Significance
The main approach to treat acute pyelonephritis is antibiotic therapy. However, the pathology is accompanied by inflammation and oxidative stress phenomena that can also be a target for intervention when direct antibacterial measures are impossible or inefficient. In our study, in vitro and in vivo models of experimental pyelonephritis were used to define the role of mitochondria in this pathology and to find a way to alleviate the kidney damage. The majority of the deleterious effects of pyelonephritis, including animal mortality in extreme cases, were prevented by the treatment with the mitochondria-targeted antioxidant, pointing to mitochondria as a therapeutic target.
Keywords: inflammation, phenoptosis, urological diseases, innate immunity, toll-like receptors
Abstract
Acute pyelonephritis is a potentially life-threatening infection of the upper urinary tract. Inflammatory response and the accompanying oxidative stress can contribute to kidney tissue damage, resulting in infection-induced intoxication that can become fatal in the absence of antibiotic therapy. Here, we show that pyelonephritis was associated with oxidative stress and renal cell death. Oxidative stress observed in pyelonephritic kidney was accompanied by a reduced level of mitochondrial B-cell lymphoma 2 (Bcl-2). Importantly, renal cell death and animal mortality were both alleviated by mitochondria-targeted antioxidant 10(6′-plastoquinonyl) decylrhodamine 19 (SkQR1). These findings suggest that pyelonephritis can be treated by reducing mitochondrial reactive oxygen species and thus by protecting mitochondrial integrity and lowering kidney damage.
Normally, the kidney and urinary tract are germ-free. However, during their lifetimes, about 40% of women and 12% of men experience urinary tract infections (UTIs) (1). Acute pyelonephritis (APN) is a potentially life-threatening complication of UTI that occurs when infection progresses to the upper urinary tract. The uropathogen most frequently associated with this disease is the pyelonephritogenic subset of Escherichia coli, which is responsible for up to 85% of both complicated and uncomplicated UTIs (2). This disease is frequently accompanied by bacterial invasion and stimulation of acute inflammatory response (3, 4). Toxin-induced epithelial damage and bladder hemorrhage contribute further to the pathogenicity of uropathogens in the kidney, with progression leading to renal damage including renal scarring and in extreme cases septicemia (5, 6). Ultimately, renal scarring is a cause of substantial morbidity (7, 8).
Leukocyte infiltration in response to bacterial invasion is an important contributor to renal tissue damage (9, 10). Production and extracellular release of reactive oxygen species (ROS) by infiltrating leukocytes can lead to kidney injury and dysfunction (11, 12). Consequently, oxidative stress in renal cells may be a critical factor in the pathogenesis of pyelonephritis whereas pharmacological management of the oxidative stress response may provide a therapeutic effect in preventing renal pathologies (13–18). However, the issue is complicated by the diversity of ROS-generating mechanisms and their differential contribution to host defense from infection and collateral tissue damage. Mitochondria and NADPH oxidases (19) are the two principle sources of ROS although their relative contribution to inflammatory pathologies is not well-defined. A new class of antioxidants that specifically target mitochondrial ROS (hereafter referred to as SkQ1 and SkQR1) have been recently developed and shown to have a beneficial effect in a number of cell pathologies (20–28).
In this study, we explored mechanisms of APN progression considering various aspects of interaction of renal cells with leukocytes and bacterial pathogens. The goal was to gain insight into the role and mechanisms of induction of oxidative stress in eukaryotic components of the system and find an approach of directed correction of the pathological oxidative changes in renal tissue. We analyzed the relevance of the strategy of protecting the kidney based on the activation of prosurvival and blockage of prodeath signaling pathways involving mitochondria. We evaluated chimeric compounds carrying an antioxidant moiety as potential agents to efficiently alleviate the deleterious consequences of APN. To facilitate the future design of directed pharmacologic interventions to normalize renal function subsequent to APN, we explored the role of mitochondria and oxidative stress in this pathology using positively charged membrane-permeable, mitochondrial-targeted compounds (29). We demonstrated that specific targeting of mitochondrial ROS by antioxidant resulted in a significant protective effect in animal models of APN. These results illustrate the role of mitochondrial ROS in renal tissue damage in the context of acute infection and suggest a therapeutic potential of mitochondrial antioxidants.
Results
Effect of Mitochondria-Targeted Antioxidant (SkQR1) on an APN-Induced Oxidative Stress In the Tissue.
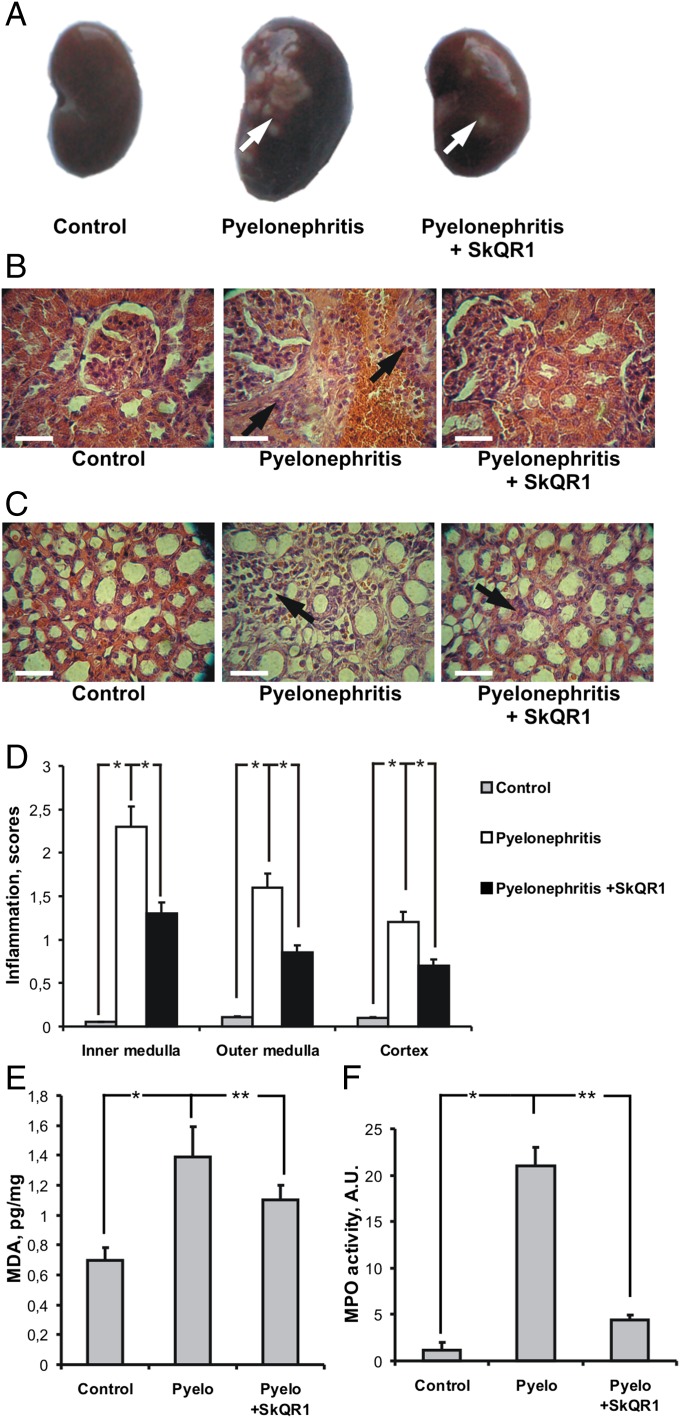
A number of signs of inflammation and oxidative kidney tissue damage were observed in animals suffering from APN (Fig. 1). The kidneys of pyelonephritic rats showed a great number of abscesses, which were significantly less prevalent in rats treated with SkQR1 (Fig. 1A). An extensive leukocyte infiltration of the kidney took place (Fig.1 B and C). Interstitial infiltrates sometimes were local but often covered a significant part of the kidney parenchyma and originated almost exclusively from infiltrating polymorphonuclear leukocytes (neutrophils). In some loci, infiltrates embraced blood vessels. Severe and characteristic changes were revealed in kidney tubules as well: they were swollen and their cells were degenerated and often peeled off to the tubular lumen. Often the tubules were filled with a pus-necrotic and bacterial content. Pathological changes were mostly observed in the medullar part of the kidney (Fig. 1 B–D). The glomeruli and cortical tubules in the majority of cases were relatively intact. Importantly, treatment with SkQR1 inhibited both leukocyte infiltration in all kidney layers and the spreading of the inflammatory process (Fig. 1 B–D).
Fig. 1.
Effects of SkQR1 on pyelonephritis-induced pathological features in renal morphology. Appearance of abscesses in kidney under pyelonephritis (A, arrows) is shown. Histological sections of renal cortex (B) and medulla (C) were stained with hematoxylin/eosin. With induction of pyelonephritis, infiltration of the cortex and medulla with leukocytes was apparent (arrows); this infiltration was prevented by SkQR1 pretreatment. Inflammation index (Materials and Methods) in different kidney layers is presented (D). Inflammation in the kidney was accompanied by oxidative stress and neutrophil infiltration, as measured by MDA accumulation (E) and an increase in myeloperoxidase activity (F), respectively. (Scale bar, 50 µm.) *P < 0.01, **P < 0.05.
The concentration of peroxidative products such as malondialdehyde (MDA), an index of oxidative stress in the whole kidney tissue, was elevated after induction of APN (Fig. 1E) demonstrating remarkable oxidative tissue damage. MDA concentration was significantly lower in animals receiving SkQR1, demonstrating the protection of the kidney tissue from oxidative stress afforded by this mitochondria-targeted antioxidant.
Tissue activity of myeloperoxidase (MPO), an index of neutrophil infiltration, was severely elevated in pyelonephritic kidneys. Again, such an effect was significantly inhibited in rats treated with SkQR1 (Fig. 1F).
Oxidative and Nitrosative Stress and Renal Cells Death.
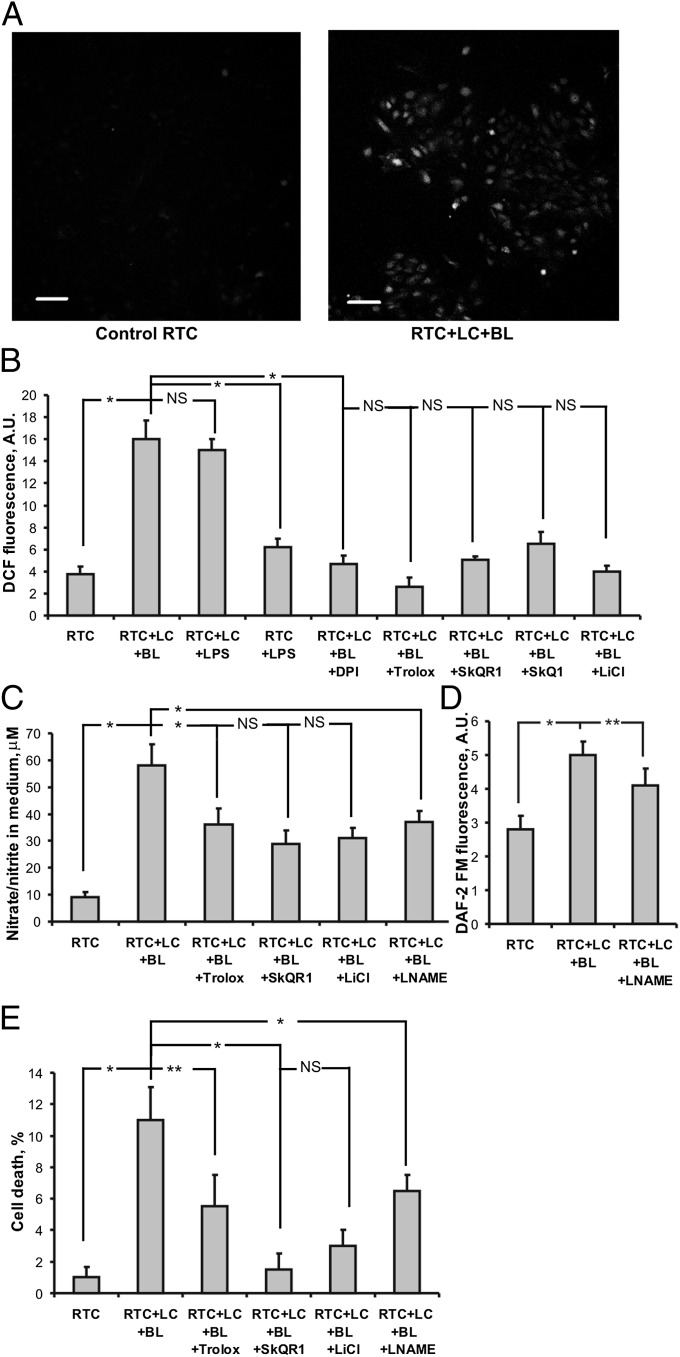
Next, we explored mitochondria-mediated oxidative stress in the cellular model of pyelonephritis, in which renal tubular cells (RTCs) are cocultured with bacterial lysate-activated leukocytes. Using this model, we observed a significantly greater ROS production as measured by fluorescent signal from DCF (2′-7′-dichlorofluorescein), compared with controls (Fig. 2A). Specifically, the ROS level was about five times higher than in the control (untreated) cells, which is in agreement with the assumption that initiation of oxidative stress in renal cells is mediated by activated leukocytes. Nonactivated leukocytes also caused the increase in ROS production in the RTC (Fig. S1A), but it was almost half as much as in the case of activated leukocytes (Fig. S1B); this difference can be explained by possible activation of leukocytes during their preparation. Confocal microscopy confirmed that the higher ROS signal detected in activated leukocyte–renal cell coculture originated mainly from the renal cells (Fig. 2A).
Fig. 2.
Oxidative stress and cell death in a cellular model of pyelonephritis in vitro. (A) Confocal microscopy of renal tubular cells (RTC) stained with DCF-DA. (Scale bar, 50 µm.) Control cells did not show any visible DCF fluorescence. After incubation with activated leukocytes (LC) plus bacterial lysate (BL), DCF fluorescence was significantly enhanced; quantitative results and effects of various treatments are shown in B. Nitrite concentration as an indicator of total NO production is shown in C. Generation of NO in RTC as measured by DAF-2FM fluorescence is shown in D. Cell death of RTC and protective effects of some drugs are presented in E. *P < 0.01, **P < 0.05.
We next compared the effects of LPS (30) and bacterial lysate to better judge the relevance of our model to previously published data. We found that application of LPS also caused leukocyte activation and greater ROS level in renal cells (Fig. 2B). Specifically, 100 ng/mL LPS had the same effect as was observed with bacterial lysate. Although the model using LPS-induced toxicity appears to be simpler, we think that utilization of bacterial lysate is more appropriate in terms of imitation of in vivo conditions after initiation of pyelonephritis. Recently, it was also shown (31) that bacterial lysates are more efficient than bacteria-derived soluble products for the induction of an activating phenotype in human dendritic cells. Experiments with an inhibitor of NADPH oxidase, diphenyleneiodonium (DPI; 0.5 µM), demonstrated that this enzyme is responsible for the primary and/or the secondary ROS production in this system because DPI inhibited the ROS production by renal cells (Fig. 2B). In addition, we evaluated ROS production in renal cells alone responding to LPS without leukocytes and observed negligible ROS production (Fig. 2B).
To prevent oxidative stress in renal cells as well as to diminish cell death, we used three drugs with potent antioxidative capacity, namely Trolox, SkQ1, and SkQR1. Treatment with each of these resulted in dramatically lowered ROS levels in renal cells (Fig. 2B). Pretreatment of renal cells with 10 nM SkQ1, 10 nM SkQR1, or 100 µM Trolox for 2 h resulted in suppression of ROS production after cocultivation with activated leukocytes to levels close to those observed on controls. Similarly, LiCl pretreatment also had an antioxidative effect (Fig. 2B). Somewhat less protection was observed when antioxidants were added to RTC and carefully washed out (Fig. S1B).
In addition to ROS, leukocytes activated by an antigen may produce a substantial amount of NO. After incubation of kidney cells with leukocytes and bacterial lysate for 24 h, the level of nitrate/nitrite (basic products of NO oxidation) in the medium was greater by more than fivefold than in controls (Fig. 2C). However, the effect of leukocyte activation on NO production in kidney cells, when measured by fluorescence of 4’,5’-diaminofluorescein diacetate-2 (DAF-2FM), seems to be much less prominent (Fig. 2D). Apparently, nitrosative stress shows a less profound response to leukocyte activation than oxidative stress. Nevertheless, preincubation with three different drugs revealed some suppression of nitrate/nitrite accumulation (Fig. 2C). A nonspecific inhibitor of NO synthase, nitro-l-arginine methyl ester (LNAME; 5 mM) partially suppresses NO production in renal cells (Fig. 2D) and nitrate/nitrite accumulation in medium (Fig. 2C). The comparison of nitrite production in pure leukocytic culture (Fig. S2) and in coculture (Fig. 2C) demonstrates the dominating role of leukocytes in NO release. Because NO production in leukocytes can be partially eliminated by antioxidants, we think that antioxidants could have an influence on both cocultivated cellular partners: on the one hand, protecting the renal cell from oxidative stress while, on the other hand, blocking activation of leukocytes. In our cellular model of pyelonephritis, we observed some renal cell death after 48 h of exposure to activated leukocytes (more than 10% of renal cells became Annexin V-positive). Preincubation with three different drugs was protective, with SkQR1 showing the highest efficiency (Fig. 2E). Comparison of the protective effects of SkQR1 and Trolox on the level of oxidative stress (Fig. 2B) and on cell death (Fig. 2E) demonstrates the quite distinct effects of these compounds. Specifically, the mitochondria-targeted antioxidant showed the highest potency in protection against renal cell death. Similarly, LiCl also protected renal cells from death, and its effect was more profound than that of Trolox. LNAME also demonstrated some protective effect (Fig. 2E), including prevention of oxidative stress-induced mitochondrial fragmentation in RTC (Fig. S3), suggesting the negative impact of NO on renal cells.
Effect of TNFα on Oxidative Stress in the Kidney.
As expected, bacterial lysate induces a robust TNFα production by leukocytes (Fig. S4A). Interestingly, TNFα release in the medium was even higher after coculture of activated leukocytes with renal cells (Fig. S4A), which points to the role of renal cell–leukocyte interaction in the induction of inflammation. Trolox, LiCl, and SkQR1 did not have a significant effect on the TNFα release in RTC–leukocyte coculture.
We tested whether released TNFα itself can affect renal cells by inducing oxidative stress. For this purpose, the medium, in which leukocytes were exposed to bacterial lysate, was centrifuged and added to renal cells. We found that TNFα-containing medium increased the ROS level in renal cells although to a lesser extent compared with that observed after cocultivation of renal cells and activated leukocytes (compare Fig. 2B and Fig. S4B). Consequently, we conclude that the direct interaction of renal cells and cocultivated leukocytes plays an important role in the induction of oxidative stress in renal cells.
To determine whether LPS targets kidney cells or leukocytes, we measured TNFα production in kidney cells after 24-h cultivation in the presence of LPS but without cocultivation with leukocytes. TNFα production is slightly increased during the incubation of RTC with LPS (Fig. S3C). Thus, LPS itself causes only a slightly increased production of TNFα in kidney cells whereas the main contribution comes from leukocytes. Interestingly, when kidney cells and leukocytes were cocultured in the presence of zymosan (10 μg/mL), another inducer of the toll-signaling cascade, we observed similar elevation of TNFα production in the medium whereas the incubation of renal cells alone with zymosan gives an insignificant increase in TNFα production (Fig. S3C). An inhibitor of leukocytic toll-signaling polymyxin B (10 μg/mL) inhibited the production of TNFα under cocultivation of renal cells with leukocytes and bacterial lysate (Fig. S3C).
Oxidative Stress and Changes in Blood Leukocytes.
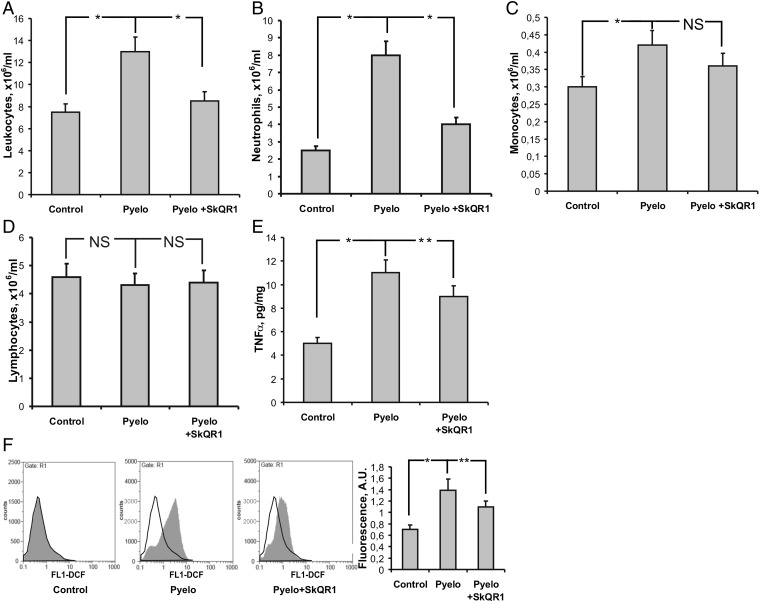
In the in vivo model of APN, the number of leukocytes in the blood 3 d after infection was increased twofold compared with the control level (Fig. 3A). Pretreatment with SkQR1 prevented an elevation in the level of leukocytes (Fig. 3A).
Fig. 3.
Elevated blood leukocyte counts are prevented by SkQR1 in pyelonephritic rats. Higher leukocyte (A), especially neutrophil (B) and monocyte (C), concentration in blood after induction of pyelonephritis with unchanged lymphocyte concentration (D). Production of proinflammatory TNFα (E) by leukocytes subsequent to pyelonephritis was elevated. Augmentation of neutrophils was accompanied by ROS burst in these cells (F). All of these inflammatory changes were prevented by SkQR1 treatment. *P < 0.01, **P < 0.05.
Leukocytosis correlated with neutrophilia (neutrophil level was three times higher; Fig. 3B). Monocyte count was also higher in pyelonephritic rats (Fig. 3C) whereas lymphocyte content in the blood was similar in all three groups (Fig. 3D). These effects were inhibited in rats treated with SkQR1, which abolished general leukocytosis and showed immune-modulating properties by reducing inflammation (Fig. 3 A–C).
The oxidative stress-dependent deleterious trend found in kidney tissue under an APN in vivo model was further confirmed in blood leukocytes. In addition to a higher level of MDA products in the pyelonephritic kidney tissue (Fig. 1E), we found that leukocytes isolated from rats with APN also had a higher level of ROS. Specifically, flow cytometry analysis demonstrated that the average intensity of DCF fluorescence in the leukocyte population from pyelonephritic rats was about twice as high as in control animals (Fig. 3F). We conclude that, in sick animals, leukocytes are in a more activated state. In fact, the addition of leukocytic cells from pyelonephritic rats to renal cell cultures caused much more profound oxidative stress in renal cells than that observed under cocultivation with leukocytes from healthy animals (Fig. S1A).
Because mitochondria-targeted antioxidants demonstrated high potency in preventing oxidative stress in the in vitro pyelonephritic model, we explored their effect under APN in rats. ROS production determined by DCF fluorescence measured in leukocytes from pyelonephritic rats receiving SkQR1 was lower than in untreated animals (Fig. 3F).
Similarly, we found that pretreatment with SkQR1 reduced the kidney tissue concentration of TNFα, which plays an essential role in inflammatory response (32). Specifically, although in our model of APN the level of TNFα was twice that of control rats, its level in the kidney tissue of SkQR1-treated pyelonephritic rats was significantly lower (Fig. 3E). As expected, the activation of TNFα production in leukocytes was mediated by an NF-κB–dependent pathway because the level of IκB in these cells was lower in untreated pyelonephritic rats but not in SkQR1-treated rats (Fig. S5).
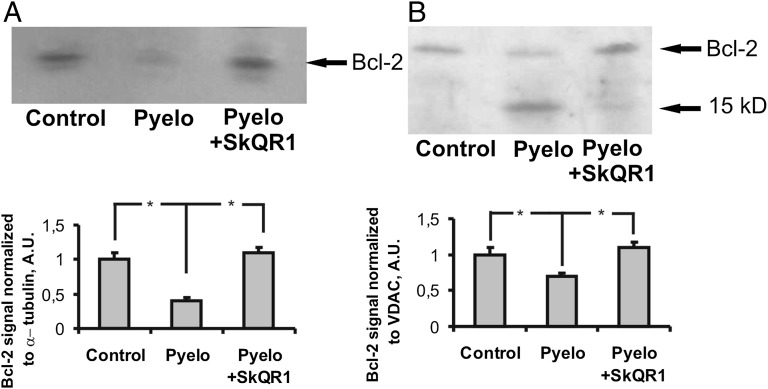
Alterations of Antiapoptotic Bcl-2 in Pyelonephritic Renal Tissue.
Considering that our main goal was to limit oxidative stress and its consequences in pyelonephritic kidney tissue, which in general may be formulated as a development of a cell protective strategy, we evaluated the balance between apoptotic and antiapoptotic activities during pyelonephritis. We found that the level of antiapoptotic protein Bcl-2 in the tubular cells of the kidney is diminished in our in vivo model of APN. In the total homogenates of the kidney, we observed a significantly lower Bcl-2 level in pyelonephritic rats, which was restored in pyelonephritic animals receiving mitochondria-targeted antioxidant SkQR1 (Fig. 4A). Also, the content of Bcl-2 in the mitochondria isolated from kidneys of pyelonephritic animals was 1.5 times lower than in control mitochondria (Fig. 4B). In parallel with a weaker band for Bcl-2 at 26 kDa, we observed a proportionally stronger band at 15 kDa positively stained for Bcl-2, suggesting cleavage of Bcl-2 under conditions of pyelonephritis. These changes were diminished after treatment with SkQR1.
Fig. 4.
Alterations of antiapoptotic Bcl-2 in control and pyelonephritic rats. In total kidney homogenates, Bcl-2 content was lower after pyelonephritis and similar to controls in pyelonephritic rats treated with SkQR1 (A). In isolated renal mitochondria, a low-weight Bcl-2-positive band was present in pyelonephritic rats (B).
Thus, we conclude that pyelonephritis accompanied by oxidative stress causes a significant depression in levels of the antiapoptotic protein Bcl-2 in mitochondria.
Role of Intercellular Contacts of Leukocytes and Renal Cells.
We next used scanning electron microscopy to analyze the morphology of renal epithelial cells cocultured with leukocytes. We found that renal cells had morphology typical for epithelial cells, which under the conditions used in this study form a semimonolayer. After 24 h cocultivation with a white blood leukocytic fraction, some leukocytic cells, monocytes according to their morphology, were found attached to the renal cells (Fig. S6A). These monocytes appeared to be attached to either cell-free plastic or directly to the renal cell. The latter interaction, we suggest, reflects a conventional interaction of an epithelial kidney cell with a leukocyte. An earlier study using the same method showed interaction of leukocytes with damaged endothelial cells (33). Apparently, in our pyelonephritic model, a similar interaction takes place. The fact that TNFα release is higher after cocultivation with renal cells than that observed in a pure culture of activated leukocytes (Fig. S4A) suggests the contribution of cell contact-dependent communication between leukocytes and renal cells. Moreover, we found that activated leukocytes died very quickly; however, the death rate was fivefold lower under conditions of coculture (Fig. S6B).
Experiments with individual leukocytic and renal cells provided additional support for the idea that direct contact of these two kinds of cells is essential to modulate the proinflammatory response and cell death. In the next set of experiments, we separated cocultured cells with a 0.4-µm porous PET membrane (cell culture insert; SPL Lifesciences) that permits communication via exchanging diffusible compounds but prevents direct contact between the cells. We found that, in this system, TNFα release is reduced compared with spatially unrestricted coculture (Table 1).
Table 1.
TNFα production and oxidative stress in RTCs under different conditions of cocultivation with leukocytes (LC)
| Measured parameters | RTC | LC | RTC + LC + bacterial lysate | RTC + LC + bacterial lysate cultivated with separating membrane |
| TNFα in the medium, pg/mL | 0 (n = 6) | 20.9 ± 0.8 (n = 6) | 16.6 ± 2.9 (n = 12) | 5.3 ± 2.2 (n = 6) |
| DCF intensity in RTC, arbitrary units | 1.4 ± 0.1 (n = 6) | N/A | 7.4 ± 0.4 (n = 12) | 5.1 ± 0.3 (n = 6) |
Survival of Animals with Acute Pyelonephritis.
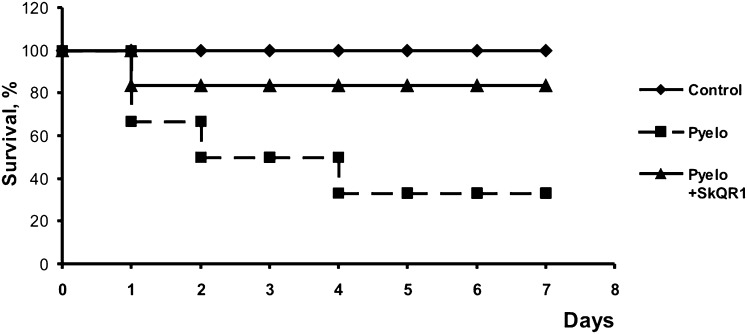
In our in vivo model of APN, the mortality of rats was substantial, apparently due to development of sepsis (34). Remarkably, treatment with SkQR1 during the first 2 d after infection resulted in significantly greater animal survival (Fig. 5). These results indicate that mitochondrial ROS production plays a crucial role in APN-associated kidney damage and subsequent septic mortality. To analyze the bacterial burden in rats at day 3 of the experiment, urine samples and kidneys were taken from rats, and the number of bacteria in both specimens was measured. In rats treated with antioxidant, the number of cfu in the kidney did not change whereas, in the urine, it was slightly reduced (Fig. S7), although SkQR1 didn’t demonstrate a bactericidal effect in vitro (Fig. S7C).
Fig. 5.
Severe pyelonephritis resulted in high animal mortality, which was prevented by SkQR1 treatment. Twelve animals were used in each group.
Discussion
Pyelonephritis is an infectious disease so the conventional strategy to cure it has been directed toward killing the bacteria causing this pathology. However, bacterial infection of both the lower and upper urinary tract is accompanied by inflammation and related oxidative stress phenomena, which can also be targeted to treat the disease by preventing or alleviating pathological consequences when direct antibacterial measures are impossible or inefficient.
The present study was designed to elucidate the role of mitochondrial ROS as a source of kidney cell damage in experimental APN using both a conventional in vivo model based on the inoculation of bacteria into the bladder and an in vitro model of inflammation based on the interaction of pathogen/endotoxin-activated leukocytes with cultured kidney epithelial cells.
In APN, we found an excessive ROS generation strongly depending on the activities of neutrophils, monocytes, and mesangial cells migrating to the primary inflammatory loci. This traffic results in elevation of the levels and activities of myeloperoxidase that persists after transformation to chronic type (35).
After activation initiated by interaction with bacterial products, leukocytes secrete ROS, NO, and TNFα. The suppression of leukocytic NAD(P)H oxidation yields a dramatic decrease of ROS within renal cells, which may serve as an argument to support the idea that leukocytes are specifically responsible for excessive ROS production in the pyelonephritic kidney.
A number of agents involved in different signaling mechanisms were tested for their ability to alleviate oxidative stress in renal cells. We found that the antioxidant Trolox reduced oxidative stress-related phenomena, apparently by diminishing transfer of ROS from leukocytes to kidney cells. Protective effects of mitochondria-targeted antioxidants (SkQ1, SkQR1) and of an inhibitor of GSK-3β (Li+) may be explained by the targeted depletion of mitochondrial ROS and the prevention of the mitochondrial permeability transition, respectively (36, 37).
The permeability transition has been shown to be a key event in a death-signaling cascade through release of proapoptotic factors (38–40). In addition, it is associated with a burst of mitochondrial ROS formation due to “ROS-induced ROS release” (41, 42), which may be a crucial factor determining the onset of a “point of no return” (43) in the path toward cell death.
Renal cell protection afforded by specialized agents (LiCl, SkQ1, and SkQR1) was more pronounced than that afforded by the traditional water-soluble antioxidant Trolox, demonstrating the critical role of mitochondrial ROS in the onset and propagation of renal pathology. Importantly, these protective agents did not have any effect on the level of TNFα production by leukocytes exposed to bacterial lysate, suggesting that their beneficial effects are due to tissue protection rather than suppression of inflammation. Antioxidants could have an influence on both cocultivated cellular partners: on the one hand, protecting the renal cell from oxidative stress (Fig. S1B), while, on the other hand, blocking activation of leukocytes (Fig. S2). Thus, targeting mitochondrial ROS may be beneficial under conditions of an ongoing infection where traditional anti-inflammatory drugs may interfere with microbial clearance.
The essential role of mitochondria-mediated oxidative stress in renal cell injury was confirmed in the in vivo model of experimental APN. MDA, which is an indicator of lipid peroxidation (44, 45), was elevated in the kidneys of rats with experimental APN, indicating oxidative renal damage. In addition, higher levels of ROS generation were observed in blood leukocytes of pyelonephritic rats. The mitochondria-targeted antioxidant SkQR1 remarkably normalized the ROS level in blood leukocytes and in kidneys of pyelonephritic animals and suppressed neutrophil infiltration in the diseased kidney as well as the level of TNFα in the tissue, indicating the high potency of mitochondria-targeted antioxidants in preventing inflammation injury in the kidney. Similarly, Sadeghi et al. (17) have shown that vitamin E injection provides significant renal protection in pyelonephritic animals.
Moreover, when the titer of bacteria injected into the bladder was high, we observed a high level of animal deaths (Fig. 5), apparently associated with septic progression. Sepsis to a large extent is recognized as a ROS-related pathology and was suggested to be a mitochondrial ROS-induced programmed death of organism (“phenoptosis”) preventing epidemics (46). Our observation that mitochondria-targeted antioxidants significantly increase survival of animals with severe APN supports this concept.
It is also recognized that the renal damage following APN is mainly caused by the inflammation associated with the infection, rather than by the direct effect of bacteria on the kidney (47–49). Bacterial invasion of the host kidney triggers the innate immune system. After recognition of bacteria, toll-like receptor signaling (50) initiates an immune response involving NF-κB and the production of cytokines and chemokines (51–53). We found that, in the leukocyte culture medium from pyelonephritic rats, the level of TNFα rises with time and that this medium itself causes oxidative stress in renal cells. The onset of APN was associated with activation of proinflammatory signaling pathways in peripheral leukocytes. This process apparently is redox-dependent because activation of NF-κB was lower in SkQR1-treated rats.
Correlation between the diminished content of the antiapoptotic (prosurvival) protein Bcl-2 in mitochondria and oxidative stress may support the hypothesis that Bcl-2 is a substrate for proapoptotic caspases (54, 55). The suppression of Bcl-2 may induce lower tolerance of the mitochondrial permeability transition to ROS, resulting in a higher rate of cell death. The protective (prosurvival) effect of antioxidants may be explained by two synergistic mechanisms: on the one hand, they might prevent the loss of Bcl-2 in pyelonephritic kidneys, while, secondly, they increase the pool of phosphorylated (inhibited) GSK-3β, which itself promotes survival by increasing the ROS threshold for the mitochondrial permeability transition, thereby increasing the tolerance of the cells to oxidative stress (56).
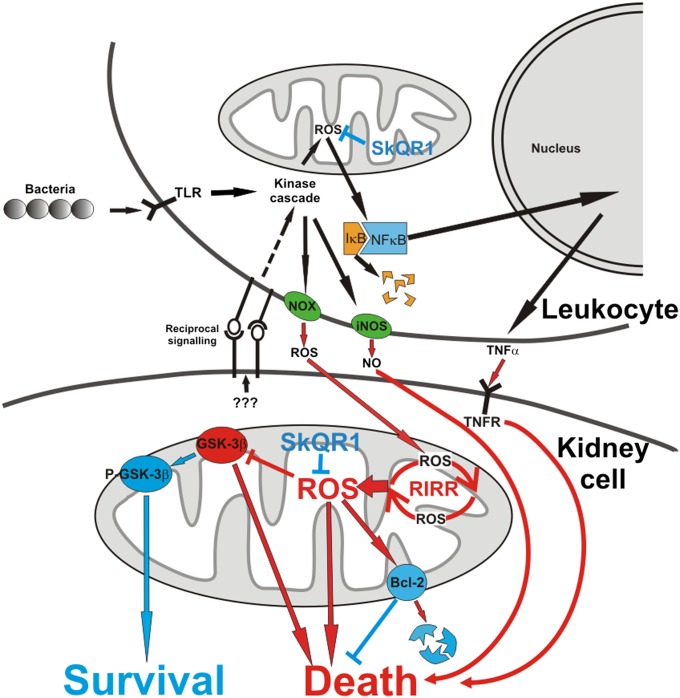
We present a tentative model of the pathological events occurring around and within the pyelonephritic renal cell (Fig. 6) that explains the incidence of inflammatory cell damage and death. A key element in this model is the essential role of mitochondrial ROS in all steps of the pathological progression. We emphasize that, in a majority of pathological steps, prodeath and prosurvival signaling is related to mitochondrial function. Mitochondria-targeted antioxidants appear to be effective antipyelonephritic drugs with high potency to prevent renal dysfunction in the cases where direct antibacterial measures are restricted (in children, pregnant women, individuals with primary immunodeficiency, etc.) or inefficient (in cases of antibiotic-resistant bacterial strains).
Fig. 6.
A tentative scheme of changes in the leukocytic and renal cells and their mitochondria after bacterial invasion. Prosurvival (blue color) and prodeath (red color) elements and pathways are shown. SkQR1, mitochondria-targeted antioxidant; NOX, NADPH oxidase; iNOS, inducible NO-synthase; TNFR, the receptor for TNF-α; RIRR, ROS-induced ROS release cascade; P-GSK-3β and GSK-3β, prosurvival (phosphorylated) and proapoptotic (dephosphorylated) forms of glycogen synthase kinase 3β.
Host defense from infection can be achieved through resistance and tolerance mechanisms (57). Our data demonstrate that mitochondrial ROS are an important contributor to inflammation-induced tissue damage and that targeting mitochondrial ROS can improve survival of the host during otherwise lethal bacterial infections.
Materials and Methods
Reagents.
DMEM and F12 culture media, FCS, and PBS were obtained from Gibco, and cell culture plasticware from Greiner. The other reagents were obtained from Sigma-Aldrich. Mitochondria-targeted antioxidants SkQ1 (10-(6′-plastoquinonyl)decyltriphenyl-phosphonium) and SkQR1 (10-(6′-plastoquinonyl) decylrhodamine 19) were synthesized (58) in the Institute of Mitoengineering (Moscow State University).
Primary Rat Kidney Cell Culture.
Kidneys from 1- to 3-d-old rats were excised under aseptic conditions. The tissue was blended and dissociated by collagenase treatment (0.5%, 30 min at 37 °C). The final suspension was centrifuged for 5 min at 50 × g. The pellet was resuspended in ∼10 mL of DMEM/F12 supplemented with 10% (vol/vol) FCS and kept for 2 min, after which the supernatant was transferred to another tube and the pellet was repeatedly resuspended. After 10 min, the renal tubules were pelleted, and dissociated cells remaining in the suspension were discarded. The pellet was resuspended in DMEM/F12 with 10% FCS and seeded onto 24-well plates or onto coverslips placed in 35-mm glass-bottom Petri dishes.
Cultivation of Bacteria.
E. coli strain no. 85 was cultured overnight in a cultivation medium containing 1% tryptone, 0.5% yeast extract, and 1% NaCl. The medium, containing roughly 109 colony-forming units (cfu) per ml, was pelleted at 300 × g for 3 min. Bacterial lysate was prepared by diluting 30 mL of an overnight culture and diluting the precipitate in 3 mL of 0.9% NaCl, followed by autoclaving for 1.5 h at 120 °C.
Preparation of Leukocytes from Peripheral Blood.
Heparinized blood (5 mL) collected from the jugular vein of adult male rats was carefully layered over 5 mL of Ficoll-Urografin (density of 1.077 g/cm3) and centrifuged for 30 min at 200 × g. This procedure results in erythrocyte pelleting while the mononuclear fraction of leukocytes forms an interphase ring on the Ficoll surface, which was carefully collected. Leukocytes were transferred into another tube and centrifuged for 3 min at 200 × g. The resultant pellet was resuspended in 5 mL of DMEM/F12. After cell counting in a hemocytometer, the cells were diluted to a final concentration of ∼100 000 cells per mL. The same procedure was applied to get leukocytes from green fluorescent protein (GFP)-transgenic mice.
For preparation of neutrophils, the proper fraction from a Ficoll gradient was transferred to another tube, mixed with 10 mL of DMEM/F12, and centrifuged for 3 min at 200 × g. The final pellet containing neutrophils was resuspended in 5 mL of DMEM/F12, and the cells were counted and then diluted to a final of ∼100 000 cells per mL.
Inflammation Modeling in Vitro.
In a 2-d-old renal cell culture, the medium was substituted with DMEM/F12 (400 µL per well, 1,600 µL per dish), supplemented with (depending on the purpose): GSK-3β inhibitor LiCl (9 mМ), water-soluble antioxidant Trolox (50 µМ, 100 µМ), and mitochondria-targeted antioxidants SkQ1 (10 nМ, 100 nМ) and SkQR1 (1 nМ, 10 nМ, or 100 nМ). Renal cells were incubated for 2 h in this medium, followed by supplementation with leukocytes or neutrophils in DMEM/F12 or DMEM/F12 alone in the control sample (400 µL per well, 1,600 µL per dish). Simultaneously, the bacterial lysate or 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich) solution in DMEM/F12 and DMEM/F12 alone in the control sample (100 µL per well, 400 µL per dish) were added. Renal cells and leukocytes (neutrophils) were cocultivated for 24 or 48 h with bacterial lysate or LPS. Cell death (both necrotic and apoptotic) rate was evaluated using Annexin-V FITC Kit (Invitrogen).
An in Vivo Rat Model of APN.
We used an in vivo experimental model of APN where bacteria are introduced in the bladder of rat (59). Experiments were performed on outbred white female rats (180–200 g) fed ad libitum. Animal protocols were approved by the Institutional Review Boards at A. N. Belozersky Institute, Moscow State University. Rats were anesthetized with chloral hydrate [300 mg/kg, intraperitoneally (i.p.)]. The animals were infected intraurethrally using a soft Intramedic non–radio-opaque polyethylene catheter (Clay Adams). The inoculum (5 mL per kg, 1 × 108 cfu/mL of rat fecal bacterial composition) was injected slowly to avoid any leakage into the bladder. Control animals were untreated. The therapeutic protocol of SkQR1 used to treat this pathology was as follows: i.p. injection of 100 nmol/kg SkQR1 1 h after injection of bacteria with subsequent injections of the same amount of SkQR1 at 12, 24, 36, and 48 h; in total, each animal received 500 nmol/kg SkQR1. On the second day after the injection, blood samples were taken, and kidneys were excised for the mitochondria isolation, determination of MDA in the tissue, Western blotting, and histopathological examination. Kidney mitochondria were isolated by homogenization and differential centrifugation in a medium containing 250 mM sucrose, 20 mM Hepes-KOH, 1 mM EGTA, and 0.1% BSA, pH 7.4. Total mitochondrial protein was determined using a bicinchoninic acid protein assay kit (Sigma-Aldrich). The mitochondria from cultured kidney cells were isolated by the same protocol.
ROS, NO, MDA, and TNFα Determination.
The ROS-sensitive fluorescent probe 2,7-DCF-DA (Molecular Probes) dissolved in DMEM/F12 without bicarbonate (final concentration 10 µМ) was added to renal cells (500 µL per well of 24-well plate, 2 mL per dish) and incubated for 15 min at 37 °C, followed by a wash with DMEM/F12 without bicarbonate. MDA was determined as in ref. 60. Fluorescent probe, DAF-2FM (Calbiochem) was used for NO determination in living cells. The procedure was the same as for 2,7-DCF-DA. The nitrite/nitrate concentration (as products of NO oxidation) in culture media was determined using the Nitrite/Nitrate Assay Kit (Sigma-Aldrich). TNFα was determined using the kit Rat TNFα ELISA Ready-SET-Go (Ebioscience).
Confocal and Scanning Electron Microscopy.
Renal cells were imaged with an LSM510 inverted confocal microscope (Carl Zeiss Inc.) with excitation at 488 nm and emission collected at 500–530 nm with a pinhole diameter of 150 µm. Images were processed using ImageJ software (National Institutes of Health).
For scanning electron microscopy, cells and bacteria were fixed in 2.5% glutaraldehyde in Ca2+ and Mg2+-free Hanks’ buffer supplemented with 5 mM EDTA, 5 mM phenylmethylsulfonyl fluoride, and 10 mM Hepes at pH 7.3. Cells were postfixed with 1% osmium tetroxide in 0.1 M sodium cacodylate with 0.1 M sucrose at pH 7.3 (without replacing a buffer containing glutaraldehyde), dehydrated in acetone series, critical-point-dried with liquid CO2 as a transitional fluid in a Balzers apparatus, sputter-coated with gold–palladium, and observed at 15 kV with a Camscan S-2 or JSM-6380 scanning electron microscope.
Renal Histology and Grading of Inflammatory Tissue Damage.
The kidney was isolated immediately after killing the animal and perfused with ice-cold PBS. It was then fixed in a 10% neutral buffered formalin solution, embedded in paraffin, and used for histopathological examination. Five-micrometer-thick sections were cut, deparaffinized, hydrated, and stained with hematoxylin/eosin. The renal sections were examined in a blinded fashion for inflammation and infiltration by leukocytes in the kidneys of all treated animals. A minimum of 10 fields for each kidney slide were examined and scored for pathologic severity.
The manifestation of inflammatory indicators (i.e., a number of infiltrated leukocytes in every layer and the presence of abscesses) under APN was evaluated using the following scale: 0, 0–4 leukocytes in the view field; 1, 5–50; 2, 51–100; 3, >100 in a view field; 4, abscesses with pus-necrotic content. The distribution of inflammatory changes (i.e., comparative analysis of infiltration and abscesses) was evaluated by the following criteria: 0, none in any layer; 1, infiltration in the medullar layer; 2, infiltration reaching the cortex.
Myeloperoxidase Activity Assay.
Kidney homogenates for MPO determination were centrifuged at 20,000 × g for 15 min, and the pellets were resuspended in 50 mM K-phosphate buffer containing 0.5% cetyltrimethylammonium bromide (CTAB). Resulting samples were frozen and thawed three times and then centrifuged at 10,600 × g for 10 min. Supernatants were analyzed for MPO activity in chromogenic reaction with o-phenylenediamine (OPD). A one hundred-microliter sample was mixed with a 100-µL substrate buffer (25 mM Na-citrate, 50 mM Na-phosphate, 0.45 mg/mL OPD, 0.1% H2O2, pH 5.0) and was incubated for 15 min at room temperature, and then OD was detected at 492 nm.
Immunocytochemistry.
Cells and kidney slices were washed in PBS, fixed for 30 min in 4% formaldehyde with PBS at 4 °C, and permeabilized in PBS containing 0.02% Triton X-100 for 60 min at 4 °C (0.5 mL per well, 5 mL per slice), followed by blocking in PBS with 0.5% BSA (PBS-BSA) for 60 min at room temperature (0.5 mL per well, 2 mL per slice). After three 15-min rinses in PBS-BSA, cells were incubated for 1 h with secondary antibodies diluted 1:200 (FITC-conjugated anti-rabbit IgG; Jackson ImmunoResearch Laboratories). The kidney slices and coverslips with attached cells were washed, placed on microscope slides with a mounting medium, and sealed beneath coverslips. Confocal microscopy images were processed using ImageJ software (NIH).
Western Blot Analysis.
Samples of kidney homogenates were loaded onto 15% Tris–glycine polyacrylamide gels (10-20 μg of total protein per lane). After electrophoresis, gels were blotted onto PVDF membranes (Amersham Pharmacia Biotech). Membranes were blocked with 5% (wt/vol) nonfat milk in PBS with 0.1% (vol/vol) Tween 20 and subsequently incubated with appropriate primary antibodies. Membranes were then treated with corresponding anti-mouse or anti-rabbit secondary antibodies. Specific bands were visualized using ECL Plus Western blotting kit (Amersham Pharmacia Biotech). After scanning, the density of the resulting staining in the membrane was measured for each band using ImageJ software (NIH).
Statistics.
All experiments were performed at least in triplicate. All data are presented as mean ± SEM. Comparisons between groups were made using a Student t test with a P value less than 0.05 taken to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank V. I. Kirpatovsky and K. W. Fishbein for help and valuable advice. The study was supported by Russian Foundation of Basic Research Grants 11-04-00771, 11-04-01307, and 12-04-31299 and the Mitoengineering Research Institute.
Footnotes
Conflict of interest statement: V.P.S. has a financial interest in SkQ.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307096110/-/DCSupplemental.
References
- 1.O'Hanley P. Prospects for urinary tract infection vaccines. In: Mobley HLT, Warren JW, editors. Urinary Tract Infections: Molecular Pathogenesis and Clinical Management. Washington, DC: American Society for Microbiology; 1996. pp. 405–425. [Google Scholar]
- 2.Hill JB, Sheffield JS, McIntire DD, Wendel GD., Jr Acute pyelonephritis in pregnancy. Obstet Gynecol. 2005;105(1):18–23. doi: 10.1097/01.AOG.0000149154.96285.a0. [DOI] [PubMed] [Google Scholar]
- 3.Trifillis AL, et al. Binding to and killing of human renal epithelial cells by hemolytic P-fimbriated E. coli. Kidney Int. 1994;46(4):1083–1091. doi: 10.1038/ki.1994.370. [DOI] [PubMed] [Google Scholar]
- 4.Uhlén P, et al. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature. 2000;405(6787):694–697. doi: 10.1038/35015091. [DOI] [PubMed] [Google Scholar]
- 5.O’Hanley P, Lalonde G, Ji G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: Efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect Immun. 1991;59(3):1153–1161. doi: 10.1128/iai.59.3.1153-1161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith YC, Rasmussen SB, Grande KK, Conran RM, O’Brien AD. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect Immun. 2008;76(7):2978–2990. doi: 10.1128/IAI.00075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011;365(3):239–250. doi: 10.1056/NEJMra1007755. [DOI] [PubMed] [Google Scholar]
- 8.Pistor K, Schärer K, Olbing H, Tamminen-Möbius T. Children with chronic renal failure in the Federal Republic of Germany. II. Primary renal diseases, age and intervals from early renal failure to renal death. Clin Nephrol. 1985;23(6):278–284. [PubMed] [Google Scholar]
- 9.Gupta A, Sharma S, Nain CK, Sharma BK, Ganguly NK. Reactive oxygen species-mediated tissue injury in experimental ascending pyelonephritis. Kidney Int. 1996;49(1):26–33. doi: 10.1038/ki.1996.4. [DOI] [PubMed] [Google Scholar]
- 10.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66(2):486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 11.Mundi H, Björkstén B, Svanborg C, Ohman L, Dahlgren C. Extracellular release of reactive oxygen species from human neutrophils upon interaction with Escherichia coli strains causing renal scarring. Infect Immun. 1991;59(11):4168–4172. doi: 10.1128/iai.59.11.4168-4172.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassar GM, Badr KF. Novel approaches to treatment of glomerulonephritis. J Nephrol. 1998;11(4):177–184. [PubMed] [Google Scholar]
- 13.Aydogdu N, et al. Protective effects of L-carnitine on myoglobinuric acute renal failure in rats. Clin Exp Pharmacol Physiol. 2006;33(1-2):119–124. doi: 10.1111/j.1440-1681.2006.04336.x. [DOI] [PubMed] [Google Scholar]
- 14.Koyner JL, Sher Ali R, Murray PT. Antioxidants: Do they have a place in the prevention or therapy of acute kidney injury? Nephron, Exp Nephrol. 2008;109(4):e109–e117. doi: 10.1159/000142935. [DOI] [PubMed] [Google Scholar]
- 15.Polo-Romero FJ, Fernández-Fúnez A, Broseta Viana L, Atienza MP, Sánchez Gascón F. Effect of N-acetylcysteine on antioxidant status in glycerol-induced acute renal failure in rats. Ren Fail. 2004;26(6):613–618. doi: 10.1081/jdi-200037115. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigo R, Bosco C, Herrera P, Rivera G. Amelioration of myoglobinuric renal damage in rats by chronic exposure to flavonol-rich red wine. Nephrol Dial Transplant. 2004;19(9):2237–2244. doi: 10.1093/ndt/gfh369. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi Z, et al. Vitamin E administration at the onset of fever prevents renal scarring in acute pyelonephritis. Pediatr Nephrol. 2008;23(9):1503–1510. doi: 10.1007/s00467-008-0853-7. [DOI] [PubMed] [Google Scholar]
- 18.Singh D, Chander V, Chopra K. Protective effect of naringin, a bioflavonoid on glycerol-induced acute renal failure in rat kidney. Toxicology. 2004;201(1-3):143–151. doi: 10.1016/j.tox.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Sanmun D, et al. Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: Implications for chronic granulomatous disease. Am J Physiol Cell Physiol. 2009;297(3):C621–C631. doi: 10.1152/ajpcell.00651.2008. [DOI] [PubMed] [Google Scholar]
- 20.Plotnikov EY, Chupyrkina AA, Pevzner IB, Isaev NK, Zorov DB. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney’s mitochondria. Biochim Biophys Acta. 2009;1792(8):796–803. doi: 10.1016/j.bbadis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Plotnikov EY, et al. New-generation Skulachev ions exhibiting nephroprotective and neuroprotective properties. Biochemistry (Mosc) 2010;75(2):145–150. doi: 10.1134/s0006297910020045. [DOI] [PubMed] [Google Scholar]
- 22.Skulachev VP, et al. An attempt to prevent senescence: A mitochondrial approach. Biochim Biophys Acta. 2009;1787(5):437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Smith RA, et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann N Y Acad Sci. 2008;1147:105–111. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- 24.Antonenko YN, et al. Protective effects of mitochondria-targeted antioxidant SkQ in aqueous and lipid membrane environments. J Membr Biol. 2008;222(3):141–149. doi: 10.1007/s00232-008-9108-6. [DOI] [PubMed] [Google Scholar]
- 25.Bakeeva LE, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke) Biochemistry (Mosc) 2008;73(12):1288–1299. doi: 10.1134/s000629790812002x. [DOI] [PubMed] [Google Scholar]
- 26.Agapova LS, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 3. Inhibitory effect of SkQ1 on tumor development from p53-deficient cells. Biochemistry (Mosc) 2008;73(12):1300–1316. doi: 10.1134/s0006297908120031. [DOI] [PubMed] [Google Scholar]
- 27.Neroev VV, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals. Biochemistry (Mosc) 2008;73(12):1317–1328. doi: 10.1134/s0006297908120043. [DOI] [PubMed] [Google Scholar]
- 28.Anisimov VN, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 5. SkQ1 prolongs lifespan and prevents development of traits of senescence. Biochemistry (Mosc) 2008;73(12):1329–1342. doi: 10.1134/s0006297908120055. [DOI] [PubMed] [Google Scholar]
- 29.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222(5198):1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S, Furusawa S, Wu J, Nagata K. Preventive effects of a biscoclaurine alkaloid, cepharanthine, on endotoxin or tumor necrosis factor-alpha-induced septic shock symptoms: Involvement of from cell death in L929 cells and nitric oxide production in raw 264.7 cells. Int Immunopharmacol. 2007;7(2):191–197. doi: 10.1016/j.intimp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Morandi B, et al. A mixture of bacterial mechanical lysates is more efficient than single strain lysate and of bacterial-derived soluble products for the induction of an activating phenotype in human dendritic cells. Immunol Lett. 2011;138(1):86–91. doi: 10.1016/j.imlet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Niño MD, et al. TNF superfamily: A growing saga of kidney injury modulators. Mediators Inflamm. 2010;2010:182958. doi: 10.1155/2010/182958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galkina SI, et al. Endothelium-leukocyte interactions under the influence of the superoxide-nitrogen monoxide system. Med Sci Monit. 2004;10(9):BR307–BR316. [PubMed] [Google Scholar]
- 34.Giamarellos-Bourboulis EJ, et al. Oleuropein: A novel immunomodulator conferring prolonged survival in experimental sepsis by Pseudomonas aeruginosa. Shock. 2006;26(4):410–416. doi: 10.1097/01.shk.0000226342.70904.06. [DOI] [PubMed] [Google Scholar]
- 35.Meylan PR, Glauser MP. Role of complement-derived and bacterial formylpeptide chemotactic factors in the in vivo migration of neutrophils in experimental Escherichia coli pyelonephritis in rats. J Infect Dis. 1989;159(5):959–965. doi: 10.1093/infdis/159.5.959. [DOI] [PubMed] [Google Scholar]
- 36.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195(2):453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 37.Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241(2):139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 39.Zamzami N, et al. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183(4):1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skulachev VP. Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cell. FEBS Lett. 1996;397(1):7–10. doi: 10.1016/0014-5793(96)00989-1. [DOI] [PubMed] [Google Scholar]
- 41.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192(7):1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: An update and review. Biochim Biophys Acta. 2006;1757(5-6):509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 43.Kroemer G, Petit P, Zamzami N, Vayssière JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9(13):1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- 44.Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005;39(7):841–852. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Sharma N, Sharma BK, Sharma S, Ganguly NK. Oxygen-dependent and -independent mechanisms of renal injury in experimental ascending pyelonephritis. FEMS Immunol Med Microbiol. 1996;13(1):35–42. doi: 10.1111/j.1574-695X.1996.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 46.Skulachev VP. Programmed death phenomena: from organelle to organism. Ann NY Acad Sci. 2002;959:214–237. doi: 10.1111/j.1749-6632.2002.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 47.Bennett RT, Mazzaccaro RJ, Chopra N, Melman A, Franco I. Suppression of renal inflammation with vitamins A and E in ascending pyelonephritis in rats. J Urol. 1999;161(5):1681–1684. [PubMed] [Google Scholar]
- 48.Haraoka M, et al. Suppression of renal scarring by prednisolone combined with ciprofloxacin in ascending pyelonephritis in rats. J Urol. 1994;151(4):1078–1080. doi: 10.1016/s0022-5347(17)35187-x. [DOI] [PubMed] [Google Scholar]
- 49.Imamoğlu M, et al. Effects of melatonin on suppression of renal scarring in experimental model of pyelonephritis. Urology. 2006;67(6):1315–1319. doi: 10.1016/j.urology.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Shirali AC, Goldstein DR. Tracking the toll of kidney disease. J Am Soc Nephrol. 2008;19(8):1444–1450. doi: 10.1681/ASN.2008010123. [DOI] [PubMed] [Google Scholar]
- 51.Li YH, Yan ZQ, Brauner A, Tullus K. Activation of macrophage nuclear factor-kappa B and induction of inducible nitric oxide synthase by LPS. Respir Res. 2002;3(1):23. doi: 10.1186/rr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ragnarsdottir B, et al. 2008. TLR- and CXCR1-dependent innate immunity: Insights into the genetics of urinary tract infections. Eur J Clin Invest 38(Suppl 2):12–20.
- 53.Tullus K, Escobar-Billing R, Fituri O, Lu Y, Brauner A. Soluble receptors to tumour necrosis factor and interleukin-6 in urine during acute pyelonephritis. Acta Paediatr. 1997;86(11):1198–1202. doi: 10.1111/j.1651-2227.1997.tb14845.x. [DOI] [PubMed] [Google Scholar]
- 54.Clem RJ, et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1998;95(2):554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng EH, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278(5345):1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 56.Juhaszova M, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113(11):1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335(6071):936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antonenko YN, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc) 2008;73(12):1273–1287. doi: 10.1134/s0006297908120018. [DOI] [PubMed] [Google Scholar]
- 59.Gupta R, Ganguly NK, Ahuja V, Joshi K, Sharma S. An ascending non-obstructive model for chronic pyelonephritis in BALB/c mice. J Med Microbiol. 1995;43(1):33–36. doi: 10.1099/00222615-43-1-33. [DOI] [PubMed] [Google Scholar]
- 60.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.