Abstract
Certain lower vertebrates like zebrafish activate proliferation of spared cardiomyocytes after cardiac injury to regenerate lost heart muscle. Here, we used translating ribosome affinity purification to profile translating RNAs in zebrafish cardiomyocytes during heart regeneration. We identified dynamic induction of several Jak1/Stat3 pathway members following trauma, events accompanied by cytokine production. Transgenic Stat3 inhibition in cardiomyocytes restricted injury-induced proliferation and regeneration, but did not reduce cardiogenesis during animal growth. The secreted protein Rln3a was induced in a Stat3-dependent manner by injury, and exogenous Rln3 delivery during Stat3 inhibition stimulated cardiomyocyte proliferation. Our results identify an injury-specific cardiomyocyte program essential for heart regeneration.
Keywords: TRAP, cardiac regeneration, interleukin, inflammation, endocardium
Cardiac regeneration eludes adult mammals, but certain nonmammalian vertebrates present models of robust cardiac repair. In particular, zebrafish maintain a high capacity for myocardial regeneration throughout life and can create new heart muscle with minimal scarring after a variety of severe genetic or mechanical injuries (1–3). Thus, studies of how heart regeneration occurs naturally can help guide approaches to stimulating adult mammalian heart regeneration, for which there are now multiple promising strategies (4–9).
Genetic fate-mapping experiments indicated that spared cardiomyocytes are the primary source tissue for zebrafish heart regeneration, with little or no contribution by a resident stem cell population (1, 10–12). Retinoic acid, Transforming growth factor beta 1, Insulin-like growth factor 2, Sonic hedgehog, and Platelet-derived growth factor ligands all are synthesized in the vicinity of proliferating cardiomyocytes, and have positive influences on muscle regeneration (13–16). Other potential factors include hypoxia, which appears to play a positive role in cardiomyocyte proliferation, whereas hyperoxia, active p38α mitogen activated protein kinase, and microRNA miR-133 have negative roles (17–19). Defining additional pathways that underlie injury-induced cardiomyocyte proliferation in zebrafish is important for better understanding heart regeneration, and is also relevant to new methodology to boost the low levels of cardiomyocyte proliferation in injured mammalian hearts (20, 21).
Novel transgenic profiling technologies have recently been developed for isolation of actively translated mRNAs from specific cell types (22, 23). For example, with translating ribosome affinity purification (TRAP) technology, an enhanced GFP (EGFP) reporter gene is fused to the N terminus of the large subunit ribosomal protein L10a, and this cassette is placed downstream of a tissue-specific promoter. Translating mRNAs can then be isolated from promoter-defined cell populations in transgenic animals by immunoprecipitation with an antibody recognizing EGFP. Although this technology has not yet been applied to tissue regeneration, TRAP avoids tissue dissociation and cell purification, and thus has clear advantages for profiling injury and regeneration responses in specific cell types. Here, we used TRAP to identify ribosome-associated RNAs in zebrafish cardiomyocytes during heart regeneration.
Results
To generate cardiomyocyte ribosome-associated RNA profiles from transgenic zebrafish, we used regulatory sequences of cardiac myosin light chain 2a to drive expression of an EGFP-L10a cassette [Tg(cmlc2:EGFP-RPL10a)pd61] (referred to as cmlc2:TRAP) (Fig. 1 A and B). Because the cmlc2a promoter is active in virtually all adult cardiomyocytes, the cmlc2:TRAP line is expected to identify cardiomyocyte gene expression during early injury responses as well as regenerative responses. The TRAP reporter was expressed in cardiomyocytes, where its presence did not inhibit heart regeneration (Fig. 1C). To assess the specificity of this approach, we immunoprecipitated ribosome-associated RNAs from ventricles of adult cmlc2:TRAP fish and examined expression of cardiac genes. These experiments detected known cardiomyocyte markers by PCR amplification, but genes with expression known to be restricted to other cell types were weak or undetectable (Fig. 1D).
Fig. 1.
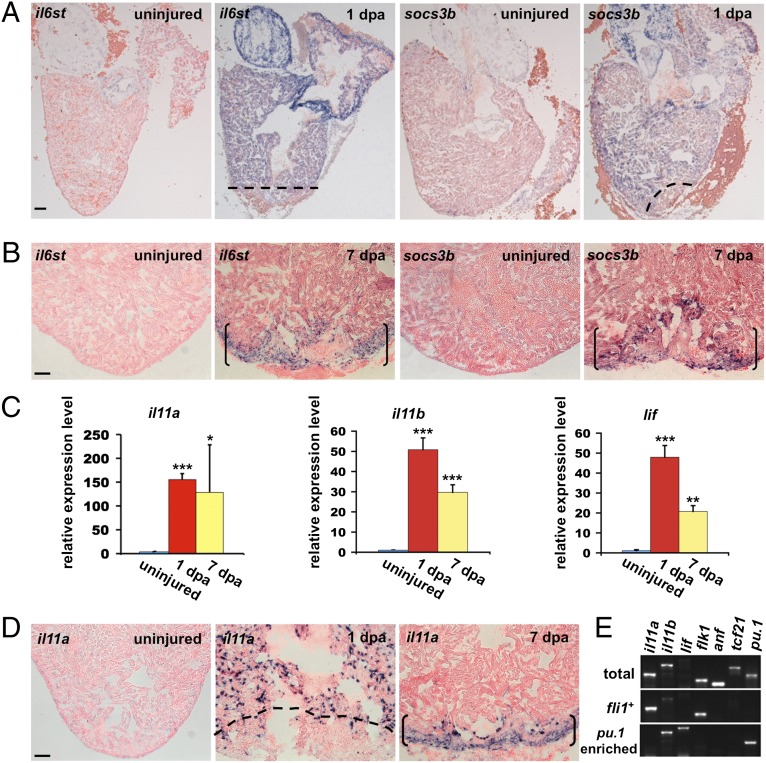
Translational profiling of cardiomyocytes during zebrafish heart regeneration. (A) Tissue section indicating localization of EGFP-L10a fluorescence to cardiomyocytes in cmlc2:TRAP hearts. (B) Higher-magnification view of an uninjured cmlc2:TRAP ventricular apex. (C) Regeneration appears normal in cmlc2:TRAP ventricles 30 d after partial resection. Dashed line indicates approximate amputation plane. (Scale bars for A–C, 50 μm.) (D) RT-PCR of total or immunoprecipitated (TRAP) RNAs isolated from adult cmlc2:TRAP ventricles. β-actin1, hand2, cmlc2, ventricular myosin heavy chain (vmhc) and anf (also known as natriuretic peptide precursor A) are expressed in cardiomyocytes (CMs), and flk1 (endocardial), gata2a (hematopoietic), and tcf21 (epicardial) are noncardiomyocyte genes (Non-CMs). (E) Heat map from microarray indicating increased levels of Jak1/Stat3 pathway members in cmlc2:TRAP RNA samples at 1 dpa. (F) qPCR using RNAs immunoprecipitated from cmlc2:TRAP ventricles, confirming up-regulation of Jak1/Stat3 pathway members after injury. Expression levels were normalized to that of β-actin2, and further normalized to that of the uninjured sample. Data are mean ± SEM n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, Student t test (unpaired, two-tailed). (G) Western Blots using total proteins extracted from uninjured and 1-dpa hearts, indicating increased protein levels of Stat3, phosphorylated Stat3 (P-Stat3), and Bcl2 at 1 dpa. Tubulin was used as a loading control (shown for Stat3 and P-Stat3 lanes).
To profile gene expression after injury, we immunoprecipitated cardiomyocyte RNAs from uninjured cmlc2:TRAP fish ventricles and ventricles at 1 and 7 d after 20% apical resection (dpa). The apical halves of ventricles were collected and pooled, and immunoprecipitated RNA was processed for microarray analysis. We identified 138 genes with significant expression differences at 1 dpa compared with uninjured ventricles, and fewer differentially expressed genes at 7 dpa (Fig. S1A and Table S1; raw data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus Web site). Levels of ion transporters and channels, such as solute carrier family 39 member 3, chloride intracellular channel 5, solute carrier family 12, and potassium voltage-gated channel subfamily H member 6, were increased by 1 dpa, supporting previously identified changes in cardiomyocyte electrical properties during regeneration (1, 11). We also found increased levels of genes relevant to cell survival and proliferation, such as B-cell lymphoma 2 (bcl2), jun B proto-oncogene a, FOS-like antigen 1a, poly(ADP-ribose) polymerase family member 3 and 4 (Fig. S1B). Compared with a published microarray dataset using whole 1-dpa ventricular tissue (24), only 10 genes with significant expression changes are shared. Possible explanations for this modest overlap include: (i) background noise from gene-expression changes in nonmuscle cells; (ii) specificity of the TRAP technique to ribosome-associated RNAs; and (iii) variation in gene coverage among microarrays. TRAP did not identify some genes previously detected by visual techniques in cardiomyocytes at 7 dpa, such as gata4 and mps1 (3, 11). We suspect that this finding is at least in part a result of dilution of signals by cardiomyocytes that are not participating in regeneration at this stage.
Most remarkably, several members of the Janus kinase 1/Signal transducer and activator of transcription 3 (Jak1/Stat3) pathway represented on the microarray, including interleukin 6 signal transducer (il6st), jak1, stat3, and the target gene suppressor of cytokine signaling 3b (socs3b), had highly elevated levels in 1-dpa cardiomyocytes (Fig. 1E), results confirmed by quantitative PCR (qPCR) (Fig. 1F). Among seven Stat factors (Stat1–4, -5a, -5b, and -6), Stat3 is activated by a variety of cytokines/growth factors and has multiple functions in embryogenesis and cell survival/proliferation (25, 26). The Jak1/Stat3 pathway was the top enriched pathway at 1 dpa as analyzed by the DAVID functional annotation clustering tool (P value: 1.4 E-4), but did not emerge as a significantly enriched pathway in previous transcriptome analyses of zebrafish heart regeneration that used whole-tissue samples (24, 27). jak2a and stat2, which is expected to provide a more restricted immunoregulatory function, were recently reported by microarray or qPCR as up-regulated in 7-dpa whole-cardiac tissue (18). However, stat2 and other stat genes did not show significant myocardial up-regulation in our experiments. To confirm that these profiles could represent increased translation products, we assessed protein levels in whole cardiac tissue by Western blotting. We detected increased levels of Stat3, phosphorylated Stat3, and Bcl2 protein at 1 dpa, consistent with rapid induction in cardiomyocytes after injury (Fig. 1G).
Jak1/Stat3 signaling is initiated by dimerization of the signal transducer Il6st upon cytokine binding to its receptor. Following Il6st dimerization, Jak is activated and phosphorylates Il6st, creating a docking site for Stat3. After phosphorylation, Stat3 dimers dissociate from the receptor, translocate to the nucleus, and activate transcription of genes involved in cell survival, cell proliferation, and other events (28, 29). Pathway up-regulation was further confirmed by in situ hybridization. Similar to dynamic expression signatures of certain epicardial and endocardial factors (15, 30), induction of Jak1/Stat3 pathway genes was first organ-wide at 1 dpa, but then localized to a region of cardiomyocytes at the site of regeneration by 7 dpa (Fig. 2 A and B, and Fig. S2A).
Fig. 2.
Induction of Jak1/Stat3 pathway members and ligands after cardiac injury. (A and B) In situ hybridization revealing Jak1/Stat3 pathway members (shown here are il6st and socs3b) induced in an organ-wide manner at 1 dpa (A), and then restricted to the injury site at 7 dpa (B). Dashed lines indicate approximate amputation plane. Brackets indicate injury site. (C) qPCR from ventricular RNA samples revealed induction of il11a, il11b, and lif at 1 and 7 dpa. Data are mean ± SEM n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, Student t test (unpaired, two-tailed). Expression levels were normalized to that of β-actin2, and further normalized to that of the uninjured sample. (D) il11a expression was induced in an organ-wide manner in endocardial cells at 1 dpa, and localized to the injury site at 7 dpa. Dashed line indicates approximate amputation plane. Brackets indicate injury site. (E) RT-PCR of samples from purified endocardial/endothelial (fli1:EGFP+) cells or pu.1-enriched cells at 1 dpa. il11a was detected in fli1:EGFP+ samples, similar to the Vegf receptor flk1. lif was detected in pu.1-enriched samples; it was undetectable when the same amount of total ventricular RNA was used for amplification (total). il11b was detected in pu.1-enriched samples and less so in fli1:EGFP+ samples. anf and tcf21 are markers for cardiomyocytes and epicardium, respectively, and were detected in total ventricular RNA samples. (Scale bars, 50 μm.)
Many ligands transduce signaling via Jak1/Stat3. Based on identification of il6st up-regulation after injury, we examined expression of cytokine receptors that are known to mediate Il6st dimerization (31). We did not detect expression of the oncostatin M receptor osmr from cmlc2:TRAP samples (Fig. S2B). Of interleukin 6 receptor alpha (il6r-α), interleukin 11 receptor alpha (il11r-α), and leukemia inhibitory factor receptor alpha a (lifra), we found that il11r-α was most abundantly expressed in uninjured cardiomyocytes, and its levels increased by 1 d following injury. lifra was the next most abundantly expressed receptor, although we could not detect increased levels after injury (Fig. S2B). Correspondingly, we detected increased levels of il11r-α ligands interleukin 11 a and b (il11a, il11b) and the lifra ligand lif from whole ventricular tissue at 1 dpa, but no increases in other potential ligands like ciliary neurotrophic factor (Fig. 2C and Fig. S2 C–E). Among these genes, il11a was the most abundant ligand at 1 dpa (Fig. S2E). By in situ hybridization, we detected induction of il11a throughout the ventricle in endocardial cells at 1 dpa (Fig. 2D), localization that was supported by histological colocalization with fli1:EGFP and by RT-PCR analysis of purified fli1:EGFP+ endothelial/endocardial cells (Fig. 2E and Fig. S2F). By 7 dpa, il11a reactivity was detected only at the injury site in endocardial cells, as well as other cell types (Fig. 2D). We did not detect the expression of il11b or lif by in situ hybridization, and by contrast with il11a, their expression was detectable at 1 dpa by RT-PCR in cells enriched for the myeloid marker pu.1 (Fig. 2E). We also detected weak expression of il11b in endothelial/endocardial cells, whereas lif was exclusively detected in pu.1-enriched cells (Fig. 2E). Thus, our data indicate that cardiac injury triggers rapid induction of Jak1/Stat3 pathway members in cardiomyocytes that is coordinated with ligand production from auxiliary cells. This program activates first in an organ-wide manner, then localizes to the injury site.
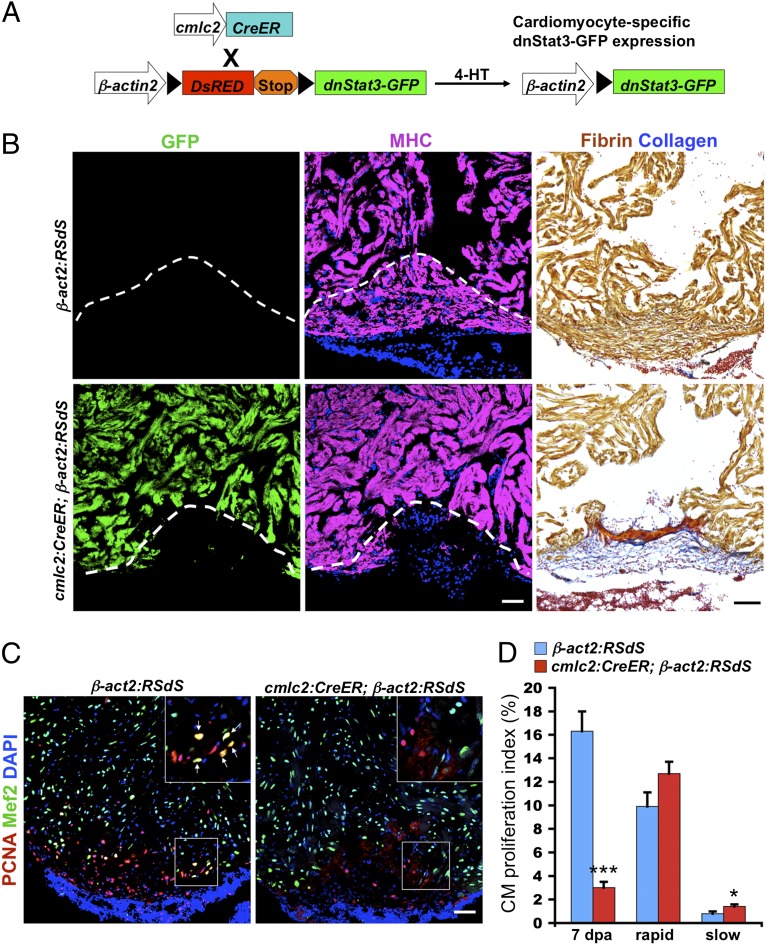
To define requirements for the Jak1/Stat3 pathway during heart regeneration, we created a transgenic line that enabled inducible expression of a dominant-negative Stat3 cassette [Tg(bactin2:loxp-DsRED-STOP-loxp-dnStat3-GFP)pd59] (referred to as β-act2:RSdS) (Fig. 3A). This cassette is based on published studies showing that mutation of a tyrosine blocks phosphorylation and dimerization, functioning as a dominant-negative to specifically inhibit Stat3 signaling (32, 33). To express dnStat3 specifically in cardiomyocytes, we crossed this line to a second transgenic line permitting 4-hydroxytamoxifen (4-HT)-inducible Cre-mediated recombination in cardiomyocytes [Tg(cmlc2:CreER)pd13], and incubated animals with 4-HT. Following 4-HT introduction and ventricular injury, there was an ∼80% reduction in expression of the target gene socs3b from whole ventricular tissue at 1 dpa, indicating reduced Stat3 activity (Fig. S3A). To examine the effects of dnStat3 on regeneration, we treated cmlc2:CreER;β-act2:RSdS and control β-act2:RSdS fish with 4-HT, injured their ventricles 3 d later, and allowed animals to regenerate for 30 d. Although the ventricular walls of control animals regenerated with little or no scarring, animals with induced dnStat3 displayed prominent cardiac muscle deficiencies and scarring (Fig. 3B). To assess how Stat3 inhibition blocks regeneration, we examined cardiomyocyte proliferation after injury. We observed ∼80% and ∼36% reductions in proliferation indices at 7 and 14 dpa, respectively, in 4-HT–treated fish expressing dnStat3 (Fig. 3 C and D, and Fig. S3 B and C), indicating a sustained requirement in normal cardiomyocyte proliferation.
Fig. 3.
Cardiomyocyte Stat3 activity is essential for heart regeneration. (A) Cartoon representation of transgenes used for inducible expression of a dominant-negative Stat3 (dnStat3-GFP) cassette in cardiomyocytes. (B) cmlc2:CreER;β-act2:RSdS (dnStat3) and β-act2:RSdS (control) clutchmates were administered 4-HT, injured 3 d later, and collected for histological analysis of muscularization and scarring at 30 dpa. Muscle regeneration was blocked, and wounds healed by fibrin retention and scar formation in dnStat3-expressing fish (n = 9). MHC, myosin heavy chain. Dashed lines indicate approximate amputation plane. (C) Confocal images of sections from 7dpa ventricles, stained with antibodies against PCNA (a proliferation marker) and Mef2 (a cardiomyocyte marker). (Insets) Higher-magnification images of the squares; arrows, proliferating cardiomyocytes. (D) Quantification of cardiomyocyte (CM) proliferation during regeneration (7 dpa) or after 10 d of rapid or slow (normal) growth conditions. Ventricular resection in adults triggered similar levels of cardiomyocyte proliferation as rapid growth conditions in juveniles/young adults. However, inhibitory effects of dnStat3 expression were apparent only during injury-induced regeneration. Data are mean ± SEM n = 6 (regeneration) or 12 (growth), *P < 0.05, ***P < 0.001. Student t test (unpaired, two-tailed). (Scale bars, 50 μm.)
We also examined whether Stat3 activity was required for cardiomyocyte proliferation that is stimulated by low population density in juvenile and young adult animals, conditions that enable rapid animal and cardiac growth. Low density-stimulated cardiomyocyte proliferation levels are typically comparable with levels of resection-induced proliferation (34). In contrast with effects on regenerative proliferation, dnStat3 induction did not reduce cardiomyocyte proliferation indices during 10 d under accelerated growth conditions, and animals grew normally (Fig. 3D and Fig. S4 A–C). We also found that zebrafish larvae survive to adulthood after myocardial induction of dnStat3 at 4 d postfertilization (Fig. S4D). Taken together, these results indicate that Stat3 requirements during cardiomyocyte proliferation are preferential or unique to the context of injury.
To determine how Stat3 levels might control cardiomyocyte proliferation, we performed a second round of unbiased screening: microarray analysis using total RNA samples collected from cmlc2:CreER;β-act2:RSdS and control ventricles at 1 and 7 dpa. We then analyzed these microarray data together with TRAP microarray data. We searched for genes encoding factors with increased expression after injury represented on both microarray datasets, and with reduced expression during Stat3 inhibition. Relaxin 3a (Rln3a), a member of the Relaxin family of secreted peptide hormones first studied in reproductive tissues but also with reported roles in other tissues, met these criteria. Relaxin ligands interact with Relaxin/Insulin-like family peptide receptors, G protein-coupled receptors that transduce signals via a cAMP/protein kinase A (PKA)-dependent pathway (35, 36). In our experiments, rln3a was up-regulated 36.2-fold in cardiomyocytes by 1 dpa (Fig. 4A), and its expression was reduced 90% by myocardial Stat3 inhibition (Fig. 4B). Analysis of rln3a upstream sequences identified multiple predicted Stat binding sites (Fig. S5). Moreover, ChIP experiments with an antibody against Stat3 showed enrichment of rln3a upstream sequences in samples from 1-dpa ventricles (Fig. 4 C and D), providing additional evidence of endogenous regulation by Stat3.
Fig. 4.
Evidence that Rln3a acts downstream of Stat3 during heart regeneration. (A) qPCR using cmlc2:TRAP RNA samples, indicating that rln3a is highly induced in cardiomyocytes (CMs) at 1 dpa. Data are mean ± SEM n = 3, **P < 0.01, Student t test (unpaired, two-tailed). Expression levels were normalized to that of β-actin2, and further normalized to that of the uninjured sample. (B) qPCR indicating reduced rln3a levels in dnStat3-expressing fish at 1 and 7 dpa. Data are mean ± SEM n = 3, ***P < 0.001, Student t test (unpaired, two-tailed). Expression level was normalized to that of β-actin2, and further normalized to that of the uninjured control sample. (C and D) Enrichment of Stat3 at its predicted binding sites in the rln3a promoter in 1-dpa ventricular tissue. ChIP was performed using anti-Stat3 or IgG antibodies. Immunoprecipitated genomic DNA was analyzed by PCR (C) and qPCR (D). Genomic DNA isolated before immunoprecipitation was also analyzed by PCR and qPCR as the input control. (E) Retro-orbital injection of human recombinant Rln3 to dnStat3-expressing fish (cmlc2:CreER;β-act2:RSdS) for 7-d stimulated cardiomyocyte proliferation at 7 dpa. No detectable effect was observed in wild-type fish (WT) when exposed to the same regimen. A one-time injection of Rln3 to dnStat3-expressing fish at 6 dpa did not significantly increase cardiomyocyte proliferation at 7 dpa. Data are mean ± SEM n = 7–9, *P < 0.05, Student t test, (unpaired, 2-tailed). (F) Confocal images indicating increased cardiomyocyte proliferation in 7 dpa ventricles of animals given seven daily injections of human recombinant Rln3. Brackets indicate injury site. (Insets) Higher-magnification images of the squares; arrows, proliferating cardiomyocytes. (Scale bar, 50 μm.)
To test if diminished Rln3a production contributed to the dnStat3-based regenerative deficits, we treated cmlc2:CreER; β-act2:RSdS zebrafish with 4-HT, injured their ventricles, and retro-orbitally introduced recombinant human Rln3 daily over a 1-wk period. Notably, a daily 100 ng Rln3 protocol increased the cardiomyocyte proliferation index by ∼61% at 7 dpa, accounting for ∼33% of the effects of Stat3 inhibition (Fig. 4 E and F). Rln3 delivery to wild-type fish did not increase 7-dpa cardiomyocyte proliferation, and a single 100-ng treatment at 6 dpa was insufficient to increase cardiomyocyte proliferation (Fig. 4E). These results indicate that the effects of myocardial Jak1/Stat3 signaling on heart regeneration are mediated at least in part by cardiac production and mitogenic activity of Rln3a.
Discussion
In summary, we have described a new transgenic line that enables the profiling of translated RNAs in zebrafish cardiomyocytes. This reagent, and similar transgenic lines representing other cardiac cells, like endocardium and epicardium, should prove a sensitive means to identify new wild-type and mutant/transgenic expression features during cardiac morphogenesis and regeneration. Our profiling during heart regeneration pointed to early myocardial activation of Jak1/Stat3 downstream mediators by tissue damage, events that occur concomitantly with cytokine production in endocardial and inflammatory cells. After an initial organ-wide response, these components are coordinately enhanced at the injury site, where their activities stimulate Rln3a production and are required for cardiomyocyte proliferation.
Notably, evidence from previous studies indicates that Stat3 and Il11 are cytoprotective for murine cardiomyocytes after ischemic injury. Il11 expression is increased in mouse hearts after myocardial infarction, and intravenous delivery of Il11 protected cardiomyocytes from apoptosis and reduced infarct size through a mechanism that requires Stat3 in cardiomyocytes. Similarly, mice with Stat3-deficient cardiomyocytes are susceptible to both age-related and injury-induced heart failure (37–39). These findings together with the current study indicate that Jak1/Stat3 signaling is activated by cardiac injury in multiple vertebrate species, but with different outcomes of cardiac regeneration (zebrafish) or nonhyperplastic remodeling (mice). It will be interesting to identify factors that interact with Jak1/Stat3 signaling and influence these outcomes. In our study, an unbiased approach found Rln3a as a direct target of Stat3 during zebrafish heart regeneration. Relaxin has been described to have antifibrotic, anti-inflammatory, or angiogenic effects in multiple tissues, including the heart (40). To our knowledge, whether Stat3 mediates some of its functions through Relaxin in mammals has not been explored.
Jak/Stat signaling was recently shown to stimulate self-renewal and differentiation of Drosophila intestinal stem cells in response to cytokine production from stressed or dying cells (41). In this tissue, Jak/Stat signaling is titrated and localized to stimulate cell replacement as necessary. Jak/Stat signaling is also critical for murine liver and intestinal epithelial regeneration, and in regenerative neural tissues of zebrafish, such as retina and hair cells (42–46). It is also interesting to note that recent studies have indicated proregenerative roles of inflammatory cytokines in tissues like zebrafish brain, axolotl limb, and adult mouse skeletal muscle (47–49). Thus, cytokine release and Jak/Stat signaling appear to represent general mechanisms for how injury induces and maintains local tissue regeneration in multiple contexts and species.
Methods
Zebrafish and Injuries.
Outbred wild-type or transgenic zebrafish of the Ekkwill strain were used for ventricular resection surgeries, as described previously (3). All transgenic strains were analyzed as hemizygotes. To induce dnStat3-GFP expression in cardiomyocytes, cmlc2:CreER;β-act2:RSdS fish were incubated with 5 μM 4-HT for 24 h. Three days later, ventricular resections were performed. Animal growth conditions were implemented as described previously (34), with 4-HT administered 3 d before placement at different densities. Recombinant human Rln3 peptide (ProSci) was dissolved in 0.9 × PBS and retro-orbitally injected once daily for 7 d after heart surgery, or once at 6 dpa (50). Experiments with zebrafish were performed in accordance with animal use guidelines at Duke University.
Generation of Transgenic Zebrafish.
To generate Tg(cmlc2:EGFP-RPL10a)pd61, the zebrafish ribosomal protein L10a (RPL10a) (NM_199636) was amplified using the primers 5′- TCCGGCCGGACTCAGATCTCGAGCTCAAGCTTCGAATTCAGCAAGGTCTCGAGG ACACGTTG -3′ and 5′- AAGTGCGGCCGCCTAGTAGAGGCGCTGTGGTTTTCCCATGGTGCT -3′, and EGFP was amplified using the primers 5′- ACAGGATCCGCCACCATGGTGAGCAAGGGCGAGGAGCTG -3′ and 5′GAATTCGAAGCTTGAGCTCGAGATCTGAGTCCGGCCGGACTTGTACAGCTCGTCCATGCCGAG -3′. The resulting PCR products were used to amplify the final EGFP-RPL10a fusion cassette using the primers 5′- ACAGGATCCGCCACCATGGTGAGCAAGGGCGAGGAGCTG -3′ and 5′- AAGTGCGGCCGCCTAGTAGAGGCGCTGTGGTTTTCCCATGGTGCT -3′. The TRAP cassette was then subcloned downstream of the ∼5-kb regulatory sequence of cmlc2.
To make the Tg(bactin2:loxp-DsRED-STOP-loxp-dnStat3-GFP)pd59 line, the zebrafish Stat3 dominant-negative mutant was created as described previously (33). Briefly, the zebrafish stat3 cDNA was isolated by PCR using primers: 5′- ATGGCCCAGTGGAATCAGTTGC -3′ and 5′- CTAAGCATTTCGGCAGGTGTCCATA -3′. A tyrosine to phenylalanine change was introduced via PCR-mediated, site-directed mutagenesis using the primers 5′- ATGTGTAACTCAACCCTTCCTGAAGACCAAGTT -3′ and 5′- AACTTGGTCTTCAGGAAGGGTTGAGTTACACAT -3′. The C terminus was fused with a 9-aa spacer followed by hrGFPII. The resultant cassette was subcloned into the previously described β-actin2:loxp-DsRED-STOP-loxp-EGFP construct (11) with Age1/NotI to replace EGFP. Plasmids were injected together with IsceI into one cell-stage embryos and founders were screened by examining fluorescence in embryos.
Tg(cmlc2:CreER)pd13 is an independent founder line similar to lines described previously (11, 51). This line displays more efficient, but also occasionally leaky, recombination compared with other published cmlc2:CreER lines. See Table S2 for primers for PCR and qPCR.
Histological Methods.
All histology was performed with 10-μm cryosections. In situ hybridization with digoxigenin-labeled cRNA probes, Acid Fuchsin-Orange G staining, and immunofluoresence staining with PCNA/Mef2 antibodies were performed, imaged, and quantified as described previously (15). Confocal images were obtained using a LSM 700 microscope (Zeiss). Primary antibodies used in this study were: anti-EGFP (rabbit; Invitrogen), anti-Myosin heavy chain (MHC; F59, mouse; Developmental Studies Hybridoma Bank), and anti-PCNA (mouse; Sigma). Alexa fluor 488-, 633-, or 594-labeled secondary antibodies were obtained from Invitrogen.
RNA Purification by TRAP, and Microarrays.
Fifty apical halves of ventricles from cmlc2:TRAP zebrafish were collected, homogenized, and subjected to polysome purification, as described previously (23). RNAs were purified with Picopure RNA isolation kit (Arcturus), and cDNAs were synthesized and amplified using the Nugen Pico WTA system (Nugen). Microarrays were performed using the zebrafish 12 × 135 Nimblegem chip at Mogene, and analyzed using the R/Bioconductor oligo and limma packages (52, 53). Genes with a false-discovery rate-adjusted P value < 0.10 were considered to be differentially expressed. For each time point, three independent microarray experiments were undertaken. Annotation was confirmed by mapping probe sequences provided by the manufacturer to the Zv9 version of the zebrafish genome. For microarray analysis of uninjured and injured ventricles from control and cmlc2:CreER;β-act2:RSdS animals, four to six apical halves of ventricles was collected for each total RNA sample (also performed in triplicate). Genes with P value < 0.05 (Student t test) and fold-changes ≥ 2 were considered as differentially expressed. See Supporting Information for additional methods.
Supplementary Material
Acknowledgments
We thank T. Wahlig for technical help; B. Liu for help with ChIP assays; A. Dickson for cell counting; J. Burris, A. Eastes, P. Williams, and N. Blake for zebrafish care; and K.D.P. laboratory members for comments on the manuscript. This work was supported in part by a postdoctoral fellowship from the American Heart Association (to Y.F.); a National Heart, Lung, and Blood Institute (NHLBI) Medical Scientist Training Program supplement (to V.G.); National Institutes of Health Training Grant T32 HL007101-35 and an American Heart Association Fellow-to-Faculty Award 12FTF11660037 (to R.K.); Grant HL081674 from NHLBI (to K.D.P.); and a grant from the Mandel Foundation (to K.D.P.). K.D.P. is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The raw data reported in this paper has been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE48914). The microarray data can be found in Table S1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309810110/-/DCSupplemental.
References
- 1.Wang J, et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138(16):3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Rosa JM, Martín V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138(9):1663–1674. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- 3.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 4.Eulalio A, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492(7429):376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 5.Jayawardena TM, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song K, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi WY, Poss KD. Cardiac regeneration. Curr Top Dev Biol. 2012;100:319–344. doi: 10.1016/B978-0-12-387786-4.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi K, et al. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138(14):2895–2902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi K, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jopling C, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi WY, et al. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development. 2013;140(3):660–666. doi: 10.1242/dev.088526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chablais F, Jazwinska A. The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling. Development. 2012;139(11):1921–1930. doi: 10.1242/dev.078543. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi K, et al. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell. 2011;20(3):397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, et al. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc Natl Acad Sci USA. 2010;107(40):17206–17210. doi: 10.1073/pnas.0915016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin VP, Lepilina A, Smith A, Poss KD. Regulation of zebrafish heart regeneration by miR-133. Dev Biol. 2012;365(2):319–327. doi: 10.1016/j.ydbio.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jopling C, Suñé G, Faucherre A, Fabregat C, Izpisua Belmonte JC. Hypoxia induces myocardial regeneration in zebrafish. Circulation. 2012;126(25):3017–3027. doi: 10.1161/CIRCULATIONAHA.112.107888. [DOI] [PubMed] [Google Scholar]
- 19.Jopling C, Suñe G, Morera C, Izpisua Belmonte JC. p38α MAPK regulates myocardial regeneration in zebrafish. Cell Cycle. 2012;11(6):1195–1201. doi: 10.4161/cc.11.6.19637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senyo SE, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 22.Doyle JP, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135(4):749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heiman M, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135(4):738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sleep E, et al. Transcriptomics approach to investigate zebrafish heart regeneration. J Cardiovasc Med (Hagerstown) 2010;11(5):369–380. doi: 10.2459/JCM.0b013e3283375900. [DOI] [PubMed] [Google Scholar]
- 25.O’Shea JJ. Jaks, STATs, cytokine signal transduction, and immunoregulation: Are we there yet? Immunity. 1997;7(1):1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 26.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 27.Lien CL, Schebesta M, Makino S, Weber GJ, Keating MT. Gene expression analysis of zebrafish heart regeneration. PLoS Biol. 2006;4(8):e260. doi: 10.1371/journal.pbio.0040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19(21):2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 29.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: The JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 30.Lepilina A, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127(3):607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 31.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 32.Kaptein A, Paillard V, Saunders M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem. 1996;271(11):5961–5964. doi: 10.1074/jbc.271.11.5961. [DOI] [PubMed] [Google Scholar]
- 33.Conway G. STAT3-dependent pathfinding and control of axonal branching and target selection. Dev Biol. 2006;296(1):119–136. doi: 10.1016/j.ydbio.2006.04.444. [DOI] [PubMed] [Google Scholar]
- 34.Wills AA, Holdway JE, Major RJ, Poss KD. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 2008;135(1):183–192. doi: 10.1242/dev.010363. [DOI] [PubMed] [Google Scholar]
- 35.Hsu SY, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295(5555):671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 36.Sudo S, et al. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem. 2003;278(10):7855–7862. doi: 10.1074/jbc.M212457200. [DOI] [PubMed] [Google Scholar]
- 37.Hilfiker-Kleiner D, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95(2):187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 38.Jacoby JJ, et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA. 2003;100(22):12929–12934. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obana M, et al. Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation. 2010;121(5):684–691. doi: 10.1161/CIRCULATIONAHA.109.893677. [DOI] [PubMed] [Google Scholar]
- 40.Du XJ, Bathgate RA, Samuel CS, Dart AM, Summers RJ. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat Rev Cardiol. 2010;7(1):48–58. doi: 10.1038/nrcardio.2009.198. [DOI] [PubMed] [Google Scholar]
- 41.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollrath J, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277(32):28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- 44.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J, et al. The stat3/socs3a pathway is a key regulator of hair cell regeneration in zebrafish. [corrected] J Neurosci. 2012;32(31):10662–10673. doi: 10.1523/JNEUROSCI.5785-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson CM, et al. Stat3 defines three populations of Müller glia and is required for initiating maximal Müller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520(18):4294–4311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyritsis N, et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338(6112):1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- 48.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA. 2013;110(23):9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heredia JE, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153(2):376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugach EK, Li P, White R, Zon L. Retro-orbital injection in adult zebrafish. J Vis Exp. 2009;(34) doi: 10.3791/1645. pii, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, et al. A dual role for ErbB2 signaling in cardiac trabeculation. Development. 2010;137(22):3867–3875. doi: 10.1242/dev.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smyth G. Limma: Linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 53.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26(19):2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.