Abstract
Previous studies revealed the existence of foreign antigen-specific memory phenotype CD8 T cells in unimmunized mice. Considerable evidence suggests this population, termed “virtual memory” (VM) CD8 T cells, arise via physiological homeostatic mechanisms. However, the antigen-specific function of VM cells is poorly characterized, and hence their potential contribution to immune responses against pathogens is unclear. Here we show that naturally occurring, polyclonal VM cells have unique functional properties, distinct from either naïve or antigen-primed memory CD8 T cells. In striking contrast to conventional memory cells, VM cells showed poor T cell receptor-induced IFN-γ synthesis and preferentially differentiated into central memory phenotype cells after priming. Importantly, VM cells showed efficient control of Listeria monocytogenes infection, indicating memory-like capacity to eliminate certain pathogens. These data suggest naturally arising VM cells display unique functional traits, allowing them to form a bridge between the innate and adaptive phase of a response to pathogens.
Keywords: homeostasis, lymphocyte
Whereas the function of memory T cells generated by exposure to foreign antigens is well studied, far less is understood about the properties of memory-like T cells, which are produced in response to homeostatic processes. Numerous studies have shown that lymphopenic conditions can drive naïve CD4 and CD8 T cells to proliferate and acquire phenotypic traits of memory cells (1–6). Furthermore, studies on lymphopenia-induced “homeostatic memory” CD8 T cells suggest they also resemble foreign antigen-primed “true” memory (TM) cells in their functional properties, including the ability to rapidly produce IFN-γ and control of bacterial and viral infections (7–9). However, differences in phenotype and function have been noted in comparison between the homeostatic memory and TM CD8 T cells, including altered kinetics of responses to pathogen and distinct expression of integrins (10, 11). Such findings indicate that homeostatic processes do not completely mimic the TM pool and suggest there may be distinct functional capacities of these populations.
In addition, our studies and others indicate that normal animals possess a population of unprimed memory-like CD8 T cells, which we termed “virtual memory” (VM) cells (to distinguish them from the induced homeostatic memory pool discussed above) (11). These cells appear soon after birth in normal mice (12) and have been found to comprise 5–20% of CD8 T cells specific for diverse foreign antigens (11–16). The finding that VM cells arise at similar frequencies in germ-free mice supports the model that such cells are produced by homeostatic mechanisms, rather than stimulation by environmental or commensal microbes (11). The fact that VM CD8 T cells appear in neonatal mice might indicate a role in protective immunity at this vulnerable stage (12), but VM cells also increase in frequency during aging, and studies on HSV responsive cells revealed that T cells with high avidity for the viral antigen were selectively maintained in the VM pool (15). Furthermore, responses by high avidity cells correlated with enhanced pathogen control in older animals, suggesting a mechanism through which VM cells may compensate for functional defects in the aging immune system (15).
However, the functional properties of naturally arising VM cells have not been clearly defined. In earlier studies, we were surprised to observe that in vivo TCR stimulation did not result in production of IFN-γ by antigen-specific VM cells (11), a result indicating that VM CD8 T cells differ from either conventional or lymphopenia-driven homeostatic memory cells (both of which are potent producers of this cytokine) (7, 8). Such findings raised the possibility that, although displaying memory phenotype, the VM pool retains naïve functional properties or may even be functionally compromised, and hence contribute little to an immune response. Furthermore, several studies suggest that memory-phenotype CD8 T cells (from unimmunized mice) exert a regulatory role, acting to inhibit CD4 and CD8 T-cell responses (17–22). Whether the antigen-specific VM population serves to restrain, rather than enhance, immune reactivity has not been tested and is especially relevant for their potential role in protective immunity against pathogens.
Here we study the functional traits of spontaneously arising VM CD8 T cells and find that this population differs from both naïve and TM CD8 T cells. In vitro studies revealed that VM CD8 T cells manifest certain functions of TM CD8 T cells (e.g., increased T-box transcription factor expression and advanced G1 cell cycle status), but also naïve-like properties, such as poor IFN-γ production after antigen stimulation. Moreover, VM CD8 T cells display qualitative and quantitative differences with naïve and TM counterparts during antigen-specific immune response in vivo yet, importantly, VM cells provide potent antigen-specific protective immunity against Listeria monocytogenes infection, similar to antigen-primed memory CD8 T cells. Together our data suggest that, despite their distinct characteristics in comparison with conventional memory and naïve CD8 T cells, VM cells display enhanced functional properties that allow them to mount a more effective immune response during primary pathogen encounter.
Results
Although VM cells constitute 5–20% of the foreign antigen-specific CD8 T-cell population in unprimed mice (11–16), the very low frequency of precursors for a given MHC/peptide ligand makes functional assessment of VM CD8 T cells challenging. To solve this problem, we used mice expressing the rearranged T cell receptor (TCR) β-chain of the ovalbumin (OVA)-specific OT-I TCR (henceforth called “Vβ5 Tg”). Pairing of this TCR chain with endogenously rearranged TCR α-chains generates a diverse, polyclonal repertoire, yet leads to an elevated precursor frequency (∼1–2%) of CD8 T cells specific for Ova/Kb in unimmunized Vβ5 Tg mice (23, 24) (Fig. S1A). Importantly, both total CD8 T-cell numbers and the frequency of memory-phenotype (CD44hi, CD122hi, CXCR3hi, and Ly6Chi) CD8 T cells are similar in Vβ5 Tg and normal B6 mice (Fig. S1 B and D). Likewise, analysis of foreign antigen-specific CD8 T cells (identified using peptide/MHC tetramers) in unprimed mice showed that the frequency and phenotype of Ova/Kb-specific VM cells in Vβ5 Tg mice was similar to polyclonal VM cells in normal B6 mice (Fig. S1D) (11). The expression of these markers on Ova/Kb tetramer-binding VM cells mirrored that of Ova/Kb-specific TM cells, which were generated by priming adoptively transferred Vβ5 Tg CD8 T cells with recombinant L. monocytogenes expressing OVA (LM-OVA) (Fig. S1D). However, whereas the TM population included both CD62L+ and CD62L− populations (i.e., central and effector memory phenotype cells, respectively), the antigen-specific VM population was uniformly CD62L+ (Fig S1D). Furthermore, Vβ5 VM cells displayed low levels of α4 integrin (CD49d) compared with antigen primed TM cells (Fig. S1 C and D), in keeping with previous studies on phenotypic differences between polyclonal VM and TM populations (11, 16). Hence, the phenotype of naïve and VM CD8 T cells within the Ova/Kb-specific population of Vβ5 mice resembled that of normal mice, yet the elevated frequency of OVA-specific cells in Vβ5 mice provided an opportunity to compare the antigen-specific responses by naïve, VM, and TM subsets.
VM CD8 T Cells Display Some but Not All in Vitro Functional Traits of True Memory CD8 T Cells.
Previous studies suggest that antigen-driven TM CD8 T cells display changes in gene expression, cytokine production, and cell cycle regulation, all of which are thought to enhance the capacity of these cells to rapidly enter an immune response (25, 26). Hence we investigated whether VM share these features with TM.
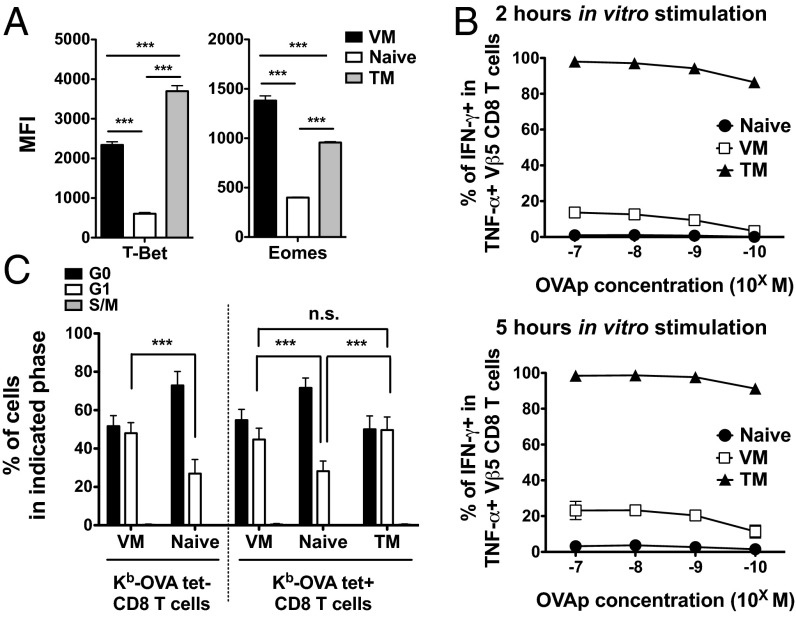
The T-box transcription factors, T-bet and Eomes, serve as critical regulators of effector functions and differentiation of memory CD8 T cells, and both factors are up-regulated in TM cells (27–29). Expression of both T-bet and Eomes was significantly increased on both VM and TM populations, compared with naïve Ova/Kb tetramer+ Vβ5 Tg CD8 T cells, although we observed reciprocal differences between the memory populations in the extent of T-bet and Eomes up-regulation (Fig. 1A and Fig. S2A). RT-PCR analysis confirmed these expression patterns at the transcriptional level (Fig. S2B). Hence, these data indicate that VM cells express not only memory phenotypic markers (Fig. S1D), but also key transcription factors characteristic of TM CD8 T cells.
Fig. 1.
Functional traits of VM, naïve, and TM CD8 T cells. (A) Mean fluorescence intensity (MFI) of T-bet and Eomes on Ova/Kb-specific VM, naïve, and TM Vβ5 CD8 T cells. Data are compiled from at least three experiments and error bars show the mean ± SD. (B) IFN-γ production in VM, naïve, and TM Vβ5 CD8 T cells upon in vitro stimulation with OVA peptide. Data show the frequency of TNF-α+, IFN- γ+ cells within the total responsive (TNF-α+) pool. The response was measure at 2 or 5 h after simulation with titrated OVA peptide doses (10−7 M–10−10 M). Graph shows compiled data from at least four independent experiments and lines show mean ± SD. (C) Analysis of cell cycle status on indicated populations. Data are compiled from at least three experiments and bars show the mean ± SD. Statistical significance is indicated (***P < 0.001; NS, not significant, is used to denote P values >0.05, Student t test).
T-box transcription factors are known to serve as positive regulators of IFN-γ production (27, 28, 30). Therefore, we next examined IFN-γ production by naïve, VM, and TM populations from Vβ5 mice, following peptide/MHC (Ova peptide) stimulation in vitro for 2 or 5 h. Because TCR engagement induces production of TNF-α in both naïve and memory CD8 T cells (8, 31), we gated on TNF-α+ cells to identify the antigen-responsive population: At 5 h, this population represented around 80% of tetramer-binding cells (Fig. S3A, allowing us to accurately assess whether TNF-α–producing cells also synthesized IFN-γ). As expected (8, 27, 28), few responding naïve phenotype CD8 T cells made IFN-γ by 5 h, whereas nearly all TM cells produced this cytokine at 2 h (Fig. 1B and Fig. S3B). Significantly, IFN-γ production by the VM CD8 T-cell population was much weaker than that of TM cells at either time point, and was only marginally increased over induction in the naïve population at limiting antigen doses (Fig. 1B and Fig. S3B). Furthermore, production of TNF-α at 2 h of stimulation was significantly greater in the TM pool compared with either VM or naïve cells (Fig. S3A). On the other hand, overall dose sensitivity was unchanged in these populations, arguing against differences in functional avidity. Such results extend and confirm our earlier in vivo studies (11) and suggest that the VM pool, despite expressing high levels of relevant T-box factors, is less competent for rapid IFN-γ production, compared with TM cells.
Finally, previous studies showed that most TM cells are maintained in G1 phase of the cell cycle, which has been proposed to allow them to progress to proliferation more quickly than naïve CD8 T cells (32–34). Hence we analyzed intracellular RNA and DNA levels (34, 35) to investigate the cell cycle status of VM, TM, and naïve CD8 T-cell populations (Fig. S4A). As expected, very few (<1%) cells of any type showed signs of active progression through cell cycle (i.e., S/G2/M phases) at steady state (Fig. S4A), but the VM and TM populations were significantly enriched in cells at G1 phase, compared with the naïve pool (Fig. 1C and Fig. S4A). Furthermore, gene expression of cell cycle regulators associated with G1 phase (CDK6 and CyclinD3) (32, 36) showed significantly elevated expression in both memory populations (Fig. S4B).
Taken together, these data support the concept that the VM population is similar to antigen-primed memory (TM) cells in some characteristics (elevated T-box factor expression, cell cycle position) yet not others (rapid, efficient IFN-γ production).
VM CD8 T Cells Preferentially Expand During the Effector Phase of Primary Immune Response Compared with Naïve CD8 T Cells.
Previous studies showed that lymphopenia-induced memory cells expanded more quickly than naïve T cells during an antigen-specific immune response (7, 8). However, such studies have not been reported for the naturally occurring VM population, due in large part to the scarcity of VM cells specific for a given antigen in normal polyclonal mice. Use of the Vβ5 system allowed us to directly compare naïve and VM cell responses to cognate antigen. Specifically, we used a dual adoptive transfer system (Materials and Methods and Fig. S5), permitting characterization of each population responding in an identical environment throughout the immune response. To avoid TCR stimulation, transferred cells were not stained with OVA/Kb tetramer (although an aliquot from each sorted sample was assessed for tetramer binding, to determine the antigen-specific precursor frequency). During early stage of the infection (0, 5 h, and 3 d postinfection), we performed Ova/Kb tetramer enrichment, to track the rare antigen-specific donor CD8 T cells.
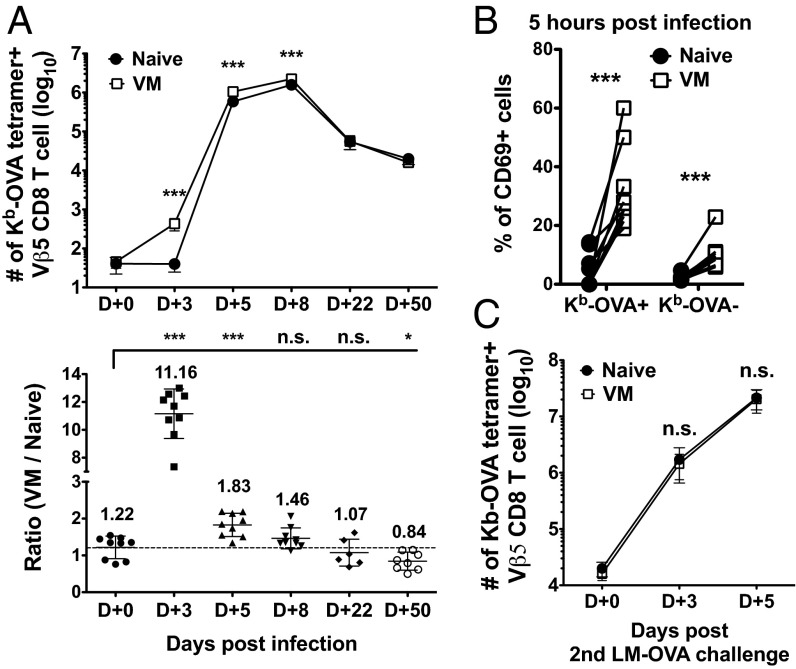
Initial engraftment of both donor populations was similar (Fig. 2A and Fig. S5B), but by day 3 of LM-OVA infection, VM CD8 T cells had undergone significant expansion, whereas the naïve pool had not increased in number, such that the VM-derived population outcompeted their naïve counterparts by more than 10-fold (Fig. 2A). The magnitude of this competitive advantage was rapidly lost, however, as the response progressed through days 5 and 8 postinfection (Fig. 2A), and at memory time points both VM- and naïve-derived populations were observed in similar numbers. Hence, these data suggest a dramatic advantage for the VM population early in the immune response to infection, whereas naïve cells “catch up” by the peak of expansion. To analyze very early antigen encounter, we examined CD69 up-regulation at 5 h postinfection. Notably, the Ova/Kb tetramer staining VM population exhibited significantly greater CD69 induction than naïve cells of the same specificity (Fig. 2B). Weaker CD69 up-regulation was observed on tetramer-negative VM cells, although whether this represents low-grade nonspecific activation or the response to other L. monocytogenes epitopes is unclear (Fig. 2B). We also examined the secondary response of cells derived from VM and naïve precursors (stimulated by infection with virulent LM-OVA at day 50) (Fig. S5). In contrast to the primary response, no numerical advantage of the VM-derived population was observed at day 3 or 5 of the recall response, which showed characteristically rapid kinetics (Fig. 2C). Such findings suggest that differences in the initial response of VM versus naïve CD8 T precursors are not carried forward into the memory pool they produce after antigen encounter.
Fig. 2.
VM CD8 T cells outcompete their naïve counterparts during the expansion phase of the primary immune response. (A) At the indicated time points following LM-OVA infection, Ova/Kb tetramer+ cells derived from VM and naïve Vβ5 donors in the spleen and superficial lymph nodes were enumerated. Data are shown as absolute numbers or ratio between VM and naïve derived cells. (B) The frequency of CD69 expressing Ova/Kb-tetramer + or Ova/Kb-tetramer− Vβ5 CD8 T cells was determined 5 h after L. monocytogenes-OVA infection. Data show CD69 expression by paired samples of naïve and VM cells in the same recipients. (C) Number of Ova/Kb-specific VM and naïve Vβ5 CD8 T cells during secondary immune respond against LM-OVA. For all experiments, the data are compiled from three independent experiments except day 22 p.i. (A), which were derived from two independent experiments (six mice total). Line graphs show mean ± SD and statistical significance is indicated (***P < 0.001; *P < 0.05; NS, not significant, is used to denote P values >0.05, Student t test).
This early proliferative advantage of VM cells could potentially be an artifact of the Vβ5 system, or specific to L. monocytogenes infections. Hence, we tested distinct model systems in which dual adoptive transfers were performed using naïve and VM populations from normal, polyclonal B6 CD8 T cells (Fig. S6). To compensate for the low precursor frequency for specific antigens, we explored the response to multiple Kb-restricted epitopes during a response to recombinant L. monocytogenes or examined the response to an immunodominant epitope (B8R) following infection with vaccinia virus (Fig. S6A). Similar to our findings with Vβ5 CD8 T cells, antigen-specific cells derived from polyclonal VM populations were present at elevated numbers compared with those derived from the naïve subset (Fig. S6 B and C). These data suggest that VM CD8 T cells activate more rapidly and expand more quickly than naïve CD8 T cells of the same specificity, leading to an initial (but transient) advantage of the VM pool, during the primary immune response.
VM CD8 T Cells Preferentially Differentiate into Short-Lived Effector Phenotype Cells and Central Memory Cells Following Priming.
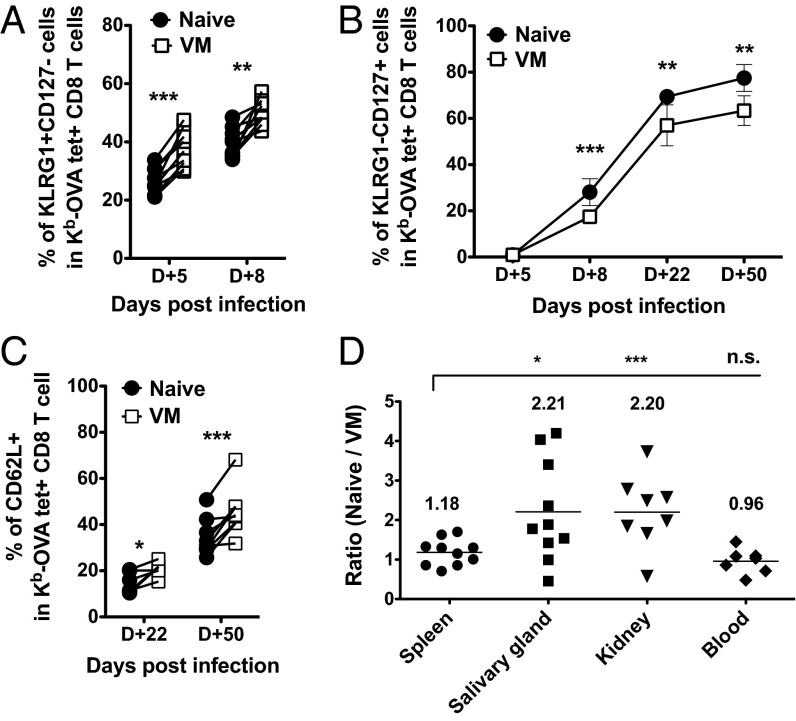
At the effector stage, naïve- and VM-derived cells showed similar capacity for cytokine production and up-regulation of T-bet and Eomes (Fig. S7 A and B), suggesting similar acquisition of effector functions and characteristics. However, the finding that initial expansion advantage of VM cells is not sustained (Fig. 2A) might suggest that a greater fraction of these cells become “short-lived effector cells” (SLECs), and hence succumb to apoptotic death as the expansion phase ends (37, 38). Indeed, we observed a modest but significant elevation in the frequency of KLRG1+ CD127lo SLEC phenotype cells among VM-derived cells at the effector stage (Fig. 3A), whereas cells derived from the naïve donor population showed a reciprocal enrichment for KLRG1− CD127hi memory phenotype cells in the late effector and memory phases (Fig. 3B). Once again, these differences between VM and naïve responder cells were only detected following priming: In the recall response, progeny of both donors gave rise to phenotypically similar secondary effector populations (Fig. S7 C and D).
Fig. 3.
Comparisons of phenotype and peripheral residency between VM and naïve CD8 T cells during primary L. monocytogenes infection. (A and B) Short-lived effector (KLRG1+ CD127lo) and memory precursor (KLRG1− CD127hi) phenotype of responding VM and naïve CD8 T cells, during effector and memory phase following LM-OVA infection. Frequencies of phenotypic subsets were determined on Ova/Kb-tetramer+ donor Vβ5 CD8 T cells at the indicated times postinfection. Line graphs show mean ± SD. (C) Central memory (CD62L+) differentiation of responding VM and naïve donor Vβ5 CD8 T cells. The frequency of CD62L+ cells in cotransfered naïve and VM populations is shown. (D) Ratio between Ova/Kb-tetramer-specific VM and naïve Vβ5 CD8 T cells in indicated tissues and blood at 50–60 d post-LM-OVA infection. (Blood contamination in each tissue was excluded as described in SI Materials and Methods). For all experiments, data were compiled from three independent experiments, except day 22 p.i. (two independent experiments; six mice total). Statistical significance between groups is indicated (***P < 0.001; **P < 0.01; *P < 0.05, whereas NS, not significant, is used to denote P values >0.05, Student t test).
We also investigated whether the VM population might also be skewed in their memory subset distribution. Two prominent memory subpopulations are CD62L+ “central memory” (TCM) and CD62L− “effector memory” (TEM) groups (39–41). Whereas TCM typically recirculate through lymphoid sites, TEM are associated with trafficking and residency in nonlymphoid tissues. Hence, we analyzed naïve- and VM-derived cells at the memory phase (days 22 and 50) to determine their phenotype and patterns of tissue distribution. Interestingly, VM-derived cells showed a significant enrichment for TCM phenotype cells compared with naïve-derived cells (Fig. 3C). Consistent with this, the progeny of naïve responder cells were significantly overrepresented in nonlymphoid tissues (salivary gland and kidney), sites where TEM are typically enriched (Fig. 3D). These data argue that, during a primary immune response, VM cells differ from naïve cells not only in their response kinetics but by qualitative changes in the generation of effector and memory subsets.
VM and TM CD8 T Cells Show Similar Response Kinetics, but Qualitative Differences in Effector Differentiation.
To this point, our studies had focused on comparing the immune response of VM cells to naïve counterparts. However, studies using lymphopenia-driven memory CD8 T cells showed that they are outcompeted by TM cells during an immune response (8, 10). To investigate this in our studies of spontaneously generated VM CD8 T cells, we again performed dual adoptive transfer experiments: As before, congenically distinct Vβ5 TM (generated by adoptive transfer of bulk Vβ5 CD8 T cells into congenic recipients, and subsequent LM-OVA infection) and VM cells were sorted and cotransferred into recipients, which were then infected with LM-OVA (Fig. S5A). Adoptive transfer efficiencies were similar for VM and TM populations (Fig. S5B).
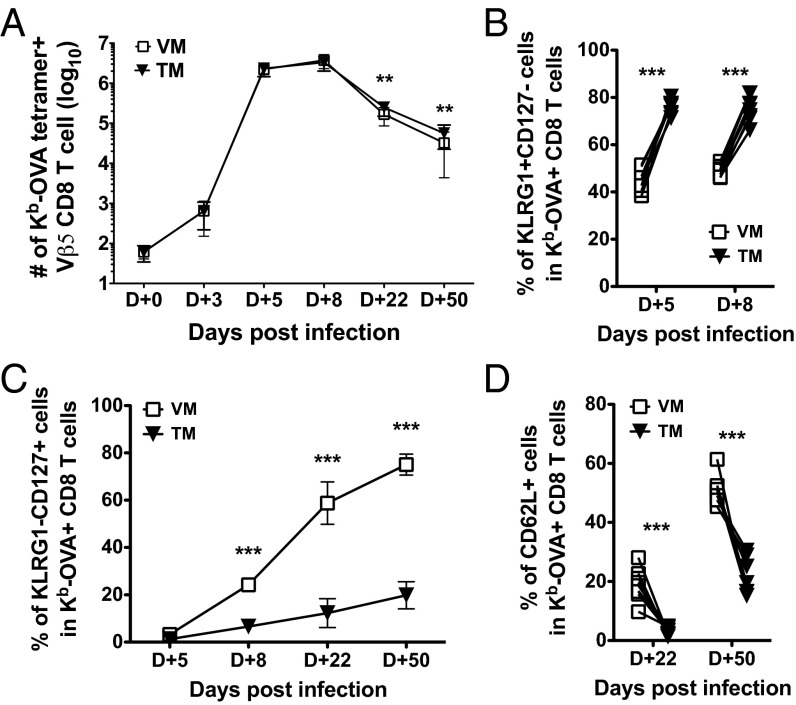
At the level of kinetics and magnitude of the response, we observed no significant differences between TM and VM populations until the memory phase, at which point the TM pool had a slight (but significant) advantage over the VM pool (Fig. 4A). In contrast to these mild effects, substantial differences were observed in the differentiation of TM- and VM-derived cells. Previous studies have shown that secondary immune responses (of true memory CD8 T cells) generate a population of effector-like CD8 T cells that are sustained into the memory phase, and a corresponding underrepresentation of TCM cells (25, 42, 43). Indeed, we observed high frequencies of KLRG-1+ CD127lo cells and a corresponding deficit in production of KLRG-1− CD127hi memory cells, for the population generated from the TM pool (Fig. 4 B and C). In contrast, the responder cells derived from the VM pool showed much more efficient production of KLRG-1− CD127hi memory-phenotype cells. Furthermore, whereas responding TM cells showed inefficient production of CD62L+ (i.e., TCM) phenotype memory cells, the VM-derived pool produced a significantly larger CD62Lhi subset (Fig. 4D), echoing similar studies using lymphopenia-induced memory OT-I CD8 T cells (10). It is important to note, however, that the bias of VM-derived cells toward the TCM phenotype did not result in their more efficient maintenance, compared with the TM-derived pool (Fig. 4A). Overall, our findings suggest that VM CD8 T cells expand similarly to true memory CD8 T cells, yet display substantially altered differentiation characteristics.
Fig. 4.
VM and TM CD8 T cells show similar kinetics in proliferation, but distinct effector differentiation. (A) Graphs show the number of cotransferred Ova/Kb-specific VM and TM Vβ5 CD8 T cells in the spleen and superficial lymph nodes at the indicated times post–L. monocytogenes-OVA infection. (B–D) Phenotypic comparison between responding VM and TM CD8 T cells, gated on OVA/Kb tetramer+ donor cells at the indicated days post–L. monocytogenes-OVA infection. Graphs show compiled data from three independent experiments and lines show mean ± SD. Statistical significance between groups is indicated (***P < 0.001; **P < 0.01; NS, not significant, is used to denote P values >0.05, Student t test).
VM Cells Provide Potent Antigen-Specific Protective Immunity Against L. monocytogenes Infection.
Our findings indicate that VM cells display only some characteristics of true memory cells. This raised the question of whether VM cells would be capable of mediating protective responses against infection. Our previous studies showed that lymphopenia-induced homeostatic memory OT-I CD8+ T cells were capable of L. monocytogenes control (8, 9), and a recent report showed similar potent protection by the VM population that arises spontaneously in intact OT-I mice (16). However, it was not clear whether these findings would correspond to the polyclonal Vβ5 CD8 T cells studied here. In particular, the poor induction of IFN-γ following TCR stimulation of Vβ5 VM (Fig. 1B and Fig. S3B) was noteworthy, because this factor is critical in control of several pathogens, including Listeria (44). Furthermore, several studies have suggested that memory-phenotype CD8 T cells from unimmunized mice have regulatory functions (17–22): Hence, the VM population might inhibit, not elicit pathogen control. To explore this issue, we performed studies to compare VM, naïve, and TM CD8 T cells for protection against virulent LM-OVA infection. Cells were isolated and sorted as before, but populations were transferred into separate hosts, and were designed to include ∼2 × 104 Ova/Kb tetramer+ cells. Host mice were subsequently challenged with a LD50 dose of virulent LM-OVA (or wild-type L. monocytogenes) and on day 5 after infection, cfu in the spleen and liver were measured, and the expansion of donor CD8 T cells was assayed as in previous reports.
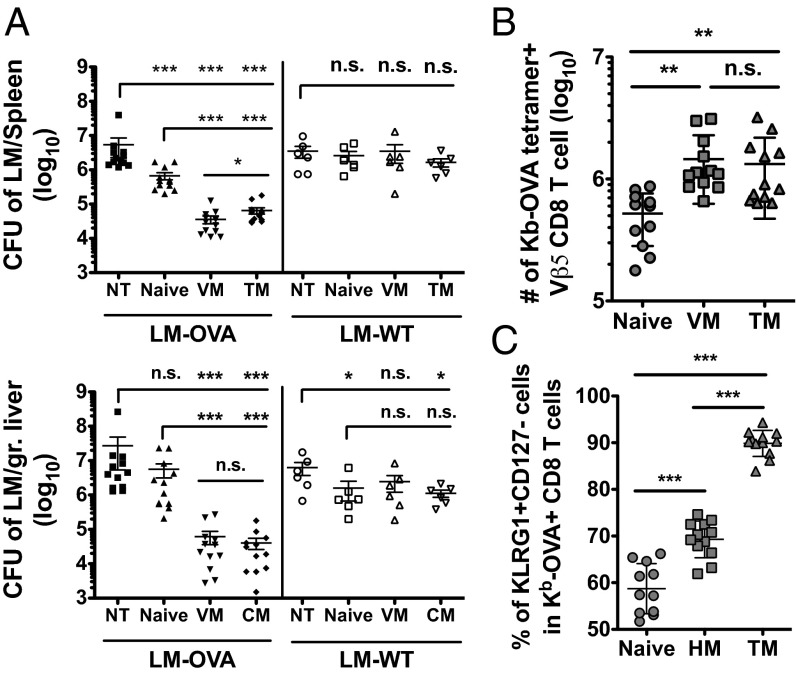
Similar to other studies using OT-I TCR transgenic CD8 T cells (8, 9, 16), naïve Vβ5 CD8 T cells offered little protection against LM-OVA infection, whereas antigen-primed TM cells induced significantly greater bacterial control, in both spleen and liver (Fig. 5A). Remarkably, the VM population was at least as potent as TM cells in mediating LM-OVA clearance (Fig. 5A), suggesting that spontaneously arising VM cells can provide efficient protective immunity. Given the low number of donor cells transferred (2 × 104 cells, corresponding to ∼2 × 103 cells with a calculated 10% take), these data suggest that, like the TM population, VM cells exhibit potent and efficient protective capacity. Because we could not purify Ova/Kb-specific VM cells before transfer (which would necessarily have involved staining with peptide/MHC tetramers, possibly affecting functional responses), it was possible that bacterial control by the polyclonal VM population involved responses to other L. monocytogenes epitopes and/or non–TCR-specific responses. For example, we previously showed that VM cells (like TM cells) elaborate IFN-γ when stimulated with IL-12 and IL-18, in the absence of TCR engagement (11, 45–47). To test whether protection in our studies was antigen specific, we conducted parallel experiments using nonrecombinant (WT) L. monocytogenes infection. In this situation, none of the transferred Vβ5 populations provided protection in the spleen, and L. monocytogenes control in the liver was insubstantial (Fig. 5A).
Fig. 5.
VM cells provide potent antigen-specific protective immunity against L. monocytogenes infection. Naïve, VM, and TM Vβ5 CD8 T cells were sorted and ∼2 × 104 Ova/Kb-specific cells were singly transferred into unprimed recipients. Host mice were infected the next day with virulent LM-OVA or wild-type L. monocytogenes (LM-WT). (A) Cfu of indicated L. monocytogenes strains in the spleens and livers of the recipient mice 5 d after infection. (B) Number of Ova/Kb-specific CD8 T cells and (C) frequency of KLRG1+CD127− CD8 T cells in the spleens of recipient mice at day 5 post–LM-OVA infection. Graphs show compiled data from four independent experiments for LM-OVA infection and two independent experiments for L. monocytogenes-WT infection and lines show mean ± SD. Statistical significance between groups is indicated (***P < 0.001; **P < 0.01; *P < 0.05; NS, not significant, is used to denote P values >0.05, Student t test).
All of the transferred populations underwent vigorous expansion after LM-OVA infection, with both memory cell populations reaching an ∼1,000-fold increase in number and significantly outexpanding naive CD8 T cells (Fig. 5B). In our earlier studies using attenuated LM-OVA, there was little difference between expansion of naïve and VM cells at this time point (day 5); hence these findings may relate to use of virulent LM-OVA for the protection assays. In keeping with our other findings, we found that the frequency of KLRG1+CD127lo effector cells was significantly different for each donor population, following the hierarchy TM > VM > naïve (Fig. 5C). Hence, these phenotypic characteristics of each responsive pool were preserved during the response to virulent LM-OVA.
These data suggest that, despite their distinct characteristics in comparison with both TM and naïve CD8 T cells, the VM pool can provide potent and antigen-specific protective immunity against pathogen infection.
Discussion
Studies over the last dozen years have shown that memory T cells are not exclusively generated through encounter with foreign antigen, but can also be induced through homeostatic pathways (1–6). Furthermore, we and others reported that a population of memory-like cells arise spontaneously in unimmunized mice and that such cells constitute a small but significant fraction of the precursors specific for a given foreign antigen, before priming (11, 12). Data in this report suggest that the functional properties of these virtual memory cells lies in between those of naïve and true memory cells. The VM pool differed from naïve cells (and resembled TM cells) in their early in vivo expansion, elevated expression of T-box factors, and position in G1 stage of the cell cycle. Perhaps most importantly, VM cells resembled true memory cells in highly efficient, antigen-specific control of the pathogen L. monocytogenes. On the other hand, we found that the VM pool differed markedly from TM cells in their preferential differentiation toward the TCM phenotype following antigen encounter in vivo, and that VM cells were much less efficient at rapid production of IFN-γ following TCR stimulation. The latter findings differ slightly from our initial study, which had concluded that VM cells behaved like naïve cells in their slow induction of IFN-γ following TCR stimulation (11). Because VM cells show strong expression of both T-box factors (T-bet and Eomes), and evidence of Tc1 differentiation (e.g., robust CXCR3 expression), their inefficient production of IFN-γ is unexpected and intriguing. Although T-bet clearly requires other factors (such as the Jmjd3 histone demethylase for chromatin remodeling of target loci) (48), such studies show good concordance for multiple T-bet targets, including CXCR3 and IFN-γ. It is possible that this trait reflects altered T-bet/Eomes expression levels (Fig. 1A), although both T-box factors are reported to be capable of promoting IFN-γ expression. Hence the selective deficiency in IFN-γ induction appears unique to VM cells.
Taken together, these data suggest homeostatic pathways only partially substitute for antigen encounter in programming full memory differentiation (although, significantly, this includes the capacity to mediate protective immunity against some infections). Interestingly, our results differ from previous studies on the properties of homeostatic memory cells generated in severely lymphopenic hosts. We and others found that such cells rapidly produce IFN-γ after TCR stimulation at levels similar to true memory cells (7, 8), whereas our current data show that the VM pool is substantially compromised in this response (Fig. 1B and Fig. S3B). On the other hand, using OT-I T cells, Cheung et al. reported that true memory cells exerted a substantial numerical advantage over lymphopenia-induced homeostatic memory cells during a competitive in vivo response to LM-OVA infection (10): In contrast, we found minimal differences in numbers of TM cells and naturally occurring VM cells during a similar response (Fig. 5 B and C). These discrepancies may reflect the distinct pathways for homeostatic memory generation entailing different intensities or mechanisms of response (1, 49). The naturally occurring VM pool arises slowly over the weeks following birth (as the T-cell compartment is filling) (12), in a process involving IL-15 presentation by CD8α+ dendritic cells (16). In contrast, acute lymphopenia provides a stronger and more sustained homeostatic signal, potentially leading to altered differentiation. Regardless, our current report suggests that the functional properties of naturally arising VM cells are not accurately reflected in studies (including our own) (8) on homeostatic memory cells artificially produced in severely lymphopenic hosts.
Interestingly, VM CD8+ T cells preferentially differentiated toward TCM phenotype after antigen priming. This was especially notable in the comparison between TM and VM, because, as expected, restimulated TM cells showed a strong bias toward TEM differentiation. Similar findings were reported in comparison of true and lymphopenia-induced memory OT-I cells (10). However, this bias toward TCM differentiation was detected even when VM cells were compared with naïve precursors, which was unexpected. Whereas the basis for this focused differentiation is unclear, a consequence is that VM cells would contribute disproportionately to the TCM pool. Because recent studies have suggested that TCM cells are especially important for control of chronic viral infections (50), these findings may suggest a particular relevance of VM cells in mounting rapid, effective responses against such pathogens.
In summary, our studies show that the spontaneously occurring virtual memory pool of CD8 T cells displays functional properties that are intermediate between naïve and true memory populations, yet mediate the sina qua non of memory cells—protection against pathogens. Although the VM pool accounts for only a fraction (∼10%) of antigen-specific precursors for a given foreign antigen, this may constitute a significant pool in the response to a complex pathogen. For example, elegant in vivo limiting dilution assays estimate that ∼14,000 CD8 T cells (in an unprimed C57BL/6 mouse) are responsive to vaccinia virus (51): this would correspond, on average, to >1,000 vaccinia-specific VM cells. Because we observed protective immunity against LM-OVA after adoptive transfer of a few thousand Ova/Kb-specific cells (Fig. 5), these calculations suggest that the naturally arising VM pool may serve a hitherto unappreciated role in “preimmune” resistance to infection.
Materials and Methods
All mice were maintained in specific pathogen-free conditions, and all animal experimental procedures were approved by the Institutional Animal Use and Care Committees at the University of Minnesota. The procedures of cell isolation, adoptive transfer, and immunization are described in SI Materials and Methods and Figs. S4 and S5A. The detailed staining conditions of surface receptors and intracellular cytokines, the IFN-γ production assay, the cell cycle analysis, the quantitative real-time PCR, the determination of resident memory CD8 T-cell in peripheral tissues, and the quantification of L. monocytogenes in spleen and liver are explained in SI Materials and Methods.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307572110/-/DCSupplemental.
References
- 1.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32(2):50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jameson SC. T cell homeostasis: Keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17(3):231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Marleau AM, Sarvetnick N. T cell homeostasis in tolerance and immunity. J Leukoc Biol. 2005;78(3):575–584. doi: 10.1189/jlb.0105050. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17(3):183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Le Campion A, et al. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc Natl Acad Sci USA. 2002;99(7):4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min B, et al. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18(1):131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 7.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192(4):557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7(5):475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton SE, Jameson SC. The nature of the lymphopenic environment dictates protective function of homeostatic-memory CD8+ T cells. Proc Natl Acad Sci USA. 2008;105(47):18484–18489. doi: 10.1073/pnas.0806487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung KP, Yang E, Goldrath AW. Memory-like CD8+ T cells generated during homeostatic proliferation defer to antigen-experienced memory cells. J Immunol. 2009;183(5):3364–3372. doi: 10.4049/jimmunol.0900641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206(2):435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188(6):2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Gruta NL, et al. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest. 2010;120(6):1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton SE, Schenkel JM, Akue AD, Jameson SC. IL-2 complex treatment can protect naive mice from bacterial and viral infection. J Immunol. 2010;185(11):6584–6590. doi: 10.4049/jimmunol.1001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudd BD, et al. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci USA. 2011;108(33):13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosinowski T, et al. CD8α+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J Immunol. 2013;190(5):1936–1947. doi: 10.4049/jimmunol.1203149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200(9):1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, et al. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci USA. 2011;108(5):2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakuraba K, Shibata K, Iwamoto Y, Yoshikai Y, Yamada H. Naturally occurring PD-1+ memory phenotype CD8 T cells belong to nonconventional CD8 T cells and are cyclophosphamide-sensitive regulatory T cells. J Immunol. 2013;190(4):1560–1566. doi: 10.4049/jimmunol.1202464. [DOI] [PubMed] [Google Scholar]
- 20.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204(10):2321–2333. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467(7313):328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai H, et al. Cutting edge: Programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185(2):803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 23.Dillon SR, Jameson SC, Fink PJ. V beta 5+ T cell receptors skew toward OVA+H-2Kb recognition. J Immunol. 1994;152(4):1790–1801. [PubMed] [Google Scholar]
- 24.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25(2):261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8(2):107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 26.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4(9):835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 27.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 28.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302(5647):1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee A, et al. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185(9):4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci USA. 2003;100(26):15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brehm MA, Daniels KA, Welsh RM. Rapid production of TNF-alpha following TCR engagement of naive CD8 T cells. J Immunol. 2005;175(8):5043–5049. doi: 10.4049/jimmunol.175.8.5043. [DOI] [PubMed] [Google Scholar]
- 32.Veiga-Fernandes H, Rocha B. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat Immunol. 2004;5(1):31–37. doi: 10.1038/ni1015. [DOI] [PubMed] [Google Scholar]
- 33.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1(1):47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 34.Munitic I, Ryan PE, Ashwell JD. T cells in G1 provide a memory-like response to secondary stimulation. J Immunol. 2005;174(7):4010–4018. doi: 10.4049/jimmunol.174.7.4010. [DOI] [PubMed] [Google Scholar]
- 35.Allam A, et al. The CD8+ memory T-cell state of readiness is actively maintained and reversible. Blood. 2009;114(10):2121–2130. doi: 10.1182/blood-2009-05-220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latner DR, Kaech SM, Ahmed R. Enhanced expression of cell cycle regulatory genes in virus-specific memory CD8+ T cells. J Virol. 2004;78(20):10953–10959. doi: 10.1128/JVI.78.20.10953-10959.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi NS, Kaech SM. Effector CD8 T cell development: A balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180(3):1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 39.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 40.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 42.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: Implications for prime-boost vaccination. J Immunol. 2006;177(2):831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 43.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203(4):919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4(10):812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 45.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198(10):1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kambayashi T, Assarsson E, Lukacher AE, Ljunggren HG, Jensen PE. Memory CD8+ T cells provide an early source of IFN-gamma. J Immunol. 2003;170(5):2399–2408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- 47.Berg RE, Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18(3):338–343. doi: 10.1016/j.coi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Miller SA, Mohn SE, Weinmann AS. Jmjd3 and UTX play a demethylase-independent role in chromatin remodeling to regulate T-box family member-dependent gene expression. Mol Cell. 2010;40(4):594–605. doi: 10.1016/j.molcel.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton SE, Jameson SC. CD8 T cell quiescence revisited. Trends Immunol. 2012;33(5):224–230. doi: 10.1016/j.it.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34(5):781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seedhom MO, Jellison ER, Daniels KA, Welsh RM. High frequencies of virus-specific CD8+ T-cell precursors. J Virol. 2009;83(24):12907–12916. doi: 10.1128/JVI.01722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.