Abstract
Tomato fruit shape varies significantly in the cultivated germplasm. To a large extent, this variation can be explained by four genes including OVATE. While most varieties with the OVATE mutation bear elongated fruits, some accessions carry round fruit, suggesting the existence of suppressors of OVATE in the germplasm. We developed three intraspecific F2 populations with parents that carried the OVATE mutation but differed in fruit shape. We used a bulk segregant analysis approach and genotyped the extreme classes using a high-throughput genotyping platform, the SolCAP Infinium Assay. The analyses revealed segregation at two quantitative trait loci (QTLs), sov1 and sov2. These loci were confirmed by genotyping and QTL analyses of the entire population. More precise location of those loci using progeny testing confirmed that sov1 on chromosome 10 controlled obovoid and elongated shape, whereas sov2 on chromosome 11 controlled mainly elongated fruit shape. Both loci were located in intervals of <2.4 Mb on their respective chromosomes.
Keywords: BSA, fruit morphology, SNP array, Solanum lycopersicum , pear shape, progeny test
Introduction
As a result of domestication and extensive selection for fruit characters, morphological variation in fruit shape and size is abundant in cultivated tomato (Paran and van der Knaap, 2007; Rodríguez et al., 2011). Unravelling the underlying molecular basis of this variation will lead to insights into the developmental regulation of fruit shape and size while also yielding insights into the domestication of this important crop. In particular, genes that control fruit elongation may underlie critical components of the proximal distal patterning process. Moreover, the relevance of the shape of vegetables is that it characterizes the culinary use and general purpose of the produce. For example, the elongated and blocky tomatoes are preferred in the processing industry because these types of fruit stay on conveyer belts during mechanical harvest and are a better fit in cans compared with round tomatoes. Large and flat tomatoes are preferred for slicing onto sandwiches and hamburgers.
Despite the tremendous diversity in tomato fruit morphology, the diversity is explained to a large extent by mutations in just four genes: SUN, OVATE, LC and/or FAS genes (Rodríguez et al., 2011). SUN (Xiao et al., 2008) and OVATE (Liu et al., 2002) control fruit elongation and encode a member of the IQ Domain and Ovate Family Protein (OFP) families, respectively. FASCIATED (FAS) (Cong et al., 2008) and LOCULE NUMBER (LC) (Muños et al., 2011) control locule number and flat fruit shape, and encode a member of the YABBY and WOX family, respectively (Mayer et al., 1998; Siegfried et al., 1999; Bowman, 2000; Goldshmidt et al., 2008; van der Graaff et al., 2009). OVATE was the first fruit shape gene to be identified by positional cloning (Ku et al., 1999; Liu et al., 2002). The changes in fruit shape as a result of the mutation in OVATE occur well before flower opening, as ovary shape is already clearly different from wild-type at anthesis (van der Knaap and Tanksley, 2001; Liu et al., 2002). OVATE encodes a member of the OFP family and its members are characterized to function as transcriptional repressors, including the expression of GA20 oxidase 1 (Liu et al., 2002; Hackbusch et al., 2005; Wang et al., 2007, 2011). However, details of the role of OFP members in diverse processes in plant growth and development are unclear as loss of function mutations in the Arabidopsis OFP genes have no or very subtle phenotypes (Wang et al., 2010; Li et al., 2011). By contrast, a loss-of-function mutation in OVATE in tomato is the basis for an elongated fruit shape (Liu et al., 2002). Transient downregulation of an OVATE-like gene (CaOvate) in pepper also showed increased fruit elongation (Tsaballa et al., 2011). Therefore, OVATE and OVATE-like genes have loss-of-function phenotypes in some vegetables and are likely to control fruit elongation in other plants.
Depending on the genetic background, the effect of the OVATE mutation is variable, suggesting that the gene interacts with others to exert its effect on shape (Gonzalo and van der Knaap, 2008). Also, while most accessions with the OVATE mutation carry fruit that is elongated, a few in our collection, namely Gold Ball Livingston (GBL) and T1693, carry round fruit, which suppresses the effects of the OVATE mutation (Rodríguez et al., 2011). The identification of genes and loci that suppress the OVATE mutation has been a challenge because of the low level of nucleotide polymorphisms within the cultivated germplasm pool. Populations derived from interspecific crosses (for example, wild relative × cultivated tomato) are typically used since they have a sufficient number of polymorphic markers (Grandillo et al., 1999; Frary et al., 2004; Gonzalo and van der Knaap, 2008). In these wide crosses, the effect of the major loci is often clearly detected, whereas minor loci may not be reproducible from experiment to experiment. Using interspecific populations that included the mapping of ovate, additional minor shape quantitative trait loci (QTLs) on chromosomes 1, 2, 8, 9, 10, 11 and 12 were identified, although none of these were evaluated in follow-up studies (Ku et al., 1999; Gonzalo and van der Knaap, 2008). More efficient detection of minor QTL may be possible when the major QTL, such as SUN or OVATE, are not segregating in the populations and when the parents are more genetically similar to one another. In parents that are closely related, a minor QTL may become a major QTL when that QTL is one of the very few loci segregating for the trait. Therefore, populations derived from more closely related parents where major QTLs are fixed are useful to detect new or previously reported minor QTLs behaving as major loci. As has been demonstrated in rice (Abe et al., 2012), crosses between genetically similar genotypes allowed the unequivocal segregation of markers closely linked to the trait and eventually the cloning of the underlying gene. Thus, to discover the complete set of genes that underlies fruit shape variation in cultivated tomato, phenotypic and molecular genetic analysis of intraspecific populations will be required.
Recently, using transcriptome data from six tomato accessions that included cultivated and wild genotypes, 62 576 non-redundant single-nucleotide polymorphisms (SNPs) were identified (Hamilton et al., 2012), permitting molecular genetic analysis of intraspecific tomato populations. From this set of SNPs, a genotyping assay was developed using the Illumina Infinium platform (Illumina, San Diego, CA, USA) containing 7720 high-quality SNPs for tomato (hereafter called SolCAP Illumina Infinium Assay; data available at http://solcap.msu.edu/). The markers on the SolCAP Illumina Infinium Assay showed that their genetic and physical position on the maps were mostly consistent with published maps and high recombination rates in the euchromatin portions of the genome (Sim et al., 2012a). The SolCAP Illumina Infinium Assay was also used to genotype accessions that represented seven subpopulations, including processing, large-fruited fresh market, large-fruited vintage, cultivated cherry, landrace, wild cherry and S. pimpinellifolium. The grouping revealed a pattern of genetic variation owing to breeding history (Sim et al., 2012b). Thus, this technology is a highly valuable tool for high-throughput, cost-effective genotyping and mapping in tomato intraspecific populations.
The aims of this research were to map suppressors of OVATE (sov) in F2 populations generated from crosses derived from cultivated varieties that carry the mutation but display a range of fruit shape from long and obovoid to round. The identification of the genes that suppress the OVATE mutation would lead to significant insights into how fruit shape is controlled by this gene, and in general the role of OFP in plant growth and development. To identify candidate regions, bulked segregant analysis (BSA) as well as genotyping of the extreme classes was conducted using the SolCAP Illumina Infinium Assay. After initial mapping experiments and progeny testing of the critical recombinants, the results demonstrate the presence of two loci sov1 and sov2 that suppress the OVATE mutation. Therefore, the results confirm the existence of modifier loci for OVATE and that minor QTLs have a large effect in populations derived from crosses between closely related parents.
Materials and methods
Plant materials and population development
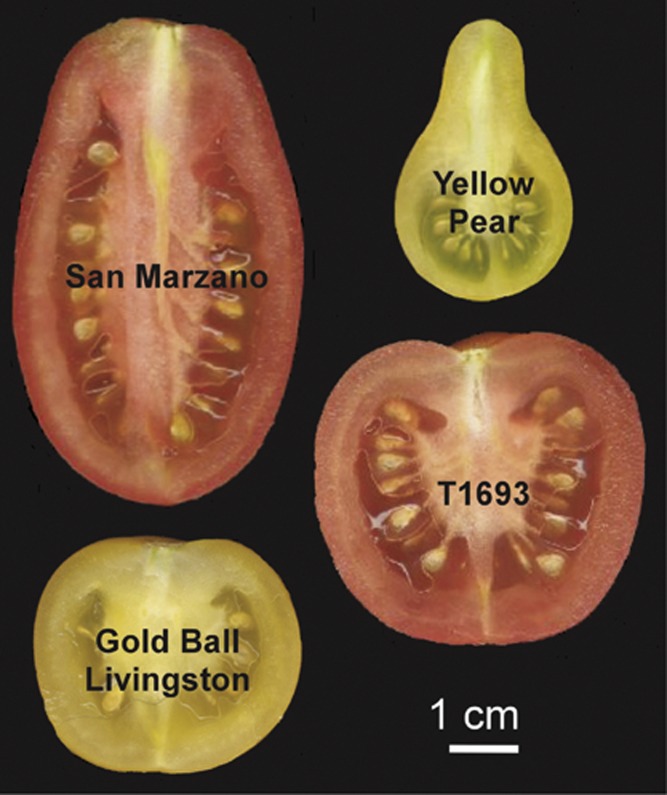
Three F2 populations were generated from crosses between S. lycopersicum varieties carrying OVATE mutation and displaying diverse fruit shape. The parental genotypes were the round fruited GBL, the obovoid variety Yellow Pear (YP), the rectangular variety San Marzano (SM) and the round Italian accession T1693 (Figure 1). The T1693 × YP F2 population (N=97) (09S95), the GBL × YP F2 population (N=100) (09S96) and the SM × GBL F2 population (N=99) (09S97), and five plants of each parental genotype as well as five plants of each F1 cross were grown in summer 2009 in the field in Wooster, OH, USA. The seed of each individual plant was saved for further experiments.
Figure 1.
Fruit images of parental cvs. of S. lycopersicum that carry the OVATE mutation and display different fruit morphology. Size bar represents 1 cm.
Evaluation of the phenotype of fruit shape in the F2 populations and F2–F3 families
Eight fruits from each plant were longitudinally cut through the center, placed cut side-down on a scanner and scanned at 300 dots per inch (dpi). The fruit images were saved as jpeg files and imported into Tomato Analyzer 3.0 for automated phenotypic measurements. The software is available at http://www.oardc.ohio-state.edu/vanderknaap/. The analyzed attributes were fruit shape index I (defined as the ratio between the maximum height and maximum width), obovoid (degree of pear shape) and the width at the widest position (position along a vertical axis of the widest width (Brewer et al., 2006). For obovoid attribute estimation, the Tomato Analyzer 3.0 calculates it as follows: if the area of the fruit is greater below mid-height than above it, then obovoid is calculated from the maximum width (W), the height at which the maximum width occurs (y), the average width above that height (w1) and the average width below that height (w2), and a scaling function scale_ob as: obovoid=1/2 × scale_ob (y) × (1−w1/W+w2/W). If obovoid>0, subtract 0.4. Otherwise, obovoid is 0. Also, from each plant 20 fruits were harvested for fruit mass evaluation. For the phenotypic analysis, mean values and standard deviations were estimated in the parental lines, the F1 and the F2 generations, for every attribute in each cross. Frequency histograms were obtained for every fruit morphological attribute in each F2 generation.
Genetic analysis and heritability estimates
The broad sense heritability (H2) of each attribute was calculated by components of variance from parental, F1 and F2 generations according to Kearsey and Pooni (1996). The variance of the F2 (VF2) represented both genetic (VG) and environmental (VE) variances. VE was estimated as 1/3 (VP1+VP2+VF1), VG was calculated as VF2−VE and H2 as VG/VF2. In each F2−F3 family, association between each marker and fruit morphological attribute was analyzed by t-test.
DNA extraction, sample preparation for the SolCAP Illumina Infinium Assay and pooling using the extreme phenotypes
Total genomic DNA was isolated from young leaves in all genotypes (parents, F1 and each individual F2 plant) as described by Bernatzky and Tanksley (1986) and Fulton et al. (1995). We selected the 30 individuals showing the most extreme values for fruit shape index based on the phenotypic distribution in each F2 population (that is, the 15 roundest individuals and the 15 most elongated individuals). The DNA of these 90 genotypes as well as the parental genotypes was quantified using the Quanti-ITPicoGreen dsDNA Assay Kit (Life Technology Corp., Grand Island, NY, USA). Each sample was diluted to achieve a final concentration of 50 ng μl and the DNA pool was made with 5 μl of each sample. The DNA extraction for the progeny selection of recombinant plants was carried out by the Geno/Grinder method as described in Zhang et al. (2012).
Genotyping using the SolCAP Illumina Infinium Assay
We set up a BSA approach in the three F2 populations (Michelmore et al., 1991). Two bulks per population were evaluated using the SolCAP Illumina Infinium Assay (Sim et al., 2012a). To validate the results of the BSA, 30 progeny on the extreme end exhibiting very round (15) or elongated (15) shapes from the GBL × YP F2 were individually genotyped using the Infinium Assay. Genotyping with the Infinium Assay was performed according to the manufacturer's instructions at the Research Technology Support Center at Michigan State, University (MI, East Lansing, USA).
SolCAP Illumina Infinium Assay data analysis
Three files were generated with the SolCAP Illumina Infinium Assay: (1) the sample sheet, which contains the samples ID information; (2) the data repository, which is a folder that contains the intensity (*.idat) files; and (3) the manifest file, which contains the description of the SNPs in the array. All files were imported into Genome Studio Genotyping Module Illumina Software (Illumina) for downstream analysis. To obtain higher overall call rates of the genotypes, we used the SolCAP_ClusterFile_v1.egt file developed by the SOLCAP Project to adjust for call rates of the different alleles of the SNPs (Sim et al., 2012a). After SNP quality adjustment, the genotyping results were exported in different formats through the Final Report Wizard tool for downstream analysis. We generated genotype data in three formats: AB matrix format, forward strand matrix format and the standard format option, in which data were obtained as θ and R values for each allele and each SNP. The missing data were removed from the genotype data files and logical functions in Excel were used to detect monomorphic and polymorphic SNPs among the parental lines of the crosses.
The Genome Studio Genotyping Module displays the genotypes in a genoplot with data points that are colour-coded for the call (red=homozygous AA; purple=heterozygous AB; and blue=homozygous BB). For each sample (point in the graph), the genotypes are called by their signal intensity (norm R value) and the allele frequency (norm θ) relative to canonical cluster position for a given SNP marker. Thus, the θ values of each created bulk (round vs elongated) were used to estimate difference in the genetic composition of each bulk for each SNP and correlated with the phenotype.
Statistical analysis of the Infinium Assay and identification of suppressors of OVATE loci (sov)
Using one-way analysis of variance (ANOVA), the scores of each polymorphic marker were evaluated for the significance of an allele belonging to the round or elongated bulks. The most significant markers in the fruit shape bulks were called sov-QTL (suppressor of OVATE QTL). The results from the bulks were confirmed using the genotype data obtained from individual plants at the extreme ends of the fruit shape index.
SNP marker development
To confirm the presence of these QTLs identified using the SolCAP Illumina Infinium Assay, dCAPS markers were developed from SNPs that appeared associated with fruit shape (Neff et al., 1998). The flanking sequence of each SNP is available in ‘Tomato Infinium SNP Annotation' at the Sol CAP website (http://solcap.msu.edu/tomato_genotype_data.shtml). This sequence was also used to confirm the physical location in the tomato genome SL2.40 (http://solgenomics.net/tools/blast/index.pl. The amplification was conducted using standard polymerase chain reaction protocols and the products were separated on 3% agarose gels.
Linkage map construction
Linkage analysis of markers on chromosomes 10 and 11 were performed using the software package MAPMAKER V.3.0b (Lander et al., 1987) at a logarithm of odds value of 3.5 and 30 cM of maximum distance. The effects of each sov-QTL on all shape and size traits were performed by both a single-point one-way ANOVA and by interval analysis using the QGene software version 4.3.10 (Joehanes and Nelson, 2008). A probability of P<0.001 was used to declare that a sov-QTL was segregating in the F2 population. The degree of dominance of the alleles at a specific locus was estimated through D/A ratio, where D=Aa−(AA+aa)/2 and A=AA−(AA−aa)/2, where AA is the phenotypic value for homozygous parent 1, aa is the phenotypic value for homozygous parent 2 and Aa the phenotypic value for the heterozygote. Two-way ANOVAs were used to detect epistatic interactions between the sov-QTLs located on the two chromosomes.
Fine mapping of sov1 and sov2
To determine more precisely the interval for each sov, F3 progeny tests were conducted from F2 plants that showed a recombination in the target region. Ten F2–F3 families were selected from the 09S95 population; 12 from the 09S96 population; and 4 from the 09S97 population. For progeny test, a selfed recombinant F2 plant for the target region (sov) segregates at the heterozygous locus by producing 1:2:1 ratio (1 homozygous as parental genotype 1:2 heterozygous:1 homozygous as parental genotype 2). From each F3 family, approximately six homozygous recombinants and six non-recombinants were identified by molecular marker-assisted selection and transplanted in the field.
Results
Fruit shape variation in the segregating populations
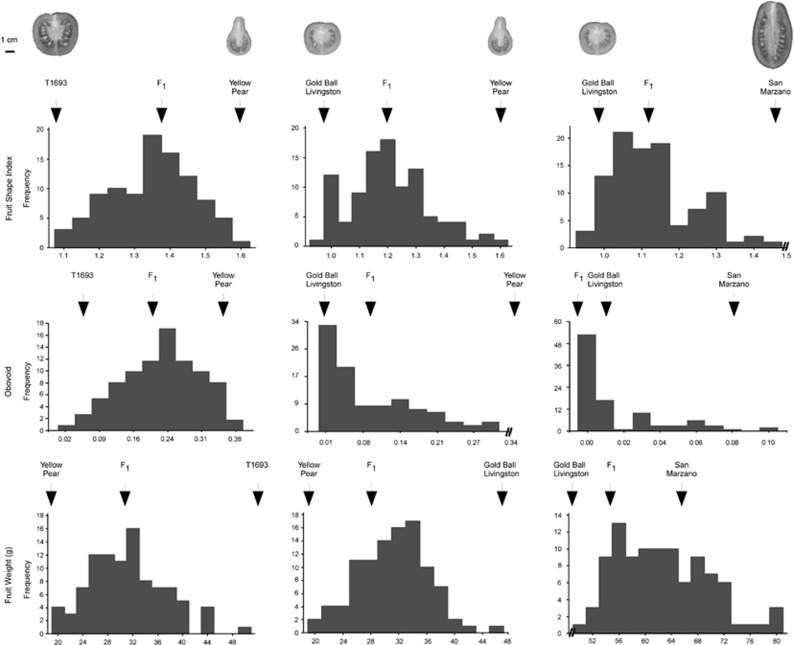
Depending on the genetic background, the OVATE mutation either results in an elongated/rectangular fruit (SM); an obovoid fruit (YP); or round fruit (GBL and T1693 varieties) (Figure 1). The F2 populations derived from the crosses between these accessions showed that fruit shape index, obovoid, width at widest position and fruit weight displayed continuous variation that is typical of a quantitative trait (Figure 2). The phenotypic variation showed a strong genetic component for most of the shape attributes, indicating that the trait was controlled by genes and less by the environment (Table 1). The highest heritability value (H2) for fruit shape index was found in the GBL × YP F2 population (78%). For fruit weight, H2 was high (0.69) in the T1693 × YP F2 population, whereas the genetic component for this trait was moderate to unnoticeable in the other F2 populations (Table 1).
Figure 2.
Phenotype distribution for fruit shape index, obovoid and fruit weight in three F2 analyzed populations. The mean values of the parental genotypes and their F1 are indicated by arrows.
Table 1. Mean fruit weight and shape attribute values in the parental genotypes.
| Attributes | GBL | SM | T1693 | YP | T1693 × YP F2 H2 | GBL × YP F2 H2 | SM × GBL F2 H2 |
|---|---|---|---|---|---|---|---|
| Fruit shape index.1 | 0.93±0.05 | 1.78±0.09 | 1.01±0.04 | 1.61±0.10 | 0.57 | 0.78 | 0.57 |
| WWP | 0.46±0.03 | 0.51±0.04 | 0.47±0.02 | 0.66±0.02 | 0.84 | 0.75 | 0.00 |
| Obovoid | 0.01±0.02 | 0.08±0.04 | 0.05±0.04 | 0.36±0.04 | 0.83 | 0.75 | 0.00 |
| Fruit weight | 46.96±6.08 | 65.60±9.15 | 58.2±3.17 | 14.36±1.52 | 0.69 | 0.29 | 0.00 |
Abbreviations: GBL, Gold Ball Livingston; SM, San Marzano; WWP, width at the widest position; YP, Yellow Pear.
Values are given as the mean (±s.d.). Broad sense heritability values (H2) for the attributes in each F2 population are given.
BSA to identify the sov loci
The genotyping of the parents demonstrated that many SNPs, 558–691, distinguish the varieties from one another (Table 2). The SNPs were distributed evenly across the genome, although some chromosomes were covered with more markers than others (Table 2). The high level of genome-wide nucleotide polymorphisms found in the parents made it feasible to genetically map the suppressors of OVATE loci onto the tomato genome. The DNA from the 15 most round and 15 most elongated genotypes in each F2 was pooled to generate two separated bulks for each population. After genotyping using the Infinium Assay, we applied quantitative analyses to evaluate the allele frequencies in the bulks by using the θ value for each SNP and estimated the absolute difference between bulks (round vs elongated) in each population. The 20 SNPs with the highest absolute differences in θ values showed that the T1693 × YP and the GBL × YP F2 populations carry two putative sov QTLs on chromosomes 10 and 11 (Supplementary Table S1). The most significantly different θ values in the SM × GBL population were found only for markers located on chromosome 11.
Table 2. Number of polymorphic SNPs among the parental lines of the analyzed crosses discriminated by chromosome.
| Chromosome | T1693 vs YP | GBL vs YP | SM vs GBL |
|---|---|---|---|
| 1 | 56 | 84 | 85 |
| 2 | 57 | 38 | 36 |
| 3 | 172 | 80 | 109 |
| 4 | 67 | 53 | 24 |
| 5 | 32 | 20 | 22 |
| 6 | 42 | 44 | 28 |
| 7 | 21 | 34 | 35 |
| 8 | 46 | 61 | 44 |
| 9 | 68 | 60 | 37 |
| 10 | 34 | 17 | 35 |
| 11 | 46 | 45 | 40 |
| 12 | 47 | 83 | 59 |
| NN | 3 | 7 | 4 |
| Total | 691 | 626 | 558 |
Abbreviations: GBL, Gold Ball Livingston; NN, unknown; SM, San Marzano; SNP, single-nucleotide polymorphism; YP, Yellow Pear.
To confirm the putative QTL on chromosomes 10 and 11, we genotyped 30 plants individually with the Infinium Assay that belonged to the most round or the most elongated category in the GBL × YP F2 population. Again, several markers that were clustered on chromosome 11 (sov2) and one locus on chromosome 10 (sov1) were significantly associated with fruit shape index (P<0.0001), thus confirming the validity of the findings from the bulked DNA analysis (Supplementary Table S2). While additional putative sov loci were found on chromosomes 2, 3, 8 and 12, further analysis did not reveal convincing associations with fruit shape (P>0.01) (Supplementary Table S3). Therefore, these potential minor loci were not pursued further.
Defining the interval of sov1 and sov2
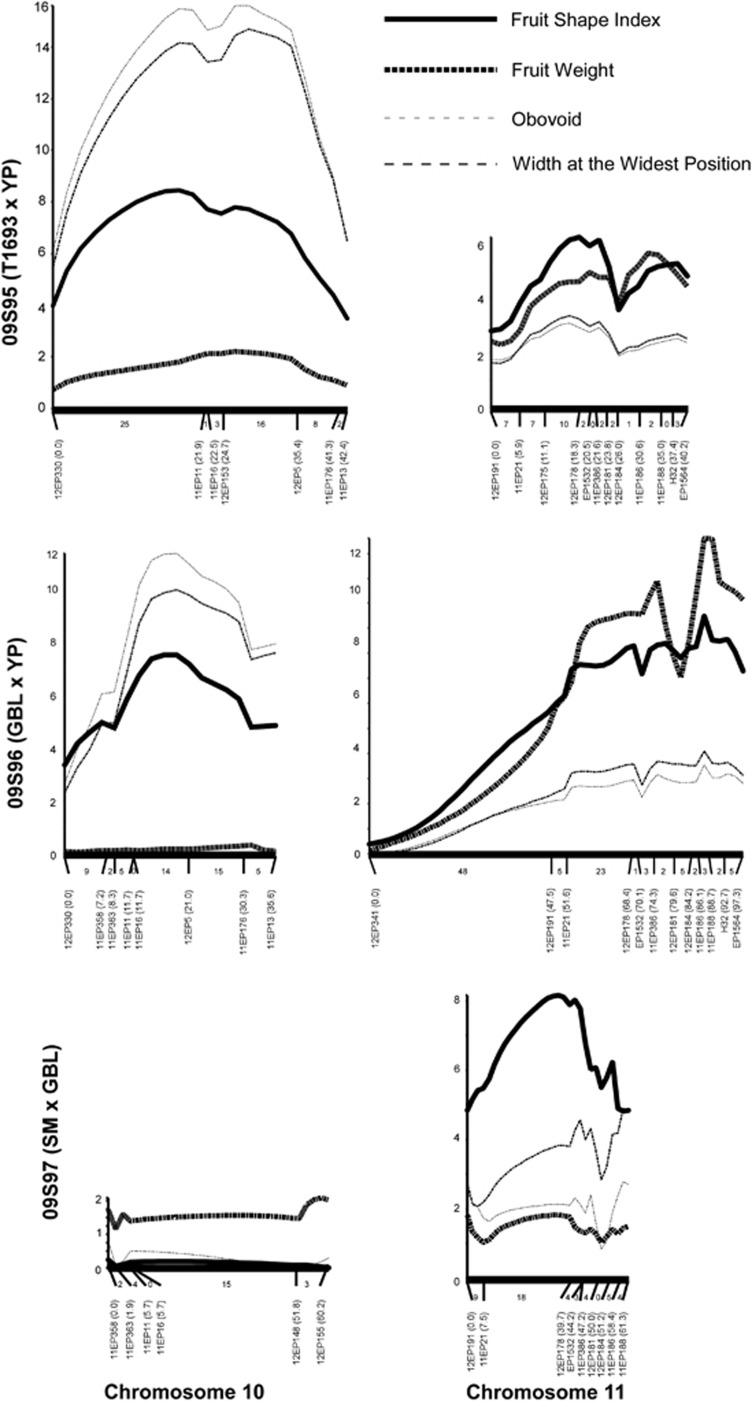
The SNPs associated with fruit shape loci were converted to dCAPS markers and genotyped in all the individuals from the three F2 populations (Supplementary Table S4). The markers showed normal Mendelian segregation indicating a lack of segregation distortion for the markers that were tested. With the dCAPS markers on chromosomes 10 and 11, a QTL map was constructed (Figure 3). For sov1, the QTL maps showed a clear effect for obovoid and width at the widest position overlapping with the QTL for fruit shape index in two of the three F2 populations (Figure 3). This result indicated that a major effect of sov1 is on the degree of pear shape originating from the YP allele. On the other hand, the major effect of sov2 on chromosome 11 is on fruit elongation, as the QTL for width at the widest position is less significant than the QTL for fruit shape index. Also, sov2 segregated in all three populations.
Figure 3.
Interval mapping analysis on the tomato chromosomes 10 and 11. On the X axis are the markers with the genetic distances within the paranthesis; the Y axis indicates the logarithm of odds score. The logarithm of odds curve was derived from F2 analysis. The number between markers indicates the number of recombinants in the interval.
Sov1 was highly associated with the markers 11EP11 and 11EP16 explaining approximately 30% of the fruit shape variation in two F2 populations (Table 3). The locus was barely associated with fruit weight variation (P>0.01). The most significant markers associated with sov2 were 12EP178 and 11EP186 and the percent phenotypic variation explained ranged from 26 to 37%. Also, this locus explained 20 and 50% of the variation in fruit weight in two of the three populations, respectively (Table 3).
Table 3. Most significant markers associated with fruit shape index in each F2 population and their effect on other fruit morphological attributes.
| Population | Marker | Ch. | Attribute | P-value | % R2 | F value | YP | N | Het | N | T1693 | N | D/A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F2 T1693 × YP | 11EP11 | 10 | Fruit shape index.1 | <0.0001 | 32 | 20.84 | 1.42 | 27 | 1.34 | 46 | 1.24 | 20 | 0.11 |
| Obovoid | <0.0001 | 51 | 47.26 | 0.30 | 0.21 | 0.14 | −0.13 | ||||||
| Width widest position | <0.0001 | 48 | 41.70 | 0.62 | 0.57 | 0.53 | −0.11 | ||||||
| Fruit weight | 0.0087 | 10 | 5.01 | 29.09 | 30.77 | 34.42 | 0.37 | ||||||
| 12EP178 | 11 | Fruit shape index.1 | <0.0001 | 26 | 15.84 | 1.42 | 20 | 1.36 | 46 | 1.27 | 27 | 0.20 | |
| Width widest position | 0.0010 | 14 | 7.49 | 0.60 | 0.58 | 0.55 | 0.20 | ||||||
| Fruit weight | <0.0001 | 20 | 11.42 | 26.60 | 31.18 | 34.11 | −0.22 | ||||||
| |
|
|
|
|
|
|
GBL
|
|
Het
|
|
YP
|
|
|
| F2 GBL × YP | 11EP16 | 10 | Fruit shape index.1 | <0.0001 | 28 | 17.13 | 1.12 | 21 | 1.20 | 55 | 1.35 | 17 | 0.30 |
| Obovoid | <0.0001 | 38 | 28.07 | 0.03 | 0.08 | 0.17 | 0.29 | ||||||
| Width widest position | <0.0001 | 35 | 23.17 | 0.49 | 0.50 | 0.56 | 0.71 | ||||||
| Fruit weight | 0.6487 | 1 | 0.43 | 31.31 | 31.43 | 30.21 | NC | ||||||
| 12EP186 | 11 | Fruit shape index.1 | <0.0001 | 37 | 25.37 | 1.07 | 23 | 1.24 | 50 | 1.30 | 18 | −0.48 | |
| Width widest position | 0.0001 | 18 | 9.90 | 0.48 | 0.52 | 0.53 | −0.60 | ||||||
| Fruit weight | <0.0001 | 50 | 44.26 | 34.93 | 31.70 | 24.94 | 0.35 | ||||||
| |
|
|
|
|
|
|
SM
|
|
Het
|
|
GBL
|
|
|
| F2 SM × GBL | 12EP178 | 11 | Fruit shape index.1 | <0.0001 | 34 | 22.31 | 1.20 | 24 | 1.13 | 43 | 1.04 | 22 | 0.12 |
| Width widest position | 0.0001 | 19 | 10.14 | 0.48 | 0.47 | 0.46 | 0.00 | ||||||
| Fruit weight | 0.0253 | 8 | 3.84 | 65.14 | 61.20 | 59.95 | −0.52 |
Abbreviations: ANOVA, analysis of variance; Ch, chromosome; D/A, degree of dominance; GBL, mean value for the homozygous Gold Ball Livingston allele; Het, mean value for the heterozygous; N, number of plants per genotypic class; NC, no calculated; % of R2, variance explained by the associated marker; SM, mean value for the homozygous San Marzano allele; T1693, mean value for the homozygous T1693 allele; YP, mean value for the homozygous Yellow Pear allele.
Data were analyzed by a single-point one-way ANOVA.
Epistatic interaction between sov1 and sov2 on tomato fruit shape
The two-way ANOVA in both T1693 × YP F2 and GBL × YP F2 populations demonstrated that sov1 and sov2 act largely independently in the control of tomato fruit shape (P>0.05). In both populations, those individuals carrying the YP alleles for sov1 and sov2 displayed the highest values for fruit shape index (Table 4). In the two populations that segregate for sov1 and sov2, the combined R2 of the loci was 50% and 64%, respectively. The genetic component estimates (H2) of the phenotypic variance for fruit shape index were 57% and 78% in the T1693 × YP and GBL × YP population, respectively (Table 1). These results suggest that it is highly unlikely that another QTL for fruit shape is segregating in the T1693 × YP and the GBL × YP populations. On the other hand, the percent phenotypic variation explained by sov2 in the SM × GBL population (34%) is below what was expected from the H2 estimates (57%). Thus, it is possible that another shape QTL is segregating in that population and was not detected from the BSA because of the possible small effect of the locus on fruit shape.
Table 4. Fruit shape index mean values (±s.d.) for the allele combinations at sov1 and sov2 in two F2 populations.
| sov1 (Ch. 10) | sov2 (Ch. 11) | T1693 × YP F 2 | GBL × YP F 2 |
|---|---|---|---|
| 1 | 1 | 1.19±0.09 a | 1.04±0.08 a |
| 1 | 2 | 1.27±0.08 ab | 1.11±0.08 a |
| 1 | 3 | 1.34±0.15 bc | 1.25±0.11 c |
| 2 | 1 | 1.29±0.08 b | 1.08±0.07 a |
| 2 | 2 | 1.36±0.07 bc | 1.23±0.10 bc |
| 2 | 3 | 1.40±0.09 cd | 1.27±0.07 c |
| 3 | 1 | 1.33±0.09 bc | 1.12±0.00 ab |
| 3 | 2 | 1.42±0.07 cd | 1.35±0.10 c |
| 3 | 3 | 1.47±0.12 d | 1.51±0.08 d |
Abbreviations: Ch, chromosome; GBL, Gold Ball Livingston cultivar; YP, Yellow Pear cultivar; 1, homozygous genotype for the T1693 or GBL alleles; 2, heterozygous; 3, homozygous for the YP alleles.
Different letters indicate significant differences at 5% between mean values according to t-test.
Progeny test from F2 plants with crossovers in the interval containing the sov loci
Progeny tests were performed to confirm the location of sov1 and sov2 and better understand their effect on fruit shape. A total of 26 recombinant plants from the 09S95, 09S96 and 09S97 populations were selected. Recombinant individuals between 11EP16 and 11EP176 on chromosome 10, and between 11EP21 and 11EP186 on chromosome 11 were progeny tested. Twenty recombinants were selected to evaluate the sov1 region: one recombinant located between 11EP16 and 12EP153; four between 12EP153 and 12EP5; nine between 11EP16 and 12EP5 and six between 12EP5 and 11EP176. Fruit shape index and obovoid attributes were statistically significant (P<0.01) between recombinant and non-recombinant progeny for family 12S50, 12S65, 12S66, 12S117, 12S121 and 12S123 (Table 5). Therefore, the results from the progeny test indicated that sov1 was delimited between the marker 12EP153 and 12EP5 in a 10.7 cM interval in 09S95 population. The physical distance between the flanking markers was approximately 1.2 Mb.
Table 5. Progeny test of sov1 (A) and sov2 (B) recombinant plants.
| (A) sov1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Genotype of parent
|
Fruit shape progeny test
|
||||||||||||||||
| Parent | Progeny |
Ch. 10
a
|
|
|
|
|
Ch. 11
b
|
Ave. 1 c | S.d. | Nd | Ave. 3 e | S.d. | N | P-valuef(FSI/obovoid) | |||
| |
|
52 872 133g11EP11 |
57 330 887
11EP16 |
57 997 266
12EP153 |
59 255 104
12EP5 |
60 348 137
11EP176 |
51 606 380
12EP178 |
|
|
|
|
|
|
|
|||
| 09S95-4 | 12S46 | 2 | 2 | 2 | 3 | 3 | 1 | 1.26 | 0.09 | 6 | 1.41 | 0.08 | 4 | */*** | |||
| 09S95-29 | 12S50 | 3 | 3 | 2 | 2 | 2 | 3 | 1.35 | 0.10 | 5 | 1.50 | 0.09 | 6 | ***/*** | |||
| 09S95-49 | 12S59 | 1 | 1 | 1 | 2 | 2 | 1 | 1.24 | 0.05 | 6 | 1.23 | 0.08 | 6 | 0.91/0.19 | |||
| 09S95-53 | 12S60h | 2 | 2 | 2 | 2 | 3 | 3 | 1.42 | 0.10 | 5 | 1.58 | 0.13 | 5 | 0.08/* | |||
| 09S95-82 | 12S65 | 2 | 2 | 2 | 1 | 1 | 3 | 1.23 | 0.10 | 6 | 1.42 | 0.10 | 6 | ****/**** | |||
| 09S95-84 | 12S66 | 3 | 3 | 3 | 2 | 2 | 1 | 1.17 | 0.07 | 6 | 1.32 | 0.08 | 6 | ***/*** | |||
| 09S95-87 | 12S88 | 3 | 3 | 3 | 3 | 2 | 3 | 1.49 | 0.09 | 6 | 1.57 | 0.09 | 5 | 0.13/0.92 | |||
| 09S95-93 | 12S89 | 1 | 1 | 1 | 1 | 2 | 1 | 1.10 | 0.07 | 6 | 1.06 | 0.07 | 6 | 0.32/0.73 | |||
| 09S96-9 | 12S91 | 2 | 2 | — | 1 | 1 | 1 | 1.09 | 0.05 | 6 | 1.27 | 0.10 | 6 | **/** | |||
| 09S96-35 | 12S95 | 1 | 1 | — | 2 | 3 | 3 | 1.28 | 0.08 | 6 | 1.54 | 0.09 | 5 | ****/* | |||
| 09S96-39 | 12S96 | 2 | 2 | — | 1 | 1 | 1 | 1.27 | 0.08 | 6 | 1.30 | 0.07 | 6 | 0.53/** | |||
| 09S96-45 | 12S117 | 2 | 2 | — | 2 | 3 | 3 | 1.24 | 0.09 | 6 | 1.43 | 0.08 | 4 | **/** | |||
| 09S96-53 | 12S118 | 1 | 1 | — | 1 | 2 | 1 | 1.10 | 0.05 | 4 | 1.04 | 0.04 | 5 | 0.09/0.36 | |||
| 09S96-62 | 12S119 | 2 | 2 | — | 1 | 1 | 1 | 1.03 | 0.05 | 6 | 1.05 | 0.04 | 2 | 0.13/0.51 | |||
| 09S96-66 | 12S120 | 2 | 2 | — | 3 | 3 | 1 | 1.05 | 0.06 | 6 | 1.24 | 0.07 | 5 | */** | |||
| 09S96-70 | 12S121 | 2 | 2 | — | 2 | 1 | 3 | 1.22 | 0.07 | 6 | 1.43 | 0.09 | 6 | ***/*** | |||
| 09S96-75 | 12S122 | 3 | 3 | — | 2 | 2 | 2 | 1.36 | 0.08 | 6 | 1.49 | 0.08 | 6 | 0.21/0.27 | |||
| 09S96-76 | 12S123 | 1 | 1 | — | 2 | 2 | 3 | 1.19 | 0.06 | 6 | 1.40 | 0.09 | 5 | ***/** | |||
| 09S96-79 | 12S124 | 2 | 2 | — | 1 | 1 | 3 | 1.41 | 0.10 | 5 | 1.62 | 0.13 | 6 | */** | |||
| 09S96-84 | 12S125 | 3 | 3 | — | 2 | 2 | 1 | 1.02 | 0.05 | 2 | 1.19 | 0.05 | 6 | 0.10/ 0.07 | |||
| (B) sov2 | |||||||||||||||||
| |
|
Genotype of parent
|
Fruit shape progeny test | ||||||||||||||
| Parent | Progeny |
Ch11
a
|
|
|
|
|
|
|
|
Ch10
b
|
|
|
|
|
|
|
|
| |
|
48 827 390
11EP21
|
49 625 802
12EP175
|
51 606 380
12EP178
|
51 876 227
EP1532
|
51 937 520
11EP386
|
52 053 483
12EP181 |
52 053 561
12EP184
|
52 126 950
11EP186
|
57 997 266
12EP153
|
Ave. 1
c
|
S.d.
|
Nd |
Ave. 3
e
|
S.d.
|
N |
P-valuef(FSI/obovoid) |
| 09S95-2 | 12S45 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1.22 | 0.07 | 4 | 1.36 | 0.09 | 6 | **/0.47 |
| 09S95-7 | 12S47 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 1.37 | 0.10 | 6 | 1.56 | 0.10 | 4 | */0.03 |
| 09S97-16 | 12S132 | 2 | — | 1 | 1 | 1 | 1 | 1 | 1 | ND | 1.08 | 0.06 | 5 | 1.05 | 0.06 | 5 | 0.44/0.95 |
| 09S97-36 | 12S135 | 2 | — | 1 | 1 | 1 | 1 | 1 | 1 | ND | 1.22 | 0.07 | 5 | 1.23 | 0.08 | 6 | 0.99/0.39 |
| 09S97-47 | 12S136 | 2 | — | 2 | 2 | 2 | 3 | 3 | 3 | ND | 1.22 | 0.09 | 5 | 1.10 | 0.07 | 6 | */0.04 |
| 09S97-59 | 12S137h | 2 | — | 2 | 2 | 2 | 2 | 2 | 1 | ND | 1.18 | 0.10 | 6 | 1.16 | 0.10 | 6 | 0.87/0.07 |
Abbreviations: Ave., average; Ch, chromosome; FSI, fruit shape index; GBL, Gold Ball Livingston cultivar; ND, not determined; YP, Yellow Pear cultivar.
sov1 locus.
sov2 locus.
Average fruit shape index for parental genotype 1 (T1693 in 09S95, GBL in 09S96 and San Marzano in 09S97).
The number of tomato plants.
Average fruit shape index for parental genotype 3 (YP in 09S95 and 09S96 and is GBL in 09S97).
P-value about FSI and obovoid. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Chromosome positions of markers. The unit is bp.
Family showed strange segregation in field test. This family needs re-progeny testing.
For the sov2 region, six recombinants were evaluated: two between 11EP21 and 12EP178, one between 12EP175 and 12EP178, two between 11EP386 and 12EP181 and one between 12EP184 and 11EP186 (Table 5). Significant differences (P<0.05) were found for the attribute fruit shape index in the 12S45, 12S47 and 12S136 families. Family 12S132 and 12S135 did not show segregation for fruit shape index. These findings implied that sov2 was located between 12EP175 and 12EP181, representing 12.7 cM in genetic map distance in family 09S95 and about 2.4 Mb for physical distance. Family 12S137, however, should have segregated for fruit shape, so the position of sov2 is less certain than sov1.
Discussion
Tomato fruit is morphologically diverse. Even though the majority of shape diversity can be explained by just four genes (Rodríguez et al., 2011), those cultivars that carry the OVATE mutation do not exhibit the same shape of the fruit, varying from elongated and pear to round. In this study, we demonstrated the existence of two regions located within ∼2 Mb interval in the tomato genome that suppressed the effect of the OVATE mutation, an important gene underlying fruit morphological diversity in the tomato germplasm (Rodríguez et al., 2011). These loci were mapped in intraspecific populations of genetically closely related parents that carried the same OVATE allele. One locus, sov1, controlled obovoid shape in addition to fruit elongation. The other locus, sov2, controlled mainly fruit elongation. Further fine mapping and cloning of the underlying genes will be an important step forward towards our understanding of the regulation of elongated fruit shape and the role of OFP in plant growth and development.
Ku et al. (1999) found a minor QTL for fruit shape variation on chromosome 10 in a population derived from a cross between YP and the wild relative LA1589. Therefore, sov1 may have been detected previously; however, the logarithm of odds score reported in that study was of low significance (Ku et al., 1999). For sov2, the story is more complex. Several QTLs that control fruit morphology have been mapped in this genomic region, including FAS (Cong et al., 2008; Huang and van der Knaap, 2011). The FAS mutation though was not present in the parents used in the study. Sov2 overlapped with a fruit weight QTL fw11.3 reported by Huang and van der Knaap (2011). However, the fruit weight and shape loci were not allelic since sov2 segregated in all three populations and fruit weight only in two populations. It is possible though that sov2 was the same QTL controlling fruit shape index in Long John cultivar (van der Knaap et al., 2002).
The sov1 and sov2 alleles from GBL and T1693 suppress OVATE, and provide two rare examples of accessions with the OVATE mutation but a round fruit phenotype. On the other hand, the presence of the SM and YP alleles of sov1 and sov2 produce more elongated fruit. Therefore, it is also possible to consider that the sov loci enhance the OVATE mutation. Regardless, the effect of sov loci is to modify OVATE as these loci was not mapped as major QTL in populations segregating for the ovate locus on chromosome 2 (Ku et al., 1999; Gonzalo and van der Knaap, 2008).
Sov1 mapped to a 1.2-Mb interval and sov2 in a 2.4-Mb interval. Only one auxin-related gene was found in the sov1 region, namely an AUX1 gene (solyc10g076790) and not PIN1. For sov2, the region contains several viable candidate genes: a Hira-like protein (Solyc11g067200) known to interact with AS1, an NAC domain transcription factor (Solyc11g068620) functioning to activate KNOX expression and a class III homeodomain leucine zipper (Solyc11g069470) involved in organ polarity (McConnell et al., 2001; Otsuga et al., 2001; Hay and Tsiantis, 2010; Byrne, 2012). In addition, the region contains two auxin response factor genes (Solyc11g069190 and Solyc11g069500) and two BELL transcription factors (Solyc11g068950 and Solyc11g069890). BEL1-like homeodomain proteins have been found to interact with OFP and KNOX, and therefore are also viable candidate genes for sov2 (Hackbusch et al., 2005).
The mapping of the sov loci was greatly facilitated by the genome-wide SNP markers that were developed by the tomato research community. In this study, we demonstrated that the SolCAP Illumina Infinium Assay is also useful in initial mapping experiments that aim to clone genes underlying important QTLs. Traditional molecular markers such as RFLP, AFLP, RAPD, transposon-based markers and SSR (Sim et al., 2012a, 2012b) are sufficient to characterize and distinguish tomato cultivars but they are not sufficient to map QTLs in intraspecific populations (van der Knaap et al., 2013). The number and distribution of the SNP in the genome show that marker coverage is quite robust. Accordingly, the quantitative BSA approach results were efficient at detecting major QTLs controlling size and morphological fruit attributes in intraspecific crosses (Supplementary Table S3). In the future, with the decreasing cost of whole genome sequencing, both major and minor QTLs will be easily detected if genotyping is carried out with a larger number of individuals per population.
In conclusion, we demonstrated that a quantitative BSA approach was highly successful at mapping two QTLs underlying fruit morphology of tomato. Sov1 and sov2 were efficiently mapped using the newly developed SolCAP Infinium Assay. The results of this study will contribute to the identification of the genes underlying these major QTLs and contribute to the understanding of the molecular basis of fruit shape variation in the cultivated germplasm of tomato. Additional experiments with sov1 and sov2 will confirm whether single or more than one gene underlies the phenotype effect of each locus on fruit morphology and provides general insights into the role of OFP in plant growth and development.
Data Archiving
Genotype and Phenotype data deposited in the Dryad repository: doi:10.5061/dryad.st020.
Acknowledgments
This work was supported by National Science Foundation IOS 0922661. We thank Rhiannon Schneider for help with DNA extraction and Jenny Moyseenko for help with the field experiment. We also thank Drs David Francis and Sung-Chur Sim for their advice with the analysis of the results from the SolCAP Infinium Assay.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat Biotechnol. 2012;30:174–178. doi: 10.1038/nbt.2095. [DOI] [PubMed] [Google Scholar]
- Bernatzky R, Tanksley SD. Toward a saturated linkage map in tomato based on isozymes and random cDNA sequences. Genetics. 1986;112:887–898. doi: 10.1093/genetics/112.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL. The YABBY gene family and abaxial cell fate. Curr Opin Plant Biol. 2000;3:17–22. doi: 10.1016/s1369-5266(99)00035-7. [DOI] [PubMed] [Google Scholar]
- Brewer M, Lang L, Fujimura K, Dujmovic N, Gray S, van der Knaap E. Development of a controlled vocabulary and software application to analyze fruit shape variation in tomato and other plant species. Plant Physiol. 2006;141:15–25. doi: 10.1104/pp.106.077867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME. Making leaves. Curr Opin Plant Biol. 2012;15:24–30. doi: 10.1016/j.pbi.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Cong B, Barrero LS, Tanksley SD. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat Genet. 2008;40:800–804. doi: 10.1038/ng.144. [DOI] [PubMed] [Google Scholar]
- Frary A, Fritz LA, Tanksley SD. A comparative study of the genetic bases of natural variation in tomato leaf, sepal, and petal morphology. Theoret Appl Genet. 2004;109:523–533. doi: 10.1007/s00122-004-1669-x. [DOI] [PubMed] [Google Scholar]
- Fulton T, Chunwongse J, Tanksley S. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol Biol. 1995;13:207–209. [Google Scholar]
- Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y. Signals derived from YABBY gene activities in organ primordia regulate growth and partitioning of Arabidopsis shoot apical meristems. Plant Cell. 2008;20:1217–1230. doi: 10.1105/tpc.107.057877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo M, van der Knaap E. A comparative analysis into the genetic bases of morphology in tomato varieties exhibiting elongated fruit shape. Theor Appl Genet. 2008;116:647–656. doi: 10.1007/s00122-007-0698-7. [DOI] [PubMed] [Google Scholar]
- Grandillo S, Ku HM, Tanksley SD. Identifying the loci responsible for natural variation in fruit size and shape in tomato. Theor Appl Genet. 1999;99:978–987. [Google Scholar]
- Hackbusch J, Richter K, Müller J, Salamini F, Uhrig JF. A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proc Natl Acad Sci USA. 2005;102:4908–4912. doi: 10.1073/pnas.0501181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Sim S-C, Stoffel K, Van Deynze A, Buell CR, Francis DM. Single nucleotide polymorphism discovery in cultivated tomato via sequencing by synthesis. Plant Genome. 2012;5:17. [Google Scholar]
- Hay A, Tsiantis M. KNOX genes: versatile regulators of plant development and diversity. Development. 2010;137:3153–3165. doi: 10.1242/dev.030049. [DOI] [PubMed] [Google Scholar]
- Huang Z, van der Knaap E. Tomato fruit weight 11.3 maps close to fasciated on the bottom of chromosome 11. Theor Appl Genet. 2011;123:465–744. doi: 10.1007/s00122-011-1599-3. [DOI] [PubMed] [Google Scholar]
- Joehanes R, Nelson JC. QGene 4.0, an extensible Java QTL-analysis platform. Bioinformatics. 2008;24:2788–2789. doi: 10.1093/bioinformatics/btn523. [DOI] [PubMed] [Google Scholar]
- Kearsey MJ, Pooni HS. Genetical Analysis of Quantitative Traits. Garland Science: UK; 1996. [Google Scholar]
- Ku H, Doganlar S, Chen K, Tanksley S. The genetic basis of pear-shaped tomato fruit. Theor Appl Genet. 1999;9:844–850. [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, et al. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Li E, Wang S, Liu Y, Chen J-G, Douglas CJ. OVATE FAMILY PROTEIN4 (OFP4) interaction with KNAT7 regulates secondary cell wall formation in Arabidopsis thaliana. Plant J. 2011;67:328–341. doi: 10.1111/j.1365-313X.2011.04595.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Van Eck J, Cong B, Tanksley SD. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc Natl Acad Sci USA. 2002;99:13302–13306. doi: 10.1073/pnas.162485999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- Michelmore RW, Paran I, Kesseli RV. Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA. 1991;88:9828–9832. doi: 10.1073/pnas.88.21.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muños S, Ranc N, Botton E, Bérard A, Rolland S, Duffé P, et al. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiol. 2011;156:2244–2254. doi: 10.1104/pp.111.173997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Neff JD, Chory J, Pepper AE. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J. 1998;14:387–392. doi: 10.1046/j.1365-313x.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001;25:223–236. doi: 10.1046/j.1365-313x.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- Paran I, van der Knaap E. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. J Exp Bot. 2007;58:3841–3852. doi: 10.1093/jxb/erm257. [DOI] [PubMed] [Google Scholar]
- Rodríguez GR, Muños S, Anderson C, Sim S-C, Michel A, Causse M, et al. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011;156:275–285. doi: 10.1104/pp.110.167577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;126:4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- Sim S-C, Durstewitz G, Plieske J, Wieseke R, Ganal MW, Van Deynze A, et al. Development of a large SNP genotyping array and generation of high-density genetic maps in tomato (T Yin, ed.) PLoS One. 2012a;7:e40563. doi: 10.1371/journal.pone.0040563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S-C, Van Deynze A, Stoffel K, Douches DS, Zarka D, Ganal MW, et al. High-density SNP genotyping of tomato (Solanum lycopersicum L.) reveals patterns of genetic variation due to breeding. PLoS One. 2012b;7:e45520. doi: 10.1371/journal.pone.0045520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaballa A, Pasentsis K, Darzentas N, Tsaftaris AS. Multiple evidence for the role of an ovate-like gene in determining fruit shape in pepper. BMC Plant Biol. 2011;11:46. doi: 10.1186/1471-2229-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff E, Laux T, Rensing SA. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009;10:248. doi: 10.1186/gb-2009-10-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Anderson C, Rodríguez G.2013Diversity within cultivated tomatoIn: Liedl BE, Labate JA, Stommel JR, Slade A, Kole C (eds.)Genetics, Genomics, and Breeding of Tomato Science Publishers,520p [Google Scholar]
- van der Knaap E, Lippman ZB, Tanksley SD. Extremely elongated tomato fruit controlled by four quantitative trait loci with epistatic interactions. Theor Appl Genet. 2002;104:241–247. doi: 10.1007/s00122-001-0776-1. [DOI] [PubMed] [Google Scholar]
- van der Knaap E, Tanksley S. Identification and characterization of a novel locus controlling early fruit development in tomato. Theor Appl Genet. 2001;103:353–358. [Google Scholar]
- Wang S, Chang Y, Guo J, Chen J-G. Arabidopsis ovate family protein 1 is a transcriptional repressor that suppresses cell elongation. Plant J. 2007;50:858–872. doi: 10.1111/j.1365-313X.2007.03096.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Chang Y, Guo J, Zeng Q, Ellis BE, Chen J-G. Arabidopsis ovate family proteins, a novel transcriptional repressor family, control multiple aspects of plant growth and development. PLoS One. 2011;6:e23896. doi: 10.1371/journal.pone.0023896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-K, Chang W-C, Liu P-F, Hsiao M-K, Lin C-T, Lin S-M, et al. Ovate family protein 1 as a plant Ku70 interacting protein involving in DNA double-strand break repair. Plant Mol Biol. 2010;74:453–466. doi: 10.1007/s11103-010-9685-5. [DOI] [PubMed] [Google Scholar]
- Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science. 2008;319:1527–1530. doi: 10.1126/science.1153040. [DOI] [PubMed] [Google Scholar]
- Zhang N, Brewer MT, van der Knaap E. Fine mapping of fw3.2 controlling fruit weight in tomato. Theor Appl Genet. 2012;125:273–284. doi: 10.1007/s00122-012-1832-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.