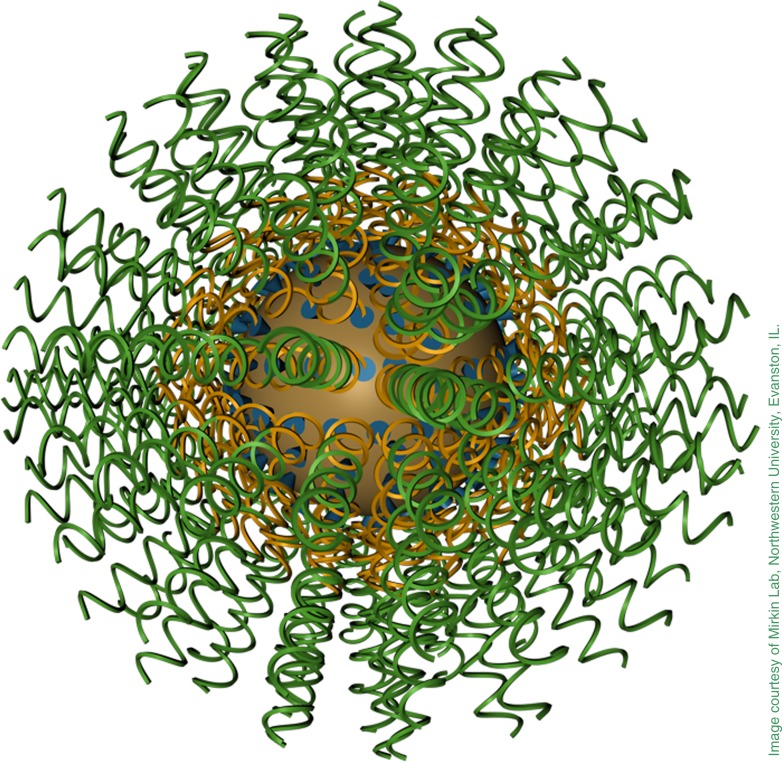
Spherical nucleic acids built from multiple strands of DNA dangling from a common center. This configuration drastically changes DNA’s physical and chemical properties.
Spherical nucleic acids are revolutionizing drug delivery, gene therapy, and diagnostics.
As the molecule that carries our genes and helps to define who we are, DNA’s double helix is one of the most recognizable symbols of science. However, when it comes to formulating genetic treatments for diseases, the iconic structure now has a rival.
On the shores of Lake Michigan, a team of scientists is championing a new shape for nucleic acids: a sphere. “You take what is arguably the most important and interesting molecule ever synthesized by chemists, and you arrange that in a three-dimensional spherical form, and on a sequence-for-sequence basis you get something that is almost night and day different,” says chemist Chad Mirkin of Northwestern University, Evanston, IL. “That new structure opens up all of these new applications,” he says.
Mirkin’s spherical nucleic acids (SNAs) are built from multiple strands of DNA, all dangling from a common center like the woolen threads of a pom-pom. Arranging DNA in this way drastically changes its physical and chemical properties. Unlike isolated double helices, the spheres can penetrate the membranes of cells, cross the skin and blood–brain barrier, and avoid attack by the human immune system.
Mirkin has spent the last two decades developing a dazzling variety of SNAs that can detect genetic abnormalities in cells, for example, or treat skin and brain cancers. He has also helped to launch three companies that are commercializing applications of the technology and is convinced that the methods will revolutionize genetic diagnostics and therapeutics.
Other scientists are just as enthusiastic. “This absolutely stands on a plateau by itself,” says chemical engineer Joseph DeSimone at the University of North Carolina at Chapel Hill. “Some of the things they’re able to do with SNAs, most other researchers would die for their platform to accomplish just one of those things,” he says.
The Ties That Bind
In the 1990s, Mirkin was deeply involved in the emerging field of nanoscience. His goal: to design nano-sized building blocks that could assemble themselves into new materials. He realized that nucleic acids—both DNA and RNA molecules—could act as linkers to tie nanoparticles together. In 1996, he published the first description of SNAs designed for this purpose (1).
Mirkin modified DNA with sulfur-bearing thiol molecules, which bind well to gold, and then coated gold nanoparticles with hundreds of the strands. Then he added more DNA sequences that were complementary to the DNA attached to the spheres. Those linkers quickly pulled the particles into a self-assembled matrix.
“As we started to look further at them, we realized the SNAs had all sorts of unique properties,” says Mirkin. “And the first important one of these was that it bound DNA much more tightly than single strands of DNA normally bound each other,” he says. With multiple strands packed together in the SNA, incoming DNA molecules were stabilized from many directions at once and held firmly in place.
That tight binding helped Mirkin tackle a problem that wasn’t originally on his agenda: how to detect low concentrations of DNA molecules. The result offers a way to quickly identify the microbes responsible for bacterial infections, so that physicians can immediately prescribe the most effective antibiotic.
Previously, doctors would need to culture the bacteria found in a blood or sputum sample for several days before there would be enough DNA to identify the culprit microbe. However, Mirkin’s SNAs bind complementary strands so well that they are extremely sensitive to trace amounts of specific DNA sequences. A company he founded, Nanosphere, has commercialized the technology as Verigene.
The system relies on a chip that carries sequences found in a particular strain of influenza, for example, or genes characteristic of antibiotic resistance in a Staphylococcus aureus infection. Any matching DNA strands in a sample stick to that part of the chip and can be highlighted by adding SNAs that
”That new structure opens up all of these new applications.“
bear more of the same complementary sequences. After the SNAs stick to the sample strands, a wash of silver coats the gold nanoparticles so that they scatter enough light to be easily detected. For Mirkin, it was the first hint of the clinical utility of SNAs, but not the last.
Special Delivery
As Mirkin was using SNAs to pull clinically relevant nucleic acids out of samples, his laboratory discovered another unexpected property of the spheres: they readily cross cell membranes. Loose DNA injected into a patient is typically blocked from entering cells, and is quickly degraded by the immune system. However, when the scientists mixed SNAs with 50 different types of cells, 99% of the spheres spontaneously entered every cell type except red blood cells (2).
The phosphate groups that help to form the backbones of nucleic acids are negatively charged, and Mirkin thinks this charge, coupled with their spherical shape, may allow SNAs to hijack positively charged proteins to help them cross the membranes. He quickly teamed up with clinical researchers who wanted to take advantage of the technology. “I immediately wanted to know whether we could use these to deliver drugs to sites in the brain,” recalls neurologist Alexander Stegh, also at Northwestern.
Stegh’s research focuses on glioblastoma multiforme (GBM), one of the most aggressive types of brain tumors. Over the last decade, projects like the Cancer Genome Atlas have revealed genetic mutations that drive the growth of GBM tumors. Stegh has discovered that by shuttling certain RNA molecules into a tumor cell, he can turn off the mutated genes and stop the tumor’s growth. However, getting these RNA molecules into the brain has been a long-standing challenge. Unique pores in the narrow blood vessels leading to the brain carefully control which molecules are allowed to pass into the organ. Most molecules—especially charged nucleic acids—cannot cross this blood–brain barrier.
To test whether SNAs might be an exception, Stegh’s and Mirkin’s teams developed spheres studded with the glioblastoma-fighting RNAs, which turn off a gene called Bcl2L12 that encourages tumor growth. They found that about 1 in 100 SNAs administered to the mouse’s blood stream could cross the blood–brain barrier and enter neurons.
The researchers have recently shown that the Bcl2L12-blocking SNAs turn off tumor signaling pathways in both isolated tumor cells and glioblastomas that are transplanted into mice. The results are not yet published. “We haven’t observed any adverse side effects at all,” says Stegh. “It really seems very effective and safe.”
Skin Deep
Stegh wasn’t the only clinician at Northwestern who was excited by Mirkin’s early results. Amy Paller, the chair of dermatology, approached Mirkin when she heard that his SNAs could spontaneously enter skin cells. “We know what genes are up-regulated in tons of skin disorders,” she says, but it is notoriously hard to change the activity of the genes. When Paller began testing Mirkin’s SNAs, she saw that they readily slipped inside skin cells and elicited no local immune response (3).
“One of the things that makes the system so beautiful is that when you make these with gold nanoparticles, you can then use the gold as a tracking mechanism,” Paller explains. Under the microscope, she could watch how quickly the SNAs entered isolated skin cells without needing additional fluorescent tags, because the gold was already visible. By mixing SNAs with a skin lotion, smearing it on mice, and studying their skin and other organs over time, she and her colleagues were able to confirm that almost all of the SNAs enter the cells within a few hours of application—and they effectively change the expression pattern of genes.
Paller is now developing SNA-based skin lotions that target genes involved in skin cancers, benign skin thickening, psoriasis, and diabetic wound healing. “What’s so important about what we’ve come up with is
”This technology is really unprecedented in terms of its promise for revolutionizing what's available for topical delivery.“
that it is a conjugate all-in-one. You don’t need a laser or an ultrasound or a needle to deliver it, and you can easily cover large body surfaces,” says Paller. “This technology is really unprecedented in terms of its promise for revolutionizing what’s available for topical delivery.” Moving SNAs to a wider patient population will take extensive clinical trials, however, and there are still basic questions about their transport mechanism. “I think there will be many of these nucleic acid delivery methods that emerge over the coming years,” says molecular biologist Phil Sharp at the Massachusetts Institute of Technology in Cambridge, who is pursuing a different method of delivering genetic therapies. “The question for each one will be how efficient they are and how safe they are.”
Back to Basics
Earlier this year, Mirkin’s laboratory answered some of the key questions about SNAs in a PNAS paper that outlined how cell uptake occurs and which cell molecules are involved (4). Microscopy studies revealed that SNAs accumulate at the outskirts of individual cells within 15 min of being added to a cell mixture. At half an hour, the spheres are accumulating inside the cells, and after an hour, they can be seen within membrane-enclosed packets called endosomes that move around the cells’ interiors.
Further cell biology experiments revealed that the SNAs bind to scavenger receptors on the outside of cells, which help to pull them through the cell membrane. These receptors do not transport loose nucleic acids, and it is the SNA’s shape, Mirkin concluded, that is largely responsible for the receptors’ willingness to play ball. However, it is still unclear how the SNAs are released from endosomes once inside the cell and whether the same scavenger receptor process happens in every cell type.
Mirkin plans to continue studying the biology of the SNAs and tweaking the formulations of nucleic acids and gold nanoparticles to optimize and expand their clinical utility. In 2012, he showed that SNAs can detect microRNAs unique to men with prostate cancer (5) and that adding antibodies to SNAs can make them favor one cell type over another (6). Meanwhile, SNAs can also help to move basic genetic research forward in the laboratory, Stegh points out. By having a tool that can enter almost every cell type and bind to nucleic acids, scientists can more quickly study the effect of turning off genes, without having to come up with a new delivery method for every cell type.
Although SNAs are delivering exciting results in biology, Mirkin doesn’t want to forget his original goals. Nucleic acid–studded nanoparticles offer a way to build functional materials by acting as “programmable atom equivalents,” with the gold nanoparticle cores playing the part of atoms and the nucleic acid linkers forming the bonds that hold them together (7). Mirkin says that he is building up a new kind of periodic table, featuring a range of SNAs with different properties. Tweaking the bonds between them allows him to fine tune the optical, magnetic, and chemical characteristics of the resulting assemblies. “The sky’s the limit when we’re building materials from the ground up,” he says.
By reshaping nucleic acids, Mirkin has also reshaped the intersection of nanotechnology,
”I think there will be many of these nucleic acid delivery methods that emerge over the coming years.“
chemistry, biology, and medicine. “When you start bringing in all the disciplines, what emerges can be quite impactful,” says DeSimone. “This is the ultimate convergence of fields.”
References
- 1.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382(6592):607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 2.Rosi NL, et al. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312(5776):1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 3.Zheng D, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci USA. 2012;109(30):11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi CHJ, Hao L, Narayan SP, Auyeung E, Mirkin CA. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc Natl Acad Sci USA. 2013;110(19):7625–7630. doi: 10.1073/pnas.1305804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhasan AH, et al. Scanometric microRNA array profiling of prostate cancer markers using spherical nucleic acid-gold nanoparticle conjugates. Anal Chem. 2012;84(9):4153–4160. doi: 10.1021/ac3004055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang K, Hao L, Hurst SJ, Mirkin CA. Antibody-linked spherical nucleic acids for cellular targeting. J Am Chem Soc. 2012;134(40):16488–16491. doi: 10.1021/ja306854d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macfarlane RJ, O’Brien MN, Hurst Petrosko S, Mirkin CA. Nucleic acid-modified nanostructures as programmable atom equivalents: Forging a new “periodic table.”. Angew Chem Int Ed. 2013;52(22):5688–5698. doi: 10.1002/anie.201209336. [DOI] [PubMed] [Google Scholar]