, relaxation rates decreased significantly from 3 to 9 months and from 9 to 36 months (p<0.001 in both cases) at strains of 1, 2, 3, 4, 5, and 6%. Error bars represent 1 SE.
TABLE 1.
Age groups mechanical results and significance.
| Elastic modulus (MPa) |
Ultimate stress (MPa) |
Ultimate strain (%) |
Relaxation rate |
% Relaxation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |||||
| 3 months | 60.78*,^ | 15.56 | 2.50*,^ | 0.79 | 9.21 | 1.96 | 0.0952*,^ | 0.0199 | 49.30*,^ | 5.71 | |||||
| 9 months | 106.86^,+ | 15.04 | 4.91+ | 0.97 | 9.89 | 1.35 | 0.0688^,+ | 0.0144 | 36.40^,+ | 1.78 | |||||
| 36 months | 87.23*,+ | 17.08 | 4.29+ | 1.34 | 9.18 | 1.52 | 0.0560*,+ | 0.0180 | 30.53*,+ | 4.98 | |||||
| ANOVA | <0.001 | <0.001 | 0.685 | <0.001 | <0.001 | ||||||||||
| Post hoc | 3–9 | 9–36 | 3–36 | 3–9 | 9–36 | 3–36 | 3–9 | 9–36 | 3–36 | 3–9 | 9–36 | 3–36 | 3–9 | 9–36 | 3–36 |
| <0.001 | 0.018 | <0.001 | <0.001 | 0.409 | <0.001 | 0.716 | 0.687 | 0.999 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.036 | |
ANOVA indicates the p values associated with an ANOVA analysis.
Post hoc indicates the pairwise analysis results (p values) following Tukey’s analysis between age groups (3, 9, and 36 months).
Symbols represent significant difference:
significant difference from 9 months
from 36 months, and
from 3 months.
FIGURE 2.
Low strain smoothed relaxation of 3 month old tendons. Results shown were normalized to peak stress for visual comparison. Relaxation rate at lowest, 0.5%, strain was noticeably faster.
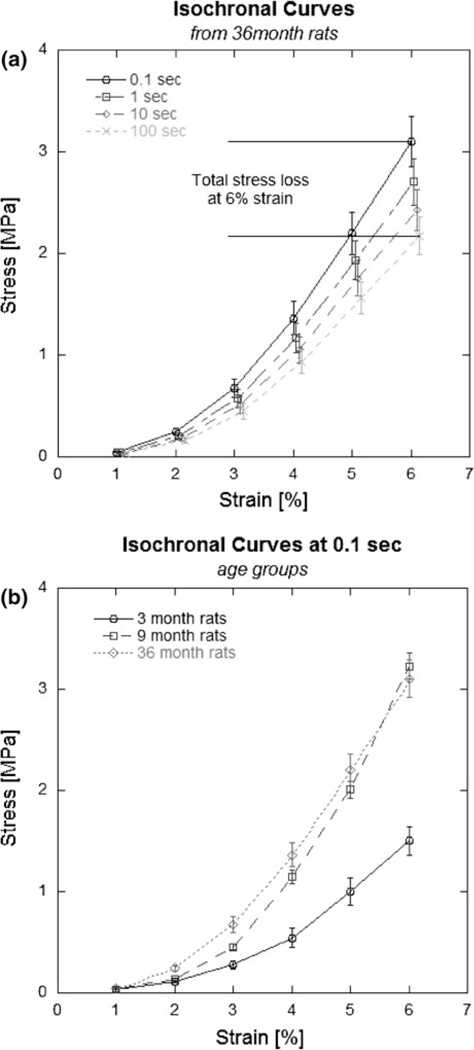
Isochronal stress–strain curves from relaxation data represent stress loss during relaxation as the difference in stress between curves at a given stress level (Fig. 3a). Significant decreases in percent stress loss were seen between age groups (p < 0.001; Table 1), with significant decreases found during maturity (3–9 month, p< 0.001; Table 1) and further aging (9–36 month, p < 0.001; Table 1). The strain-stiffening behavior of tendon can also be visualized using the isochronal curves. The effects of maturation on the loading behavior of tendon are demonstrated in Fig. 3b, as 3 month tendons display different strain-stiffening behavior than older (9 or 36 month) tendons.
FIGURE 3.
Representative isochronal results obtained from relaxation tests (a) showing strain-stiffening behavior typical of tendon. A comparison of age groups (b) shows that young (3 month) tendons exhibit different mechanical behavior than adult (9 month) and elderly (36 month) tendons. Maturation causes the most dramatic changes in tendon mechanical properties. Error bars represent 1 SE.
Cross sectional area for 3, 9, and 36 month rats was 10.51 ± 1.9, 11.80 ± 1.9, and 9.66 ± 2.41 mm (mean ± SD), respectively. Area measurements in the sedentary, moderate, and high exercise groups were 8.64 ± 1.77, 9.65 ± 1.98, and 10.12 ± 1.98 mm2, respectively. No significant effect of aging (p = 0.364) or exercise (p = 0.407) was seen.
Results from pull-to-failure testing also demonstrated age-dependent results. Elastic modulus was significantly affected by age (p < 0.001; Table 1), with a significant increase during maturity (3–9 month, p < 0.001; Table 1) followed by a significant decrease during further aging of the matured tendon (9–36 month, p = 0.018). Though the aged (36 month) tendons had a decreased modulus, they remained significantly higher than the young (3 month) control (p < 0.001; Table 1). Ultimate stress also noted a significant effect of aging (p < 0.001) with a significant increase with maturity (3–9 month, p < 0.001; Table 1), but demonstrated no significant change with further aging (9–36 month, p = 0.409; Table 1).
Mechanical results for exercise groups are shown in Table 2. No statistically significant effects of exercise were noted for any parameter, but a decreasing trend was seen for elastic modulus (p = 0.057; Table 2). A decreasing trend in elastic modulus values from the sedentary to the moderate exercise groups (p = 0.062) and from the sedentary to the high exercise group (p = 0.146) was shown. A decreasing trend in ultimate stress was also seen from the sedentary to the moderate exercise group (p = 0.149).
TABLE 2.
Exercise groups mechanical results and significance.
| Elastic modulus (MPa) |
Ultimate stress (MPa) |
Ultimate strain (%) |
Relaxation rate |
% Relaxation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |||||
| Sedentary | 98.01 | 15.33 | 4.88 | 1.29 | 9.05 | 1.30 | 0.0540 | 0.0141 | 32.45 | 5.46 | |||||
| Moderate | 80.16 | 20.58 | 3.70 | 1.18 | 8.81 | 1.76 | 0.0584 | 0.0192 | 29.87 | 4.91 | |||||
| High | 83.53 | 10.21 | 4.30 | 1.45 | 9.76 | 2.73 | 0.0554 | 0.0207 | 29.27 | 4.72 | |||||
| ANOVA | 0.057 | 0.174 | 0.667 | 0.582 | 0.472 | ||||||||||
| Post hoc | S–M | M–H | S–H | S–M | M–H | S–H | S–M | M–H | S–H | S–M | M–H | S–H | S–M | M–H | S–H |
| 0.062 | 0.892 | 0.146 | 0.149 | 0.593 | 0.607 | 0.972 | 0.662 | 0.795 | 0.564 | 0.777 | 0.944 | 0.613 | 0.973 | 0.481 | |
S sedentary control group, M moderate intensity exercise group, H high intensity exercise group.
ANOVA indicates the p values associated with an ANOVA analysis.
Post hoc indicates the pairwise analysis results (p values) following Tukey’s analysis between exercise groups (sedentary, moderate, and high).
Mechanical results for weight groups are shown in Table 3. No statistically significant effects of weight group were noted for any parameter (p > 0.150 for all parameters). A nonsignificant increase in both elastic modulus (p = 0.164) and ultimate stress (p = 0.175) was noted between the lowest (~400 g) and middle (~470 g) weight groups.
TABLE 3.
Weight groups mechanical results and significance.
| Elastic modulus (MPa) |
Ultimate stress (MPa) |
Ultimate strain (%) |
Relaxation rate |
% Relaxation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |||||
| ~400 g | 78.70 | 6.30 | 3.60 | 1.15 | 8.97 | 2.64 | 0.0569 | 0.0186 | 29.69 | 4.41 | |||||
| ~470 g | 94.87 | 12.04 | 4.84 | 0.82 | 9.10 | 1.40 | 0.0540 | 0.0196 | 29.42 | 6.37 | |||||
| ~540 g | 89.69 | 27.06 | 4.59 | 1.80 | 9.65 | 1.72 | 0.0569 | 0.0157 | 32.93 | 3.70 | |||||
| ANOVA | 0.175 | 0.167 | 0.17 | 0.741 | 0.394 | ||||||||||
| Post hoc | 400–470 g | 470–540 g | 400–540 g | 400–470 g | 470–540 g | 400–540 g | 400–470 g | 470–540 g | 400–540 g | 400–470 g | 470–540 g | 400–540 g | 400–470 g | 470–540 g | 400–540 g |
| 0.164 | 0.838 | 0.443 | 0.175 | 0.936 | 0.343 | 0.990 | 0.883 | 0.812 | 0.767 | 0.796 | 0.999 | 0.994 | 0.433 | 0.467 | |
ANOVA indicates the p values associated with an ANOVA analysis.
Post hoc indicates the pairwise analysis results (p values) following Tukey’s analysis between weight groups (400, 470, and 540 g).
DISCUSSION
In this study, we examined the mechanical behavior of tail tendons in young, adult, and (exercised or sedentary) elderly rats in an effort to quantify the systemic effects of maturity, aging, and exercise on the mechanical and viscoelastic properties of a non-weight bearing tendon. We show significant age-dependent differences in modulus, ultimate stress, relaxation rate, and percent relaxation. Relaxation rate and percent relaxation decreased with age, while modulus and ultimate stress increased from 3 to 9 months (not from 9 to 36 months). Trends of lower elastic modulus and ultimate stress in exercise data between sedentary and moderately exercised animals (p < 0.150 in each case) suggest that with exercise, tendons may assume the elastic characteristics of younger rats. Additionally, no significant differences were found between weight groups, suggesting that the differences seen with maturity and aging were not due to animal size alone. Finally, we demonstrate a unique, nonlinear strain-dependent relaxation rate in 3 month rats which is higher at low and high strain rates than intermediate strains.
Tendon modulus and ultimate stress increase with maturity (from 3 to 9 months), which is consistent with results in the literature demonstrating dramatic increases in tendon strength and stiffness.2,8,19,30,35,38 Suggested structural reasons for this effect include anabolic processes leading to increases in both collagen fibril diameter and volume fraction,7,9,19 and enzymatic collagen crosslinking1,2 which increases resistance to collagenase and protease degradation.21
Further aging (9–36 months) resulted in a significantly decreased elastic modulus, which is consistent with results by Vogel,38 Dressler et al.,12 Johnson et al.,25 and Onambele et al.31 Ultimate stress was not significantly changed from 9 to 36 months (p = 0.302). This agrees with previous results by Flahiff et al.17 and Hubbard and Soutas-Little,24 who found no significant changes in strength with tendon aging. Structural assessments attempting to explain age-related changes report conflicting results. Crosslinked collagen (correlated to greater elastic modulus) continues to increase with age,21 while fibril volume fraction and stiffness both decreased in a highly correlated manner with age19 and SEM observations of collagen demonstrate the imbalance between anabolic production of collagen and catabolic mechanisms.12,19,27 Collagen fibers appear highly organized as they are being produced in early to middle age, but in all cases begin to disorganize with further aging as somatic repair mechanisms become less dominant.
Viscoelastic parameters relaxation rate and percent relaxation decreased with maturity (from 3 to 9 months), confirming a reduction in viscoelastic behavior previously indicated in the literature.26,34,35,38 Factors involved in tendon maturation that may be responsible for this decrease in viscoelasticity include changes in collagen crosslinking,26 glycosaminoglycans (GAGs),9,29,34 water content,4,20,36 or collagen fibril diameter.7,9,12,27
A further decrease in viscoelastic parameters between 9 and 36 month rats indicates that viscoelastic behavior continues to decrease with age. This is in agreement with the study by Vogel,38 which demonstrated a decrease in hysteresis parameters with aging, and with the study by Andreassen et al.,1 which demonstrated a slower relaxation rate in an accelerated aging model. However, our result is in contrast with the study by Johnson et al.,25 which showed no significant difference in viscoelastic properties with aging in human patellar tendon. The observed age-related reductions in viscoelasticity may be due to compositional changes in proteoglycans or water content, which have both been shown to change with age.3,10,26 Proteoglycan loss has been shown to affect relaxation rate15 and hysteresis,29 and an increase in proteoglycan content increased strain rate sensitivity in mouse tendon.34 Likewise, increased water content has been reported to correlate with greater stress relaxation.4,20 Thus, changes in these and other matrix components with age likely contribute to age-related changes in viscoelasticity.
No significant effect of exercise level was demonstrated in this study. Elastic modulus was the only parameter displaying notable exercise effect, with a nearly significant decrease in elastic modulus values from the sedentary to the moderate exercise groups (p = 0.062) and a decreasing trend in modulus values from the sedentary to the high exercise group (p = 0.146). A decreasing trend in ultimate stress was also seen from the sedentary to the moderate exercise group (p = 0.149). Nielsen et al.30 previously reported a similar decrease in ultimate stress and modulus with exercise in the Achilles tendons of older rats, which they associated with “younger” mechanical properties. The trends observed in the elastic properties in the current study suggest that exercise may have a weak systemic effect which causes older tendons to behave more like younger tendons. However, this effect is not as strong as that seen in a weight-bearing tendon such as the Achilles.30
No significant effect of animal weight was demonstrated in the study. A nonsignificant trend in both elastic modulus and ultimate stress was noted between the lowest (~400 g) and middle (~470 g) weight groups; this is likely due to the highly significant difference between the behavior in the 3 month (generally smaller) and older (generally larger) groups.
The higher relaxation rates observed at low and at high strain suggest both tendon-like and ligament-like characteristics. Specifically, in prior studies, ligament relaxation rate was higher at low strain14,22,32 while in tendon, rate was higher at high strain.13,14 This behavior may be considered in the context of tail tendon anatomy; rat tail tendon has partial constraint from the vertebrae, so it partakes of ligament-like anatomy. Relaxation at low strain levels may be a probe into proteoglycan-induced behavior and/or the interface between collagen fibers. This relaxation rate behavior impacts future modeling by constitutive equations, as strain dependence of the relaxation rate is consistent with nonlinear superposition or with Schapery models, in which modulus is dependent on both time and strain, but not with quasilinear viscoelasticity (QLV), in which the relaxation modulus may be written as a product of time dependent and strain dependent functions.
One limitation in this study was the treatment of the tail tendon as a non-loadbearing tendon. While the tail is not weight-bearing, animals lift and move their tails during running and thus changes in running intensity may result in larger and more frequent forces being placed on the tail tendons. Trends associated with exercise may therefore not purely result from systemic effects. This study only examined the effects of aging and exercise in the context of mechanical changes, with no investigation of the biology of these effects. Previous reports in the literature (discussed in previous paragraphs) suggest that changes in collagen structure (i.e., fibril diameter, crosslink density), proteoglycan content, and water content may be altered as a result of age and/or exercise level, but neither these nor other biological effects (i.e., cell behavior) are quantified in this study. Further examination is necessary to determine whether aging and systemic exercise effects are demonstrated in the context of these biological changes.
Methodology limitations also exist for this study. For example, the hydration method (covering the tendon in PBS-soaked gauze and keeping the gauze saturated by continually dripping PBS) improved gripping of the tissue ends, but prevented the measurement of localized, on-tissue strain as we were unable to capture images of on-tissue markers. While the tendon remained hydrated throughout experiments, this hydration technique may induce more variability than commonly used PBS fluid baths both due to hydration consistency and potentially due to the use of grip-to-grip rather than localized on-tissue strain. Another limitation was the signal-to-noise ratio in the load data, particularly at the lowest strain. In our test system, 0.5% was the lowest strain level at which relaxation rate could be reliably inferred without excess noise. Maximum loads at this strain were ~0.15 N, measured on a 222 N load cell (~0.07% of the maximum load). Because this is near the preload level (0.1 N), it is likely that the strain dependence in the low-strain region is underestimated. Similarly, mechanical tests were conducted with a 1000 lb load cell (with a sensitivity of 0.06 N), which is much larger than loads seen on the tendons. With the exception of the extreme low strains, however, system noise was much lower than the load signal, as demonstrated by clarity in the raw data at strains of 2% and higher in Fig. 2 (and a clearly discernable relaxation pattern at even 1% strain).
In this study, we investigated the effects of age on the elastic and viscoelastic properties of rat tail tendon, as well as the systemic effects of exercise on tendon properties. We demonstrate significant increases in ultimate stress and modulus, and significant decreases in relaxation rate and percent relaxation with maturity. Conversely, we show decreases in viscoelastic parameters with aging. While age-dependent differences in modulus and ultimate stress have been previously reported, few previous studies examined the effects of both maturation and aging on viscoelastic parameters such as relaxation rate and percent relaxation. We also demonstrate a unique strain-dependence behavior in the rat tails (more ligament-like at extremely low strains, tendon-like at moderate–high strains); the strain-dependent relaxation rate is consistent with nonlinear superposition or Schapery models but not with QLV. Though rat tail tendons have been extensively studied in the past, the strain-dependent viscoelastic behavior has been largely neglected. We report no significant differences between exercise groups, but trends in exercise data suggest a weak systemic effect on the elastic properties (which decreased toward values seen in younger rats). Further experimentation is needed to determine which mechanisms are responsible for the changes associated with aging, and which factors are systemically altered with exercise. Additionally, the ability of exercise to rejuvenate older, weight-bearing tendons (both elastic and viscoelastic parameters) remains to be determined. Such information will contribute to our understanding of structure–function relationships, remodeling, mechano-transduction, and damage accumulation in aged tendons.
ACKNOWLEDGMENTS
Support by the National Science Foundation (award 0553016) and National Institutes of Health (awards EB008548 and AG030423) is gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Andreassen TT, Seyer-Hansen K, Bailey AJ. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim. Biophys. Acta. 1981;677(2):313–317. doi: 10.1016/0304-4165(81)90101-x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech. Ageing Dev. 2001;122(7):735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 3.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J. Appl. Physiol. 2008;105(6):1907–1915. doi: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chimich D, Shrive N, Frank C, Marchuk L, Bray R. Water content alters viscoelastic behaviour of the normal adolescent rabbit medial collateral ligament. J. Biomech. 1992;25(8):831–837. doi: 10.1016/0021-9290(92)90223-n. [DOI] [PubMed] [Google Scholar]
- 5.Chung E, Diffee GM. Moderate intensity, but not high intensity, treadmill exercise training alters power output properties in myocardium from aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2012 doi: 10.1093/gerona/gls146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjær M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J. Appl. Physiol. 2009;107(3):880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- 7.Curwin SL, Roy RR, Vailas AC. Regional and age variations in growing tendon. J. Morphol. 1994;221(3):309–320. doi: 10.1002/jmor.1052210306. [DOI] [PubMed] [Google Scholar]
- 8.Danielsen CC, Andreassen TT. Mechanical properties of rat tail tendon in relation to proximal–distal sampling position and age. J. Biomech. 1988;21(3):207–212. doi: 10.1016/0021-9290(88)90171-6. [DOI] [PubMed] [Google Scholar]
- 9.Derwin KA, Soslowsky LJ. A quantitative investigation of structure–function relationships in a tendon fascicle model. J. Biomech. Eng. 1999;121(6):598–604. doi: 10.1115/1.2800859. [DOI] [PubMed] [Google Scholar]
- 10.Derwin KA, Soslowsky LJ, Kimura JH, Plaas AH. Proteoglycans and glycosaminoglycan fine structure in the mouse tail tendon fascicle. J. Orthop. Res. 2001;19(2):269–277. doi: 10.1016/S0736-0266(00)00032-2. [DOI] [PubMed] [Google Scholar]
- 11.Diffee GM, Seversen EA, Titus MM. Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes. J. Appl. Physiol. 2001;91:309–315. doi: 10.1152/jappl.2001.91.1.309. [DOI] [PubMed] [Google Scholar]
- 12.Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J. Orthop. Res. 2002;20(6):1315–1322. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 13.Duenwald SE, Vanderby R, Lakes RS. Viscoelastic relaxation and recovery of tendon. Ann. Biomed. Eng. 2009;37(6):1131–1140. doi: 10.1007/s10439-009-9687-0. [DOI] [PubMed] [Google Scholar]
- 14.Duenwald SE, Vanderby R, Lakes RS. Stress relaxation and recovery in tendon and ligament: experiment and modeling. Biorheology. 2010;47(1):1–14. doi: 10.3233/BIR-2010-0559. [DOI] [PubMed] [Google Scholar]
- 15.Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, Soslowsky LJ. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann. Biomed. Eng. 2003;31(5):599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- 16.Fitzsimons DP, Diffee GM, Herrick RE, Baldwin KM. Effects of endurance exercise on isomyosin patterns in fast- and slow-twitch skeletal muscles. J. Appl. Physiol. 1990;68:1950–1955. doi: 10.1152/jappl.1990.68.5.1950. [DOI] [PubMed] [Google Scholar]
- 17.Flahiff CM, Brooks AT, Hollis MJ, Vander Shilden LJ, Nicholas RW. Biomechanical analysis of patellar tendon allografts as a function of donor age. Am. J. Sports Med. 1995;23(3):354–358. doi: 10.1177/036354659502300319. [DOI] [PubMed] [Google Scholar]
- 18.Galeski A, Kastelic J, Baer E, Kohn RR. Mechanical and structural changes in rat tail tendon induced by alloxan diabetes and aging. J. Biomech. 1977;10:775–782. doi: 10.1016/0021-9290(77)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Goh KL, Holmes DF, Lu HY, Richardson S, Kadler KE, Purslow PP, Wess TJ. Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J. Biomech. Eng. 2008;130:21011–21018. doi: 10.1115/1.2898732. [DOI] [PubMed] [Google Scholar]
- 20.Haut TL, Haut RC. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J. Biomech. 1997;30(1):79–81. doi: 10.1016/s0021-9290(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 21.Haut RC, Lancaster RL, DeCamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J. Biomech. 1992;25(2):163–173. doi: 10.1016/0021-9290(92)90273-4. [DOI] [PubMed] [Google Scholar]
- 22.Hingorani RV, Provenzano PP, Lakes RS, Escarcega A, Vanderby R., Jr Nonlinear viscoelasticity in rabbit medial collateral ligament. Ann. Biomed. Eng. 2004;32(2):306–312. doi: 10.1023/b:abme.0000012751.31686.70. [DOI] [PubMed] [Google Scholar]
- 23.Huang T-F, Perry SM, Soslowsky LJ. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann. Biomed. Eng. 2004;32(3):336–341. doi: 10.1023/b:abme.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard RP, Soutas-Little RW. Mechanical properties of human tendon and their age dependence. J. Biomech. Eng. 1984;106:144–150. doi: 10.1115/1.3138471. [DOI] [PubMed] [Google Scholar]
- 25.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J. Orthop. Res. 1994;12(6):796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- 26.Lam TC, Frank CB, Shrive NG. Changes in the cyclic and static relaxations of the rabbit medial collateral ligament complex during maturation. J. Biomech. 1993;26:9–17. doi: 10.1016/0021-9290(93)90609-i. [DOI] [PubMed] [Google Scholar]
- 27.Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J. Biomech. 2005;38(1):69–75. doi: 10.1016/j.jbiomech.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Legerlotz K, Schjerling P, Langberg H, Brügge-mann GP, Niehoff A. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J. Appl. Physiol. 2007;102(2):564–572. doi: 10.1152/japplphysiol.00767.2006. [DOI] [PubMed] [Google Scholar]
- 29.Millesi H, Reihsner R, Hamilton G, Mallinger R, Menzel E. Biomechanical properties of normal tendons, normal palmar aponeuroses, and tissues from patients with Dupuytren’s disease subjected to elastase and chondro-itinase treatment. Clin. Biomech. 1995;10(1):29–35. doi: 10.1016/0268-0033(95)90434-b. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen HM, Skalicky M, Viidik A. Influence of physical exercise on aging rats. III. Life-long exercise modifies the aging changes of the mechanical properties of limb muscle tendons. Mech. Ageing Dev. 1998;100(3):243–260. doi: 10.1016/s0047-6374(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 31.Onambele GL, Narici MV, Maganaris CN. Calf muscle–tendon properties and postural balance in old age. J. Appl. Physiol. 2006;100(6):2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- 32.Provenzano P, Lakes R, Keenan T, Vanderby R., Jr Nonlinear ligament viscoelasticity. Ann. Biomed. Eng. 2001;29(10):908–914. doi: 10.1114/1.1408926. [DOI] [PubMed] [Google Scholar]
- 33.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J. Physiol. 2003;548(3):971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J. Biomech. Eng. 2004;126(2):252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- 35.Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J. Appl. Physiol. 1990;68:1033–1040. doi: 10.1152/jappl.1990.68.3.1033. [DOI] [PubMed] [Google Scholar]
- 36.Svensson RB, Hassenkam T, Grant CA, Magnusson SP. Tensile properties of human collagen fibrils and fascicles are insensitive to environmental salts. Biophys. J. 2010;99(12):4020–4027. doi: 10.1016/j.bpj.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viidik A, Nielsen HM, Skalicky M. Life-long exercise delays aging of tail tendon collagen. Mech. Ageing Dev. 1996;88(3):139–148. doi: 10.1016/0047-6374(96)01729-0. [DOI] [PubMed] [Google Scholar]
- 38.Vogel H. Age dependence of mechanical properties of rat tail tendons (hysteresis experiments) Aktuelle Gerontol. 1983;13(1):22–27. [PubMed] [Google Scholar]