Abstract
The Proterozoic-Cambrian transition records the appearance of essentially all animal body plans (phyla), yet to date no single hypothesis adequately explains both the timing of the event and the evident increase in diversity and disparity. Ecological triggers focused on escalatory predator–prey “arms races” can explain the evolutionary pattern but not its timing, whereas environmental triggers, particularly ocean/atmosphere oxygenation, do the reverse. Using modern oxygen minimum zones as an analog for Proterozoic oceans, we explore the effect of low oxygen levels on the feeding ecology of polychaetes, the dominant macrofaunal animals in deep-sea sediments. Here we show that low oxygen is clearly linked to low proportions of carnivores in a community and low diversity of carnivorous taxa, whereas higher oxygen levels support more complex food webs. The recognition of a physiological control on carnivory therefore links environmental triggers and ecological drivers, providing an integrated explanation for both the pattern and timing of Cambrian animal radiation.
Keywords: evolution, hypoxia, Ediacaran, Metazoa
Cambrian fossils chronicle the appearance of essentially all high-level animal body plans, as measured by cumulative first appearances of metazoan phyla and classes, in a geologically brief interval between ∼540 and 500 million years ago (1, 2). Hypotheses to explain this event have commonly focused on either external controls, such as increasing oxygenation of the atmosphere–ocean system (1, 3–5), or internal controls based on an evolutionary, ecological, or genomic breakthrough (2, 6–11). Recently, hypotheses in the latter category have emphasized the importance of macropredation in facilitating observed increases in diversity and disparity across the Proterozoic-Cambrian transition (6–10).
External and internal controls have distinct attractions as triggers for Cambrian radiation. External controls relating to environmental oxygenation can explain the timing of the radiation—in other words, why animals radiated so dramatically beginning ∼540 Ma, and not earlier or later. Indeed, the appearance of large, complex animals in the fossil record seems to follow directly on the heels of an Ediacaran increase and stabilization of marine oxygen levels as inferred from a number of different proxy records (12, 13). Such an environmental shift could remove a barrier to animal evolution, but aside from direct links to maximum permissible body size (14), it lacks an explicit mechanism to generate diversity (new species) and disparity (new body plans). There is no theoretical reason why ocean redox change should generate the evolutionary novelties—specifically the fundamentally new bauplans—seen in the Cambrian fossil record (15).
In contrast, ecological hypotheses focused on predation contain a clear driving mechanism for morphological innovation, namely selection pressures in evolving food webs. They can also explain the origin and maintenance of high-level body plan disparity through the principle of frustration: organisms optimally suited to one task will be less well suited for another, leading to a roughening of the fitness landscape and isolation of distinct fitness peaks (16). Consistent with this hypothesis, the origin of carnivory itself appears to be temporally correlated with the Proterozoic-Cambrian transition (Fig. 1), a prerequisite if predator–prey “arms races” are to be viewed as the driving forces behind morphological innovation. In this discussion, we distinguish carnivory as mobile animal–animal interactions, as opposed to predation, which more broadly refers to one organism consuming another and may be as ancient as Eukarya (17). The oldest paleontological evidence for carnivory comes from circular perforations interpreted as drill holes in the lightly biomineralized metazoan fossil Cloudina from upper Ediacaran rocks in China (18). Strong evidence for carnivory can further be found in chaetognath fossils—voracious predators in the modern ocean—including the widespread early Cambrian skeletal fossil Protohertzina, interpreted as chaetognath grasping spines (19), and early Cambrian body fossils (20). Fossil aggregates and preserved gut contents in Cambrian Lagerstätten (e.g., hyolith shells in priapulid guts) provide additional fossil evidence for carnivory in early Cambrian oceans (21).
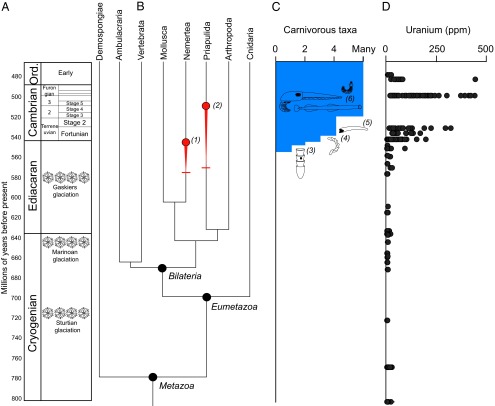
Fig. 1.
The temporal origins of carnivory in animals. (A) Geological time scale for the Cryogenian-Ordovician (Ord.). (B and C) Origins of carnivorous metazoans as inferred from the molecular (2, 23) and fossil records. In B, red horizontal whiskers represent maximum estimates for the evolution of carnivory in nemerteans and priapulids as constrained by molecular clock ages for their divergence from noncarnivorous sister groups. Red circles represent minimum ages for carnivory as constrained by the nemertean crown group (1), as all extant nemerteans are carnivores, and early Cambrian priapulid fossils (2) with gut contents indicating a carnivorous habit. Carnivory evolved between these minimum and maximum age estimates. In C, fossil evidence for carnivory around the Precambrian-Cambrian transition includes apparent drill holes in the fossil Cloudina (3); the trace fossil Treptichnus pedum, if it represents the burrowing activities of carnivorous priapulids (47) (4); and the widespread early Cambrian small shelly fossil Protohertzina, interpreted as grasping spines of chaetognaths (19) (5). By series 2 and 3 of the Cambrian, fossil Lagerstätten record numerous carnivores including a variety of arthropods, priapulids with hyolith gut contents, and chaetognath body fossils (6) (reviewed by ref. 21). (D) The origin of carnivory coincides with a major increase in the concentration of uranium and other redox-sensitive trace elements in organic-rich, fine-grained sedimentary rocks (data replotted from ref. 48). Higher values indicate higher seawater concentrations of U and more widespread oxygenation. Other redox proxies for this interval, including patterns similar to uranium for molybdenum and vanadium, are reviewed by refs. 12 and 13.
Support for the origin of carnivory near the Proterozoic-Cambrian boundary also comes from the qualitative mapping of feeding strategies onto a time-calibrated metazoan phylogeny (2). This suggests that the last common ancestor of bilaterians and the last common ancestors of the bilaterian superclades Deuterostomia, Ecdysozoa, and Lophotrochozoa were unlikely to have been carnivorous. Evolution of the carnivorous habit cannot be constrained with confidence on the tree until the origin of crown group Nemertea (Fig. 1). Priapulids are another clade that likely evolved carnivory around the Proterozoic-Cambrian transition, although the timing can only currently be constrained between their divergence from kinorhynchs and the appearance of early Cambrian predatory forms (21), as the Priapulida crown group is undated and it is unclear whether the small nonpredatory forms at the base of this clade in morphological cladistics trees (22) represent the primitive form or are derived from a larger, potentially carnivorous ancestor. Molecular clock ages for nodes constraining the evolution of carnivory (2, 23) clearly suggest that bilaterians originated long before carnivory evolved within the clade around the Proterozoic-Cambrian transition (Fig. 1). It is worth noting that the derived nature of carnivory and the logic of phylogenetic systematics requires such a conclusion at some level regardless of the accuracy of molecular clock ages. A carnivory-based ecological hypothesis, then, can explain the pattern of morphological diversification seen in the Cambrian fossil record but does not directly address its timing.
Based on a global study of feeding strategies in modern oxygen minimum zones (OMZs), we argue here that environmental and ecological hypotheses for Cambrian animal diversification are not decoupled, but can be linked through the lens of physiological constraint. Modern OMZs impinge on more than 106 km2 of seafloor (as determined at the <0.5 mL/L or 22 µM O2 level) (24) and can serve as an analog for ancient low-oxygen oceans (4, 25). In modern OMZs, the low-oxygen conditions are important in structuring the diversity and abundance of benthic communities (26–29). Some studies have considered how changing oxygen levels affect feeding strategies in individual basins, especially among polychaetes, but unlike studies of diversity (27), a global synthesis analysis of oxygen effects on carnivory has not been conducted.
Oxygen and Carnivory in Modern OMZs
We assembled a data set comprising polychaete occurrences in low-oxygen (<2 mL/L O2; <89 µM) settings from below 150 m water depth. Water depth was constrained to be essentially beneath storm wave base, thus removing the effects of atmospheric mixing, which cause fluctuating oxygen levels not necessarily represented during sampling. Only studies using a sieve size ≤500 µm were used (30). Polychaetes were chosen as the study taxon for five reasons. First, they are the dominant macrofaunal taxon in deep-sea sediments and are especially abundant at low bottom-water oxygen concentrations, constituting up to 90% of the total fauna by abundance (26). Second, they exhibit a diversity of feeding strategies, including surface- and subsurface-deposit feeding, detritivory, filter feeding, carnivory, and even chemosymbiosis. Third, their feeding biology is relatively well understood (31), and although the feeding of most deep-sea polychaetes has not been observed in vivo, it is possible to code feeding strategies with reference to shallow water relatives. Fourth, polychaetes are more tolerant of low-oxygen levels than most other bilaterians, including arthropods and vertebrates (26, 32) and so constitute a conservative choice for our study. Finally, the use of a single group ensures that all taxa will have broadly similar physiologies and body plans, and observed trends will not be the result of ecological replacement by a different taxon with a fundamentally different bauplan. Similar correlations between oxygen and feeding ecology were found for the entire fauna in a single basin investigation of the western Indian OMZ (33), suggesting that the results extend beyond polychaetes and are unlikely to be specific to this clade.
A total of 10 published studies were identified that met the oxygen, depth, and sieve size requirements outlined above, resulting in a full data set that includes 962 polychaete species occurrences from 68 stations worldwide (Table 1 and Dataset S1). In addition to the 10 studies analyzed quantitatively for the relationship between oxygen and carnivory, a new data set from the Bay of Bengal, which contains additional very low-oxygen sites, was analyzed qualitatively for the presence–absence of carnivores. All species occurrences were coded for their likely feeding mode based on the literature, incorporating both classical observations and gut content analyses, as well as new insights from tracer studies, stable isotopes, and fatty acid analysis (see SI Materials and Methods for full coding details). In some cases, there was uncertainty in coding due to either contradictory information in the literature or low taxonomic resolution in faunal lists for higher taxa that are known to feed heterogeneously. In these cases, as well as for omnivorous taxa, we followed the logic of ref. 34 in counting these taxa as one-half carnivore for the purposes of calculating the number of carnivorous individuals in a fauna and the number of carnivorous taxa. To test the effect of this coding strategy, sensitivity analyses were conducted wherein all uncertain and omnivorous taxa were coded as either entirely carnivorous or noncarnivorous.
Table 1.
Analyzed studies
| Study | Reference | Margin | Number of stations | Oxygen range (mL/L) |
| Diaz-Casteñada and Harris, 2004 | 49 | Baja California, Mexico | 6 | 1.0–1.4 |
| Vetter and Dayton, 1998 | 50 | Southern California | 5 | 0.45–1.39 |
| Levin et al., 2010; this study | 51 | California and Oregon | 9 | 0.22–0.66 |
| Levin et al., 2000 | 52 | Oman | 5 | 0.13–0.52 |
| Hughes et al., 2009 | 53 | Pakistan | 8 | 0.1–1.78 |
| Gallardo et al., 2004 | 54 | Central Chile | 2 | 0.13–0.52 |
| Palma et al., 2005 | 55 | Chile | 11 | 0.06–1.93 |
| Levin et al., 2009 | 56 | Pakistan | 16 | 0.117–0.2 |
| Levin et al., 1991 | 57 | Volcano 7, off Mexico | 3 | 0.09–0.81 |
| Ingole et al., 2010 | 27 | Western Indian | 3 | 0.08–1.35 |
| A. Raman, this study | Bay of Bengal | 38 | 0.01–1.19 |
Number of stations refers to the stations within the specified depth and O2 range investigated here and not the total number of stations in a given study. Oxygen concentrations reported as in the published studies (mL/L O2); for reference, 0.2 mL/L ∼ 9 μmol/kg ∼ 0.29 mg/L ∼ 9 matm (also see ref. 35).
The percentage of carnivorous individuals in an assemblage and number of carnivorous taxa in an assemblage were binned for four different oxygen levels: suboxia (0–0.2 mL/L O2, or 0–9 µM; 26 stations), severe hypoxia (0.2–0.5 mL/L O2, or 9–22 µM; 13 stations), moderate hypoxia (0.5–1.0 mL/L O2, or 22–45 µM; 9 stations), and mild hypoxia (1.0–2.0 mL/L O2, or 45–89 µM; 20 stations). Binning of stations was guided by table 1 of ref. 35, which describes various O2 thresholds currently used in the low-O2 literature. We acknowledge that there are several different definitions for these thresholds, and, in particular, any definition of suboxia based on dissolved O2 concentration will encompass a variety of biogeochemical environments including many dominated by purely aerobic metabolisms (36) (see SI Materials and Methods for binning details).
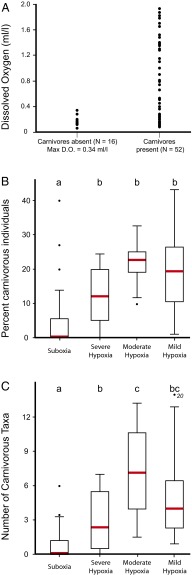
Presence–absence data for carnivores across the data set illustrate a clear relationship to oxygen. Some carnivores can survive at low oxygen levels—but carnivores are only absent from an assemblage when oxygen is <0.34 mL/L (∼15 µM; Fig. 2A). A similar pattern was seen in the Bay of Bengal (Table S1). The other investigated metrics for carnivory also show a relationship with oxygen level (Fig. 2 B and C). To test for significant differences in percent of carnivorous individuals and number of carnivorous taxa among oxygen levels, the data were log-transformed and compared using ANOVA. Post hoc Tukey-Kramer tests (α = 0.05) were used to further explore significant differences among oxygen levels. Percent carnivorous individuals (Fig. 2B) increased dramatically between suboxic and hypoxic environments (F3,64 = 14.25; P < 0.0001). Indeed, half the suboxic stations had no carnivores at all. The striking relationship between oxygen and feeding ecology is further shown by comparing the number of carnivorous taxa present, a measure of food web complexity, against oxygen (Fig. 2C). The number of carnivorous polychaete taxa in suboxic conditions was significantly lower than at higher oxygen settings (F3,64 = 20.4; P < 0.0001). These results are robust with respect to assumptions regarding feeding mode uncertainty (Tables S2 and S3). Although these analyses focus on oxygen, we recognize that other environmental parameters and physiological stressors may be important in shaping the biology of modern OMZs (26). Many potentially important variables, such as lower pH or high ammonium and sulfide levels in the sediment, can be ameliorated physiologically, although this typically requires an energetic expenditure (37). Because aerobic respiration is the means by which animals regenerate the majority of their ATP, the ability to cope with these stressors thus largely remains linked to oxygen availability.
Fig. 2.
Relationship between oxygen and carnivory in modern oxygen minimum zones. (A) Bottom-water oxygen concentrations at stations with carnivores present and absent. D.O., dissolved oxygen. (B and C) Standard box-and-whisker plots of percent carnivorous individuals (B) and number of carnivorous taxa (C) against four oxygen bins: suboxia (0–0.2 mL/L O2, or 0–9 µM; 26 stations), severe hypoxia (0.2–0.5 mL/L O2, or 9–22 µM; 13 stations), moderate hypoxia (0.5–1.0 mL/L O2, or 22–45 µM; 9 stations), and mild hypoxia (1.0–2.0 mL/L O2, or 45–89 µM; 20 stations). For box plots, the box encompasses the first and third quartiles, thick bar depicts the median, and whiskers depict true minimum and maximum values except where outliers (dots) greater than 1.5 times the interquartile range were identified. Means with the same lowercase letter at the top of the graph are not significantly different based on Tukey HSD test (α = 0.05). Outlier in mild hypoxia bin in C not to scale.
The global analysis of feeding strategies demonstrates a strong relationship between oxygen and the presence–absence of carnivores, the percentage of carnivores in an assemblage, and food web complexity as measured by species diversity of carnivores. The precise reasons why carnivores are excluded from low-oxygen environments are not fully understood, but several (nonexclusive) possibilities can be considered. The ability to be a successful carnivore will relate fundamentally to the energy expended while catching and digesting prey vs. the total energy gained. In general, macrofaunal abundances remain steady, or even increase (28), with respect to declining oxygen until it reaches very low levels, below which organismal densities drop precipitously (26). The lack of predators at the very lowest oxygen stations may therefore simply be a function of extremely low prey densities. This possibility is countered by the lack of polychaete carnivores feeding on abundant meiofaunal nematodes that characterize OMZ cores (26). A physiological cause is likely required.
As there is a direct relationship between oxygen and maximum permissible body size (14), a second possibility is the higher oxygen requirement of larger body size. Given that many predators are larger than their prey, decreasing size with lower oxygen levels (26, 38) may place a physiological limit on the ability of a carnivore to be larger than its prey. We suggest that the most likely reason for the dearth of carnivores in modern low-oxygen benthic environments is an inability to pay off the accumulated oxygen debt of a carnivorous lifestyle. Active carnivory, specifically capturing and subduing prey, is energetically costly (equals high oxygen demand) compared with either deposit or suspension feeding. In deposit and suspension feeding the mechanical costs of feeding are low and constant relative to total metabolism (39, 40). The metabolic cost of digestion (41) may also be important for low-oxygen settings. Filter and deposit feeders can maintain a continuous food input and thereby a more or less constant reduced carbon load in their gut. In contrast, many carnivores (especially those that engulf prey whole) are episodically faced with very large reduced carbon loads. Indeed, this observation forms the foundation of feeding mode inference from gut content analysis (31): deposit feeders and detritivores almost continuously have guts filled with sedimentary particles and detritus, whereas intermittently feeding carnivores are usually characterized by empty guts. Together, active and muscular predation followed by digestion of a relatively large food item results in episodically high oxygen demand. The pelagic realm of modern OMZs is characterized by a diversity of carnivores that, in the cores of OMZs, usually migrate to more oxygenated water to pay off this oxygen debt (42). Interestingly, however, some permanently pelagic OMZ residents such as the vampire squid have adapted to low-oxygen levels by losing the carnivorous feeding habit that is primitive for cephalopods and becoming detritivores (43). The inability to pay off an oxygen debt in the chronically low-oxygen benthos, where organisms are unable to migrate to higher-O2 waters, represents the most likely explanation for the observation that polychaete carnivore species diversity and percentage of the total fauna correlate with oxygen level.
Conclusions
The observation that oxygen and feeding ecology are linked by physiology in the modern ocean suggests that ecological and environmental triggers for the Cambrian radiation can be integrated in a new causal hypothesis for Cambrian animal diversification. Modern OMZs indicate that low Proterozoic oxygen levels could have supported diploblastic animals and small, thin bilaterians (25), but also that any such animals in Neoproterozoic oceans would have been constrained to small sizes and to lifestyles and feeding modes that incur little oxygen debt. This would have limited both their fossilization potential and their capacity to drive evolutionary arms races via carnivory. Rising oxygen levels would have allowed larger body sizes, but more importantly from a macroevolutionary standpoint, the first active, muscular carnivores. The establishment of stable redox conditions (44) (even if pO2 remained relatively low) may have been as important as the absolute magnitude of change itself, as unpredictable redox conditions would be deleterious to carnivores with the fluctuating oxygen demands described above. Escalatory arms races driven by these newly evolved carnivores could then explain the relatively rapid expansion of metazoan diversity and disparity near the beginning of the Cambrian Period.
An Ediacaran transition in the availability of oxygen, allowing widespread carnivory, can thus explain both the timing and recorded biological pattern of Cambrian animal diversification. Specifically, the key physiological threshold in regard to the Cambrian radiation was likely not one of body size alone but rather ecological feeding strategy. Given evolving physiologies through time, the specific oxygen levels at which ecological effects are seen in the modern may not directly relate to Proterozoic oceans. However, because carnivory in polychaetes is limited at low oxygen levels despite their being among the most low oxygen-tolerant taxa in the modern ocean (26, 32), with high-affinity respiratory pigments and good acid-base regulation—and 500 million years of natural selection doubtfully made carnivores less fit—it seems inescapable that low Proterozoic oxygen levels would have limited early animal food webs. Other factors besides carnivory and oxygen may have been important, but many of them are related to carnivore evolution itself (e.g., the evolution of sensory apparatus and vision) (11). This focus does not obviate a role for developmental genetics, but because most gene families that govern bilaterian development originated well before Cambrian body plan diversification (2, 45), the prime role of development was in assembling these preexisting genes into coherent networks to build body plans suited to the evolving Cambrian fitness landscape. The primary question in this integrated causal hypothesis now remains the timing and absolute magnitude of hypothesized late Neoproterozoic oxygenation. Continued exploration of the causes, timing, and magnitude of oxygenation will provide further insight into the role of oceanographic change in the evolution of carnivory and this unique geobiological event. Further study of the relationship between feeding ecology and oxygen in modern OMZs, as well as the coevolutionary history of animals and ocean redox state in deep time, may also help us predict future changes associated with ocean deoxygenation and expanding oxygen minimum zones (46).
Supplementary Material
Acknowledgments
We thank D. Johnston, F. Macdonald, C. Neira, K. Peterson, G. Rouse, J. Strauss, and J. Vinther for discussion; B. Runnegar, G. Narbonne, and P. Jumars for comments on an earlier draft of this paper; and B. Ingole, S. Sautya, D. Hughes, L. Harris, E. Vetter, G. Mendoza, and C. Partin for contributing raw data tables from published papers. A.H.K. thanks the National Aeronautics and Space Administration Astrobiology Institute. A.V.R. thanks T. Ganesh and Y. K. V. Rao for help in sample collection and Ministry of Earth Science, New Delhi, for funding. E.A.S. was supported by Agouron Geobiology and National Aeronautics and Space Administration Astrobiology Institute postdoctoral fellowships. We acknowledge National Science Foundation Ocean Sciences (NSF OCE) 1041062 for support of L.A.L. and NSF OCE 0927445 for support of C.A.F. and L.A.L.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312778110/-/DCSupplemental.
References
- 1.Knoll AH, Carroll SB. Early animal evolution: Emerging views from comparative biology and geology. Science. 1999;284(5423):2129–2137. doi: 10.1126/science.284.5423.2129. [DOI] [PubMed] [Google Scholar]
- 2.Erwin DH, et al. The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science. 2011;334(6059):1091–1097. doi: 10.1126/science.1206375. [DOI] [PubMed] [Google Scholar]
- 3.Cloud PE., Jr Atmospheric and hydrospheric evolution on the primitive earth. Both secular accretion and biological and geochemical processes have affected earth’s volatile envelope. Science. 1968;160(3829):729–736. doi: 10.1126/science.160.3829.729. [DOI] [PubMed] [Google Scholar]
- 4.Rhoads DC, Morse JW. Evolutionary and ecologic significance of oxygen-deficient marine basins. Lethaia. 1971;4(4):413–428. [Google Scholar]
- 5.Runnegar B. The Cambrian explosion: Animals or fossils? J Geol Soc Aust. 1982;29(3-4):395–411. [Google Scholar]
- 6.Stanley SM. An ecological theory for the sudden origin of multicellular life in the late Precambrian. Proc Natl Acad Sci USA. 1973;70(5):1486–1489. doi: 10.1073/pnas.70.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield NJ. Macroevolution and macroecology through deep time. Palaeontology. 2007;50(1):41–55. [Google Scholar]
- 8.Butterfield NJ. Animals and the invention of the Phanerozoic Earth system. Trends Ecol Evol. 2011;26(2):81–87. doi: 10.1016/j.tree.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Dzik J. In: The Rise and Fall of the Ediacara Biota. Vickers-Rich P, Komarower P, editors. London: Geological Society London; 2005. pp. 405–414. [Google Scholar]
- 10.Peterson KJ, McPeek MA, Evans DAD. Tempo and mode of early animal evolution: Inferences from rocks, Hox, and molecular clocks. Paleobiology. 2005;31(Suppl):36–55. [Google Scholar]
- 11.Parker A. In the Blink of an Eye. Cambridge, MA: Perseus Publishing; 2003. [Google Scholar]
- 12.Kah LC, Bartley JK. Protracted oxygenation of the Proterozoic biosphere. Int Geol Rev. 2011;53(11-12):1424–1442. [Google Scholar]
- 13.Och LM, Shields-Zhou GA. The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth Sci Rev. 2012;110(1-4):26–57. [Google Scholar]
- 14.Payne JL, et al. The evolutionary consequences of oxygenic photosynthesis: A body size perspective. Photosynth Res. 2011;107(1):37–57. doi: 10.1007/s11120-010-9593-1. [DOI] [PubMed] [Google Scholar]
- 15.Erwin DH. Novelties that change carrying capacity. J Exp Zoolog B Mol Dev Evol. 2012;318(6):460–465. doi: 10.1002/jez.b.21429. [DOI] [PubMed] [Google Scholar]
- 16.Marshall CR. Explaining the Cambrian “Explosion” of animals. Annu Rev Earth Planet Sci. 2006;34:355–384. [Google Scholar]
- 17.Cavalier-Smith T. Predation and eukaryote cell origins: A coevolutionary perspective. Int J Biochem Cell Biol. 2009;41(2):307–322. doi: 10.1016/j.biocel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Bengtson S, Zhao Y. Predatorial borings in late precambrian mineralized exoskeletons. Science. 1992;257(5068):367–369. doi: 10.1126/science.257.5068.367. [DOI] [PubMed] [Google Scholar]
- 19.Szaniawski H. New evidence for the protoconodont origin of chaetognaths. Acta Pal Pol. 2002;47(3):405–419. [Google Scholar]
- 20.Vannier J, Steiner M, Renvoisé E, Hu S-X, Casanova J-P. Early Cambrian origin of modern food webs: Evidence from predator arrow worms. Proc Biol Sci. 2007;274(1610):627–633. doi: 10.1098/rspb.2006.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vannier J, Chen J. Early Cambrian food chain: New evidence from fossil aggregates in the Maotianshan Shale Biota, SW China. Palaios. 2005;20(1):3–26. [Google Scholar]
- 22.Harvey THP, Dong X, Donoghue PCJ. Are palaeoscolecids ancestral ecdysozoans? Evol Dev. 2010;12(2):177–200. doi: 10.1111/j.1525-142X.2010.00403.x. [DOI] [PubMed] [Google Scholar]
- 23.Rota-Stabelli O, Daley AC, Pisani D. Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr Biol. 2013;23(5):392–398. doi: 10.1016/j.cub.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Helly JJ, Levin LA. Global distribution of naturally occurring marine hypoxia on continental margins. Deep Sea Res Part I Oceanogr Res Pap. 2004;51(9):1159–1168. [Google Scholar]
- 25.Sperling EA, Halverson GP, Knoll AH, Macdonald FA, Johnston DT. A basin redox transect at the dawn of animal life. Earth Planet Sci Lett. 2013;371–372:143–155. [Google Scholar]
- 26.Levin LA. Oxygen minimum zone benthos: Adaptation and community response to hypoxia. Oceanogr Mar Biol Annu Rev. 2003;41:1–45. [Google Scholar]
- 27.Levin LA, Gage JD. Relationship between oxygen, organic matter and the diversity of bathyal macrofauna. Deep Sea Res Part II Top Stud Oceanogr. 1998;45(1-3):129–163. [Google Scholar]
- 28.Mullins HT, Thompson JB, McDougall K, Vercoutere TL. Oxygen- minimum zone edge effects: Evidence from the central California coastal upwelling system. Geology. 1985;13(7):491–494. [Google Scholar]
- 29.Gooday AJ, et al. Habitat heterogeneity and its influence on benthic biodiversity in oxygen minimum zones. Mar Ecol (Berl) 2010;31(1):125–147. [Google Scholar]
- 30.Gage JD, Hughes DJ, Gonzalez Vecino JL. Sieve size influence in estimating biomass, abundance and diversity in samples of deep-sea macrobenthos. Mar Ecol Prog Ser. 2002;225:97–107. [Google Scholar]
- 31.Fauchald K, Jumars PA. The diet of worms: A study of polychaete feeding guilds. Oceanogr Mar Biol Annu Rev. 1979;17:193–284. [Google Scholar]
- 32.Diaz RJ, Rosenberg R. Marine benthic hypoxia: A review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanogr Mar Biol Annu Rev. 1995;33:245–303. [Google Scholar]
- 33.Ingole BS, Sautya S, Sivadas S, Singh R, Nanajkar M. Macrofaunal community structure in the western Indian continental margin including the oxygen minimum zone. Mar Ecol (Berl) 2010;31(1):148–166. [Google Scholar]
- 34.Jumars PA, Fauchald K. In: Ecology of Marine Benthos. Coull B, editor. Columbia, SC: Univ. of South Carolina Press; 1977. pp. 1–20. [Google Scholar]
- 35.Hofmann A, Peltzer E, Walz P, Brewer P. Hypoxia by degrees: Establishing definitions for a changing ocean. Deep Sea Res Part I Oceanogr Res Pap. 2011;58(12):1212–1226. [Google Scholar]
- 36.Canfield DE, Thamdrup B. Towards a consistent classification scheme for geochemical environments, or, why we wish the term ‘suboxic’ would go away. Geobiology. 2009;7(4):385–392. doi: 10.1111/j.1472-4669.2009.00214.x. [DOI] [PubMed] [Google Scholar]
- 37.Hochacka PW, Somero GN. Biochemical Adaptation. Oxford, UK: Oxford Univ Press; 2002. [Google Scholar]
- 38.Gooday AJ, et al. Faunal responses to oxygen gradients on the Pakistan margin: a comparison of foraminiferans, macrofauna and megafauna. Deep Sea Res Part II Top Stud Oceanogr. 2009;56(6-7):488–502. [Google Scholar]
- 39.Taghon GL. The benefits and costs of deposit feeding in the polychaete Abarenicola pacifica. Limnol Oceanogr. 1988;33(5):1166–1175. [Google Scholar]
- 40.Jorgensen C, Mohlenberg F, Sten-Knudsen O. Nature of relation between ventilation and oxygen consumption in filter feeders. Mar Ecol Prog Ser. 1986;29:73–88. [Google Scholar]
- 41.Secor SM. Specific dynamic action: A review of the postprandial metabolic response. J Comp Physiol B. 2009;179(1):1–56. doi: 10.1007/s00360-008-0283-7. [DOI] [PubMed] [Google Scholar]
- 42.Childress JJ, Seibel BA. Life at stable low oxygen levels: Adaptations of animals to oceanic oxygen minimum layers. J Exp Biol. 1998;201(Pt 8):1223–1232. doi: 10.1242/jeb.201.8.1223. [DOI] [PubMed] [Google Scholar]
- 43.Hoving HJT, Robison BH. Vampire squid: Detritivores in the oxygen minimum zone. Proc Biol Sci. 2012;279(1747):4559–4567. doi: 10.1098/rspb.2012.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston DT, et al. Late Ediacaran redox stability and metazoan evolution. Earth Planet Sci Lett. 2012;335:25–35. [Google Scholar]
- 45.Domazet-Loso T, Brajković J, Tautz D. A phylostratigraphy approach to uncover the genomic history of major adaptations in metazoan lineages. Trends Genet. 2007;23(11):533–539. doi: 10.1016/j.tig.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Stramma L, Schmidt S, Levin LA, Johnson GC. Ocean oxygen minima expansions and their biological impacts. Deep Sea Res Part I Oceanogr Res Pap. 2010;210(4):587–595. [Google Scholar]
- 47.Vannier J, Calandra I, Gaillard C, Zylinska A. Priapulid worms: Pioneer horizontal burrowers at the Precambrian-Cambrian boundary. Geology. 2010;38(8):711–714. [Google Scholar]
- 48.Partin CA, et al. Large-scale fluctuations in Precambrian atmospheric and oceanic oxygen levels from the record of U in shales. Earth Planet Sci Lett. 2013;369-370:284–293. [Google Scholar]
- 49.Diaz-Castañeda V, Harris L. Biodiversity and structure of the polychaete fauna from soft bottoms of Bahia Todos Santos, Baja California, Mexico. Deep Sea Res Part II Top Stud Oceanogr. 2004;51(6-9):827–847. [Google Scholar]
- 50.Vetter E, Dayton P. Macrofaunal communities within and adjacent to a detritus-rich submarine canyon system. Deep Sea Res Part II Top Stud Oceanogr. 1998;45(1-3):25–54. [Google Scholar]
- 51.Levin LA, Mendoza GF, Gonzalez JP, Thurber AR, Cordes EE. Diversity of bathyal macrofauna on the northeastern Pacific margin: The influence of methane seeps and oxygen minimum zones. Mar Ecol (Berl) 2010;31(1):94–110. [Google Scholar]
- 52.Levin LA, Gage JD, Martin C, Lamont PA. Macrobenthic community structure within and beneath the oxygen minimum zone, NW Arabian Sea. Deep Sea Res Part II Top Stud Oceanogr. 2000;47(1-2):189–226. [Google Scholar]
- 53.Hughes DJ, et al. Macrofaunal communities and sediment structure across the Pakistan Margin oxygen minimum zone, north-east Arabian Sea. Deep Sea Res Part II Top Stud Oceanogr. 2009;56(6-7):434–448. [Google Scholar]
- 54.Gallardo VA, et al. Macrobenthic zonation caused by the oxygen minimum zone on the shelf and slope off central Chile. Deep Sea Res Part II Top Stud Oceanogr. 2004;51(20-21):2475–2490. [Google Scholar]
- 55.Palma M, et al. Macrobenthic animal assemblages of the continental margin off Chile (22º to 42º S) J Mar Biol Assoc UK. 2005;85(2):233–245. [Google Scholar]
- 56.Levin LA, Whitcraft CR, Mendoza GF, Gonzalez JP, Cowie G. Oxygen and organic matter thresholds for benthic faunal activity on the Pakistan margin oxygen minimum zone (700-1100 m) Deep Sea Res Part II Top Stud Oceanogr. 2009;56(6-7):449–471. [Google Scholar]
- 57.Levin LA, Huggett CL, Wishner KF. Control of deep-sea benthic community structure by oxygen and organic-matter gradients in the eastern Pacific Ocean. J Mar Res. 1991;49(4):763–800. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.