Abstract
Mechanical stimuli, including tactile and sound signals, convey a variety of information important for animals to navigate the environment and avoid predators. Recent studies have revealed that Drosophila larvae can sense harsh or gentle touch with dendritic arborization (da) neurons in the body wall and can detect vibration with chordotonal organs (Cho). Whether they can also detect and respond to vibration or sound from their predators remains an open question. Here we report that larvae respond to sound of wasps and yellow jackets, as well as to pure tones of frequencies that are represented in such natural sounds, with startle and burrowing behaviors. The larval response to sound/vibration requires Cho neurons and, to a lesser extent, class IV da neurons. Our calcium imaging and electrophysiological experiments reveal that Cho neurons, but not class IV da neurons, are excited by natural sounds or pure tones, with tuning curves and intensity dependence appropriate for the behavioral responses. Furthermore, our study implicates the transient receptor potential (TRP) channels NOMPC, NANCHUNG, and INACTIVE, but not the dmPIEZO channel, in the mechanotransduction and/or signal amplification for the detection of sound by the larval Cho neurons. These findings indicate that larval Cho, like their counterparts in the adult fly, use some of the same mechanotransduction channels to detect sound waves and mediate the sensation akin to hearing in Drosophila larvae, allowing them to respond to the appearance of predators or other environmental cues at a distance with behaviors crucial for survival.
In the life of insects, sound signals mediate important information that is used in various contexts, ranging from courtship to detection of predators or prey (1, 2). Insects are equipped with sensitive receptor organs for detection of sounds and the underlying neural network enabling recognition and localization of the targets in a complex environment (3). Chordotonal organs (Cho), the main hearing organs for insects, are specialized mechanosensitive organs found in the Insecta and Crustacea (4–7) that may serve as proprioceptors or as cutaneous mechanoreceptor organs (8).
In the adult fruit fly Drosophila melanogaster, Cho neurons of the Johnston organ in the antenna mediate both hearing and sensing of gravity and wind (9, 10). Larval Cho have similar developmental programs (11) and structures as their adult counterparts, suggesting that they might be capable of sensing sound/vibration. Previous studies have shown that larval Cho play a role in low-temperature sensation (12, 13). Moreover, mistargeting of Cho axons in the ventral nerve cord (VNC) impairs the larval response to tactile vibration (14), suggesting that Cho neurons might be involved in vibration sensing as well.
The mechanotransduction channels in sound sensation have been studied extensively for decades. Several transient receptor potential (TRP) channels and proteins have been implicated in mechanotransduction in the Cho neurons of adult flies. TRP channels usually form nonselective cation channels, which have been found in many types of sensory neurons as well as other cell types. They function as sensors for both intrinsic signals and external stimuli (15, 16). The TRP channel NOMPC (TRPN1) is thought to be a component of the transduction complex (17). Loss of NOMPC eliminates mechanical response in touch-sensitive neurons, and amino acid substitutions in the putative pore domain of the channel can alter ion selectivity (18–21). The loss of NOMPC does not entirely eliminate sound-evoked response in the adult Drosophila auditory nerve, however. Two other Drosophila TRP channels, NANCHUNG (NAN) and INACTIVE (IAV), likely function as heteromers (22), and mutations of either channel render the fly deaf (22, 23). These TRPV family members also could be part of the transduction complex. The precise roles of NOMPC/NAN/IAV in the mechanotransduction of Cho neurons remain unclear. PAINLESS, a Drosophila TRPA channel (24), along with dmPIEZO, one of the first mechanotransduction channels identified in Drosophila, are involved in mechanical nociception (25); however, whether they have a role in sound sensing is unknown.
In the present study, by combining in vivo recording and behavioral tests, we are able to study the capabilities of Drosophila larvae to detect and respond to sound and to evaluate the role of Cho neurons in this behavior. We also explored the potential roles of different TRP channels and dmPIEZO channels in Cho neurons for sound sensation.
Results
Drosophila Larvae Are Capable of Sensing Sound.
Many animals, including insects, display startle-freeze behaviors, which serve as a readout of the hearing response (14). In the natural environment, larvae encounter many types of sounds, including those made by predators. We found that larvae responded to the natural sound of a wasp with a startle behavior (Movie S1). Spectral analysis of those natural sounds revealed that the frequency centered around 400 Hz (Fig. S1).
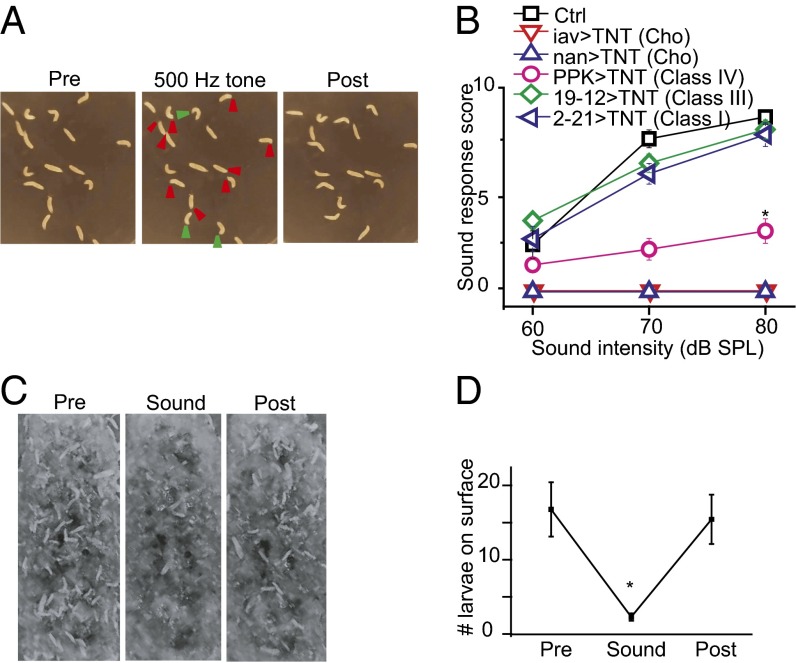
Freely moving WT Drosophila larvae also froze in response to sound stimulation triggered by a pure tone of 500 Hz, within the range of the frequencies of natural sounds made by wasps and yellow jackets. Larvae normally explore the open field on an agar plate with a rhythmic locomotion pattern (26). When exposed to a sound, they immediately stopped crawling, retracted their mouth hooks, and made excessive turning movements (Fig. 1A and Movie S2), as reported previously (14). To quantify this startle behavior, we scored the number of times that each larva responded to sound stimuli presented 10 times in each trial. The WT larva had a score close to 8 when stimulated with a 70-dB pure tone; the greater the sound intensity, the higher the response score (Fig. 1B).
Fig. 1.
The sound-triggered startle response requires normal function of Cho. (A) Sound-induced startle behaviors in WT larvae. (Left) Freely moving larvae. (Center) Larvae showing a startle response to vibration (contraction and stop indicated by red arrowheads; turning, by green arrowheads). (Right) Larvae recovered at 4 s after discontinuation of sound. (B) Blocking the synaptic output of Cho neurons impairs larval response to vibration induced by a 500-Hz tone. Blockage of class I and III da neurons did not change the response of larvae to sound. Blockage of class IV da neurons partially reduced the response. n = 7, 7, 7, 5, 8, and 7 (Top to Bottom). *P < 0.001, unpaired t test comparing control larvae and larvae with class IV neuron blockage. (C) Top view of larvae on the food surface before (Left), during (Center), and after (Right) sound stimulation. (D) Summary plot of the number of larvae on the food surface (those with more than half their body length exposed). n = 7. *P < 0.001, unpaired t test. Error bars represent SEM.
We then observed the behavior of third instar larvae of the wandering stage in regular cornmeal fly food on exposure to prolonged sound stimulation. Normally, the larvae moved about on the surface or slightly below the surface of the food medium (Fig. 1C). On exposure to a pure tone of 500 Hz (1-s duration/3-s interval), most of the larvae on the surface began to dig and burrowed inside the food within 5 s. Shortly after termination of the sound stimulation, these larvae reemerged at the surface (Fig. 1 C and D).
By expressing the tetanus toxin light chain (TNT) in different classes of dendritic arborization (da) neurons or Cho neurons to interfere with their synaptic transmission, we found that TNT expression in Cho neurons eliminated the startle response (Fig. 1B), whereas blockage of class IV da neurons’ synaptic transmission reduced the response (Fig. 1B). In contrast, this behavioral response to sound was not significantly altered by TNT expression in class I or III da neurons (Fig. 1B). This finding indicates that the startle response to sound requires signaling of Cho neurons, as suggested previously (14), and, to a lesser degree, also involves synaptic transmission from class IV da neurons.
Sound-Induced Action Potential Firing in Cho Neurons.
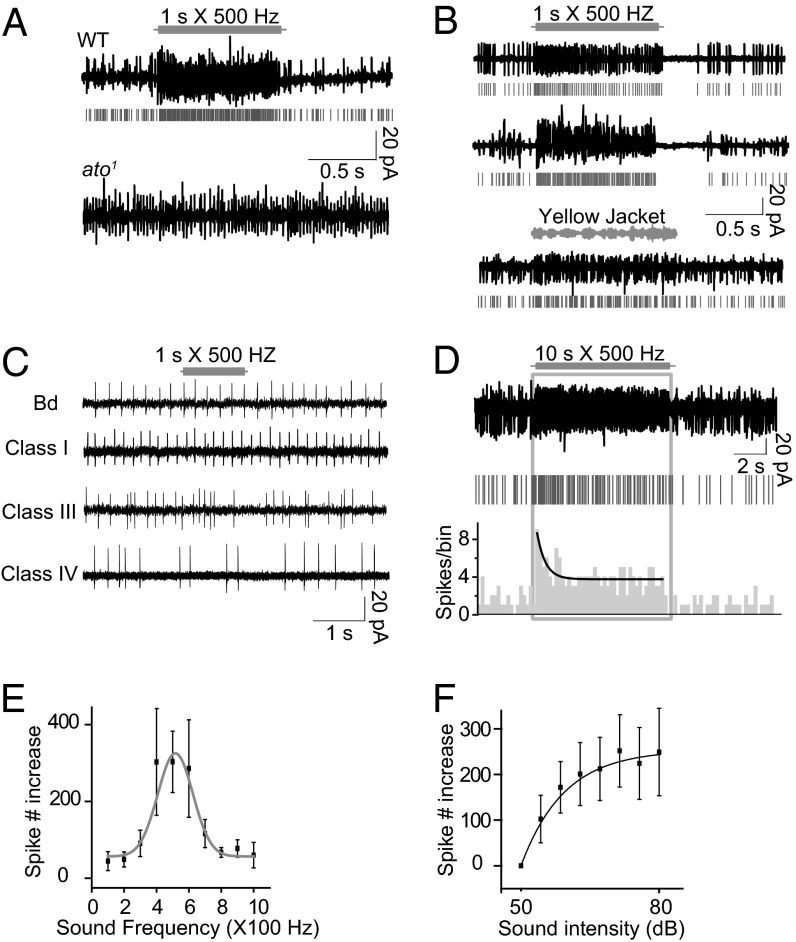
To identify the sensory neurons that can respond to sound directly, we used suction electrodes for extracellular recording from the distal end of a nerve bundle. The nerve bundle was severed from the VNC and included axons of sensory neurons, allowing us to monitor the action potentials of most of the sensory neurons in a single hemisegment. We found an increase in the firing rate in response to a pure tone of 500 Hz/70 dB, indicating the presence of sound sensors within the body wall (Fig. 2A).
Fig. 2.
Cho neurons respond to sound with increased action potential firing. (A) Action potentials of body wall neurons responding to a 500-Hz pure tone vibration. A raster plot of the spikes is shown in gray. The ato1 mutant shows no response. (B) (Top and Middle) Response to a 500-Hz pure tone of singlet (Top) and grouped (Middle) Cho neurons. Raster plots of the spikes are in gray. (Bottom) Response of Cho neurons to the sound of a yellow jacket. (C) bd and class I, III, and IV da neurons do not respond to sound. (D) Response of a Cho neuron bundle to a prolonged 500-Hz pure tone showing the response dynamics. (Top) Original trace. (Middle) Raster plot. (Bottom) Histogram of spikes. (E) Tuning curve of action potential firing response to pure tone (n = 7). (F) Intensity-dependent curve of sound response; samples from nine recordings. Error bars represent SEM.
We next performed similar recording of nerve bundles from ato1 mutant larvae, which lack Cho neurons and a subset of multiple dendritic (md) neurons (11, 27). We found no electrophysiological response to sound in the ato1 mutants (Fig. 2A), suggesting that Cho and/or md neurons might serve as the sound sensors.
To test whether Cho and/or md neurons mediate the response to sound, we directly recorded from different types of sensory neurons labeled with GFP. We found no sound response in class I, III, and IV da neurons and bipolar dendritic (bd) neurons (Fig. 2C). Through focal recording on the soma of a Cho neuron (Fig. S2), we were able to monitor the action potential firing of an individual Cho neuron. Each of the abdominal hemisegments contains three singlet Cho neurons (vchA, vchB, and lch1) and a cluster of five Cho neurons (lch5) (28) (Fig. S2). Both the vch and lch Cho neurons exhibited spontaneous firing, and responded to a 500-Hz pure tone of 70 dB with increased action potential firing (Fig. 2B). With longer stimulation (10 s), the sound response displayed rapid adaptation (Fig. 2D). We also tested the Cho neuronal response to natural sounds. Cho neurons responded to the sound of yellow jacket, a common predator of Drosophila in nature (29), with increased firing (Fig. 2B).
To characterize this response, we evaluated the Cho neuronal response to different frequencies of sound at the same intensity. The Cho neuronal response to sound was broadly tuned in the frequency range tested (100–1,000 Hz), and showed the highest sensitivity to a pure tone of 500 Hz (Fig. 2E). At the same frequency, the louder the sound, the stronger the response from individual Cho neurons (Fig. 2F). In most cases, the termination of a sound stimulus was followed by reduced firing (Fig. 2 A and B), as has been observed in the mammalian auditory system as well (30).
Ca2+ Increases in Cho Neurons Responding to Sound.
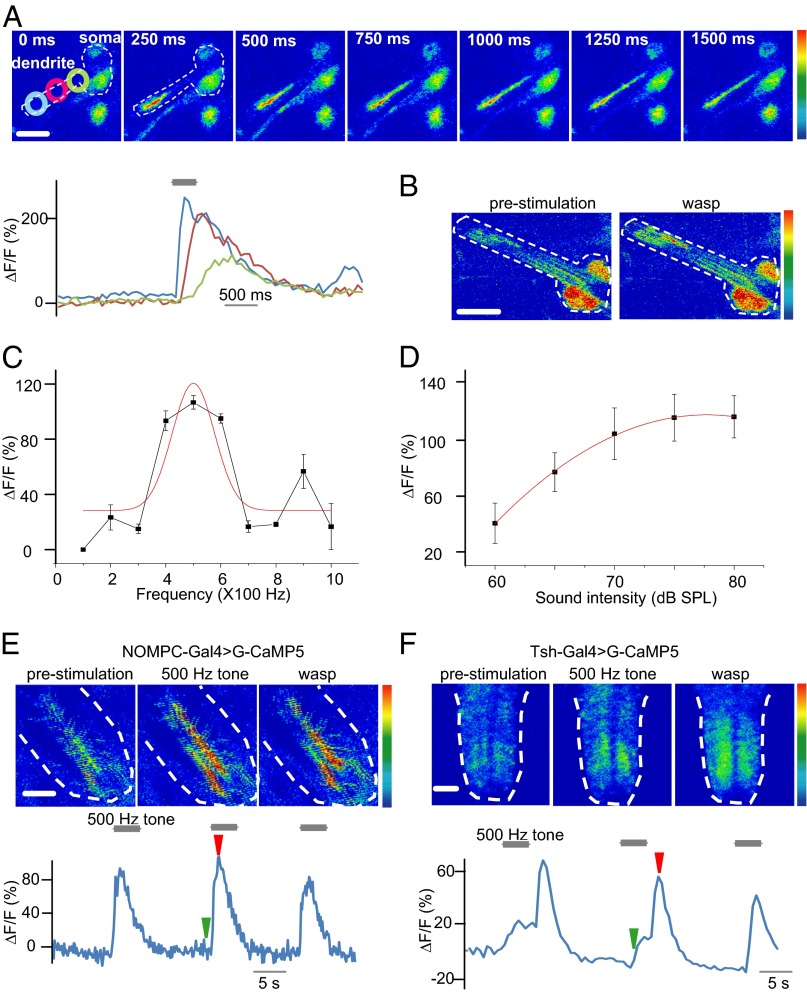
We performed in vivo Ca2+ imaging to provide cellular characterization of the sound response of Cho neurons in intact larvae. The Ca2+ indicator GCaMP5 (31), driven by Cho-specific Gal4, was used to monitor the Ca2+ response in these neurons. We found dramatically increased GCaMP fluorescence in Cho neurons on exposure to a 500-Hz sound of 70 dB (Fig. 3A). In contrast, class IV da neurons did not respond to a similar sound stimulation with increased Ca2+ levels (Fig. S3). The GCaMP fluorescence was increased throughout the Cho neuron, with the greatest increases seen at the dendritic tip and the initial segment of axons (Fig. 3A and Movie S3).
Fig. 3.
Ca2+ imaging revealed spatial activation of Cho neurons to sound. (A) Ca2+ levels in Cho neurons responding to sound. The dashed line represents the border of the Cho. (Rainbow color range: 0–255; scale bar: 10 µm.) The graph shows Ca2+ propagation over time along dendrites in Cho neurons responding to vibration (blue, dendritic tip; magenta, middle dendrite; aqua, basal dendrite). The gray bar represent the tone stimulation. (B) Ca2+ levels in Cho neurons responding to the wasp sound. (Color range: 0–255; scale bar: 10 µm.) (C) Tuning curve for pure tones of 100–1,000 Hz. (D) Intensity curve of responses to a 500-Hz pure tone. Error bars represent SEM. (E) Ca2+ responses to the pure tone and natural sound in the axon termini of Cho neurons. The white dashed lines indicate the border of the VNC; the green arrow indicates the time of the prestimulation image; the red arrow indicates the 500-Hz tone. (Color range, 0–255; scale bar: 100 µm.). (F) Ca2+ responses to the pure tone and natural sound in VNC projection neurons. The white dashed lines indicate the border of the VNC; the green arrow indicates the time of the prestimulation image; the red arrow indicates the 500-Hz tone. (Color range: 0–255; scale bar: 100 µm.)
Using faster imaging to resolve the spatial distribution of Ca2+ response, as indicated by GCaMP fluorescence within Cho neurons during the sound response, we found that the Ca2+ signal was initiated at the distal end of the dendritic tip and propagated to the cell body over a course of hundreds of milliseconds (Fig. 3A). These Ca2+ dynamics within Cho neurons might play a role in signal transduction or adaptation to sound. In vertebrate hair cells, the spatial distribution of Ca2+ has been found to be important for adaptation to sound (32, 33).
Ca2+ signals also could be elicited by natural sounds from wasps or yellow jackets (Fig. 3B). We also tested the Cho response to high-frequency sounds, which serve as a danger signal for some insect species (34), and found that the Drosophila larval Cho neurons were insensitive to a pure tone of 10 kHz (Fig. S4).
The Ca2+ response of Cho neurons to sound stimuli in vivo exhibited a tuning curve similar to that demonstrated by electrophysiological recording. Quantification of the Ca2+ signal in Cho dendrites revealed that this tuning curve was broadly tuned in the frequency range tested (100–1,000 Hz), with the maximal response at 500 Hz (Fig. 3C). The Ca2+ response increased in a stimulus-dependent manner with increasing sound intensity (Fig. 3D).
The axons of Cho neurons merge with axons of other sensory neurons and project to the midlateral VNC. We also imaged the Ca2+ signals in the axon termini of Cho neurons in the VNC, and found an increase in Ca2+ in response to both the pure tones and natural sounds (Fig. 3E).
By expressing G-GaMP5 with teashirt-Gal4, which labels most of the neurons in the VNC (35), we found that the central neurons exhibited an increase in Ca2+ in response to both pure tones and natural sounds (Fig. 3F), indicating that the sound information conveyed by Cho neurons is relayed to central neurons.
TRP Channels Are Involved in the Cho Sound Response.
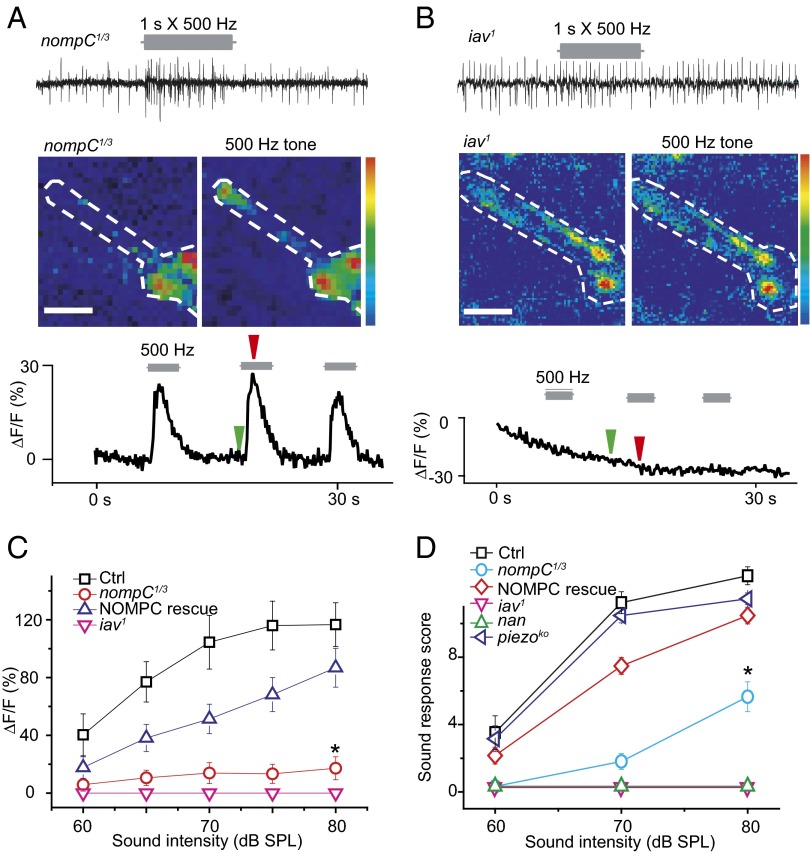
To begin to uncover the molecular mechanisms underlying the Cho neuronal response to sound, we next tested the Ca2+ response to sound of larval Cho neurons in several different mutants. TRP channels have been implicated in the mechanotransduction of adult Cho neurons. The TRPN channel NOMPC is important for hearing in adult Drosophila, as well as in larval locomotion and gentle touch behavior (18–21, 36–38). Immunostaining revealed expression of NOMPC protein in the cilium of larval Cho neurons (Fig. S5), similar to its localization in adult Johnston organs (19). Cho neurons of the nompC mutant did not respond to a low level of sound that was sufficient to trigger a Ca2+ response in Cho neurons of WT larvae (Fig. 4C); however, a higher level of sound stimulation elicited a response revealed by extracellular recording and Ca2+ imaging (measured at a 500-Hz pure tone) of Cho neurons lacking NOMPC. This response was weaker in the nompC mutant than in WT Cho neurons (Fig. 4 A and C), but the tuning curve appeared to be normal (Fig. S6). The defect in Ca2+ response could be rescued by expressing WT NOMPC in Cho neurons (Fig. 4C), indicating that NOMPC is involved in the sound response in Cho neurons.
Fig. 4.
TRP channel mutants affect the response of Cho neurons to sound. (A) nompC mutant exhibited reduced action potential firing and Ca2+ response to pure tone sound. The green arrow indicates the time of the prestimulation image; the red arrow, the 500-Hz tone. (Color range: 0–255; scale bar: 10 µm.) (B) Cho neurons of iav1 showed no detectable action potential firing or Ca2+ response to sound. The green arrow indicates the time of the prestimulation image; the red arrow, the 500-Hz tone. (Color range: 0–255; scale bar: 10 µm.) (C) The Ca2+ response of Cho neurons in TRP channel mutants to sounds of varying intensity. WT NOMPC partially rescued the response of nompC mutant. Ctrl, NOMPC-Gal4; UAS-GCaMP5, UAS-mCherry. nompC1/3:nompC1/nompC3; NOMPC-Gal4, UAS-GCaMP5, UAS-mCherry. NOMPC rescue: nompC1/3; NOMPC-Gal4, UAS-NOMPC/UAS-GCaMP5, UAS-mCherry. iav: iav1/Y;;NOMPC-Gal4, UAS-GCaMP5, UAS-mCherry. n = 7, 7, 7, 8 (Top to Bottom). *P < 0.001, unpaired t test between Ctrl and nompC1/3. (D) Startle response of TRP channel and dmpiezo mutants to varying sound intensities. iav and nan mutants larval showed no response to sound. The nompC mutant demonstrated reduced sensitivity to sound, which could be rescued by the expression of WT NOMPC in Cho neurons. The dmpiezo mutant exhibited a normal response. Ctrl: w1118. nompC1/3: nompC1/nompC3. NOMPC rescue: nompC1/3; NOMPC-Gal4,UAS-NOMPC. iav: iav1. nan: nan36. dmpiezo: dmpiezoKO. n = 7, 7, 7, 8, 6 (Top to Bottom). *P < 0.001, unpaired t test comparing Ctrl and nompC1/3. Error bars represent SEM.
Two other Drosophila TRP channels, IAV and NAN, are required for hearing in adult Drosophila, and the function of one depends on that of the other (22, 23). We found that Cho neurons in iav1 mutant larvae exhibited a lower spontaneous firing rate and no increase in action potential firing in response to sound (Fig. 4B). Ca2+ imaging also revealed no response of iav1 mutant Cho neurons to sound (Fig. 4 B and C).
Importantly, the larval startle response to sound was reduced or eliminated in TRP channel mutants. The nompC mutant larvae exhibited greatly reduced response, whereas the iav1 and nan mutant larvae were unresponsive to sound stimulation (Fig. 4C). With a stronger stimulation intensity (80 dB), the nompC mutants demonstrated a detectable sound response. The defect in the nompC mutants was largely rescued by expression of WT NOMPC in the Cho neurons (Fig. 4D). The dmPIEZO channel, which is required for nociception of Drosophila larvae, apparently is not required for the sound response even though the expression pattern of dmPiezo-Gal4 suggests expression of dmPIEZO in Cho neurons (25), given that the dmpiezoko mutant exhibited a behavioral response to sound similar to that of WT larvae (Fig. 4D).
Discussion
In this study, we have explored the role of Cho neurons and mechanosensitive channels of Drosophila larvae in the sound/vibration sensation. Our results support the previous suggestion that Cho are essential for the startle response of larvae to vibration (14). In addition, we report several heretofore unreported findings: (i) Along with exhibiting the the startle response, the larvae burrow into soft food in response to sounds, including those of predators; (ii) the larval response to sound requires Cho neurons and, to a lesser extent, class IV da neurons; (iii) Cho neurons, but not class IV da neurons, are excited by natural sounds or pure tones, with tuning curves and intensity dependence appropriate for the behavioral responses; and (iv) TRP channels (including NOMPC, NAN, and IAV), are required for Cho neurons to sense sound, but the dmPIEZO channel is not.
The ability to sense mechanical stimuli that indicate potential harm is important for survival (39, 40). Drosophila larvae use their mechanosensory neurons to sense the mechanical pain caused by a predator attack (29). The da neurons on the body wall are capable of sensing gentle and harsh touch, allowing larvae to move away from harm (20, 25, 41). Their survival could be further enhanced if larvae could detect signals such as sound from predators at a distance. Our results show that Drosophila larvae exhibit startle behavior in response to certain frequencies of sound, including the sound from predators such as wasps and yellow jackets. This startle behavior and ensuing escape or avoidance behavior may increase a larva’s chance of survival. Interestingly, Drosophila larvae are highly sensitive to low-frequency sounds but not to high-frequency sounds, unlike some other insects that can detect high-frequency sounds including ultrasonic sounds (1, 8). This diversity in hearing might reflect evolutionary adaptation to different predators for organisms ranging from insects to bats, and might entail interspecies differences at both structural and molecular levels.
We found that although both Cho neurons and class IV da neurons are involved in the sound-triggered startle response, only Cho neurons are sensitive to sound. Class IV da neurons may have modulatory effects on the neural circuits activated by the Cho neuronal response to sound—a likely scenario, considering that class IV da neurons mediate avoidance behaviors to several noxious stimuli (25, 29). The startle response and avoidance of sound also may depend on this neural circuit for avoidance behaviors. Alternatively, class IV da neurons may contribute to the behavioral response through their involvement in peristalsis (29).
Several TRP channels have been implicated in hearing and touch sensation in Drosophila, although the roles of these channels in mechanotransduction may differ in different sensory neurons. For example, NOMPC is critical for touch sensation but IAV and NAN are not (20), whereas IAV and NAN are important for adult hearing (9, 42, 43). With respect to larval Cho neurons, it appears that IAV and NAN are required for sound transduction, whereas NOMPC function is important, but not essential, for the detection of loud sound. A possible model is one in which NOMPC serves as one of the primary sensors for sound and enhances the movement of the Cho neuronal cilium to activate IAV and NAN, which may be able to sense loud sound on their own in the absence of NOMPC. An alternative model has been suggested for the adult Johnston organs, which may use IAV and NAN rather that NOMPC as the primary sensor (44).
Given that the cytoplasmic G-CaMP5 might not be localized to the small structure within the tip of the cilium, the Ca2+ imaging method in our experiments might not be sufficiently sensitive to detect Ca2+ influx at the site of mechanotransduction. Thus, the absence of a Ca2+ signal in Cho neurons might be attributed to the lack of downstream amplification. dmPIEZO, one of the first mechanotransduction channels identified for mechanical nociception in Drosophila larvae, appears to have no involvement in hearing, suggesting that larvae make use of different channels for different modalities of mechanosensation.
Recent microarray studies have identified hundreds of genes implicated in the hearing of adult flies. Many of these genes also have been implicated in other sensory modalities besides hearing (42). A major challenge is the difficulty of recording from a single neuron in the adult antenna. The larval Cho neurons are accessible to electrophysiological recording at single-cell resolution. Moreover, the entire structure of a Cho neuron can be imaged simultaneously in vivo. In conjunction with the extensive genetic resources available, larval Cho neurons lend themselves to mechanistic studies of mechanotransduction for hearing in Drosophila.
Materials and Methods
Fly Stocks.
The nompC mutant lines were provided by C. Zuker (Columbia University, New York). The UAS-GCaMP5 line was a gift from G. Rubin and L. Looger (Howard Hughes Medical Institute, Ashburn, VA). The UAS-TNT line was provided by U. Heberlein (University of California, San Francisco). The iav1 and nan1 mutants and IAV-Gal4 and NAN-Gal4 were obtained from the Bloomington Stock Center. The dmpiezo mutant line was provided by A. Patapoutian (Scripps Institute, La Jolla, CA).
Behavior Assay.
Animals were raised at 25 °C in an incubator with a 12-h light/dark cycle and humidity control (Darwin Chamber Co.). For the startle assay, at 96 h after egg-laying, 10–20 third instar larvae were gently removed from the vial, washed twice with PBS, and then transferred to a 50-mm agar plate. The larvae were allowed to crawl freely at room temperature. The plate was placed on top of a speaker, and the behaviors of the larvae were videotaped with a camera placed atop the setup. For evaluating larval sound response, the larvae were stimulated with a 1-s sound pulse, repeated 10 times. The sum of responses in 10 trials served as the response score. A larva was scored as responsive when exhibiting startle behavior, including pausing, mouth-hook retraction, excessive turning, and/or backward locomotion, in response to the sound stimulation.
For the avoidance assay, a spoonful of food (5 mm × 20 mm × 5 mm) with approximately 100 third instar larvae was taken from a bottle and spread on a Petri dish, which was then placed on top of a speaker. The entire food surface was videotaped. The surface number was determined by counting the larvae with at least half of their full length exposed. A custom-made MATLAB program was used to generate sound waves with different patterns. Natural sounds were obtained from the JungleWalk Web site (www.junglewalk.com).
In Vivo Ca2+ Imaging.
In vivo calcium imaging of larval Cho neurons was performed with third instar larvae. A freely moving larva was pressed between two coverslips with a drop of PBS to reduce its movement. The imaging data were acquired with a Zeiss LSM510 confocal microscope. The calcium indicator GCaMP5 was used to measure the Ca2+ signal. GCaMP and red fluorescent proteins (as references) were excited by a 488-nm and a 543-nm laser, respectively, and the fluorescent signals were measured. The GCaMP fluorescence demonstrated a dramatic increase on sound stimulation, whereas the red fluorescent proteins showed no change in fluorescence when the same stimulus was delivered to the neurons. A region of interest covering the distal one-third end of the Cho was selected to measure GCaMP fluorescence intensity. The average GCaMP signal from the first 5 s before application of stimulus was taken as fluorescence (F)0, and ΔF/F0 was calculated for each data point. G-CaMP signals from the soma or the distal dendritic tips were analyzed.
Electrophysiological Recording.
Third instar larvae were pinned on the recording chamber. Fillets were prepared by dissecting larvae in hemolymph-like saline solution with minor modifications (45), containing 103 mM NaCl, 3 mM KCl, 5 mM 2-([1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino)ethanesulfonic acid, 10 mM trehalose, 10 mM glucose, 7 mM sucrose, 26 mM NaHCO3, 1 mM NaH2PO4, and 4 mM MgCl2, adjusted to pH 7.25 and 310 mOsm. Before use, 2 mM Ca2+ (in the form of CaCl2) was added to the saline solution. Major muscles covering the body wall were gently removed with fine forceps, and the da neurons were exposed. Extra muscles covering the Cho neurons were removed by electrodes under microscopy. Cho neurons were visualized and identified by fluorescent markers driven by IAV-Gal4 or NOMPC-Gal4 drivers. Glass electrodes for electrophysiological recording were pulled with a P-97 puller (Sutter Instruments) from thick wall borosilicate glass to a diameter of 10 µm, fire-polished. and filled with external solution. Action potentials were recorded extracellularly at a sample rate of 10 kHz and then low-pass filtered at 2 kHz. Data were acquired and processed with a Multiclamp 200B amplifier, a Digidata 1440A data acquisition system, and Clampex 10.3 software (Molecular Devices). Neurons with spontaneous activity were chosen for further stimulation.
Immunostaining.
Immunostaining of Drosophila larvae was performed as described previously (46). In brief, third instar larvae were dissected in PBS, fixed in 4% paraformaldehyde solution for 20 min at room temperature, and treated with the primary antibody overnight at 4 °C and secondary antibody for 2 h at room temperature. The primary antibody was a mouse mAb against NOMPC (1:100; a gift from J. Howard, Max Planck Institute, Dresden, Germany), and the secondary antibodies were appropriate fluorescence conjugated anti-mouse IgG (1:200; Jackson ImmunoResearch). Images were acquired with a Leica SP5 confocal microscope.
Data Analysis and Statistical Methods.
The extracellularly recorded action potentials of Cho neurons were detected by a threshold-based search of the single-unit recordings with custom-made MATLAB programs. A time window of fixed duration (1 s) was used before and during stimulation. The response firing number was calculated as the difference between the two time windows. A poststimulus time histogram for a single cell was plotted for the number of spikes in a 50-ms bin. The unpaired t test was used for significance testing.
Supplementary Material
Acknowledgments
We thank C. Zuker, U. Heberlein, G. Rubin, A. Patapoutian, and C. Montell for fly lines; J. Howard for the NOMPC antibody; and L. Looger for G-CaMP5 flies. We also thank C. Schreiner and B. Seybold for assistance with sound intensity measurements; S. Younger, S. Barbel, and T. Cheng for technical support; L. Cheng and Y. Q. Song for reading the manuscript; and members of the L.Y.J. and Y.N.J. laboratory for discussion. Z.Y. is a recipient of a Long-Term Fellowship from the Human Frontier Science Program. This work was supported by the National Institutes of Health (Grants R37NS040929 and 5R01MH084234, to Y.N.J.). L.Y.J. and Y.N.J. are investigators at the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312477110/-/DCSupplemental.
References
- 1.Fullard JH, Yack JE. The evolutionary biology of insect hearing. Trends Ecol Evol. 1993;8(7):248–252. doi: 10.1016/0169-5347(93)90200-9. [DOI] [PubMed] [Google Scholar]
- 2.Wyttenbach RA, Farris HE. Psychophysics in insect hearing. Microsc Res Tech. 2004;63(6):375–387. doi: 10.1002/jemt.20054. [DOI] [PubMed] [Google Scholar]
- 3.Yack JE. The structure and function of auditory chordotonal organs in insects. Microsc Res Tech. 2004;63(6):315–337. doi: 10.1002/jemt.20051. [DOI] [PubMed] [Google Scholar]
- 4.Moran DT, Rowley JC, 3rd, Varela FG. Ultrastructure of the grasshopper proximal femoral chordotonal organ. Cell Tissue Res. 1975;161(4):445–457. doi: 10.1007/BF00224135. [DOI] [PubMed] [Google Scholar]
- 5.Burns MD. Structure and physiology of the locust femoral chordotonal organ. J Insect Physiol. 1974;20(7):1319–1339. doi: 10.1016/0022-1910(74)90236-4. [DOI] [PubMed] [Google Scholar]
- 6.Corbière-Tichané G. Fine structure of the chordotonal organs of the head appendages of Speophyes lucidulus larva. Z Zellforsch Mikrosk Anat. 1971;117(2):275–302. (French) [PubMed] [Google Scholar]
- 7.Kinzelbach-Schmitt B. [Data on antennal chordotonal organs in thysanurans (Thysanura, Insecta)] Z Naturforsch B. 1968;23(2):289–291. [PubMed] [Google Scholar]
- 8.Eberl DF. Feeling the vibes: Chordotonal mechanisms in insect hearing. Curr Opin Neurobiol. 1999;9(4):389–393. doi: 10.1016/S0959-4388(99)80058-0. [DOI] [PubMed] [Google Scholar]
- 9.Kamikouchi A, et al. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458(7235):165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell JC, Eberl DF. Towards a molecular understanding of Drosophila hearing. J Neurobiol. 2002;53(2):172–189. doi: 10.1002/neu.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73(7):1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- 12.Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci. 2010;30(31):10465–10471. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell JC, Miller MM, Wing S, Soll DR, Eberl DF. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc Natl Acad Sci USA. 2003;100(26):16053–16058. doi: 10.1073/pnas.2535546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, et al. A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS. Neuron. 2011;70(2):281–298. doi: 10.1016/j.neuron.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82(2):429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 16.Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2(6):387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 17.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287(5461):2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 18.Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67(3):373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang X, Madrid J, Saleh HS, Howard J. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton (Hoboken) 2011;68(1):1–7. doi: 10.1002/cm.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Z, et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493(7431):221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Effertz T, Wiek R, Göpfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011;21(7):592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 22.Gong Z, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24(41):9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450(7167):294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 24.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113(2):261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 25.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483(7388):209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busto M, Iyengar B, Campos AR. Genetic dissection of behavior: Modulation of locomotion by light in the Drosophila melanogaster larva requires genetically distinct visual system functions. J Neurosci. 1999;19(9):3337–3344. doi: 10.1523/JNEUROSCI.19-09-03337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121(7):2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- 28.Orgogozo V, Grueber WB. FlyPNS, a database of the Drosophila embryonic and larval peripheral nervous system. BMC Dev Biol. 2005;5:4. doi: 10.1186/1471-213X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang RY, et al. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17(24):2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen GD. Effects of stimulus duration on responses of neurons in the chinchilla inferior colliculus. Hear Res. 1998;122(1-2):142–150. doi: 10.1016/s0378-5955(98)00103-8. [DOI] [PubMed] [Google Scholar]
- 31.Akerboom J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32(40):13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eatock RA. Adaptation in hair cells. Annu Rev Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- 33.Fettiplace R, Ricci AJ. Adaptation in auditory hair cells. Curr Opin Neurobiol. 2003;13(4):446–451. doi: 10.1016/s0959-4388(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 34.Goerlitz HR, Greif S, Siemers BM. Cues for acoustic detection of prey: Insect rustling sounds and the influence of walking substrate. J Exp Biol. 2008;211(Pt 17):2799–2806. doi: 10.1242/jeb.019596. [DOI] [PubMed] [Google Scholar]
- 35.Soller M, et al. Sex peptide–regulated female sexual behavior requires a subset of ascending ventral nerve cord neurons. Curr Biol. 2006;16(18):1771–1782. doi: 10.1016/j.cub.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN (= NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE. 2010;5(6):e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang X, et al. A NOMPC-dependent membrane-microtubule connector is a candidate for the gating spring in fly mechanoreceptors. Curr Biol. 2013;23(9):755–763. doi: 10.1016/j.cub.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 38.Gong J, Wang Q, Wang Z. NOMPC is likely a key component of Drosophila mechanotransduction channels. Eur J Neurosci. 2013;38(1):2057–2064. doi: 10.1111/ejn.12214. [DOI] [PubMed] [Google Scholar]
- 39.Marshall KL, Lumpkin EA. The molecular basis of mechanosensory transduction. Adv Exp Med Biol. 2012;739:142–155. doi: 10.1007/978-1-4614-1704-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413(6852):194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- 41.Tsubouchi A, Caldwell JC, Tracey WD. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr Biol. 2012;22(22):2124–2134. doi: 10.1016/j.cub.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senthilan PR, et al. Drosophila auditory organ genes and genetic hearing defects. Cell. 2012;150(5):1042–1054. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Kim C. TRPV family ion channels and other molecular components required for hearing and proprioception in Drosophila. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, Frontiers in Neuroscience. Boca Raton, FL: CRC Press; 2007. [PubMed] [Google Scholar]
- 44.Lehnert BP, Baker AE, Gaudry Q, Chiang AS, Wilson RI. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron. 2013;77(1):115–128. doi: 10.1016/j.neuron.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303(5656):366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 46.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129(12):2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.