Abstract
Purpose
To evaluate the use of global indices summarizing the current status of a patient’s visual field as predictors of their future rate of change.
Methods
Ninety-five subjects with early or suspected glaucoma were studied, of whom 50 exhibited glaucomatous optic neuropathy (GON) at baseline. Subjects underwent seven annual visual field tests. Results from the first test in the sequence were used to predict their subsequent rate of change. Two global indices were considered: mean deviation (MD) and pattern standard deviation (PSD).
Results
Using multiple linear regression, baseline MD predicted subsequent slope of MD significantly better than baseline PSD predicted subsequent slope of PSD (p = 0.017). Using multiple logistic regression, a worse initial MD was predictive of being in the worst tertile for subsequent change in MD (pseudo-R2 = 0.33 for subjects with GON at baseline and 0.31 for those without). Worse initial PSD was not predictive of being in the worst tertile for subsequent change in PSD (pseudo-R2 = 0.09 with GON at baseline, 0.10 without).
Conclusions
Among patients with otherwise similar clinical profiles, a worse visual field at baseline, as measured by the global index MD, is predictive of a more rapid future rate of change. This should be taken into account when clinical decisions are made concerning management of patients who already have some visual field damage at presentation.
Keywords: glaucoma, perimetry, visual field, progression, data analysis
The aim of glaucoma management is to protect a patient’s visual function and maintain their vision-related quality of life. Consequently, predicting whether a patient’s vision will deteriorate rapidly or remain relatively stable is a critical part of this endeavor. Although glaucoma is commonly diagnosed and followed up based on a combination of tonometric, structural, and functional tests, the most important issue for patients is loss of their visual function. It has been estimated that within their lifetime, 15% of patients with glaucoma will have visual fields that are so badly damaged that they can no longer be monitored with standard automated perimetry (SAP),1 with associated consequences for their quality of life. It is desirable to identify these patients as early as possible so that appropriate management strategies can be implemented. Deriving better predictive models of glaucomatous progression is also a key aim for future clinical trials of glaucoma medications.2 However, predicting the rate of functional change for a subject remains challenging. Subjects may progress in very different manners based on their physiology and glaucomatous pathology. In addition, the currently available testing methods such as SAP suffer from high variability.
Various factors have been reported to be associated with an increased probability of future progression and/or the rate of future progression. These include higher intraocular pressure (IOP),3–6 IOP fluctuation,4,7 increased age,1,3–6 and having certain racial backgrounds1,5,8 (although some of these factors have been disputed).6,8,9 It could be posited that patients who already display functional loss indicative of glaucoma are more likely to have a rapid rate of progression in the future, as there is a decreased probability that they have a “false positive” diagnosis and do not have glaucoma. It may also be expected that patients whose disease has already progressed to a stage where functional loss is clinically detectable would be more likely to progress further. However, empirical evidence for the predictability of future change based on the current visual field is weak, and estimates of the predictive value of initial perimetry vary. In the Early Manifest Glaucoma Treatment (EMGT) trial, mean deviation (MD) at baseline was found to be significantly associated with the probability of progression (hazard ratio 1.58, p = 0.01)3; the effects of other visual field indices were not reported. In the Ocular Hypertension Treatment Study (OHTS),5 baseline pattern standard deviation (PSD) was found to have significant predictive value for reaching a primary open-angle glaucoma endpoint, but baseline MD was not. In the Collaborative Normal-Tension Glaucoma Study (CNTGS),8 baseline MD was not found to be predictive of future progression. Similarly, in the Advanced Glaucoma Intervention Study (AGIS),3 baseline visual field status (field score) was not found to be predictive of future progression. Each of these studies had different inclusion and exclusion criteria, making it difficult to generalize their conclusions. In particular, the AGIS cohort had more severe glaucoma at baseline than the other studies cited above.
The majority of work to date, including the clinical studies cited above, has sought to classify subjects as “glaucoma/normal” or as “stable/progressing.” However, in a clinical situation, it is also desirable to determine the likely rate of progression.10 The management strategies for subjects with ocular hypertension, stable glaucoma, or slowly progressing glaucoma could be similar, with the exception perhaps of follow-up intervals. However, a rapidly progressing subject would likely require a more aggressive management strategy, possibly including an earlier move to laser or incisional surgery, to prevent a significant reduction in vision-related quality of life and/or eventual visual disability.
The current study has two aims. First, in view of the equivocal findings from different studies, we seek to determine the extent to which summary measures from one SAP test could be used to predict which subjects with early or suspected glaucoma are most likely to undergo a rapid subsequent rate of functional change. Second, we seek to determine which of the currently available global indices of the visual field provide the best means to monitor and predict future rapid progression.
METHODS
Data
Data for this study were obtained from an ongoing longitudinal investigation of progression in subjects with early and suspected glaucoma at Devers Eye Institute in Portland, Oregon. The study adheres to the tenets of the Declaration of Helsinki and complies with the Health Insurance Portability and Accountability Act of 1996, and the protocol was approved by the Institutional Review Board at Legacy Health. Each subject provided their informed consent, after having the risks and benefits of participation explained to them.
Subjects were tested annually with a variety of structural and functional tests.11,12 For nearly all cases, testing took place within 1 month of the same date each year. At study entry, subjects had either a clinical diagnosis of early glaucoma or ocular hypertension (untreated IOP ≥22 mmHg) plus one or more additional possible risk factors for glaucoma (e.g., age >70 years, systemic hypertension, diet-controlled diabetes, peripheral vasospasm, African ancestry, or selfreported family history of glaucoma) and/or previously diagnosed glaucomatous optic neuropathy (GON) or suspicious optic nerve head appearance (cup-disc ratio asymmetry >0.2, neuroretinal rim notching or narrowing, and disc hemorrhage).11,12 Subjects with other diseases or taking medications known to affect the visual field or who had undergone ocular surgery (except for uncomplicated cataract surgery) were excluded. To avoid bias when evaluating the use of visual field parameters, the status of the subject’s visual field was not a criterion for inclusion in the study, except that subjects with visual acuity worse than 20/40 in either eye or with SAP MD worse than −6 dB at enrollment were excluded to remove subjects with advanced glaucoma.
SAP visual field tests were performed using a Humphrey Field Analyzer II13 (Carl Zeiss Meditec, Dublin, CA), using the 24-2 testing pattern, SITA standard algorithm,14 and conventional test procedures. During testing, an optimal full aperture lens correction was placed before the tested eye, while the fellow eye was occluded with a translucent eye patch. All subjects had previously undergone at least one visual field test to determine eligibility before study entry, and many had performed multiple previous tests. Unreliable fields (>33% fixation losses or >15% false positives) were repeated if possible and otherwise excluded from analysis. In addition, stereo-optic nerve head photographs (3-Dx; NIDEK, Gamagori, Japan) taken at the initial visit were evaluated by two masked experienced clinicians to determine whether they exhibited GON, with adjudication by a third clinician in case of disagreement.15 This was recorded along with age at baseline, IOP at baseline measured using Goldmann Applanation Tonometry (Haag-Streit, Bern, Switzerland) with the subject seated at a slitlamp, and treatment status (whether the patient had been prescribed and claimed to be taking IOP-lowering medication). Visual acuity was measured at each visit after autorefraction with a Humphrey Automated Refractor, with results reported using Snellen notation converted to logMAR equivalent.
For inclusion in this study, subjects were required to have performed visual field tests at seven or more annual visits (i.e., covering approximately a 6-year period). All such subjects were included. If results from more than seven annual visual field tests were available, data from the seven most recent were used. To enrich the sample with a wider range of disease severities and rates of progression, and hence make the conclusions more generalizable, the most rapidly changing eye of each subject was chosen as detailed below. In total, 95 subjects satisfied these entry criteria. These were split for analysis into two cohorts: 50 subjects exhibiting GON at baseline (who are therefore more likely to have already developed glaucoma) and 45 subjects with an optic nerve head appearance that was considered within normal limits at baseline (who are assumed to have either very early glaucoma or high-risk ocular hypertension). Splitting the data in this way, rather than using GON status as an additional predictor, allows findings from one group to be evaluated in an independent dataset at a different stage of the disease process.
Global Indices
Two summary measures of the visual field were used.
Mean deviation (MD): The amount of loss, defined at each test location as the difference between the measured sensitivity and the age-matched normal sensitivity, averaged over the entire visual field.16
Pattern standard deviation (PSD): This provides a measure of localized loss and is based on the difference at each test location between the measured sensitivity and the sensitivity that would be expected if the visual field corresponded with the normal hill of vision but with the same average sensitivity.
For each index, “subsequent slope” was defined as the slope of a linear regression of that index against time, based on fields 2 to 7 of the series. These slopes for MD and PSD will be referred to as SlMD and SlPSD, respectively, and give measures of the rate of functional change. The aim of the analyses in this study is to predict these subsequent slopes based on the values of the global indices at baseline, i.e., using field 1 of the series to predict the change during the interval from field 2 to field 7. Although the implicit assumption that the indices decline linearly over time is debatable (and is unlikely to be true in subjects with more severe glaucoma than those included in this study, especially for PSD), the assumption is the same for both indices, and so there is no resultant meaningful bias in the results. Note that the value of the index at baseline should not be included when determining the rate of subsequent change, because that would cause a systematic bias in the results when using its value to predict the subsequent slope. For consistency, the most rapidly changing eye for each subject that was used for this study was defined as being the eye exhibiting the most rapid rate of change in MD (i.e., the eye with the lowest value of SlMD was used).
Parametric Analyses
Linear regression was used to predict SlMD using the initial value of MD plus IOP, treatment status, and age (all measured at baseline). Stepwise backward elimination was used to exclude predictors at each stage, so as to maximize the adjusted R2 of the final model. The baseline value of the index being considered as a predictor was not eligible for elimination from the regression during this process. Of the 95 subjects in the two cohorts, three had missing IOP readings at baseline and, so, were excluded from this analysis. Once backward elimination had been completed, the adjusted R2 value for the final model was recorded, as a measure of how well the index predicts SlMD after accounting for the other factors. The adjusted R2 is defined as , where R2 is the square of the Pearson correlation coefficient, n is the sample size, and p is the number of predictors in the model. It is used in preference to the standard R2 because it penalizes over-fitting of the model by uninformative predictors. This was then repeated using PSD as the predictor index instead of MD and then using both MD and PSD individually to predict the slope SlPSD. These analyses were performed on the GON and non-GON cohorts separately.
To determine whether there were statistically significant differences among the models, the ability of PSD to predict both its own subsequent slope and the subsequent slope of MD was tested against the equivalent correlation for MD predicting its own subsequent slope. The correlations between the actual subsequent slope and that predicted by each model for each subject were calculated and compared. This comparison was done using the Z2* test statistic, which follows an asymptotic standard normal distribution, N(0,1), under the null hypothesis that the two correlations are equal.17 Although this reduces the power of the test to detect a difference when compared with using the simple Z statistic, it is more valid because it accounts for the fact that the correlations come from data drawn from the same subjects.
Nonparametric Analyses
Second, it was sought to compare the indices in terms of their ability to predict that which subjects would progress most rapidly in the future (the most crucial to identify in a clinical situation). To do this, the baseline values of MD and PSD were used to predict whether a subject would be in the worst tertile of subsequent rate of change. This analysis also negates a potential problem of the parametric analysis above; although it is desirable to predict the actual subsequent slope, doing so relies on assumptions about the relation between the predictor and the outcome. In the above analysis, it is implicitly assumed not only that the error term in the regression is normally distributed (which is a reasonable assumption in this case when averaging over a number of test locations, due to the central limit theorem) but also that there is a linear relation between the predictor and outcome, as opposed to a logarithmic or some other relation.
For each index in turn, the subjects were divided into tertiles based on their subsequent rate of progression (as defined by Slxx for an index xx). Tertiles were chosen over other potential divisions of the data to ensure a sufficient number of subjects being placed in each group.18
Logistic regression was performed to predict whether a subject would be in the worst tertile for subsequent rate of change. As before, potential predictors included in the model were the baseline values of each index, age, IOP, and treatment status. When the predictor is the baseline value of index Ind, the probability of a subject being in the worst tertile for subsequent rate of change based on the multivariate analysis is given by:
Stepwise backward elimination was used to minimize the Akaike information criterion.19 Nagelkerke’s coefficient of determination (a pseudo-R2 index for use in binomial logistic regression) was used to assess the strength of the association in the final model.20 A regression technique is preferred over receiver-operating characteristic analyses, because using the area under an receiver-operating characteristic curve as the measure of predictability gives equal weight to all sensitivities and specificities, despite the fact that only decision criteria with high specificity are useful clinically due to the relatively low prevalence of rapid glaucomatous progression.
To ensure that effects were truly caused by glaucomatous progression rather than development of cataract (which would also adversely affect MD), visual acuity was analyzed. The acuity at baseline and the rate of change of acuity over the subsequent six tests (as obtained by linear regression of the logMAR equivalent acuity over time) were compared between subjects inside and outside the worst tertile as defined by SlMD, for both the GON and non-GON cohorts, using the Wilcoxon rank sum test.
RESULTS
Table 1 compares subjects in the tertiles as defined by their rate of change of MD (i.e., SlMD). For each variable shown, the median value within each of the tertiles is given, followed by the p value of a Wilcoxon rank sum test comparison between subjects within and outside the worst tertile. In the GON cohort, subjects in the worst tertile for subsequent change tended to be older (note that this is accounted for in the subsequent multiple regression analyses as age is one of the predictors used). There was no difference between tertiles for either the baseline visual acuity or (more importantly when assessing progression) the rate of change of visual acuity. Note that when Snellen fraction is used for visual acuity, the same conclusions are obtained (indeed, the p values for the comparisons of baseline visual acuities are unchanged because a nonparametric test was used). This finding makes it unlikely that the results below showing the predictability of change in MD are due to developing cataracts.
TABLE 1.
Descriptive statistics for subjects in each tertile of subsequent change, as defined by rate of change of MD over years 2–7
| Worst tertile | Middle tertile | Best tertile | Comparison p |
|
|---|---|---|---|---|
| Baseline MD (dB) | −0.59 (1.82) | 0.31 (1.09) | 0.92 (1.79) | 0.029 (0.991) |
| Baseline PSD (dB) | 5.77 (5.82) | 5.89 (5.35) | 5.82 (5.81) | 0.688 (0.116) |
| Rate of change of MD (SlMD; dB/yr) | −0.64 (−0.42) | −0.35 (−0.19) | −0.12 (−0.07) | <0.001 (<0.001) |
| Age (yr) | 66 (55) | 55 (56) | 56 (53) | 0.006 (0.372) |
| IOP (mm Hg) | 18.5 (21.0) | 18.5 (21.0) | 20.0 (20.0) | 0.881 (0.234) |
| LogMAR equivalent baseline visual acuity | 0.051 (0.009) | −0.035 (−0.044) | −0.009 (0.021) | 0.050 (0.349) |
| Rate of change of visual acuity (logMAR/yr) | 0.002 (0.002) | 0.006 (0.003) | 0.003 (0.002) | 0.701 (0.971) |
Values given are the median among subjects falling into each tertile, followed by the p value of a comparison between subjects in the worst tertile and the remainder of the subjects in the dataset. For baseline visual acuity and change in visual acuity, to distinguish between tertiles when a large proportion of subjects have logMAR acuity of zero, means are reported rather than medians. In each cell, the upper number is the result for the GON cohort, and the number in brackets is the equivalent result for the non-GON cohort.
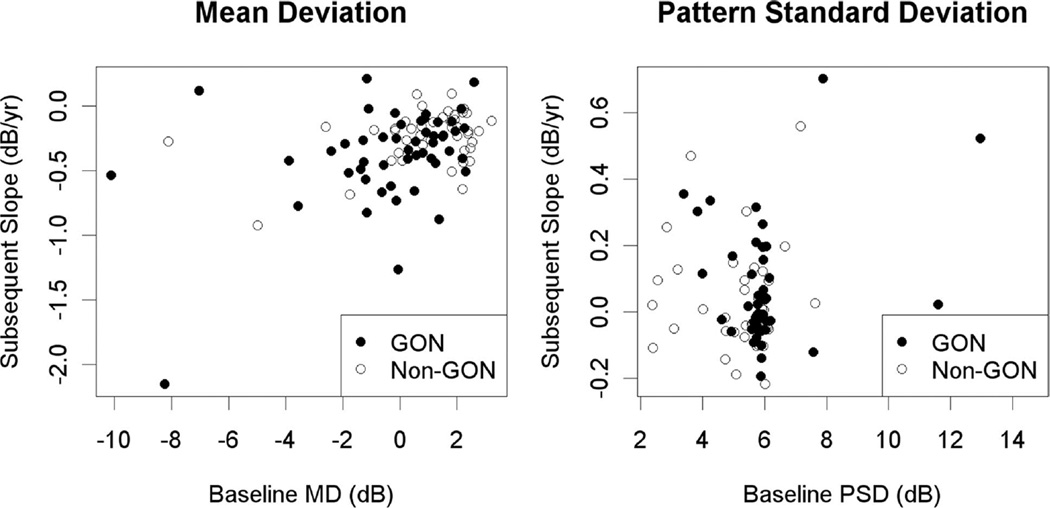
Table 2 shows results of the parametric analyses for each combination of the predictor index and the index used for subsequent slope. In the GON cohort, with the baseline index value as the sole predictor, the two models predicting SlPSD both showed significantly worse fits than the model using MD to predict SlMD (for MD as the predictor, p = 0.037; for PSD, p = 0.017). Fig. 1 shows plots of the baseline value against subsequent change in the same index, for both indices.
TABLE 2.
Results of parametric analyses
| Predictor | |||
|---|---|---|---|
| Outcome | MD | PSD | |
| GON cohort | SlMD | 0.143 | 0.131 |
| SlPSD | 0.084 | 0.088 | |
| Non-GON cohort | SlMD | 0.166 | 0.154 |
| SlPSD | 0.018 | −0.011 | |
The table shows the adjusted R2 values obtained when using the baseline value of each global index to predict the subsequent slopes of each of the two indices using a linear regression model, accounting for other factors using backward-elimination regression model as outlined in Methods section. Results are reported for both cohorts, as indicated.
Slxx, the subsequent slope of index xx over tests 2–7 of the series.
FIGURE 1.
The subsequent rate of change over years 2 to 7 of the sequence (in dB/yr), plotted against baseline value in year 1 (in dB), for each of the global indices considered in this study. Shading of the data points indicates whether the subject was considered to have GON at their initial visit.
Each index was used in turn as a predictor in a logistic regression model, to predict whether a subject will be in the worst tertile for subsequent change (as defined by SlMD and SlPSD in turn), for both cohorts. Values of SlMD in the worst tertile ranged from −2.16 dB/yr to −0.44 dB/yr in the GON cohort and from −0.92 dB/yr to −0.27 dB/yr in the non-GON cohort. Table 3 shows the effect size (odds ratios, giving the multiplicative effect on the probability of being in the worst tertile for subsequent change when MD or PSD is increased by 1 dB) and goodness of fit (pseudo-R2 values) for these models. As before, MD is more predictable than PSD. The only odds ratio whose 95% confidence interval did not include unity (indicating a significant predictive effect with p < 5%) occurred when MD was used to predict tertiles of SlMD in the GON cohort. In the non-GON cohort, PSD performed slightly better as a predictor of SlMD than MD. Even though this latter finding did not reach significance, it would conform to the hypothesis that PSD may be useful for detecting the very earliest glaucomatous damage whereas MD may be better for following up the progress of existing damage.
TABLE 3.
Results of logistic regression to predict whether a subject will be in the worst tertile for subsequent rate of change (defined according to each of the two slopes in turn)
| Predictor | |||||
|---|---|---|---|---|---|
| MD | PSD | ||||
| Outcome | OR | R2 | OR | R2 | |
| GON cohort | SlMD | 0.76 (0.58–0.99) | 0.332 | 1.25 (0.91–1.72) | 0.278 |
| SlPSD | 0.94 (0.72–1.22) | 0.052 | 1.06 (0.76–1.48) | 0.086 | |
| Non-GON cohort | SlMD | 0.72 (0.46–1.12) | 0.309 | 1.99 (0.88–4.48) | 0.321 |
| SlPSD | 0.89 (0.66–1.20) | 0.103 | 1.27 (0.71–2.25) | 0.098 | |
The baseline value of each global index is used to predict the probability of being in the worst tertile, using a backward-elimination regression model as outlined in the Methods section. The table shows the odds ratio, with 95% confidence interval in brackets (the multiplicative change in the probability of being in the worst tertile when the baseline index value increases by 1dB) and the Nagelkerke’s pseudo-R2 coefficient obtained (a measure of the goodness of fit of the model).
DISCUSSION
Identifying patients who are most likely to undergo rapid progression is a key aim for clinicians and researchers.10 The findings in this study indicate that subjects with worse baseline visual field (by either MD or PSD) are likely to undergo more rapid subsequent loss of function, presumably caused by glaucomatous progression. This agrees with the findings of the EMGT study,3 whose population was similar to our GON cohort, and the OHTS,5 whose population was more similar to our non-GON cohort, but differs from results of CNTGS8 and AGIS3 studies, whose populations consisted of subjects with normal tension glaucoma and advanced glaucoma, respectively. The visual field provides additional prognostic information that is not available from examination of the optic disc alone. Although GON at baseline was predictive of the rate of future functional change when participants with and without GON were combined (p = 0.02 when performing linear regression to predict SlMD with GON status as the sole predictor), MD performed notably better as a predictor. Comparing models using the Z2* test statistic as before, GON status was a worse predictor of SlMD than MD (p = 0.016) or PSD (p = 0.045). We would suggest that examination of optic nerve head photographs in isolation might not be sufficient when assessing patients with early stage glaucoma. It should be noted that this patient population consisted of subjects with early glaucomatous damage or risk factors for development of glaucoma and that our findings may not be generalizable to patients with more advanced glaucoma.
The subjects in this study had a clinical diagnosis of early glaucoma or were high-risk ocular hypertensives with either GON or at least one additional possible risk factor for glaucoma. In a previous study of this subject group, using liberal criteria for the definition of glaucoma (repeatable GON and/or progressive GON and/or a visual field defect consistent with glaucomatous damage), 84% of the subjects were found to have glaucoma in at least one eye.21 More stringent criteria would obviously reduce this percentage. Therefore, it is possible that the reason for our findings is that the visual field indices are simply separating those subjects with glaucoma, who are more likely to suffer progressive change, from subjects who only have ocular hypertension and, so, may never develop glaucoma and will only change at the normal age-related rate. However, this is exactly the problem often faced by clinicians: namely determining whether a patient with elevated IOP and other risk factors for glaucoma is likely to progress rapidly, without definitive knowledge of whether they truly have glaucoma. The fact that our conclusions extend to predicting the future functional change of subjects who already exhibit GON strengthens this motivation. We believe that this makes the conclusions highly relevant both to clinicians and researchers, even if they may not yet be generalizable to subjects with moderate or advanced disease.
Mean sensitivity, a simple arithmetic mean of the sensitivity values at each of the 52 visual field locations (excluding the blind spot) in the 24-2 field, was also considered as an alternative to MD or PSD. In some of the analyses, mean sensitivity performed slightly better for predicting change than MD, although the improvement was not statistically significant. This may indicate that the age-correction implicit in the calculation of MD is imperfect.22 One reason for this could be that age-related decline occurs at substantially different rates even in normal/healthy eyes. A range of rates of change of MD over time among normal control subjects has been reported,23 although most results regarding age-related changes have been based on cross-sectional rather than longitudinal data24,25 with few longitudinal studies having been carried out in normal subjects.26
PSD was significantly less predictable than MD and may also be a poorer predictor of subsequent change (although this difference was not found to be significant based on our data). The comparison between MD and PSD is consistent with a previous report that pattern deviation analysis may underestimate progression,23 although that study did not look at MD and PSD directly. Although indices based on pattern deviation such as PSD and the recently released visual field index27 detect localized visual field defects, they do not detect the generalized loss of sensitivity that is also observed in glaucoma28,29 and have been found to perform no better than MD at detecting and predicting progression.30 A further possible explanation is that the PSD is a measure of the spread of sensitivity values in the field, rather than an average (as with MD), and such measurements may be more affected by measurement noise.
IOP and treatment status (i.e., whether the subject had been prescribed IOP-lowering therapy) were rarely found to be predictive of the future rate of functional change in the regression models. This would seem to be in contrast with the principal results of previous clinical studies,31 performed on subjects with similar levels of damage at baseline. However, this finding needs to be considered with caution. No protocol was in place that dictated how the subjects in this study were to be treated based on study results, with all treatment decisions left to the subject’s physician (who was provided with a copy of all test results collected in this study). Perhaps more importantly, IOP measurement is variable both diurnally and over longer periods of time, and so a single measurement of IOP, as in the data used in this study, may not capture enough information to provide much predictive power. Also, it has been suggested that fluctuation in IOP is a better predictor of glaucomatous change than the mean IOP over the same time period,4 although this is disputed.9 Adequate data to address this hypothesis is not available in our testing protocol. The issue may not be resolved until more continuous modes of IOP monitoring such as IOP telemetry become available.32 Although we would posit that these findings demonstrate that our main conclusions are not artifacts caused by IOP-related factors differing among subjects, it should not be inferred from our results that lowering IOP does not favorably affect the future rate of progression. Similarly, the nonsignificance of treatment status does not take into account the fact that because treatment decisions were left to their clinician, management strategies were not consistent across subjects or the fact the issue of the compliance among those participants. The variety of treatment regimens between subjects makes it impractical to make useful assertions about the effect of particular treatments or management strategies.
One possible explanation for the results could be that the predictors are identifying subjects with early cataract and that the subsequent decrease in MD is due to worsening of their cataract.27 However, there was no evidence of any difference between tertiles in the rate of change of Snellen visual acuity over years 2 to 7, the period in which visual field change was measured. Although it cannot be ruled out, this makes it less likely that cataract progression, rather than glaucomatous progression, is responsible for the findings.
The second, nonparametric analysis in this study does not try to predict the actual rate of change but instead to predict which tertile of rate of change the subject fell into. Because of the variability inherent in perimetry, predicting the actual rate of change (for example, by using a linear regression model as in this study) is more challenging and involves making assumptions about the course of the disease, which may or may not be valid. Although methods are available to reduce the variability using spatial filtering,33,34 this filter was designed to not to affect the global measure of mean sensitivity. Temporal filtering35 may be more useful in this regard, although the techniques have not yet been sufficiently developed. Therefore, accurately predicting the actual rate of change instead of seeking to classify subjects into groups with differing likelihoods of undergoing rapid progression would require a larger sample size and more frequent testing to counteract the variability inherent in the clinical tests currently being used. Indeed, performing three tests per year has been recommended for early clinical identification of those subjects undergoing the most rapid progression.36 Currently, we only have data available from series of seven tests, without reducing the sample size to an extent where we feel that useful conclusions cannot reliably be drawn. Collecting and analyzing such data are certainly a desirable aim for the future, along with (as always in clinical research) validating these results in an independent dataset and extending the analyses to subjects with more advanced glaucomatous damage.
The principal conclusion to be drawn from this study is that a worse visual field at baseline, as measured by the global indices MD or PSD, is predictive of a more rapid future rate of change among subjects with early or suspected glaucoma, especially among subjects who already exhibit GON. This should be taken into account when clinical decisions are made concerning management of patients who already have some visual field damage at presentation.
ACKNOWLEDGMENTS
This project was funded in part by NIH grants NEI R01-EY-03,424 (to author CAJ) and NEI R01-EY-19,674 (to author SD).
Footnotes
No authors have any financial/conflicting interests to disclose.
Contributor Information
Stuart K. Gardiner, Discoveries in Sight Laboratories, Devers Eye Institute, Legacy Health, Portland, Oregon.
Shaban Demirel, Discoveries in Sight Laboratories, Devers Eye Institute, Legacy Health, Portland, Oregon.
Chris A. Johnson, Department of Ophthalmology and Visual Sciences, University of Iowa, Iowa City, Iowa.
REFERENCES
- 1.Broman AT, Quigley HA, West SK, Katz J, Munoz B, Bandeen-Roche K, Tielsch JM, Friedman DS, Crowston J, Taylor HR, Varma R, Leske MC, Bengtsson B, Heijl A, He M, Foster PJ. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49:66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Kaufman PL. The glaucoma research community and FDA look to the future: a report from the NEI/FDA CDER Glaucoma Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2009;50:1497–1505. doi: 10.1167/iovs.08-2843. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 4.Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, Caprioli J. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–1635. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, II, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004;138:S19–S31. doi: 10.1016/j.ajo.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 7.Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. 2007;125:1010–1013. doi: 10.1001/archopht.125.8.1010. [DOI] [PubMed] [Google Scholar]
- 8.Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 9.Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:205–209. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 10.Caprioli J. The importance of rates in glaucoma. Am J Ophthalmol. 2008;145:191–192. doi: 10.1016/j.ajo.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Spry PG, Johnson CA, Mansberger SL, Cioffi GA. Psychophysical investigation of ganglion cell loss in early glaucoma. J Glaucoma. 2005;14:11–19. doi: 10.1097/01.ijg.0000145813.46848.b8. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner SK, Johnson CA, Cioffi GA. Evaluation of the structure-function relationship in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3712–3717. doi: 10.1167/iovs.05-0266. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DR, Patella VM. Automated Static Perimetry, 2nd ed. St. Louis, MO: Mosby; 1999. [Google Scholar]
- 14.Bengtsson B, Olsson J, Heijl A, Rootzen H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol Scand. 1997;75:368–375. doi: 10.1111/j.1600-0420.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 15.Fortune B, Demirel S, Zhang X, Hood DC, Patterson E, Jamil A, Mansberger SL, Cioffi GA, Johnson CA. Comparing multifocal VEP and standard automated perimetry in high-risk ocular hypertension and early glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1173–1180. doi: 10.1167/iovs.06-0561. [DOI] [PubMed] [Google Scholar]
- 16.Johnson CA, Sample PA, Cioffi GA, Liebmann JR, Weinreb RN. Structure and function evaluation (SAFE): I. criteria for glaucomatous visual field loss using standard automated perimetry (SAP) and short wavelength automated perimetry (SWAP) Am J Ophthalmol. 2002;134:177–185. doi: 10.1016/s0002-9394(02)01577-5. [DOI] [PubMed] [Google Scholar]
- 17.Steiger J. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. [Google Scholar]
- 18.Kass MA, Gordon MO, Gao F, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JK, Miller JP, Parrish RK, Wilson MR the Ocular Hypertension Treatment Study Group. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol. 2010;128:276–287. doi: 10.1001/archophthalmol.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- 20.Nagelkerke NJ. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 21.Demirel S, Fortune B, Fan J, Levine RA, Torres R, Nguyen H, Mansberger SL, Gardiner SK, Cioffi GA, Johnson CA. Predicting progressive glaucomatous optic neuropathy using baseline standard automated perimetry data. Invest Ophthalmol Vis Sci. 2009;50:674–680. doi: 10.1167/iovs.08-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spry PG, Johnson CA. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001;78:436–441. doi: 10.1097/00006324-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Artes PH, Nicolela MT, LeBlanc RP, Chauhan BC. Visual field progression in glaucoma: total versus pattern deviation analyses. Invest Ophthalmol Vis Sci. 2005;46:4600–4606. doi: 10.1167/iovs.05-0827. [DOI] [PubMed] [Google Scholar]
- 24.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–1549. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- 25.Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008;49:4437–4443. doi: 10.1167/iovs.08-1753. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan BC, House PH, McCormick TA, LeBlanc RP. Comparison of conventional and high-pass resolution perimetry in a prospective study of patients with glaucoma and healthy controls. Arch Ophthalmol. 1999;117:24–33. doi: 10.1001/archopht.117.1.24. [DOI] [PubMed] [Google Scholar]
- 27.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–353. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Henson DB, Artes PH, Chauhan BC. Diffuse loss of sensitivity in early glaucoma. Invest Ophthalmol Vis Sci. 1999;40:3147–3151. [PubMed] [Google Scholar]
- 29.Airaksinen PJ, Drance SM, Douglas GR, Mawson DK, Nieminen H. Diffuse and localized nerve fiber loss in glaucoma. Am J Ophthalmol. 1984;98:566–571. doi: 10.1016/0002-9394(84)90242-3. [DOI] [PubMed] [Google Scholar]
- 30.Casas-Llera P, Rebolleda G, Munoz-Negrete FJ, Arnalich-Montiel F, Perez-Lopez M, Fernandez-Buenaga R. Visual field index rate and event-based glaucoma progression analysis: comparison in a glaucoma population. Br J Ophthalmol. 2009;93:1576–1579. doi: 10.1136/bjo.2009.158097. [DOI] [PubMed] [Google Scholar]
- 31.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 32.Downs J, Burgoyne CF, Liang Y, Sallee VL. A new implantable system for telemetric IOP monitoring in nonhuman primates (NHP) Invest Ophthalmol Vis Sci. 2008;49 E-abstract 2043. [Google Scholar]
- 33.Gardiner SK, Crabb DP, Fitzke FW, Hitchings RA. Reducing noise in suspected glaucomatous visual fields by using a new spatial filter. Vision Res. 2004;44:839–848. doi: 10.1016/S0042-6989(03)00474-7. [DOI] [PubMed] [Google Scholar]
- 34.Strouthidis NG, Scott A, Viswanathan AC, Crabb DP, Garway-Heath DF. Monitoring glaucomatous visual field progression: the effect of a novel spatial filter. Invest Ophthalmol Vis Sci. 2007;48:251–257. doi: 10.1167/iovs.06-0576. [DOI] [PubMed] [Google Scholar]
- 35.Spry PG, Johnson CA, Bates AB, Turpin A, Chauhan BC. Spatial and temporal processing of threshold data for detection of progressive glaucomatous visual field loss. Arch Ophthalmol. 2002;120:173–180. doi: 10.1001/archopht.120.2.173. [DOI] [PubMed] [Google Scholar]
- 36.Chauhan BC, Garway-Heath DF, Goni FJ, Rossetti L, Bengtsson B, Viswanathan AC, Heijl A. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]