Abstract
Aims
Although the focus of therapeutic intervention has been on neurohormonal pathways thought to be harmful in heart failure (HF), such as the renin–angiotensin–aldosterone system (RAAS), potentially beneficial counter-regulatory systems are also active in HF. These promote vasodilatation and natriuresis, inhibit abnormal growth, suppress the RAAS and sympathetic nervous system, and augment parasympathetic activity. The best understood of these mediators are the natriuretic peptides which are metabolized by the enzyme neprilysin. LCZ696 belongs to a new class of drugs, the angiotensin receptor neprilysin inhibitors (ARNIs), which both block the RAAS and augment natriuretic peptides.
Methods
Patients with chronic HF, NYHA class II–IV symptoms, an elevated plasma BNP or NT-proBNP level, and an LVEF of ≤40% were enrolled in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortailty and morbidity in Heart Failure trial (PARADIGM-HF). Patients entered a single-blind enalapril run-in period (titrated to 10 mg b.i.d.), followed by an LCZ696 run-in period (100 mg titrated to 200 mg b.i.d.). A total of 8436 patients tolerating both periods were randomized 1:1 to either enalapril 10 mg b.i.d. or LCZ696 200 mg b.i.d. The primary outcome is the composite of cardiovascular death or HF hospitalization, although the trial is powered to detect a 15% relative risk reduction in cardiovascular death.
Perspectives
PARADIGM-HF will determine the place of the ARNI LCZ696 as an alternative to enalapril in patients with systolic HF. PARADIGM-HF may change our approach to neurohormonal modulation in HF.
Trial registration
Keywords: Chronic heart failure, Renin–angiotensin, ACE inhibitor, Angiotensin receptor blocker, Natriuretic peptides, Neprilysin, Neutral endopeptidase, Angiotensin receptor neprilysin inhibitor, LCZ696
Introduction
Neurohormonal pathways are thought to be fundamentally important in the pathophysiology of heart failure.1,2 The belief that sustained activation of certain neurohumoral pathways such as the renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system (SNS) is detrimental in heart failure underpins the basis of therapy.3 The crucial importance of the RAAS is supported by the beneficial effects of ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists.4 Likewise, the benefits of beta-blockers argue for a key role of the SNS.5 Although the focus of therapeutic intervention has been on blocking these pathways thought to be harmful in heart failure, potentially beneficial counter-regulatory systems are also activated in heart failure. These pathways variously promote vasodilatation and natriuresis, inhibit abnormal growth, suppress the RAAS and SNS, inhibit the release and actions of vasopressin, and augment the parasympathetic nervous system. The best understood mediators exerting these actions are the natriuretic peptides. The first of these to be described, A-type natriuretic peptide (ANP), is secreted in response to atrial distension, activates the ANPR-A receptor, increasing intracellular cyclic guanylate monophosphate (cGMP), and is cleared by the ANPR-C receptor and by the action of the enzyme neutral endopeptidase (NEP), also known as neprilysin.6–10 B-type natriuretic peptide, secreted predominantly by the ventricles in response to increased wall stress, exerts similar actions and is cleared in the same way.10
Augmentation of natriuretic peptides has been considered as a therapeutic strategy in heart failure for over two decades.11,12 Because oral delivery of these peptides is ineffective and long-term parenteral delivery is problematic, blockade of natriuretic peptide breakdown, by inhibition of neprilysin, has been developed as an alternative approach to increasing natriuretic peptides. Oral neprilysin inhibitors, used in combination with ACE inhibitors, had favourable short-term effects on haemodynamic measurements and exercise time in patients with heart failure, but were not developed further.12–14 Subsequently, a molecule that was both a neprilysin and an ACE inhibitor, omapatrilat, was studied in both hypertension and heart failure, but its development was terminated because of an unacceptable incidence of angioedema.15–18 This adverse effect was thought to occur because both ACE and neprilysin break down bradykinin, which directly or indirectly can cause angioedema, and because omapatrilat may also inhibit a third enzyme metabolizing bradykinin, aminopeptidase P.19 Angiotensin receptor neprilysin inhibitors (ARNIs) are a new class of drug developed both to block the RAAS and augment natriuretic peptides.20,21 By so doing, ARNIs have the potential to modulate favourably the neurohormonal imbalance that characterizes heart failure. Because RAAS blockade is achieved by antagonism of the angiotensin II type 1 receptor, and not by inhibition of ACE (or aminopeptidase P), it is hypothesized that the risk of angioedema will not be increased.22 LCZ696 is the first ARNI to be tested in patients, and here we describe the design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortalilty and morbidity in Heart Failure trial (PARADIGM-HF).
Trial design and methods
PARADIGM-HF is a randomized, double-blind, parallel group, active-controlled, two-arm, event-driven trial comparing the long-term efficacy and safety of enalapril and LCZ696 in patients with chronic symptomatic heart failure and reduced EF (HF-REF). The trial was designed by the academic members of the Executive Committee in collaboration with Novartis personnel.
Patients
Entry criteria were as follows: (i) age 18 years or older and able to give written informed consent; (ii) NYHA functional class II–IV; (iii) LVEF ≤ 35% (initially this was ≤ 40% but changed in a protocol amendment dated 15 December 2010); (iv) plasma BNP ≥150 pg/mL (or NT-proBNP ≥600 pg/mL) at the screening visit (Visit 1, Figure 1) or a BNP ≥100 pg/mL (or NT-proBNP ≥400 pg/mL) and a hospitalization for heart failure within the last 12 months; (v) treatment with a stable dose of an ACE inhibitor or an ARB equivalent to enalapril 10 mg/day (see Table 1) for at least 4 weeks before the screening visit; and (vi) treatment with a stable dose of a beta-blocker for at least 4 weeks prior to the screening visit, unless contraindicated or not tolerated. Although not required, the protocol specified that an aldosterone antagonist should also be considered in all patients, taking account of renal function, serum potassium, and tolerability. If given, the dose of aldosterone antagonist should be stable for at least 4 weeks prior to the screening visit. The key exclusion criteria are listed in Table 2.
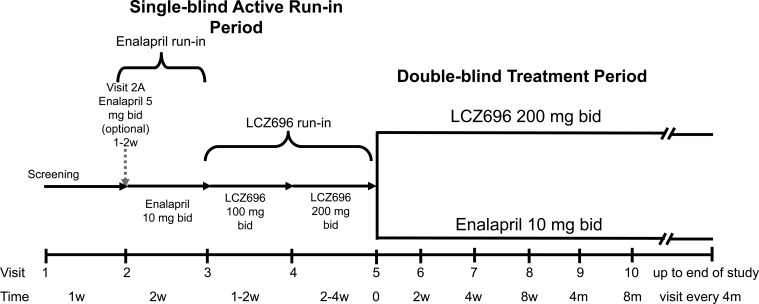
Figure 1.
PARADIGM-HF study schema.
Table 1.
Minimum required pre-study daily doses of commonly prescribed angiotensin-converting enzyme inhibitors and angiotensin receptor blockers
| ACE inhibitors | Minimum daily dose | ARBs | Minimum daily dose |
|---|---|---|---|
| Enalapril | 10 mg | Candesartan | 16 mg |
| Captopril | 100 mg | Eprosartan | 400 mg |
| Cilazapril | 2.5 mg | Irbesartan | 150 mg |
| Fosinopril | 20 mg | Losartan | 50 mg |
| Lisinopril | 10 mg | Olmesartan | 10 mg |
| Moexipril | 7.5 mg | Telmisartan | 40 mg |
| Perindopril | 4 mg | Valsartan | 160 mg |
| Quinapril | 20 mg | ||
| Ramipril | 5 mg | ||
| Trandolapril | 2 mg | ||
| Zofenopril | 30 mg |
Table 2.
Key exclusion criteria
| 1. History of hypersensitivity or allergy to any of the study drugs, drugs of similar chemical classes, ACE inhibitors (ACEIs), ARBs, or neprilysin inhibitors, as well as known or suspected contraindications to the study drugs. |
| 2. Previous history of intolerance to recommended target doses of ACEIs or ARBs. |
| 3. Known history of angioedema. |
| 4. Requirement for treatment with both ACEIs and ARBs. |
| 5. Current acute decompensated heart failure (exacerbation of chronic heart failure manifested by signs and symptoms that may require intravenous therapy). |
| 6. Symptomatic hypotension and/or a systolic blood pressure <100 mmHg at Visit 1 (screening) or <95 mmHg at Visit 3 or at Visit 5 (randomization). |
| 7. Estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 at Visit 1 (screening), Visit 3 (end of enalapril run-in), or Visit 5 (end of LCZ696 run-in and randomization) or >35% decline in eGFR between Visit 1 and Visit 3 or between Visit 1 and Visit 5. |
| 8. Serum potassium >5.2 mmol/L at Visit 1 (screening) or >5.4 mmol/L at Visit 3 or Visit 5 (randomization). |
| 9. Acute coronary syndrome, stroke, transient ischaemic attack, cardiac, carotid, or other major cardiovascular surgery, PCI, or carotid angioplasty within the 3 months prior to Visit 1. |
| 10. Coronary or carotid artery disease likely to require surgical or percutaneous intervention within the 6 months after Visit 1. |
| 11. Implantation of a CRT device within 3 months prior Visit 1 or intent to implant a CRT. |
| 12. History of heart transplant or on a transplant list or with LV assistance device. |
| 13. History of severe pulmonary disease. |
| 14. Diagnosis of peripartum- or chemotherapy-induced cardiomyopathy within the 12 months prior to Visit 1. |
| 15. Documented untreated ventricular arrhythmia with syncopal episodes within the 3 months prior to Visit 1. |
| 16. Symptomatic bradycardia or second- or third-degree atrioventricular block without a pacemaker. |
| 17. Presence of haemodynamically significant mitral and/or aortic valve disease, except mitral regurgitation secondary to LV dilatation. |
| 18. Presence of other haemodynamically significant obstructive lesions of the LV outflow tract, including aortic and subaortic stenosis. |
| 19. Any surgical or medical condition which might significantly alter the absorption, distribution, metabolism, or excretion of study drugs, including, but not limited to, any of the following: |
| History of active inflammatory bowel disease during the 12 months before Visit 1. |
| Active duodenal or gastric ulcers during the 3 months prior to Visit 1. |
| Evidence of hepatic disease as determined by any one of the following: aspartate aminotransferase or alanine aminotransferase values exceeding 2× upper limit of normal at Visit 1, history of hepatic encephalopathy, history of oesophageal varices, or history of porto-caval shunt. |
| Current treatment with cholestyramine or colestipol resins. |
| 20. Presence of any other disease with a life expectancy of <5 years. |
Study design
The study consists of four phases: (i) screening; (ii) single-blind enalapril run-in; (iii) single-blind LCZ696 run-in; and (iv) randomized double-blind treatment (Figure 1). The rationale for the active run-in periods is explained in the Discussion.
Screening (Visit 1)
At the screening visit, patient eligibility was assessed according to the inclusion/exclusion criteria (including the criteria in Table 3). Any local measurement of LVEF within the eligibility range made within the past 6 months was acceptable provided there was no subsequent LVEF measurement above it. Eligibility BNP (and NT-proBNP), serum potassium, and estimated glomerular filtration rate (eGFR) were measured in a central laboratory.
Table 3.
Safety monitoring criteria that need to be met at screening, at the end of the enalapril run-in and at randomization
| Parameter | Screening visit (V1) | End of enalapril run-in/randomization visit (V3 and 5) |
|---|---|---|
| Hyperkalaemia | K+ ≤5.2 mmol/L | K+ ≤5.4 mmol/L |
| Renal dysfunction | eGFR ≥30 mL/min/1.73 m2 | eGFR ≥30 mL/min/1.73 m2 |
| No decrease of eGFR of >35% from Visit 1 | ||
| Blood pressure | No symptomatic hypotension | No symptomatic hypotension |
| SBP ≥100 mmHg | SBP ≥95 mmHg | |
| Adverse events | No postural symptoms or other AEs that preclude continuation according to the investigator's judgement | No postural symptoms or other AEs that preclude continuation according to the investigator's judgement |
AE, adverse effect/event; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Enalapril active run-in period (Visit 2)
At Visit 2, most eligible patients started 2 weeks of single-blind treatment with enalapril 10 mg b.i.d. A lower dose of enalapril (5 mg b.i.d.) was allowed for patients currently treated with an ARB and for those taking a low dose of ACE inhibitor (see Table 1) if the investigator was concerned that switching directly to enalapril 10 mg b.i.d. might not be tolerated (e.g. because of hypotension, renal dysfunction, and/or hyperkalaemia). These patients were up-titrated to enalapril 10 mg b.i.d. after 1–2 weeks. Patients tolerating enalapril 10 mg b.i.d. as defined by the criteria in Table 3 were eligible for Visit 3.
LCZ696 active run-in period (Visits 3 and 4)
At Visit 3, patients started single-blind treatment with LCZ696 100 mg b.i.d. After 1–2 weeks, the dose was up-titrated to 200 mg b.i.d., for a further 2–4 weeks.
Other heart failure medication (except for an ACE inhibitor or ARB) was continued during the run-in periods.
Randomization to double-blind treatment (Visit 5)
Patients tolerating both enalapril 10 mg b.i.d. and LCZ696 200 mg b.i.d., as defined by the criteria in Table 3, were randomized in a 1:1 ratio to double-blind treatment with either enalapril 10 mg b.i.d. or LCZ696 200 mg b.i.d. Study visits occur every 2–8 weeks during the first 4 months of the double-blind period and every 4 months thereafter (with additional unscheduled visits, at the discretion of the investigator).
There were two short washout periods during the run-in periods to minimize the potential risk of angioedema due to overlapping ACE inhibition and NEP inhibition at Visit 3 and Visit 5: (i) enalapril was stopped a day prior to starting LCZ696 at Visit 3 and (ii) LCZ696 was stopped a day prior to starting randomized study drug at Visit 5.
Monitoring of safety and tolerability during double-blind period
Patients are assessed at each study visit for hyperkalaemia, symptomatic hypotension, increase in serum creatinine, angioedema, and other adverse events (AEs) and serious AEs. Patients who can no longer tolerate the target dose of study drug can be down-titrated to the lower dose at the investigator's discretion (after considering whether any other relevant non-disease-modifying therapy can be discontinued, e.g. a calcium channel or alpha-adrenoceptor blocker in a hypotensive patient). The dose of background disease-modifying drugs, such as beta-blockers, should not be reduced to facilitate maintenance of study drug. Every attempt should be made to re-challenge the patients so as to maintain as many patients as possible on the target dose of study drug.
Collection and adjudication of potential angioedema events
Potential angioedema cases are identified in two ways: (i) proactive reporting of any events that resemble angioedema by site investigators; and (ii) routine safety monitoring by the sponsor for signs or symptoms suggestive of potential angioedema. All identified cases are submitted to an independent angioedema adjudication committee for a final decision.
Study objectives
Primary objectives
The purpose of this study is to evaluate the effect of LCZ696 200 mg b.i.d. compared with enalapril 10 mg b.i.d., in addition to conventional heart failure treatment, in delaying time to first occurrence of either cardiovascular (CV) death or heart failure hospitalization.
Secondary objectives
Secondary endpoints were to test whether LCZ696, compared with enalapril, is superior: (i) in improving the Kansas City Cardiomyopathy Questionnaire (KCCQ) clinical summary score for heart failure symptoms and physical limitations at 8 months;23 (ii) in delaying the time to all-cause mortality; (iii) in delaying time to new onset atrial fibrillation; and (iv) in delaying the time to first occurrence of either: (a) a 50% decline in eGFR relative to baseline (i.e. Visit 5); (b) >30 mL/min/1.73 m2 decline in eGFR relative to baseline to a value <60 mL/min/1.73 m2; or (c) reaching end-stage renal disease.
Exploratory objectives
These are listed in Table 4.
Table 4.
Exploratory objectives
| To compare the effects of LCZ696 and enalapril on: |
| first occurrence of a composite of CV death, hospitalization for heart failure, non-fatal myocardial infarction, non-fatal stroke, or resuscitated sudden death |
| the number of patients hospitalized and number of hospital admissions (all-cause and cause-specific) |
| days alive out of hospital at 12 months |
| the rate of decline in eGFR |
| time to ‘treatment failure’, defined as: the addition of a new drug, intravenous treatment, or a persistent increase in dose of diuretic dose (>1 month) for the treatment of worsening HF |
| a ‘clinical composite score’ (assessed by NYHA classification and patient global assessment) at 8 months |
| time to new-onset diabetes mellitus |
| health-related quality of life, assessed by total score and individual scores of the subdomains of the KCCQ and the EuroQol 5-dimensions scale24 |
| the incidence of coronary revascularization procedures |
| the profile of pre-specified biomarkers (e.g. vascular, renal, collagen, metabolism, and inflammatory biomarkers) from baseline to pre-defined time points |
| health resource utilization, i.e. number of days/stays in the intensive care unit and number of emergency room visits. |
| The pharmacokinetics of valsartan, AHU377, and LBQ657 will also be characterized at steady state in patients receiving LCZ696 using population modelling and/or non-compartmental based methods. |
CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Table 5.
Large trials in heart failure using enalapril
| Trial | n | Target dose (mg) | Mean/median daily dose (mg) |
|---|---|---|---|
| CONSENSUS (1987)a | 127 | 20 b.i.d. | 18.429 |
| SOLVD-T (1991)b | 1285 | 10 b.i.d. | 16.628 |
| SOLVD-P (1992) | 2111 | 10 b.i.d. | 16.730 |
| V-HeFT II (1991) | 403 | 10 b.i.d. | 15.031 |
| NETWORK (1998)c | 516 | 10 b.i.d. | 17.932 |
| Nanas et al. (2000)d | 122 low | 10 b.i.d. | 17.933 |
| 126 high | 30 b.i.d. | 19.333 | |
| OVERTURE (2002) | 2884 | 10 b.i.d. | 17.717 |
| CARMEN (2004) | 190 E only | 10 b.i.d. | 16.834 |
| 191 E + C | 10 b.i.d. | 14.934 | |
| CIBIS-3 (2005)e | 505 B first | 10 b.i.d. | 15.835 |
| 505 E first | 10 b.i.d. | 17.235 |
B, bisoprolol; C, carvedilol; E, enalapril.
aThe trial had no EF entry criterion. Of patients randomized to enalapril, 22% were titrated to the target dose of 20 mg b.i.d.
bThe trial had an active (enalapril) run-in period; 49% reached the target dose.
cThe trial had no EF entry criterion. All patients had to tolerate a test dose of 2.5 mg enalapril.
dBy 3 months, 72.5% of patients in the 10 mg b.i.d. group reached target dose compared with 32.5% in the 30 mg b.i.d. group.
eDuring the ‘enalapril-first’ monotherapy phase, 84% of patients were titrated to the target dose.
Study management and committees
PARADIGM-HF is conducted by Novartis AG under the guidance and leadership of the Executive Committee. The National Leaders of participating countries are listed in Appendix 1.
An independent, external Data Monitoring Committee (DMC) (listed in Appendix 1) has been appointed to oversee the safety of the patients and review the results of the interim analyses. An Endpoint Adjudication Committee (listed in Appendix 1) is responsible for classifying all deaths and for determining whether pre-specified endpoint criteria are met for the non-fatal events. Another committee is adjudicating suspected cases of angioedema in this and other trials using LCZ696 (listed in Appendix 1).
Statistical considerations
The estimated annual event rate for the primary endpoint in the control (enalapril) arm of PARADIGM-HF was based on the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM)-Added trial where the annual event rate for the same composite was 16.6% in the placebo group (and 14.1% in the candesartan group).25 Because patients are treated with a higher dose of ACE inhibitor (or LCZ696 equivalent) in PARADIGM-HF and a greater proportion were expected to receive a beta-blocker and a mineralocorticoid antagonist, a more conservative expected annual event rate of 14.5% was chosen for sample size calculation. The annual CV mortality rate of 7% was estimated from the CHARM-Added trial in a similar manner. We also anticipated that the requirement for an elevated BNP or NT-proBNP level at enrolment would ensure an adequate event rate.
The sample size is based upon the CV mortality (although the trial will continue until the required number of patients have experienced CV death or heart failure hospitalization—see below). A total of 1229 CV deaths are required to give 80% power to detect a relative risk reduction of 15% in the LCZ696 group, compared with the enalapril group. A total of ∼8000 patients are required to accrue this number of events, assuming an annual CV death rate of 7% in the enalapril group, with a mean follow-up of ∼34 months.
Assuming an annual rate of CV death or heart failure hospitalization in the enalapril group of 14.5%, and the same sample size and follow-up period, at least 2410 patients are expected to experience a primary event. This means that PARADIGM-HF should have >97% power to detect a relative risk reduction of 15% in this composite.
A final statistical analysis plan will be developed prior to the end of the study and treatment unblinding.
Study duration, interim analyses, and early termination
As PARADIGM-HF is an event-driven trial, all randomized patients will remain in the trial until 2410 patients have experienced a CV death or heart failure hospitalization, unless the DMC recommends that the study be stopped earlier for efficacy or safety reasons. The total length of the trial will depend on the duration of the patient recruitment period and the time taken to accrue the pre-specified number of patients with a primary event. Currently, three interim efficacy analyses are planned when approximately one-third, one-half, and two-thirds of the primary events have occurred. The one-sided significance level of α to be used for the final analysis will be adjusted for the interim efficacy analyses to control the overall type I error at 2.5% (one-sided). A futility analysis will also be performed at each of these interim analyses, permitting early termination of the trial for lack of efficacy.
Safety considerations
In view of the limited exposure of patients with heart failure to LCZ696 before the start of PARADIGM-HF, the DMC performed a safety assessment after the first 100, 300, and 600 patients completed the run-in period and the number of patients exposed to study drug was also limited to 600 until the DMC had evaluated safety in the first 200 patients to complete 4 weeks of double-blind therapy. Thus, this study employed a seamless design, incorporating ‘Phase II’ into a ‘Phase III’ outcomes trial, as considered further in the Discussion. Further interim safety assessments are planned twice a year.
Current status
The protocol was approved by the Ethics Committee/Institutional Review Board affiliated to each investigative site. Patients started enrolling in PARADIGM-HF on 8 December 2009. As of 17 January 2013, the study was fully enrolled, with 8436 validly randomized patients at 985 centres in 47 countries distributed across all major geographical regions.
The study is being conducted in accordance with Good Clinical Practice, Declaration of Helsinki 2002. The trial has been registered on Clinicaltrials.gov, NCT01035255.
Discussion
A strategy of dual RAAS blockade and natriuretic peptide augmentation is theoretically attractive in heart failure and was tried previously with the dual neprilysin/ACE inhibitor omapatrilat in the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events trial (OVERTURE).17 Although omapatrilat did not reduce the primary endpoint of death or hospitalization for heart failure requiring intravenous treatment, compared with enalapril 10 mg b.i.d., it was superior to enalapril in relation to the secondary endpoint of CV death or CV hospitalization. Furthermore, when the effect of omapatrilat on the primary endpoint was evaluated, retrospectively, using the same definition of hospitalization for heart failure as had been used in the Studies of Left Ventricular Dysfunction Treatment-trial (SOLVD-Treatment), where the use of intravenous therapy was not required for positive adjudication, omapatrilat was superior to enalapril. Furthermore, administration of a single, large, dose of omapatrilat once daily may have on the one hand caused excessive post-dose hypotension and on the other not provided complete 24 h RAAS blockade or 24 h neprilysin inhibition. Ultimately, however, because omapatrilat caused an unacceptable incidence of angioedema in patients with hypertension, its development was halted.18
LCZ696 is a first-in-class ARNI.20–22 After ingestion, LCZ696 delivers systemic exposure to AHU377, a neprilysin inhibitor pro-drug, and valsartan, an ARB. AHU377 is then rapidly metabolized by non-specific esterases to the active neprilysin inhibitor LBQ657. LCZ696 causes dose-dependent increases in ANP, plasma and urinary cGMP, plasma renin activity, and angiotensin II, effects consistent with activation of the NPR-A receptor and blockade of the angiotensin II type 1 receptor.20–22 In healthy volunteers and patients with heart failure, a total daily dose of 400 mg of LCZ696 gives systemic exposure to valsartan similar to Diovan® 320 mg daily.20,26
Several features of the design of PARADIGM-HF are worthy of discussion.
The patients included in PARADIGM-HF are similar to those enrolled in SOLVD-Treatment, i.e. in NYHA class II–IV and with an LVEF ≤ 35% (in most cases). However, PARADIGM-HF also had natriuretic peptide entry criteria. We used a BNP entry threshold of ≥150 pg/mL (or NT-proBNP ≥600 pg/mL) at Visit 1 or a BNP ≥100 pg/mL (or NT-proBNP ≥400 pg/mL) if the patient was hospitalized for heart failure within the last 12 months. This is because BNP and NT-proBNP are powerful predictors of CV events in patients with heart failure and are, therefore, useful in helping select a higher risk population.27,28 This was necessary, in the light of recent improvements in outcome in patients with heart failure as a result of new drug and device therapies, to ensure a realistic trial size, and a trial with adequate power to show a modest but still important treatment effect. Note that patients in PARADIGM-HF are required to be treated with a beta-blocker unless contraindicated or not tolerated, and investigators are encouraged to use a mineralocorticoid (aldosterone) receptor antagonist, as indicated.
The choice of active comparator was based upon the SOLVD-Treatment trial which was a pivotal ACE inhibitor mortality/morbidity study in a broad spectrum of patients with HF-REF.29 In that trial, the target dose of enalapril was 10 mg b.i.d. and the mean daily prescribed dose in patients taking enalapril was 16.6 mg. The same target dose of enalapril was used in at least seven other large heart failure trials (Table 5).30–36 These trials and another large community study of >17 500 patients achieved a mean daily dose of enalapril of between 15 and 18 mg.37 Two trials, which had higher target doses (20 mg b.i.d. and 30 mg b.i.d.), achieved only slightly greater average doses, with less than half of patients titrated to target. Therefore, from a regulatory perspective, the ‘gold standard’ comparator for LCZ696 is enalapril 10 mg b.i.d., the most tested ACE inhibitor in HF-REF. Moreover, we anticipate that achievement of a similar average dose to that attained in the SOLVD-Treatment trial will be required, although in two more recent trials where enalapril was used in addition to a beta-blocker (which was rarely used in SOLVD-Treatment), a lower average achieved dose of enalapril was attained.35,36
The choice of dose of LCZ696 was first and foremost based on the need to achieve serum concentrations of valsartan equivalent to those obtained with the Diovan® formulation of valsartan 160 mg b.i.d., the dose shown to be effective in Val-HeFT and VALIANT.38,39 Secondly, the dose of LCZ696 also had to achieve adequate inhibition of neprilysin. LCZ696 200 mg b.i.d. achieves systemic exposure similar to valsartan 160 mg b.i.d. and, at that dose, also achieves 90% of its maximum inhibition of neprilysin and leads to sustained increases in plasma cGMP concentrations in healthy volunteers.20,21 In addition, in patients with hypertension, LCZ696 200 mg once daily lowered sitting systolic (difference 5.3 mmHg) and diastolic (difference 3.0 mmHg) blood pressure more than valsartan 160 mg, an incremental blood pressure-lowering effect attributed to neprilysin inhibition.21 Furthermore, in a proof-of-concept study in patients with HF-PEF, the Prospective comparison of ARNi with ARB on Management Of heart failUre with preserved ejectioN fraction trial (PARAMOUNT), LCZ696 200 mg b.i.d., compared with valsartan 160 mg b.i.d., led to a sustained reduction over 36 weeks in plasma NT-proBNP concentration (a marker of LV diastolic wall stress and not a substrate for neprilysin) and left atrial (LA) size (as a potential marker of LA and LV end-diastolic pressure).40
The active run-in period of PARADIGM-HF served two main functions. First, both the enalapril and LCZ696 run-in periods maximized attainment of the target doses of each drug which is important as outlined above. The LCZ696 run-in period also provided investigator-unblinded, albeit short-term, safety information on the ARNI, which was important in light of the limited data on tolerability of LCZ696 in patients with heart failure available before PARADIGM-HF commenced—in essence the development programme for LCZ696 in systolic heart failure bypassed the typical ‘Phase II’ development stage. The decision to do this, rather than conduct an initial ‘stand-alone’ Phase II trial, was based on substantial and reassuring safety experience with LCZ696 in hypertension and prior experience with omapatrilat. Furthermore, Phase II studies rarely predict success in Phase III trials in heart failure and simply prolong the time and increase the cost involved in making a definitive determination of the efficacy and safety of a new treatment, which always requires a large mortality/morbidity trial.
For safety reasons, there are also two short washout periods during the run-in. These washout periods are designed to minimize concomitant neprilysin and ACE inhibition which is likely to increase the risk of angioedema. Although the risk of angioedema is expected to be low, for the reasons outlined earlier,22 a special committee of experts are adjudicating all cases of suspected angioedema in PARADIGM-HF and other studies with LCZ696 (see Appendix 1).
The primary outcome of CV death or heart failure hospitalization was chosen as the one that best reflects the major mortality and morbidity burden of this syndrome and has been used in other recent trials including CHARM-Added, SHIFT, and EMPHASIS-HF.25,40,41 However, uniquely, PARADIGM-HF is powered on the basis of CV mortality giving both adequate power to detect a reduction in CV mortality and >97% power to detect a clinically meaningful reduction in the primary composite outcome. Two of the secondary and exploratory endpoints merit a special mention. Decline in renal function is of interest given that natriuretic peptides and neprilysin inhibition have been reported to increase glomerular filtration and because renal impairment as an adverse effect was less common with omaptrilat than with enalapril in OVERTURE.17,41,42 Similarly, development of diabetes is of interest, given the recent suggestion that low natriuretic peptides levels may be associated with the development of this disease and omapatrilat improved glucose utilization peripherally.45,46
In summary, PARADIGM-HF is addressing, within a major clinical outcome trial, the place of the ARNI LCZ696 as an alternative to the ‘gold-standard’ ACE inhibitor enalapril in patients with chronic systolic heart failure. As the largest clinical trial in heart failure to date, PARADIGM-HF may change our approach to neurohumoral modulation in heart failure.
Funding
PARADIGM-HF is funded by Novartis Pharma AG.
Conflict of interest: all Executive Committee members or their institutions have received consulting fees as well as travel and research support in the planning and conduct of PARADIGM-HF. J.G., M.P.L., A.R.R., and V.C.S. are employees of Novartis.
Appendix 1. Complete list of the PARADIGM-HF Committees and Investigators
Executive Committee: John McMurray (Co-Chair), Milton Packer (Co-Chair), Jean Rouleau, Scott Solomon, Karl Swedberg, Michael Zile.
Data Monitoring Committee: Henry Dargie (Chair), Robert Foley, Gary S. Francis, Michele Komajda, Stuart Pocock.
Angioedema Adjudication Committee: Allen P. Kaplan (Chair), Nancy Brown, Bruce Zuraw.
Endpoint Adjudication Committee: Scott Solomon (Chair), Akshay S. Desai (Co-Chair), Peter Finn, Larry Weinrauch, Howard Hartley, Ebrahim Barkoudah, Chau Duong
Investigators: NL = National Leader.
Argentina
Felipe Martinez (NL), J. Albisu, A. Alvarisqueta, M. Amuchastegui, A. Astesiano, E. Avila, J. Beloscar, M. Berli, C. Borrego, B. Bustos, P. Calella, J. Carbajales, G. Caruso, O. Caruso, H. Casabé, J. Cimbaro Canella, F. Colombo Berra, R. Colque, R. Costello, R. Dran, F. Ferre Pacora, A. Gabito, J. Glenny, P. Guzman, J. Ibañez, R. Ingaramo, C. Kotliar, R. Leon de la Fuente, A. Liberman, G. Liniado, J. Llanos, L. Lobo Marquez, H. Luquez, I. Mackinonn, M. Mallagray, R. Martingano, D. Mercado, G. Moises Azize, E. Petenian, M. Rodriguez, C. Rojas, A. Romano, A. Salvatierra Ruiz, A. Sanchez, R. Sarjanovich, A. Sarries, L. Schiavi, H. Sessa, J. Soler, M. Vico, A. Vives Galeano, C. Zaidman.
Belgium
Johan Vanhaecke (NL), P. Decroly, P. Dendale, B. Ector, A. Friart, A. Heyse, L. Missault, T. Mulleners, W. Smolders, H. Vandekerckhove, M. Vincent, C. Weytjens, B. Wollaert.
Brazil
Felix Ramires (NL), W. Barroso, R. Bassan, F. Borelli, R. Botelho, J. Braga, M. Braile, C. da Costa, F. da Costa, C. de Andrade, N. Duda, M. Garcia, O. Greco, M. Hernandez, C. Jaeger, I. Kohler, E. Mesquita, J. Moraes Jr, J. Neto, F. Neuenschwander, M. Paiva, A. Rabelo, S. Rassi, R. Rech, G. Reis, H. Reis, P. Rossi, W. Saporito, M. Simoes, F. Vilas Boas.
Bulgaria
Tzvetana Katova (NL), H. Benov, P. Chobanska, B. Chompalova, S. Denchev, B. Dimov, T. Donova, P. Georgiev, D. Gotchev, A. Goudev, T. Gruev, V. Hergeldjieva, P. Lazov, I. Manukov, L. Mihov, M. Milanova, A. Mileva, V. Mincheva-Kabakchieva, Z. Parvanova, G. Pencheva, S. Petranov, K. Ramshev, A. Staneva, S. Tisheva, G. Todorov, M. Tokmakova, M. Tzekova, P. Valchanova, Y. Yotov, V. Zhelev.
Canada
Malcolm Arnold (NL), S. Bergeron, R. Bourgeois, S. Bourgeois, J. Cha, M. DeGrâce, D. Delgado, F. Deslongchamps, D. Dion, N. Giannetti, T. Huynh, J. Johnston, P. Klinke, J. Kornder, R. Labonte, C. Lauzon, S. Lepage, G. Moe, D. Murthy, S. Pandey, J. Parker, M. Rajda, S. Robinson, D. Rupka, G. Sabe-Affaki, F. Sestier, R. Sheppard, L. Yao.
Chile
F. Albornoz, P. Avendano, L. Cobos, E. Escobar, M. Fernandez, J. Jalil, F. Lanas, P. Sepulveda, B. Stockins, P. Yovaniniz.
China
Jun Huang (NL), M. Gui, H. Hu, Y. Ke, L. Li, X.D. Li, X.L. Li, W. Li, X. Liu, S. Liu, G. Ma, N. Sun, G. Xu, K. Yang, Z. Yuan, J. Zhang, R. Zhao.
Colombia
Efrain Gomez (NL), J. Accini, A. Almanzar, J. Coronel, C. Cotes, L. Echeverria, F. Manzur, E. María, M. Rodriguez, A. Sotomayor, M. Urina, J. Velasquez, S. Vélez, B. Vesga.
Czech Republic
Jan Belohlavek (NL), H. Burianova, J. Carda, V. Cech, M. Cepelak, A. Hanustiakova, I. Horny, K. Kolar, J. Krupicka, J. Kvasnicka, P. Lindovsky, Z. Lorenc, F. Malek, J. Malik, A. Mandovec, R. Nechanicky, I. Petrova, B. Podzemska, P. Povolny, M. Radvan, M. Richter, V. Riha, J. Slaby, J. Svejda, P. Telekes, J. Ulman, Z .Vomacka.
Denmark
Ole Lederballe (NL), H. Bjerregaard-Andersen, N. Bruun, K. Egstrup, H. Elming, O. Goetzsche, F. Hald Steffensen, L. Koeber, O. May, H. Rickers.
Dominican Republic
Angel Gonzalez (NL), P. Martinez, A. Paulino.
Ecuador
Yan Carlo Duarte (NL), C. Delgado, Y. Duarte, L. Hidalgo, C. Mariscal, R. Marmol.
Estonia
Arvo Rosenthal (NL), A. Kaasik, J. Kaik, E. Laane.
Finland
Keijo Peuhkurinen (NL), T. Jääskeläinen, K .Kiilavuori, J. Taurio.
France
Alber-Alain Hagege (NL), A. Alexeeva-Kovalchuck, F. Bauer, P. Berdague, J. Berneau, J. Bouvier, T. Damy, J. Davy, E. Decoulx, N. El Mansour, C. Etchecopar, M. Galinier, P. Gibelin, P. Gosse, J. Labeque, B. Livarek, D. Logeart, M. Martelet, P. Nazeyrollas, Y. Neuder, J. Poulard, R. Rihani, R. Sabatier, N. To, F. Zannad.
Germany
Michael Böhm (NL), V. Adelberger, A. Al-Zoebi, A. Bastian, S. Behrens, H. Bessler, R. Braun, M. Buhr, G. Cieslinski, W. Daut, H. Demmig, S. Denny, H. Ebert, C. Fechtrup, S. Fischer, S. Genth-Zotz, U. Gerbaulet, H. Germann, S. Gessner, G. Gola, G. Groenefeld, A. Hagenow, J. Hampf, A. Hartmann, G. Hauf, C. Hegeler-Molkewehrum, P. Hegemann, S. Hermes, A. Himpel-Boenninghoff, S. Hoeltz, R. Jerwan-Keim, C. Kadel, C. Karle, I. Kindermann, M. Knapp, K. Krause, B. Krosse, U. Kuehne, M. Kuhrs, M. Leicht, M. Loebe, H. Loos, H. Mehling, K. Melchior, F. Menzel, M. Natour, I. Naudts, R. Naumann, R. Nischik, H. Olbrich, W. Pohl, M. Prohaska, N. Proskynitopoulos, S. Regner, D. Reimer, S. Roeder, R. Rummel, P. Salbach, T. Schaefer, T. Schaum, I. Schenkenberger, A. Schindler, A. Schmidt, E. Schmidt, A. Schnabel, W. Schneider, R. Schneider, A. Schreckenberg, F. Schreibmueller, M. Schreiner, T. Schroeder, M. Schumacher, A. Segner, H. Seibert, K. Siao, H. Sohn, R. Stoehring, G. Tangerding, G. Taubert, A. Terhorst, C. Tesch, N. Toursarkissian, K. Tyler, P. Uebel, J. vom Dahl, K. Weyland, A. Wilke, A. Yilmaz, R. Zotz.
Guatemala
Juan Luis Arango (NL), J. Arriola, V. Corona, S. Leal, E. Lopez, R. Munoz, A. Ovando, A. Paniagua, D. Rodriguez, L. Velasquez, F. Wyss.
Hong Kong
S. Li, H. Tse, B. Yan, G. Yip.
Hungary
Bela Merkely (NL), G. Andrassy, P. Andreka, J. Bakai, K. Csapo, A. Cziraki, I. Edes, T. Forster, E. Hajko, A. Illes, L. Janoskuti, A. Kalina, Z. Kovacs, Z. Laszlo, G. Lupkovics, A. Matoltsy, G. Muller, A. Nagy, E. Noori, N. Nyolczas, A. Papp, C. Salamon, G. Szantai, A. Szocs, J. Tomcsanyi, K. Toth, I. Varju, G. Veress, A. Vertes, K. Zamolyi, Z. Zilahi.
Iceland
Karl Andersen (NL), T. Gudnason, A. Sigurdsson, G. Thorgeirsson.
India
M. Srinivasa Rao (NL), A. Abhyankar, D. Agarwal, R. Aggarwal, D. Banker, V. Bisne, P. Bohra, V. Chopra, S. Dani, A. Dharmadhikari, M. Fulwani, M. Gadkari, N. Ghaisas, B. Gowdappa, S. Gupta, S. Hiremath, P. Jagtap, V. Jain, A. Jain, R. Jindal, S. Joseph, P. Kerkar, M. Kumbla, B. Malipeddi, G. Mathan, A. Mehta, M. Mohan, L. Murthy, A. Nair, V. Pai, A. Pandey, V. Prakash, B. Raghuraman, S. Rao, N. Reddy, P. Sarma, P. Shah, K. Shamsudden, K. Sharma, S. Sinha, B. Thakkar, S. Thanvi, P. Trivedi, V. Vijan, B. Yugandhar.
Israel
D. Aronson, T. Ben Gal, S. Goland, A. Katz, A. Keren, B. Lewis, A. Marmor, S. Mayler, M. Shochat.
Italy
Michele Senni (NL), L. Anastasio, M. Baldin, C. Brunelli, G. Casolo, C. Coppolino, F. Cosmi, G. Danzi, M. Destro, T. Di Napoli, A. D'Ospina, A. Fucili, G. Lembo, E. Mannarino, D. Marchese, C. Minneci, M. Modena, L. Mos, C. Opasich, G. Pajes, F. Perticone, P. Pileri, E. Ronchi, P. Saba, J. Salerno Uriarte, M. Sicuro, F. Silvestri, V. Spagnuolo, S. Taddei, P. Terrosu, M. Tespili, W. Vergoni, M. Volterrani.
Latvia
Andrejs Erglis (NL), G. Dormidontova, R. Eglite, T. Lvova, G. Rancane, I. Sime.
Lithuania
Zaneta Petrulioniene (NL), D. Luksiene, R. Mazutavicius, R. Miliuniene, R. Slapikas.
Malaysia
D. Chew, O. Ismail, T. Ong, A. Wan.
Mexico
Edmundo Bayram Llamas (NL), M. Aguilera, J. Arenas, J. Carrillo, J. Gonzalez Guerra, S. León, G. Llamas, A. Macias, O. Orihuela, A. Pavía, T. Rodriguez, I. Rodríguez, E. Salcido, G. Solache, R. Velasco.
The Netherlands
Arend Mosterd (NL), D. Basart, L. Bellersen, H. Brunner-La Rocca, C. de Nooijer, F. Den Hartog, A. Derks, R. Dijkgraaf, P. Dunselman, J. Eck, G. Hoedemaker, R. Kaplan, J. Koolen, J. Milhous, A. Pronk, H. Roeters van Lennep, E. Ronner, H. Swart, G. Tjeerdsma, F. Willems.
Panama
E. Aviles, G. Frago, B. Gonzalez, R. Nieto.
Peru
Walter Cabrera (NL), R. Alegre, R. Azanero, J. Garcia, A. Godoy, L. Lu, B. Orihuela, A. Rodriguez, P. Torres, J. Urquiaga.
Philippines
Antonio S. Sibulo Jr (NL), J. Anonuevo, A. Atilano, A. Borromeo, R. Castillo, P. Chua, A. Ferrolino, A. Guerrero, S. Locnen, B. Manlutac, G. Rogelio, R. Rosita, A. Ruales, G Vilela.
Poland
Marta Negrusz-Kawecka (NL), K. Cymerman, M. Dabrowska, E. Ewa Mirek-Bryniarska, M. Foczpaniak, E. Jazwinska-Tarnawska, A. Kabara, G. Kania, P. Kolaczyk, W. Kucharski, K. Landa, M. Piepiorka, Z. Pijanowski, R. Sciborski, B. Sobkowicz, M. Szpajer, M. Tyminski, P. Weglarz, C. Wojciechowska, D. Wronska.
Portugal
José Silva-Cardoso (NL), F. Almeida, A. Andrade, N. Braganca, S. Carvalho, J. Ferreira Santos, C. Fonseca, L. Oliveira, F. Padua, R. Soares.
Romania
Dragos Vinereanu (NL), M. Andor, D. Bartos, G. Basarab, I. Coman, I. Copaci, M. Cristea, S. Dragulescu, D. Enache, A. Fruntelata, D. Gaita, L. Iliescu, O. Istratoaie, D. Lighezan, C. Militaru, T. Nanea, C. Nechita, M. Puschita, M. Tomescu, M. Tudoran.
Russia
Sergey Boytsov (NL), A. Yakovlev, F. Ageev, A. Akimov, O. Averkov, M. Balliuzek, E. Baranov, E. Baranova, O. Barbarash, O. Berkovich, S. Berns, N. Bessonova, M. Boyarkin, O. Bulashova, V. Chernetsov, I. Chukaeva, I. Dovgalevskiy, S. Dovgolis, D. Dupliakov, L. Ermoshkina, S. Fitilev, A. Galiavich, G. Gendlin, A. Gofman, B. Goloschekin, T. Gomova, A. Gordienko, I. Kamenskiy, Y. Karpov, A. Kastanayan, O. Khromtsova, O. Kisliak, Z. Kobalava, A. Konrady, M. Korolev, E. Kosmacheva, V. Kostenko, N. Koziolova, A. Kuimov, E. Kulibaba, P. Lebedev, V. Lesnov, R. Libis, Y. Lopatin, V. Makukhin, I. Masterov, Y. Moiseeva, T. Morozova, I. Motilev, S. Murashkina, V. Nosov, V. Oleinikov, T. Palatkina, E. Parmon, L. Pimenov, D. Privalov, V. Rafalskiy, A. Rebrov, I. Reznik, M. Ruda, R. Saifutdinov, S. Sayganov, S. Shoustov, L. Shpagina, E. Shutemova, Y. Shvarts, M. Sitnikova, J. Sizova, O. Smolenskaya, A. Solovieva, I. Staroverov, R. Struk, A. Svistov, N. Tarasov, E. Tarlovskay, S. Tereschenko, V. Trofimov, Y. Uspenskiy, E. Vasilieva, N. Vesikova, A. Vishnevsky, D. Volkov, D. Yakhontov, V. Yakusevich, A. Yavdosuk, A. Zateyshchikova, O. Zharkov, E. Zhilaev, D. Zotov, K. Zrazhevskiy.
Singapore
Raymond Wong (NL), C. Lee, H. Ong, D. Yeo.
Slovakia
Andrej Dukat (NL), L. Antalik, A. Banikova, D. Demesova, M. Dvorzak, F. Fazekas, D. Foldiova, P. Fulop, P. Kabaivanov, J. Kovacs, V. Macek, I. Majercak, J. Mazur, A. Mihalikova, P. Olexa, J. Pacherova, M. Palinsky, J. Palka, D. Pella, S. Remisova, J. Schichorova, R. Smik, B. Sokolova, S. Such, D. Vinanska.
South Africa
Lesley Burgess (NL), F. Ahmed, D. Basson, F. Bester, A. Bruning, E. Delport, F. Dindar, J. Foccart, M. Gani, T. Gerntholtz, E. Hellstrom, A. Horak, S. Ismail, L. Jamjam, C. Kapp, G. Latiff, J. Lombard, P. Manga, M. Mkhwanazi, Z. Mohamed, M. Mpe, D. Naidoo, T. Padayachee, N. Ranjith, J. Saaiman, B. Sebopa, M. Tayob, H. Theron, B. Thomas, T. Vally, N. van der Merwe, D. van Rensburg, L. van Zyl, T. Venter, H. Wellmann.
South Korea
Kee-Sik Kim (NL), S. Baek, S. Kang, D. Kang, D. Kim, U. Kim, B. Kim, D. Park, J. Shin, B. Yoo, J. Zo.
Spain
Carlos Calvo (NL), J. Comin, J. Cosin, M. Crespo, L. De Teresa, R. Freixa, E. Galve Basilio, M. García, R. Gomez, M. Gomez Bueno, M. Jimenez, F. Martinez, M. Martinez Selles, D. Marzal, B. Muñoz Calvo, J. Nuñez Villota, D. Pascual, G. Peña, A. Reyes, M. Sanmartin, F. Torres, M. Vida.
Sweden
Michael Fu (NL), P. Ahlstroem, I. Hagerman, A. Hajimirsadeghi, A. Hansson, A. Kempe, C. Thorsén, M. Zethson-Halldén.
Taiwan
C-H Chen (NL), C.P. Chen, P. Chen, K. Hsu, L. Lin, P. Pai, H. Tsao, B. Tzeng.
Thailand
Songsak Kiatchoosakun (NL), K. Hengrussamee, D. Piyayotai, S. Sanguanwong, T. Thongsri.
Turkey
Omer Kozan (NL), M. Aktoz, A. Birdane, A. Camsari, C. Ermis, Y. Guray, H. Kudat, D. Ural, O. Yavuzgil, M. Yenigun, Z. Yigit, M. Yilmaz, M. Yokusoglu.
UK
Iain Squire (NL), P. Banerjee, C. Barr, V. Bhatia, R. Bogle, C. Boos, G. Brigden, N. Brown, S. Bulugahapitiya, M. Dayer, D. Dutka, M. El-Harari, M. Fisher, M. Gaballa, N. Gandhi, J. Glover, R. James, H. Kadr, P. Kalra, A. Kardos, C. Lang, S. Leslie, T. Levy, M. Lynch, R. MacFadyen, W. Martin, S. MeGarry, R. Mohindra, R. More, A. Moriarty, J. Murphy, R. Muthusamy, L. Neyses, A. Nightingale, L. O'Toole, D. Price, J. Purvis, A. Ryding, D. Smith, J. Sobolewska, L. Soo, D. Strain, J. Trelawny, J. Trevelyan, R. Watkin, F. Witherow, S. Woldman, Z. Yousef.
USA
Randall Starling (NL), John Teerlink (NL), P. Adamson, O. Akinboboye, A. Akyea-Djamson, A. Amkieh, A. Amos, I. Anand, V. Awasty, D. Banish, A. Bank, D. Barnard, M. Beacom, M. Berk, J. Best, S. Bilazarian, A. Bouchard, B. Bozkurt, W. Breisblatt, L. Brookfield, C. Brown, K. Browne, R. Canadas-Zizzias, K. Carr, D. Chapman, A. Chu, E. Chung, D. Colan, N. Dang, B. Davis, V. Desai, C. Dharma, J. Edwards, S. Efstratiadis, H. Eisen, P. Fattal, B. Fenster, J. Fernandez, A. Flores, E. Flores, J. Floro, G. Frivold, B. Fuhs, D. Goldscher, R. Gould, L. Grazette, G. Haas, K. Habet, T. Hack, A. Haidar, S. Halpern, J. Hargrove, J. Harris, T. Hart, B. Hattler, M. Hazelrigg, K. Heilman III, M. Heiman, A. Heroux, W. Herzog, M. Hoffman, D. Hotchkiss, C. Hunter, J. Hunter, B. Iteld, D. Jackson, N. Jaffrani, M. Janik, M. Jardula, J. Joseph, A. Kaneshige, M. Khan, M. Koren, J. Kostis, N. Laufer, K. Lee, M. Leonen, I. Lieber, M. Liu, J. Maher, A. Maisel, F. Maislos, M. Malkowski, G. Mallis, M. Mandviwala, C. Mani, M. Maurer, W. McKenzie, A. Mehrle, J. Mendez, A. Miller, R. Miller, J. Mishkin, J. Mitchell, F. Mody, B. Montgomery, D. Murray, A. Murray, J. Naidu, J. Neutel, M. Oberoi, T. O'Brien, S. Olsen, H. Ooi, R. Orchard, C. Parrott, J. Petersen II, T. Poling, J. Prodafikas, M. Ptacin, E. Quinlan III, T. Quinn, B. Rama, K. Ramanathan, D. Rawitscher, J. Rosado, S. Rosenthal, A. Samal, S. Schaefer, C. Schmalfuss, S. Schwartz, A. Seals, Y. Selektor, T. Seto, S. Shah, J. Shanes, S. Singh, S. Sooudi, R. Sotolongo, D. Suiter, S. Sunderam, U. Thadani, J. Thrasher, B. Trichon, W. Tuan, R. Vicuna, R. Vranian, N. Ward, D. Weinstein, T. Wells, W. Wickemeyer, J. Wight, L. Wu, Z. Xu, J. Zebrack.
Venezuela
Ivan Mendoza (NL), M. Alvarez, A. Avendaño, E. Silva, G. Vergara.
References
- 1.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 2.Swedberg K. Importance of neuroendocrine activation in chronic heart failure. Impact on treatment strategies. Eur J Heart Fail. 2000;2:229–233. doi: 10.1016/s1388-9842(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 3.Packer M. Beta-blockade in the management of chronic heart failure. Another step in the conceptual evolution of a neurohormonal model of the disease. Eur Heart J. 1996;17:21–23. doi: 10.1093/eurheartj/17.suppl_b.21. (Suppl B) [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ. CONSENSUS to EMPHASIS: the overwhelming evidence which makes blockade of the renin–angiotensin–aldosterone system the cornerstone of therapy for systolic heart failure. Eur J Heart Fail. 2011;13:929–936. doi: 10.1093/eurjhf/hfr093. [DOI] [PubMed] [Google Scholar]
- 5.Shibata MC, Flather MD, Wang D. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail. 2001;3:351–357. doi: 10.1016/s1388-9842(01)00144-1. [DOI] [PubMed] [Google Scholar]
- 6.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 7.Lee ME, Jr., Miller WL, Edwards BS, Burnett JC., Jr. Role of endogenous atrial natriuretic factor in acute congestive heart failure. J Clin Invest. 1989;84:1962–1926. doi: 10.1172/JCI114385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada A, Tsutamoto T, Matsuda Y, Kinoshita M. Cardiorenal and neurohumoral effects of endogenous atrial natriuretic peptide in dogs with severe congestive heart failure using a specific antagonist for guanylate cyclase-coupled receptors. Circulation. 1994;89:2232–2240. doi: 10.1161/01.cir.89.5.2232. [DOI] [PubMed] [Google Scholar]
- 9.Brandt RR, Jr., Redfield MM, Aarhus LL, Lewicki JA, Burnett JC., Jr. Clearance receptor-mediated control of atrial natriuretic factor in experimental congestive heart failure. Am J Physiol. 1994;266:R936–R943. doi: 10.1152/ajpregu.1994.266.3.R936. [DOI] [PubMed] [Google Scholar]
- 10.Mangiafico S, Jr., Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC., Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs262. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray J, Struthers AD. Significance of atrial natriuretic factor in chronic heart failure. Br J Hosp Med. 1988;40:55–57. [PubMed] [Google Scholar]
- 12.Northridge DB, Jardine AG, Alabaster CT, Barclay PL, Connell JM, Dargie HJ, Dilly SG, Findlay IN, Lever AF, Samuels GM. Effects of UK 69 578: a novel atriopeptidase inhibitor. Lancet. 1989;2:591–593. doi: 10.1016/s0140-6736(89)90714-9. [DOI] [PubMed] [Google Scholar]
- 13.Münzel T, Kurz S, Holtz J, Busse R, Steinhauer H, Just H, Drexler H. Neurohormonal inhibition and hemodynamic unloading during prolonged inhibition of ANF degradation in patients with severe chronic heart failure. Circulation. 1992;86:1089–1098. doi: 10.1161/01.cir.86.4.1089. [DOI] [PubMed] [Google Scholar]
- 14.Newby DE, McDonagh T, Currie PF, Northridge DB, Boon NA, Dargie HJ. Candoxatril improves exercise capacity in patients with chronic heart failure receiving angiotensin converting enzyme inhibition. Eur Heart J. 1998;19:1808–1813. doi: 10.1053/euhj.1998.1201. [DOI] [PubMed] [Google Scholar]
- 15.McClean DR, Ikram H, Garlick AH, Richards AM, Nicholls MG, Crozier IG. The clinical, cardiac, renal, arterial and neurohormonal effects of omapatrilat, a vasopeptidase inhibitor, in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:479–486. doi: 10.1016/s0735-1097(00)00741-5. [DOI] [PubMed] [Google Scholar]
- 16.Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, Porter CB, Proulx G, Qian C, Block AJ. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]
- 17.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 18.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Fryer RM, Segreti J, Banfor PN, Widomski DL, Backes BJ, Lin CW, Ballaron SJ, Cox BF, Trevillyan JM, Reinhart GA, von Geldern TW. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. 2008;153:947–955. doi: 10.1038/sj.bjp.0707641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor–neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 21.Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 22.Hegde LG, Yu C, Renner T, Thibodeaux H, Armstrong SR, Park T, Cheruvu M, Olsufka R, Sandvik ER, Lane CE, Budman J, Hill CM, Klein U, Hegde SS. Concomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the rat. J Cardiovasc Pharmacol. 2011;57:495–504. doi: 10.1097/FJC.0b013e318210fc7e. [DOI] [PubMed] [Google Scholar]
- 23.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 24.The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 26.Prasad PP, Yeh C-M, Gurrieri P, Glazer R, McLeod J. Pharmacokinetics of multiple doses of valsartan in patients with heart failure. J Cardiovasc Pharmacol. 2002;40:801–807. doi: 10.1097/00005344-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Maisel A, Mueller C, Adams K, Jr, Anker SD, Aspromonte N, Cleland JG, Cohen-Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Wedel H, McMurray JJ, Lindberg M, Wikstrand J, Cleland JG, Cornel JH, Dunselman P, Hjalmarson A, Kjekshus J, Komajda M, Kuusi T, Vanhaecke J, Waagstein F CORONA Study Group. Predictors of fatal and non-fatal outcomes in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): incremental value of apolipoprotein A-1, high-sensitivity C-reactive peptide and N-terminal pro B-type natriuretic peptide. Eur J Heart Fail. 2009;11:281–291. doi: 10.1093/eurjhf/hfn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 30.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 31.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 32.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, Bhat G, Goldman S, Fletcher RD, Doherty J, Hughes CV, Carson P, Cintron G, Shabetai R, Haakenson C. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 33.The NETWORK Investigators. Clinical outcome with enalapril in symptomatic chronic heart failure;a dose comparison. Eur Heart J. 1998;19:481–489. doi: 10.1053/euhj.1997.0839. [DOI] [PubMed] [Google Scholar]
- 34.Nanas JN, Alexopoulos G, Anastasiou-Nana MI, Karidis K, Tirologos A, Zobolos S, Pirgakis V, Anthopoulos L, Sideris D, Stamatelopoulos SF, Moulopoulos SD. Outcome of patients with congestive heart failure treated with standard versus high doses of enalapril:a multicenter study. High Enalapril Dose Study Group. J Am Coll Cardiol. 2000;36:2090–2095. doi: 10.1016/s0735-1097(00)01025-1. [DOI] [PubMed] [Google Scholar]
- 35.Komajda M, Lutiger B, Madeira H, Thygesen K, Bobbio M, Hildebrandt P, Jaarsma W, Riegger G, Rydén L, Scherhag A, Soler-Soler J, Remme WJ CARMEN investigators and co-ordinators. Tolerability of carvedilol and ACE-inhibition in mild heart failure. Results of CARMEN (Carvedilol ACE-Inhibitor Remodelling Mild CHF EvaluatioN) Eur J Heart Fail. 2004;6:467–475. doi: 10.1016/j.ejheart.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, Krum H, Ponikowski P, Skene A, van de Ven L, Verkenne P, Lechat P CIBIS III Investigators. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426–2435. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- 37.Messner Pellenc P, Rudnicki A, Leclercq F, Grolleau R. Enalapril in the treatment of mild-to-moderate heart failure in general medical practice: a prospective and multicentre study concerning 17,546 patients. Acta Cardiol. 1995;50:187–201. [PubMed] [Google Scholar]
- 38.Cohn JN, Tognoni G Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 39.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 40.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 41.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 42.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 43.Lafferty HM, Gunning M, Silva P, Zimmerman MB, Brenner BM, Anderson S. Enkephalinase inhibition increases plasma atrial natriuretic peptide levels, glomerular filtration rate, and urinary sodium excretion in rats with reduced renal mass. Circ Res. 1989;65:640–646. doi: 10.1161/01.res.65.3.640. [DOI] [PubMed] [Google Scholar]
- 44.van der Zander K, Houben AJ, Hofstra L, Kroon AA, de Leeuw PW. Hemodynamic and renal effects of low-dose brain natriuretic peptide infusion in humans: a randomized, placebo-controlled crossover study. Am J Physiol Heart Circ Physiol. 2003;285:H1206–H1212. doi: 10.1152/ajpheart.00085.2003. [DOI] [PubMed] [Google Scholar]
- 45.Jungmann E, Konzok C, Höll E, Fassbinder W, Schöffling K. Effect of human atrial natriuretic peptide on blood glucose concentrations and hormone stimulation during insulin-induced hypoglycaemia in healthy man. Eur J Clin Pharmacol. 1989;36:593–597. doi: 10.1007/BF00637742. [DOI] [PubMed] [Google Scholar]
- 46.Pfister R, Sharp S, Luben R, Welsh P, Barroso I, Salomaa V, Meirhaeghe A, Khaw KT, Sattar N, Langenberg C, Wareham NJ. Mendelian randomization study of B-type natriuretic peptide and type 2 diabetes:evidence of causal association from population studies. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001112. e1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]