Abstract
Climate change may disrupt interspecies phenological synchrony, with adverse consequences to ecosystem functioning. We present here a 40-y-long time series on 10,425 dates that were systematically collected in a single Russian locality for 97 plant, 78 bird, 10 herptile, 19 insect, and 9 fungal phenological events, as well as for 77 climatic events related to temperature, precipitation, snow, ice, and frost. We show that species are shifting their phenologies at dissimilar rates, partly because they respond to different climatic factors, which in turn are shifting at dissimilar rates. Plants have advanced their spring phenology even faster than average temperature has increased, whereas migratory birds have shown more divergent responses and shifted, on average, less than plants. Phenological events of birds and insects were mainly triggered by climate cues (variation in temperature and snow and ice cover) occurring over the course of short periods, whereas many plants, herptiles, and fungi were affected by long-term climatic averages. Year-to-year variation in plants, herptiles, and insects showed a high degree of synchrony, whereas the phenological timing of fungi did not correlate with any other taxonomic group. In many cases, species that are synchronous in their year-to-year dynamics have also shifted in congruence, suggesting that climate change may have disrupted phenological synchrony less than has been previously assumed. Our results illustrate how a multidimensional change in the physical environment has translated into a community-level change in phenology.
Keywords: global warming, mismatch, trophic interactions, boreal forest
The timing of phenological events is shifting as a result of climate change (1–5). Together with other adaptive mechanisms, plasticity in phenology is essential for maintaining many aspects of biodiversity in a changing environment (5–7), such as species’ demography (8), species interactions (3), and species distributions (9). As different species within a community may show different responses to climate variation (10, 11), many studies have speculated on the possibility that phenological synchrony within ecological communities may be extensively disrupted (12–15). Conversely, other studies including observational evidences, theoretical considerations, and small-scale experiments have suggested that the maintenance of synchrony in terrestrial and aquatic systems may be common (16–19). Therefore, the extent to which the stability and persistence of natural systems will be hampered as a result of loss of phenological synchrony remains largely an open question. Addressing this pertinent question is challenging because of the complex, dynamic, and often poorly understood structure of ecological interaction networks.
To date, most studies evaluating the maintenance or disruption of phenological synchrony have examined whether long-term phenological shifts have been congruent between interacting species and environmental conditions (18, 20). The majority of these studies have described and generated predictions for phenology shifts in terms of thermal conditions (12, 21), although different organisms, and even the different phenological events of a single organism, may be sensitive to other kinds of climate cues as well (22, 23), such as snowmelt (24) or frost (25). As different aspects of climate are shifting at dissimilar rates (26), phenological synchrony among species may be disrupted even if the species individually keep pace with environmental change. Furthermore, whether dissimilarity in phenological shift leads to disruption of synchrony depends on whether the phenological events were initially synchronous (27). However, the link between synchrony and divergence in shift rate still remains poorly studied.
To examine how community-level phenological change is built up from species-level responses, there is a need for long-term data on multispecies assemblages of species that differ in their taxonomic and ecological aspects. Such data are rare (but see, e.g., ref. 28) because most studies on the effect of climate change on species interactions have been based on short-term observational or experimental data (29) or have combined data from heterogeneous sources such as different study locations or different periods (18, 30). In this article, we assess a community-level phenological response to climate change by using a 40-y-long time series that is unique in three aspects. First, the data have been systematically collected, mainly by six of the authors, in a single locality, and thus variability in observation effort is of much less concern than usual. Second, the data involve multiple taxonomic groups; namely, birds, plants, insects, arthropods, amphibians, reptiles, and fungi. Third, in addition to five weather covariates that were recorded daily (mean, minimum, and maximum temperature; precipitation; and snow cover; Fig. 1), the data include a large number of climatic events (recorded as calendar dates when those events took place) related to temperature, snow, ice, frost, and atmospheric phenomena. Thus, these data allow one to impose community-wide phenological patterns on a multidimensional description of the physical environment.
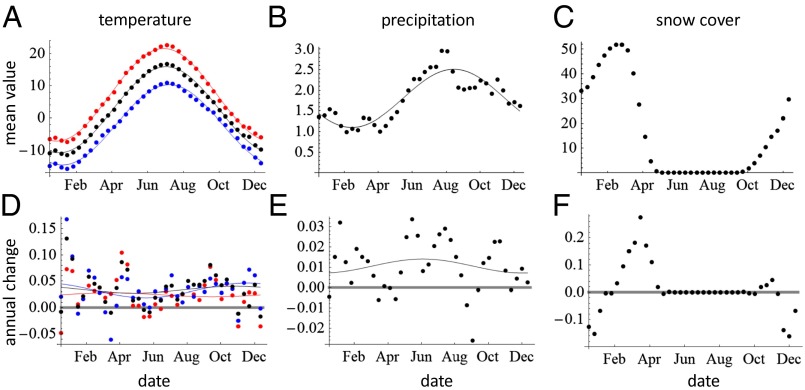
Fig. 1.
Seasonal patterns of temperature, precipitation, and snow cover. The upper panels (A–C) show averages over the study period, and the lower panels (D–F) show the mean annual change (the slope of linear regression for climatic variable vs. year) for temperature (°C; A and D), precipitation (mm/day; B, E), and snow cover (cm; C, F). For temperature, black corresponds to daily mean, blue to daily minimum, and red to daily maximum. All data averaged over 10-d periods. The lines show periodic regressions. To measure the rate of thermal change in the units of days, we examined the time derivative of the periodic regression line for mean temperature (A). We defined the mean date of spring and the mean date of autumn as the times at which the derivative was minimized and maximized, respectively. We computed the change in spring (autumn) temperature by averaging the slope (D) during the period of 0.3 y centered at the mean date of spring and autumn, yielding 0.027 °C and 0.039 °C, respectively. Given the rate at which spring and autumn advance (slope of periodic regression, 0.23 °C per day for both), the changes in temperature correspond to the shifts of −0.12 and 0.17 d/y for spring and autumn, respectively.
Following a large number of previous studies (e.g., refs. 14 and 20), we first measure long-term changes in phenology (to be called phenological shifts) by the slopes of linear models for date against year (Figs. 2A and 4A) to assess, first, how similar the phenological shifts have been among the different taxonomic groups, and second, how they relate to the seasonal timings of those events. Second, we consider short-term phenological fluctuations (i.e., year-to-year variation), which we define as residual variation around the long-term shifts. Here our aims are to study what kind of climate variation (and at which temporal scale) each of the taxonomic groups are influenced by and to compare the levels of phenological synchrony among species within and between taxonomic groups. Finally, to connect the long-term changes to the short-term fluctuations (i.e., to study the link between synchrony and divergence in shift rate), we assess whether or not synchronous events have shifted in a congruent manner.
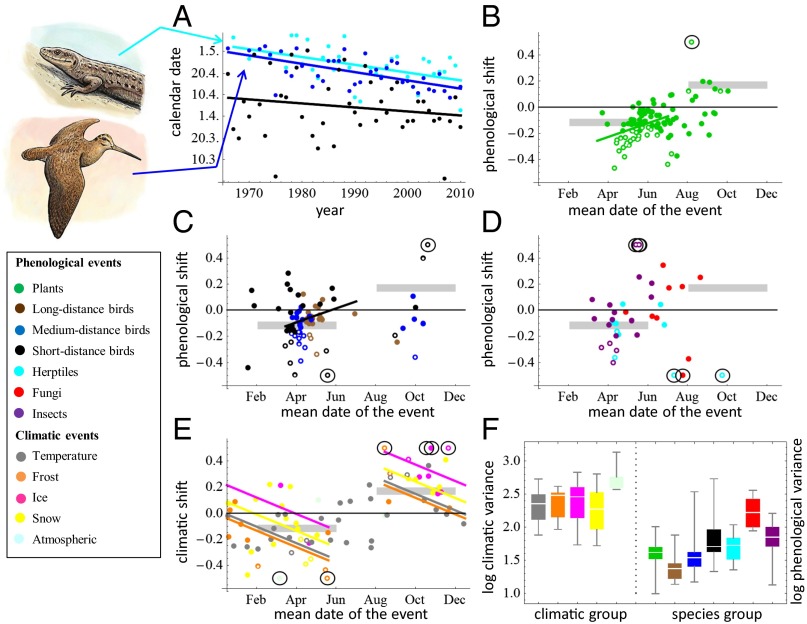
Fig. 2.
Patterns of climatic and phenological shift and variance. (A) Phenological shifts for the first occurrence of the common lizard (Zootoca vivipara; cyan), the start of the display flight of the Eurasian woodcock (Scolopax rusticola; blue), and the climatic event of daily average temperature moving above 0 °C (black). The lizard, bird, and temperature events have shifted at rates of −0.36, −0.39, and −0.19 (day/year), and their phenological variances are 7.32, 5.82, and 13.92 (day2), respectively. (B–E) Dots depict shifts (day/year) in plant phenology (B); bird phenology (C); insect, fungal, and herptile phenology (D); and climatic events (E). (F) Distributions of residual variances. The lines in B, C, and E show linear regression models through the part of the year with the most data (spring for bird and plant events and winter for weather events). The circles indicate shifts greater than 0.5 for which the location of the dot has been truncated in the figure. Significant shifts (P < 0.05; 61/213 phenological events and 15/77 climatic events) are indicated with a white center. The thick gray lines depict the pace of climate change from the point of view of average temperature (Fig. 1). (Left) Color key of the different taxonomical and climatic groups.
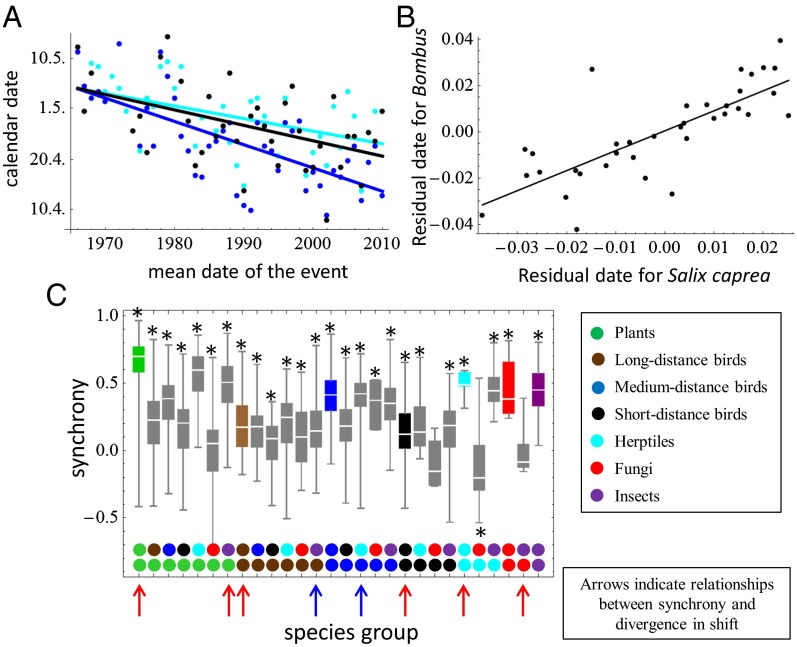
Fig. 4.
Patterns of phenological synchrony and divergence in shift. (A) Date of first appearance of bumblebees (Bombus spp.; black) and the blooming of the plants goat willow Salix caprea (cyan) and coltsfoot Tussilago farfara (blue). (B) Illustration of the high level of phenological synchrony (with a value of 0.77) between the appearance of bumblebees and the blooming of goat willow, measured as the correlation coefficient for the residuals from the regression of A. (C) Bars showing distributions of within- and between-group phenological synchronies. The stars indicate cases for which the median correlation was significantly (P < 0.05) greater than zero (star above the bar) or smaller than zero (star below the bar). The arrows point out cases with a statistically significant (P < 0.05) negative (red arrows) or positive (blue arrows) correlation between synchrony and divergence in shift (see Datasets S3 and S4 for the full results and Materials and Methods for details on the randomization tests). Pairs of events of the same species are excluded for these analyses.
Results
As elsewhere in Northern Europe (26, 31), weather covariates show that our study area has experienced an increase in temperature (on average +0.034 °C per year) and precipitation (on average +3.9 mm per year) during the study period (Fig. 1). Contrary to previous reports (32), we found a decrease in snow cover in autumn but an increase in spring, possibly because of the increase in snowfall during winter (Fig. 1; see Dataset S1 for the raw data). Our time-series data on climatic events illustrate that climate change has indeed been a multidimensional phenomenon (26), as all aspects of climate have not changed coherently (Fig. 2E). For example, the date when the rapidly flowing part of the Suna river gains ice cover has been delayed by as much as 2 mo (from early November in the 1970s to the middle of January in the 2010s), whereas the overall change in temperature suggests that the beginning of winter has been delayed by 1 wk during the same time. In general, the winter period has become shorter, as the arrival of winter has been delayed while its end has been advanced. As illustrated by the lines in Fig. 2E, the rate of shift has been different for the different groups of climatic variables (best model included different intercepts; df = 3; P = 0.003), but the seasonal pattern is similar in all groups (the slopes do not differ; df = 3; P = 0.61). Climatic events related to ice have been especially delayed in early winter, whereas events related to frost and temperature have been delayed less in early winter and, correspondingly, advanced more in late winter than events in the other groups.
Variation in Phenological Shifts Across Taxa and Seasons.
As expected from the shift in temperature, most phenological events have advanced in spring and been delayed in autumn (Fig. 2 B–D and Dataset S2). However, there is a high degree of variation among and within the taxonomic groups. Focusing on those phenological events for which we have the most statistical power (i.e., spring events for plants and birds), the best model included different intercepts (df = 1; P < 0.001) but similar slopes (df = 1; P = 0.47) for these two groups. In both plants and birds, events that take place early in the spring have advanced their phenology more than late spring events. Plants have advanced their early spring phenology in a consistent manner, and even faster than suggested by the increase in mean temperature (Fig. 2B), whereas migratory bird species have shown more diverge responses and have shifted their phenology, on average, less (Fig. 2C). We found no further differences in the responses of these taxonomic groups (P > 0.13 for all associated tests) when classifying the species into subgroups of similar life-history characteristics (herbaceous, shrubs or trees for plants; long-, medium-, or short-distance dispersers for birds).
Variation in Short-Term Phenological Fluctuations.
There was high variation among the different taxonomic groups in their short-term phenological fluctuations. In our data, year-to-year variation was greatest for fungal phenology and smallest for migratory birds traveling long or medium distances (Fig. 2F). Year-to-year variation in phenological events of different taxonomic groups was typically explained by one and sometimes two climatic variables, with the exception of short-distance birds and fungi, for which only a minority of cases were significantly explained by climatic variation (Fig. 3A and Dataset S3). Most taxonomic groups responded to variation in temperature, but only a few of them responded to the variation in precipitation (Fig. 3B), even though the latter climatic variable also was measured throughout the year and was, thus, available for all candidate models (Fig. S1).
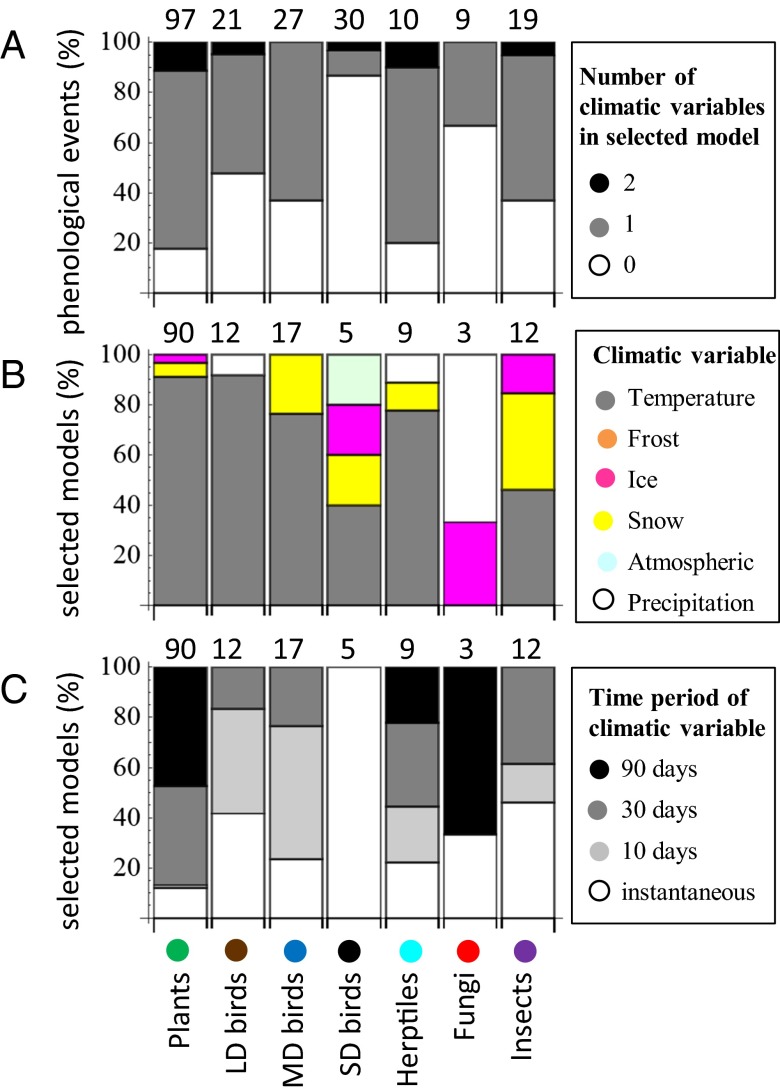
Fig. 3.
Year-to-year variation in phenological timing explained by climatic variation. (A) Number of climatic variables included in the selected model of each phenological event. (B and C) Kinds of climatic variables included in the models: (B) type of climatic variable and (C) period during which the climatic variable was averaged. Above the bars are shown the numbers of data points from which the percentages were calculated.
A number of phenological events were associated with snow cover (Fig. 3B and Dataset S3), including the onset of blooming of several plants (e.g., the lady’s slipper orchid Cypripedium calceolus), the first spring appearance of several birds and insects (e.g., the Eurasian woodcock Scolapax rusticola, the mosquitoes Culex spp. and Aedes spp., the butterfly brimstone Gonepterix rhamni, the tick Ixodes persulcatus, and stoneflies Plecoptera spp.), and the onset of the croaking of frogs (Rana temporaria), as well as the last autumn occurrence of geese (Anser spp.). Events associated with the melting of ice from the Suna river included the arrival of the waterbird goldeneye Bucephala clangula and the time when black flies (Simuliidae spp., which spend their larval stage in a river environment) were first observed biting the observers, as well as other events for which it is difficult to find a causal explanation (e.g., the onset of blooming of the cloudberry Rubus chamaemorus and the first occurrence of the butterfly orange tip Anthocharis cardamines). As expected, the occurrence of mushrooms (Lactarius spp. and Leccinum spp.) was associated with precipitation.
Phenological events of birds and insects were mainly triggered by climate cues occurring during short periods (e.g., temperature crossing a certain threshold value), whereas plants, herptiles, and fungi were often affected by long-term climatic averages (Fig. 3C). We expected to find a positive correlation between climatic conditions and phenology; that is, that phenological events would take place early or late in years in which climatic events took place early or late, respectively. This was indeed the case for 92 of the 93 models in which we could test this prediction. The exception was that the leaf fall of silver birch (Betula pendula) ended early in autumns that lasted longer in terms of temperature remaining high, probably because of warm summer days leading to the drying of birch leaves.
Phenological Synchrony and Divergence in Shift.
Focusing on phenological events that occur at the same time of year (the mean date of events being within 2 wks of each other), we examined which kinds of pairs of events covary in synchrony. A high level of synchrony suggests that the events may be in a causal relationship or that they may respond to the same climatic factors. An example of a likely causal link is the one between the first appearance of bumblebees (Bombus spp.) and the blooming of the plants goat willow (Salix caprea) and coltsfoot (Tussilago farfara), which represent for bumblebees the basic source of forage in the first days after their emergence. In some years, goat willow blooms earlier than coltsfoot, whereas in other years, the situation is the opposite (Fig. 4A), bringing temporal variability to the realized interactions, as is common in plant pollinator networks (33). The appearance of bumblebees has shifted in parallel with the blossoming of goat willow, whereas the blooming of coltsfoot has shifted faster (Fig. 4A). All three events are in high synchrony with each other (Fig. 4B), and they are also positively correlated with the mean temperature during a preceding 30-d period (Dataset S3).
Despite the large number of species included in our data, it is difficult to pinpoint other groups of events that would be causally tightly linked to each other. Thus, we considered a more general, community-level approach in which we asked to what extent events within and among the taxonomical groups are synchronized. The results, shown in Fig. 4C, show that plants had the highest level of within-group synchrony, but herptiles, insects, fungi, and medium-distance migratory birds were also highly positively synchronized within their taxonomical groups. Although plants, herptiles, and insects also were in synchrony between the taxonomical groups, fungi formed a distinctive group that was not in synchrony with any other group, possibly because they depend largely on precipitation (Fig. 3B). Pairs of phenological events that are in synchrony have shifted at more similar rates than random pairs of events in all seven taxonomic groups, with a statistically significant effect for four groups (Fig. 4 and Dataset S4). A similar analysis across pairs of taxonomic groups (Fig. 4 and Dataset S5) showed such a statistically significant pattern for two pairs of taxonomic groups, whereas in two cases, events that are in synchrony have actually diverged more than expected for random pairs of events (Fig. 4).
Discussion
Our analyses illustrate that taxonomic groups in the “Kivach” community have shifted their phenological windows at dissimilar rates. Dissimilar rates of shift among phenological events in different taxonomic groups have been observed before (12–14, 27, 30) and are to be expected because the mechanisms underlying phenological timing are likely to differ from case to case. For example, dissimilar sensitivities to climate may at least partly be dependent on the particular life-history strategies of the species in question (10, 11, 34). However, only assessing congruence in shift tells us very little about whether different phenological events are triggered by the same mechanisms. To compare the rates at which climatic and phenological events have been changing, it is essential to resolve which climatic factors are the most relevant for species phenology (14). Such information may be at least partially extracted from year-to-year variation, which is likely to reflect the attempt of the species to adjust their phenology to short-term variation in climatic conditions (35). We found that different taxonomic groups are responding to different climatic factors, which in turn have shifted at dissimilar rates. These results parallel a number of earlier studies by suggesting that climate change has the potential to disrupt phenological synchrony within communities (12–14, 27, 30). Variation in climatic shifts may result in a mismatch between the species’ decision-making environment (the climatic cues that trigger specific behaviors; e.g., the start of breeding) and the selection environment (favorable conditions for survival, growth, and reproduction; e.g., availability of food resources), with obvious ecological and evolutionary consequences (14).
Phenological synchrony among species may be created both by shared responses to climatic variation and by ecological interactions. Although our data do not enable one to quantify the relative roles of these two causes, the patterns in Fig. 4 allow for some speculation. To start with, species within the same taxonomical group can be expected to have a high probability of interacting with each other (e.g., through competitive interactions for the same resources) but also to be influenced by shared environmental factors. Indeed, species within all taxonomical groups were found to be positively synchronized (Fig. 4). Between the taxonomical groups, we observed a high level of synchrony among plants, birds, insects, and herptiles. These groups are likely to have a number of direct and indirect ecological interactions. For example, most flowers depend on insects for pollination, whereas some fruits and seeds are dispersed by birds. Many links between plants, birds, herptiles, and insects are also created by feeding relationships. The fungi considered in our data (except Gyromitra spp.) form mycorrhizal interactions with plants, but we did not expect or observe (Fig. 4) these interactions to lead to the synchronization between plant phenology and the formation of fungal fruit-bodies, as fruiting is much influenced by precipitation (Fig. 3B).
Our data set is based on first dates instead of mean population event dates. Previous studies (36, 37) have stressed that population size, observation effort, and observability may affect the first dates of phenological events. According to our observations, the majority of the study species have not changed drastically in their abundance in Kivach during 1970–2010. For those species for which notable changes in abundances were observed, we examined post hoc whether the results could be explained by a change in population size. We observed an advance in the first occurrences of whooper swans (Cygnus сygnus) and black birds (Turdus merula) (Dataset S2), which may be partly explained by their increase in abundance in Northwest Russia (including Karelia) during the last few decades. Similarly, the first occurrence of dragonflies (Aeschnidae) has shifted to a later date (Dataset S2), most likely because of a considerable decrease in their abundance during the study period.
Conversely, despite the observed reductions in population sizes of chiffchaff (Phylloscopus colibita), woodpigeon (Columbo palumbus), and lapwing (Vanellus vanellus), their first phenological dates were not delayed (Dataset S2). Given these mixed results, and the lack of quantitative data on changes in population size, we did not attempt to correct the phenological dates for changes in abundance but consider such variation to lead to additional noise that is likely to weaken the signal strength in our results, but not to create systematic biases. Although we agree that, for example, daily count data would provide a more robust assessment of phenological change than dates on first occurrences (36), such data are simply not available at the community level. Variation in observation effort is of major concern, especially in studies based on volunteer observations. In our data set, the phenological dates were collected in a systematic manner, with sampling effort remaining approximately constant. As the variation in sampling effort was relatively minor and distributed over the study period, we considered this to create additional noise in the data, rather than a systematic bias.
Earlier studies have suggested that species may respond individualistically to climate, creating mismatches between species, with potentially devastating consequences for communities and ecosystem services (5, 10). This is expected to be especially the case if there are systematic differences in species’ responses to climate change among different groups or trophic levels (10). However, dissimilar long-term shifts can only create a mismatch in phenological timing if there was a match to start with (27), and a mismatch only has important implications for causally interrelated events. Our results demonstrate that synchronous species have tended to shift in congruence, and thus climate change may disrupt community-level synchrony less than has been assumed in previous studies. However, as species are embedded in complex networks of interactions, the exact consequences of climate change for a focal species are also dependent on the effects on interacting species and on the focal species’ potential to adapt to novel community composition (11, 38). For example, climate change can directly cause subtle organismal or population changes, and these changes can affect other members of a community via species interactions, which may be more important than direct climate effects (8). Small changes in the phenology of interacting species may result in changes of the dynamics of the community as a whole (13, 14). Furthermore, climate is changing in a multidimensional fashion (5), with some factors shifting at dissimilar rates (Fig. 2E) and others, such as the photoperiod, remaining stable (39). As the effect of one climate factor may be exacerbated or mitigated by another, and as interacting species are dependent on the interaction of different climate cues, it remains a challenge to predict whether species are or are not able to adapt to unpredictable new combinations of environmental conditions.
Materials and Methods
The study was performed in Nature Reserve “Kivach” in Russian Karelia (62° 17' N, 33° 55' E). The reserve was established in 1931 and has a total area of 10,900 ha, consisting of boreal forest, middle taiga subzone. During the period 1960–2010, the permanent research staff of the reserve conducted daily observations to record the dates at which a predefined list of weather-related and phenological events took place (most data types start around 1970; Dataset S6). The plant data are acquired along established routes, and the bird data are acquired at fixed observatories near bird settlement areas. The main deviation from constant sampling effort is that in 1960–1975, 1987–1988, and 1991–1993, only a single ornithologist worked in the reserve, whereas during the other periods there were two to three ornithologists.
The data include 77 weather-related events, which we classified into five groups: temperature (30 events; e.g., temperature crossing a certain threshold value), snow (21 events; e.g., the first winter snowfall), ice (9 events; e.g., the first winter day with ice on ponds), frost (13 events; e.g., first day with frost after the summer), and atmospheric phenomena (4 events; e.g., the first fog of the year). The phenological events were classified into plants (97 events on 66 species), birds (78 events on 52 species, which we classified into three groups on the basis of their migration distance; Dataset S6), insects (including ticks, 19 events on 17 species), herptiles (amphibians and reptiles, 10 events on 3 species), and fungi (9 events on 6 species). The majority of the phenological events in these data are highly dependent on external conditions, as they relate, for example, to plant flowering, phenology of poikilothermic animals, and birds’ spring arrival dates (Dataset S6). After removing redundant event types (i.e., those essentially based on the same observation, see Dataset S6 for the list of included and excluded events and Dataset S7 for the raw data), the data consist of 10,425 dates representing 77 weather-related events and 213 phenological events.
We studied the shift of each climatic and phenological event by fitting the linear regression dt = α + β(t − 1990) + εt, which models day of the year dt by year t. The model prediction α for 1990 (the middle of the study period) is called the mean date of the event, whereas the slope β is called the shift. To remove the effect of exceptional events, we excluded from all analyses the data points for which the residual εt exceeded three times the SD (29/10,425 data points).
We examined how shifts relate to mean dates by fitting linear models to cases with sufficient data: bird and plant events in summer and meteorological events in winter. In the models, we allowed both the slope and the intercept to be dependent on the taxonomic (climatic for meteorological events) group. In addition, we subdivided the different species of plants and birds into groups with similar life-history characteristics (herbaceous, shrubs or trees for plants; long-, medium- or short-distance dispersers for birds) and used the same approach to test whether these groups had an effect.
We assessed short-term fluctuations (year-to-year variation) from shift-corrected phenological dates defined as 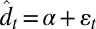 . We measured phenological variance for each event as the variance among shift-corrected dates. We used linear models to explain variation in shift-corrected phenological dates by shift-corrected climatic variables. As candidate climatic variables, we included those climatic events with a mean date no earlier than 1 mo before the phenological event. We further included five weather covariates (mean, minimum, and maximum temperatures; precipitation; and snow cover) averaged for the periods of 10, 30, or 90 d before the mean date of the phenological event. For each phenological event, this resulted in 14–26 (mean, 21.2) candidate explanatory variables, which were classified as temperature, precipitation, snow, ice, frost, or atmospheric phenomena. To account for the fact that temperature-related factors were overrepresented among the explanatory variables and that many of the explanatory variables were correlated, we clustered the explanatory variables within each class so that within-cluster correlation was at least 0.8. We represented each cluster by the average of standardized (mean zero, variance one) explanatory variables. To avoid the problem of multiple testing and the variation in number of clusters within the classes, we used Bonferroni correction in a hierarchical manner. Thus, we adjusted the P values by first multiplying them by the number of classes and then further by multiplying them by the number of clusters within each class. We selected the best cluster, using 0.05 as the threshold value for adjusted P values, and then the best variable within the cluster (if multiple present) by the smallest P value. We continued by examining whether the residual variance could be explained by the remaining variables until no significant cluster was found.
. We measured phenological variance for each event as the variance among shift-corrected dates. We used linear models to explain variation in shift-corrected phenological dates by shift-corrected climatic variables. As candidate climatic variables, we included those climatic events with a mean date no earlier than 1 mo before the phenological event. We further included five weather covariates (mean, minimum, and maximum temperatures; precipitation; and snow cover) averaged for the periods of 10, 30, or 90 d before the mean date of the phenological event. For each phenological event, this resulted in 14–26 (mean, 21.2) candidate explanatory variables, which were classified as temperature, precipitation, snow, ice, frost, or atmospheric phenomena. To account for the fact that temperature-related factors were overrepresented among the explanatory variables and that many of the explanatory variables were correlated, we clustered the explanatory variables within each class so that within-cluster correlation was at least 0.8. We represented each cluster by the average of standardized (mean zero, variance one) explanatory variables. To avoid the problem of multiple testing and the variation in number of clusters within the classes, we used Bonferroni correction in a hierarchical manner. Thus, we adjusted the P values by first multiplying them by the number of classes and then further by multiplying them by the number of clusters within each class. We selected the best cluster, using 0.05 as the threshold value for adjusted P values, and then the best variable within the cluster (if multiple present) by the smallest P value. We continued by examining whether the residual variance could be explained by the remaining variables until no significant cluster was found.
We defined synchrony between pairs of phenological events as the correlation between shift-corrected dates [unitless, range in (−1,1)] and measured their divergence in shift as the absolute difference in shifts. We computed the Spearman Rank Correlation coefficient between synchrony and divergence in shift for all pairs of phenological events, separately for all pairs of taxonomic groups, excluding event pairs that were related to the same species (relevant only for self-pairs). As the same events appeared multiple times in the data (in different combinations for pairs of events), we assessed statistical significance by randomizing the slopes among the events and computing the Spearman Rank Correlation coefficients for 1,000 replicates of such randomized data sets. We tested whether median synchrony (within a taxonomical group or between two taxonomical groups) was different from zero by computing the synchrony values for 1,000 replicates in which the order of shift-corrected dates was randomly permuted for each event.
Supplementary Material
Acknowledgments
We thank Hannu Pietiäinen, Henrik de Knegt, and Raila Hekkanen for valuable comments on the manuscript; Seppo Leinonen for the drawings in Fig. 2; and Juri Kurhinen for initiating the collaboration between O.O. and S.S., M.Y., A.S., A.K., N.K., and A. Shcherbakov. Special thanks to the colleagues who helped with data collection, especially T. V. Kiseleva, M. M. Romanovskaya, S. V. Sazonov, A. A. Tikhomirov, L. S. Zakharova, V. B. Zimin, and F. S. Yakovlev. The work was financially supported by the Academy of Finland, Grants 250243 (to O.O.) and 250444 and 14037 (to M.d.M.D.); European Research Council (ERC) Starting Grant 205905 (to O.O.); and Junta of Andalucía Excellence Project Grant RNM-5090 (to M.d.M.D.). The field work was conducted as part of the monitoring program of Russian nature reserves, Chronicles of Nature.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305533110/-/DCSupplemental.
References
- 1.Walther GR, et al. Ecological responses to recent climate change. Nature. 2002;416(6879):389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst . 2006;37:637–669. [Google Scholar]
- 3.Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland O. How does climate warming affect plant-pollinator interactions? Ecol Lett. 2009;12(2):184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 5.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecol Lett. 2008;11(12):1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 6.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15(4):365–377. doi: 10.1111/j.1461-0248.2011.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287(5459):1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 8.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. The effects of phenological mismatches on demography. Philos Trans R Soc Lond B Biol Sci. 2010;365(1555):3177–3186. doi: 10.1098/rstb.2010.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menzel A, Sparks TH, Estrella N, Roy DB. Altered geographic and temporal variability in phenology in response to climate change. Glob Ecol Biogeogr. 2006;15(5):498–504. [Google Scholar]
- 10.Voigt W, et al. Trophic levels are differentially sensitive to climate. Ecology. 2003;84(9):2444–2453. [Google Scholar]
- 11.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends Ecol Evol. 2010;25(6):325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Harrington R, Woiwod I, Sparks T. Climate change and trophic interactions. Trends Ecol Evol. 1999;14(4):146–150. doi: 10.1016/s0169-5347(99)01604-3. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly A, Caffarra A, O’Neill BF. A review of climate-driven mismatches between interdependent phenophases in terrestrial and aquatic ecosystems. Int J Biometeorol. 2011;55(6):805–817. doi: 10.1007/s00484-011-0426-5. [DOI] [PubMed] [Google Scholar]
- 14.Visser ME, Both C, Lambrechts MM. Global climate change leads to mistimed avian reproduction. In: Moller AP, Fielder W, Berthold P, editors. Birds and Climate Change, Advances in Ecological Research. Vol 35. London: Academic Press Ltd; 2004. pp. 89–110. [Google Scholar]
- 15.McKinney AM, et al. Asynchronous changes in phenology of migrating Broad-tailed Hummingbirds and their early-season nectar resources. Ecology. 2012;93(9):1987–1993. doi: 10.1890/12-0255.1. [DOI] [PubMed] [Google Scholar]
- 16.Aebischer NJ, Coulson JC, Colebrook JM. Parallel long-term trends across 4 marine trophic levels and weather. Nature. 1990;347(6295):753–755. [Google Scholar]
- 17.Seebens H, Einsle U, Straile D. Copepod life cycle adaptations and success in response to phytoplankton spring bloom phenology. Glob Change Biol. 2009;15(6):1394–1404. [Google Scholar]
- 18.Bartomeus I, et al. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci USA. 2011;108(51):20645–20649. doi: 10.1073/pnas.1115559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iler AM, et al. Maintenance of temporal synchrony between syrphid flies and their floral resources desbite differential phenological responses to climate. Glob Change Biol. 2013;19(8):2348–2359. doi: 10.1111/gcb.12246. [DOI] [PubMed] [Google Scholar]
- 20.Visser ME, Holleman LJM, Gienapp P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia. 2006;147(1):164–172. doi: 10.1007/s00442-005-0299-6. [DOI] [PubMed] [Google Scholar]
- 21.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421(6918):57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 22.Crimmins TM, Crimmins MA, Bertelsen CD. Complex responses to climate drivers in onset of spring flowering across a semi-arid elevation gradient. J Ecol. 2010;98(5):1042–1051. [Google Scholar]
- 23.Diez JM, et al. Forecasting phenology: From species variability to community patterns. Ecol Lett. 2012;15(6):545–553. doi: 10.1111/j.1461-0248.2012.01765.x. [DOI] [PubMed] [Google Scholar]
- 24.Iler AM, Høye TT, Inouye DW, Schmidt NM. Nonlinear phenological responses to climate: Evidence for snowmelt, temperature, and photoperiodic flowering cues. Philos Trans R Soc Lond B. 2013;368(1624):20120489. doi: 10.1098/rstb.2012.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inouye DW. The ecological and evolutionary significance of frost in the context of climate change. Ecol Lett. 2000;3(5):457–463. [Google Scholar]
- 26.IPCC . The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge Univ Press; 2007. [Google Scholar]
- 27.Singer MC, Parmesan C. Phenological asynchrony between herbivorous insects and their hosts: Signal of climate change or pre-existing adaptive strategy? Philos Trans R Soc Lond B Biol Sci. 2010;365(1555):3161–3176. doi: 10.1098/rstb.2010.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Primack RB, et al. Spatial and interspecific variability in phenological responses to warming temperatures. Biol Conserv. 2009;142(11):2569–2577. [Google Scholar]
- 29.Sheriff MJ, et al. Phenological variation in annual timing of hibernation and breeding in nearby populations of Arctic ground squirrels. Proc Biol Sci. 2011;278(1716):2369–2375. doi: 10.1098/rspb.2010.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser ME, Holleman LJM. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc Biol Sci. 2001;268(1464):289–294. doi: 10.1098/rspb.2000.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steltzer H, Post E. Ecology. Seasons and life cycles. Science. 2009;324(5929):886–887. doi: 10.1126/science.1171542. [DOI] [PubMed] [Google Scholar]
- 32.Vehviläinen B, Lohvansuu J. The effects of climate change on discharges and snow cover in Finland. Hydrol. Sci. J.-J. Sci. Hydrol. 1991;36(2):109–121. [Google Scholar]
- 33.Dupont YL, Padron B, Olesen JM, Petanidou T. Spatio-temporal variation in the structure of pollination networks. Oikos. 2009;118(8):1261–1269. [Google Scholar]
- 34.Cleland EE, Chiariello NR, Loarie SR, Mooney HA, Field CB. Diverse responses of phenology to global changes in a grassland ecosystem. Proc Natl Acad Sci USA. 2006;103(37):13740–13744. doi: 10.1073/pnas.0600815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charmantier A, et al. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science. 2008;320(5877):800–803. doi: 10.1126/science.1157174. [DOI] [PubMed] [Google Scholar]
- 36.Miller-Rushing AJ, Inouye DW, Primack RB. How well do first flowering dates measure plant responses to climate change? The effects of population size and sampling frequency. J Ecol. 2008;96(6):1289–1296. [Google Scholar]
- 37.Linden A. Using first arrival dates to infer bird migration phenology. Boreal Environ. Res. 2011;16:49–60. [Google Scholar]
- 38.Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant-pollinator interactions. Ecol Lett. 2007;10(8):710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 39.Körner C, Basler D. Plant science. Phenology under global warming. Science. 2010;327(5972):1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.