Abstract
Isolated methylmalonic acidemia (MMA), caused by deficiency of the mitochondrial enzyme methylmalonyl-CoA mutase (MUT), is often complicated by end stage renal disease that is resistant to conventional therapies, including liver transplantation. To establish a viable model of MMA renal disease, Mut was expressed in the liver of Mut−/− mice as a stable transgene under the control of an albumin (INS-Alb-Mut) promoter. Mut−/−;TgINS-Alb-Mut mice, although completely rescued from neonatal lethality that was displayed by Mut−/− mice, manifested a decreased glomerular filtration rate (GFR), chronic tubulointerstitial nephritis and ultrastructural changes in the proximal tubule mitochondria associated with aberrant tubular function, as demonstrated by single-nephron GFR studies. Microarray analysis of Mut−/−;TgINS-Alb-Mut kidneys identified numerous biomarkers, including lipocalin-2, which was then used to monitor the response of the GFR to antioxidant therapy in the mouse model. Renal biopsies and biomarker analysis from a large and diverse patient cohort (ClinicalTrials.gov identifier: NCT00078078) precisely replicated the findings in the animals, establishing Mut−/−;TgINS-Alb-Mut mice as a unique model of MMA renal disease. Our studies suggest proximal tubular mitochondrial dysfunction is a key pathogenic mechanism of MMA-associated kidney disease, identify lipocalin-2 as a biomarker of increased oxidative stress in the renal tubule, and demonstrate that antioxidants can attenuate the renal disease of MMA.
Keywords: cobalamin, chronic renal failure, megamitochondria, organic acidemia
Renal tubular dysfunction with progression into chronic tubulointerstitial nephritis (CTIN) and end stage renal disease (ESRD) is a cardinal manifestation of methylmalonic acidemia (MMA), a common and severe organic acidemia characterized by metabolic instability, multisystemic complications, and high mortality (1, 2). Isolated MMA is primarily caused by mutations in the vitamin B12-dependent, mitochondrial matrix-localized methylmalonyl-CoA mutase (MUT), an enzyme that mediates the entry of carbon skeletons derived from branched-chain amino acid, odd-chained fatty acid, and cholesterol oxidation into the Krebs cycle (3). Although the MUT enzyme is expressed ubiquitously, the clinical features observed, such as pancreatitis, metabolic “stroke” of the globus pallidus, optic nerve atrophy, immune dysfunction, and especially renal disease, indicate a tissue-specific vulnerability in this metabolic disorder. There are no treatments available for MMA other than dietary management and symptomatic care, although liver, kidney, and combined liver–kidney transplantation have been used in the severely affected patients (3). Kidney disease in MMA can manifest with proximal and/or distal tubular dysfunction and is associated with progressive tubulointerstitial disease (1, 4), eventually leading to ESRD in >50% of those with severe forms of MMA by the age of 8 y (5). Although renal disease can be present as early as 18 mo (6), the decreased muscle mass seen in the patients (1, 7) renders routine laboratory markers, such as creatinine, poorly predictive of kidney involvement early in disease evolution.
Patients with MMA who have been treated by orthotopic liver transplantation display metabolic stability yet develop extrahepatic disease, including ESRD, and, therefore, provide a clinical paradigm for the development of animal models to study the renal pathophysiology of MMA (8). In the present work, we have used transgenesis to create Mut−/− mice that express Mut in hepatocytes under the control of the mouse albumin promoter (Mut−/−;TgINS-Alb-Mut). These mice are protected from the neonatal lethality that characterizes the Mut−/− mice (9, 10) but manifest CTIN and a decreased glomerular filtration rate (GFR) associated with megamitochondria formation (11) and decreased cytochrome c oxidase (COX) activity in the proximal tubules. The murine studies prompted the search for similar pathology in kidney biopsies from MMA patients and suggested a therapeutic approach directed at alleviating mitochondrial dysfunction. Genomic and biochemical characterization of disease progression in the Mut−/−;TgINS-Alb-Mut mice identified >50 biomarkers associated with MMA renal disease, including lipocalin-2 (Lcn2), that was subsequently validated in a large cohort of MMA patients. A therapeutic regimen, directed at reducing oxidant injury with CoQ10 and vitamin E (VitE), ameliorated the loss of GFR in the Mut−/−;TgINS-Alb-Mut mice and was predicted by plasma Lcn2 concentrations. Our studies establish LCN2 as a biomarker of oxidative stress and renal mitochondrial dysfunction and define an approach for the treatment and monitoring of kidney disease in patients with MMA.
Results
Hepatic Expression of Mut Provides Phenotypic Attenuation in Mut−/− Mice.
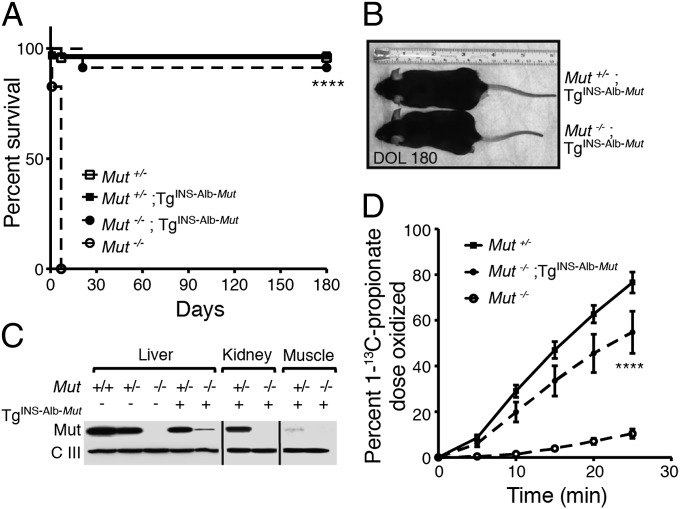
A construct was engineered to express the Mut gene under the control of the chimeric murine minimal albumin promoter and α-fetoprotein enhancer (Fig. S1 A and B). Three transmitting C57BL/6 TgINS-Alb-Mut founder lines were established and crossed to C57BL/6 Mut+/− mice (9, 12). To accelerate the development of extrahepatic manifestations, the lowest expressing line was selected for further study. Mut−/−;TgINS-Alb-Mut mice were uniformly rescued from neonatal lethality (Fig. 1A) and appeared comparable to their heterozygote littermates (Fig. 1B). Immunoreactive Mut protein was detected only in the liver of the Mut−/−;TgINS-Alb-Mut mice and was expressed at levels comparable to mRNA expression (Fig. 1C and Fig. S1C). [1-13C]Propionate oxidative capacity (13) was measured to evaluate the in vivo metabolic capacity provided by the INS-Alb-Mut transgene. The Mut−/−;TgINS-Alb-Mut mice metabolized 54.7 ± 9.2% of the administered [1-13C]propionate isotopomer in 25 min, compared with 76.5 ± 4.5% in the Mut+/− and 10 ± 2% in the Mut−/− mice (P < 0.0001) (Fig. 1D). Despite the grossly normal phenotype, the animals displayed elevated plasma MMA levels (825.7 ± 331.4 µmol/L) compared with their Mut+/−;TgINS-Alb-Mut littermates (5.8 ± 1.4 µmol/L; n = 7 and 5; P < 0.001). The histology of various organs showed no significant changes, whereas electron microscopic examination showed increased number of mitochondria and multiple matrix granules/deposits in the renal tubular cytosol of Mut−/−;TgINS-Alb-Mut kidneys (Fig. S2A).
Fig. 1.
Phenotypic characterization of the Mut−/−;TgINS-Alb-Mut mice. (A) Mut−/−;TgINS-Alb-Mut mice (n = 23) exhibit normal survival compared with the immediate neonatal lethality of the knockout strain (n = 29; P < 0.0001) and comparable to their heterozygote littermates with or without the transgene (n =32 and 24, respectively; P = NS for both). (B) At 6 mo of age, Mut−/−;TgINS-Alb-Mut mice appear normal and are 14 ± 0.56% smaller than their littermates (n = 4 per group; P < 0.001). (C) Mut (78 kDa) was detected in whole-tissue lysates from Mut−/−;TgINS-Alb-Mut liver but not in kidney or muscle. (D) [1-13C]Propionate oxidation showed a significant improvement of the in vivo oxidative capacity by the hepatic transgene-mediated Mut expression. (n = 11, 9 and 6 for Mut+/−, Mut−/−;TgINS-Alb-Mut, and Mut−/−, respectively; values are means ± SEM, ****P < 0.0001 between Mut−/−;TgINS-Alb-Mut vs. Mut−/− mice.)
Acceleration of MMA Renal Disease by Ingestion of a High-Protein Diet.
To recapitulate the renal disease observed in some patients post-liver transplantation with liberalized protein intake (8), the mice were fed a high-protein (HP) (casein) chow. A dietary challenge study for 6 mo (n = 8) was performed in female Mut−/−;TgINS-Alb-Mut mice, because only rarely did male littermates survive beyond 2 mo of age on the same diet. On the HP chow, the Mut−/−;TgINS-Alb-Mut mice experienced a rapid weight loss of 14 ± 4% within the first month and failed to regain their weight (Fig. S3A). Plasma methylmalonic acid concentration in the Mut−/−;TgINS-Alb-Mut mice was 863 ± 288 µmol/L at baseline, 1,500 ± 620 µmol/L after 2 mo, and 1,938 ± 418 µmol/L after 6 mo on a HP diet (P = 0.013 for Mut−/−;TgINS-Alb-Mut mice compared with baseline values and P < 0.0001 compared with heterozygotes) (Fig. S3B). Studies in plasma vs. brain tissue extracts, obtained 2 mo after HP diet in a separate group of mice, showed significantly higher methylmalonic acid concentrations in the brain tissue compared with mice fed regular chow (RD) (Fig. S3 C and D; n = 6 per group; P = 0.007), similar to the trend in plasma. These concentrations closely approximate methylmalonic acid levels observed in our MMA patient cohort at baseline and after the onset of kidney failure in the older subgroup, respectively (Table S1).
Severe Proximal Tubular Mitochondrial Ultrastructural Changes in MMA Mice Replicate the Renal Pathology Seen in MMA Patients.
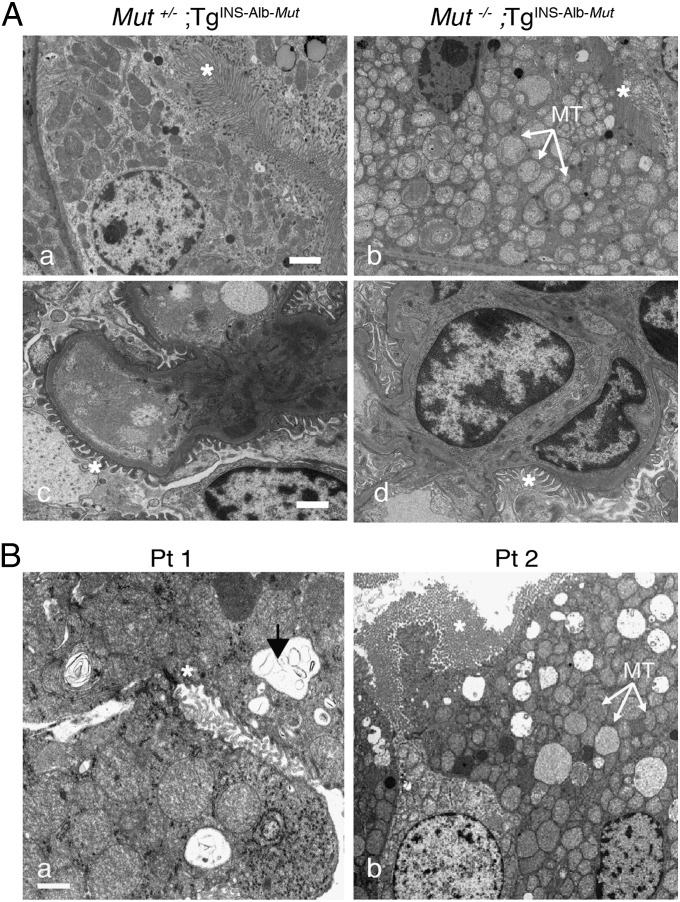
Mut−/−;TgINS-Alb-Mut mice that ingested a HP diet for 6 mo developed severe multifocal tubulointerstitial nephritis (Fig. S3 Ea vs. Eb), causing the kidney cortex in the Mut−/−;TgINS-Alb-Mut mice to have a granular appearance (Fig. S3Eb, black arrows), whereas tubules in the convoluted proximal segment had fine microvesicular cytoplasmic changes (Fig. S3Eb, Inset). Ultrastructural examination of the kidneys in the Mut−/−;TgINS-Alb-Mut mice revealed a large number of mitochondria with electron-dense matrix and abnormal cristae in the proximal tubules (Fig. 2 Aa vs. Ab). Of note, the glomeruli in the Mut−/−;TgINS-Alb-Mut mice appeared normal, and the podocyte foot processes were not effaced (Fig. 2 Ac vs. Ad). In contrast to the striking renal pathology observed in Mut−/−;TgINS-Alb-Mut mice, there were no light or electron microscopic abnormalities in the liver, despite the prolonged exposure to HP and the massive (greater than 1,000× increased) plasma methylmalonic acid concentrations (Fig. S2B). To determine whether kidneys have a different susceptibility to methylmalonic acid than liver, we administered 20 mg/100 g body weight of methylmalonic acid via daily i.p. injections for 2 mo to wild-type mice. Nephrotoxicity was not observed (Fig. S4 A and B).
Fig. 2.
Dietary challenge with HP in Mut−/−;TgINS-Alb-Mut mice and patient correlations. Mut−/−;TgINS-Alb-Mut mice experienced significant growth failure and plasma MMA concentrations of 1,938 ± 418 μmol/L after 6 mo on HP diet (Fig. S3). (A) Transmission EM showed enlarged mitochondria with whorl-like cristae (Aa vs. Ab; white arrows, mitochondria; white asterisks, brush border of the proximal tubule). Glomeruli appeared normal (Ac and Ad; white asterisks, podocyte foot processes). (B) Transmission EM of patient kidneys showed enlarged mitochondria with disorganized cristae. There were also large remnant vacuoles that contained amorphous membranous inclusions (white arrows, mitochondria; white asterisks, brush border of the proximal tubule; black arrow, vacuoles). (Scale bars: 1 μm.)
In parallel to the murine studies, we collected kidney biopsies or explants from three MMA patients enrolled in a natural history study (ClinicalTrials.gov identifier: NCT00078078), who were undergoing solid-organ transplantation. The microscopic findings in the MMA patient kidneys closely resembled the changes seen in the Mut−/−;TgINS-Alb-Mut mice (Fig. S3 E vs. F), whereas electron microscopic examination revealed similarly enlarged mitochondria in the proximal tubule, with increased electron-dense matrix and shortened, abnormal cristae (Fig. 2B).
Abnormalities of Glomerular Filtration and Tubular Reabsorption Are Early Manifestations of the Renal Disease in Mut−/−;TgINS-Alb-Mut Mice.
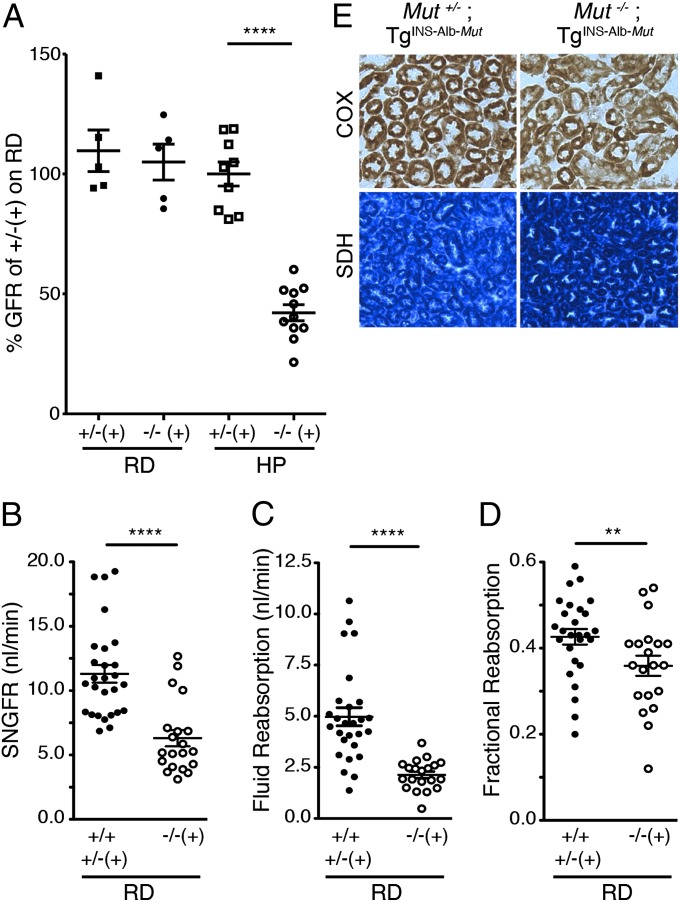
After ingesting a HP diet for 2 mo, the Mut−/−;TgINS-Alb-Mut mice manifested significant weight loss, increased mortality, and massively elevated plasma methylmalonate concentrations. We, therefore, selected this time point to measure the GFR using FITC-labeled inulin clearance and to assess proximal tubular function. Mut−/−;TgINS-Alb-Mut animals showed a significant reduction in GFR (42.1 ± 3.35%; n = 11; P < 0.0001) compared both to the heterozygotes on HP and to the Mut−/−;TgINS-Alb-Mut mice maintained on RD (Fig. 3A). We observed no differences between male and female GFRs (Fig. S4E); however, an increase in serum creatinine was evident in male (P = 0.003) but not in female Mut−/−;TgINS-Alb-Mut mice on HP. The overall survival in a larger group of age- and sex-matched Mut−/−;TgINS-Alb-Mut mice fed a HP diet was 52% (P < 0.0001 compared with their Mut+/− littermates with or without the transgene; Fig. S5A), with males being more severely affected (P = 0.005 between sexes, Fig. S5B). Histology and ultrastructure of the kidneys in both Mut−/−;TgINS-Alb-Mut male and female mice did not reveal any significant differences compared with heterozygotes at this time point (Fig. S5E).
Fig. 3.
Renal function at the whole-kidney and single-nephron level and COX/SDH staining in the kidney sections of Mut−/−;TgINS-Alb-Mut mice. (A) Whole-animal GFR in the Mut−/−;TgINS-Alb-Mut mice fed a HP diet (n = 11; 3 male and 8 female) was 42.1 ± 3.35% of the average GFR in heterozygote mice on RD (n = 5; P < 0.0001). No change was observed in mutants on RD (n = 5) or heterozygotes fed a HP diet (n = 9). (B) SNGFR (nanoliters per minute) in male Mut−/−;TgINS-Alb-Mut mice on RD was lower than in Mut+/−;TgINS-Alb-Mut littermates and Mut+/+ control animals (n = 20 vs. 27 SNGFR determinations on 6 Mut−/−;TgINS-Alb-Mut and 6 control, 2 Mut+/−;TgINS-Alb-Mut littermates, and 4 Mut+/+ mice, respectively; P < 0.0001). (C and D) The proximal tubule fluid reabsorption (C) and fractional reabsorption (D) obtained from the SNGFR studies were significantly impaired in the male Mut−/−;TgINS-Alb-Mut mice (P < 0.0001and P = 0.026, respectively). No differences were observed in arterial pressure (93 ± 2.2 mm Hg in Mut−/−;TgINS-Alb-Mut mice vs. 95 ± 2.6 mm Hg in Mut+/− and Mut+/+ mice; n = 6 per group) or kidney weight (385 ± 8.5 mg in Mut−/−;TgINS-Alb-Mut vs. 395 ± 24 mg in the Mut+/− and Mut+/+ mice; n = 6 per group). (E) Immunohistochemistry for COX and SDH on kidney sections of mutant mice fed a HP diet revealed patchy/focal decrease in COX staining (20×) in the presence of increased staining for the nuclear-encoded SDH (10×) in sections of the proximal tubules. Data are means ± SEM. **P < 0.01; ****P < 0.0001.
Measurements of single-nephron (SN) GFR and tubular function were performed on anesthetized male mice fed RD because male Mut−/−;TgINS-Alb-Mut mice fed the HP diet did not survive the procedure. SNGFR was significantly lower in the Mut−/−;TgINS-Alb-Mut mice (Fig. 3B), and this reduction was accompanied by a parallel diminution of the proximal tubule fluid reabsorption rate (Fig. 3C). However, there was also a significantly lower fractional fluid reabsorption in the Mut−/−;TgINS-Alb-Mut mice, indicating reduced reabsorptive capacity of the proximal tubule, that was independent of the GFR (Fig. 3D). Blood and urine collected during the micropuncture SNGFR studies also showed a lower plasma bicarbonate and higher chloride concentration in the Mut−/−;TgINS-Alb-Mut mice compared with controls, consistent with chronic hyperchloremic metabolic acidosis. Despite the metabolic acidosis, urinary ammonium excretion was not increased (Table S2), an expected observation in the setting of renal tubular acidosis (14). Whole-body GFR studies on RD were not significantly decreased up until 9 mo to 1 y of age (Fig. S4 C and D), suggesting that liver-restricted expression of Mut may offer protection from renal failure unless the pathway is challenged by an increased propiogenic load.
Bioenergetic Profiling of the Proximal Tubule.
The abnormal ultrastructure and function of the proximal tubules prompted an investigation of respiratory-chain enzyme expression and function. Using in situ immunohistochemistry, we compared COX (complex IV) and succinate dehydrogenase (SDH) (complex II) staining patterns in kidneys from Mut+/− compared with Mut−/−;TgINS-Alb-Mut mice fed a HP diet for 2 mo. Mut−/−;TgINS-Alb-Mut kidneys demonstrated a mixture of COX-positive and COX-negative tubules with uniformly SDH-hyperreactive staining compared with the heterozygotes (Fig. 3E). The enzymatic activities of the respiratory-chain complexes in both liver and kidney protein extracts showed no significant differences (Table S3). Furthermore, we measured levels of CoQ, isoforms 9 and 10, to examine whether a primary or secondary synthetic defect might be associated with the mitochondrial abnormalities observed. Mut−/−;TgINS-Alb-Mut mice fed a HP diet for 2 mo showed significantly lower CoQ10 levels than their heterozygote littermates (P < 0.001) (Table S4); however, CoQ9 levels were comparable, suggesting that the biosynthetic capacity for CoQ was preserved in the mutant kidneys.
Expression Profiling of MMA Renal Disease in Mut−/−;TgINS-Alb-Mut Kidneys.
We compared gene-expression profiles of whole-kidney mRNA samples between four female Mut+/+, Mut+/−, and Mut−/−;TgINS-Alb-Mut mice after they ingested a HP diet for 2 mo. Fifty-five genes fulfilled criteria for twofold differential expression. Significant enrichment was identified in 10 pathways, including those involved in immune response, lipid, branched-chain amino acid and ketone metabolism, and cell survival (Tables S5 and S6). Genes showing highly discordant expression between the Mut−/−;TgINS-Alb-Mut mice and both Mut+/+ or Mut+/−;TgINS-Alb-Mut littermates included lipocalin-2 (Lcn2, or neutrophil-gelatinase-associated lipocalin) (P = 6.74 × 10−5) (15), as well as other transcripts expressed in the setting of kidney damage such as Fabp1 (P = 0.0024) (16) and Havcr1 [kidney injury molecule (Kim)-1; P = 0.0012] (17). Targets of PPARα were up-regulated in the Mut−/−;TgINS-Alb-Mut kidneys, including Cyp4a12a and Cyp4a12b, two subtypes of a cytochrome P450 system that are involved in the metabolism of arachidonic acid in the proximal tubule and result in the generation of 20-hydroxyeicosatetraenoic acid (20-HETE), a powerful vasoconstrictor (18). A sexually dimorphic expression was observed for Cyp4a isoforms (Fig. S5 F–I).
Lipocalin-2 As a Biomarker of Renal Dysfunction and Oxidative Stress in Mut−/−;TgINS-Alb-Mut Mice and MMA Patients.
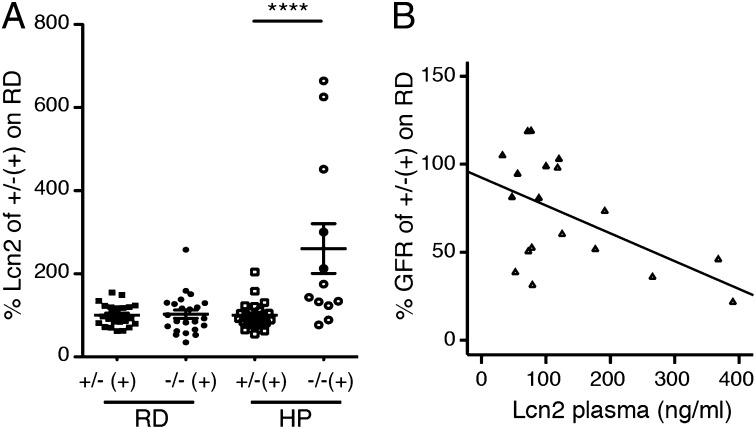
To confirm the induction of Lcn2 observed in the microarray studies, Lcn2 was measured in both mice and patients. Plasma concentrations of Lcn2 in the Mut−/−;TgINS-Alb-Mut mice after 2 mo of ingesting a HP chow were elevated (260.7 ± 59.7% of the mean Lcn2 in heterozygotes on RD) compared with their littermates (100 ± 6.05%; P = 0.0004) (Fig. 4A). Moreover, an increased expression of Lcn2 correlated with the severity of chronic kidney disease, as demonstrated by the inverse correlation of Lcn2 plasma levels with GFR (r = −0.533; n = 19; P = 0.016) (Fig. 4B). Lcn2 correlated with the decreased GFR even in female animals [r = −0.52; P = not significant (NS)], despite the normal creatinine levels and a much milder phenotype, suggesting that Lcn2 is an early biomarker of the renal dysfunction in Mut−/−;TgINS-Alb-Mut mice. To investigate the relation between the Lcn2 induction and the abnormal tubular mitochondrial ultrastructure and focally decreased COX activity evident in the Mut−/−;TgINS-Alb-Mut mouse kidneys, we evaluated for increased radical oxygen species (ROS) production as a byproduct of disturbed mitochondrial function. Urinary F2-isoprostanes (F2iPs) are a well-characterized marker of ROS-induced lipid peroxidation and were found to positively correlate with urine Lcn2 concentrations in Mut−/−;TgINS-Alb-Mut mice (r = 0.712; n =14; P = 0.0063). We next sought to validate these biomarkers in a cohort of patients with MMA (n = 46), enrolled in a dedicated natural history study. We documented significant correlations between LCN2 and serum markers of renal function including creatinine (Fig. S6A), cystatin-C (Fig. S6B), and estimated GFR (Fig. S6C), as well as markers of oxidant stress, including plasma oxidized LDL (Fig. S6D) and urinary F2iPs (Fig. S6E). No correlation was observed between LCN2 and dietary protein intake (grams per kilogram body weight per day) of complete or deficient protein and serum total protein or albumin concentrations (grams per deciliter) (Fig. S6 F and G).
Fig. 4.
Lipocalin-2 correlates with kidney disease progression in Mut−/−;TgINS-Alb-Mut mice. (A) There was no difference in plasma Lcn2 (nanograms per milliliter) between heterozygote [n = 24 (12 male and 12 female); solid square] and Mut−/−;TgINS-Alb-Mut [n = 23 (11 male and 12 female) solid circle] mice on RD or heterozygotes fed a HP diet [n = 26 (17 male, 9 female); open square]. However, Lcn2 was elevated in Mut−/−;TgINS-Alb-Mut mice [n = 12 (5 male and 7 female); open circle] after ingesting a HP diet (P = 0.0007). (B) Plasma Lcn2 (nanograms per milliliter) showed a negative correlation with measured GFR in mice on RD or HP [n = 19; 11 Mut−/−;TgINS-Alb-Mut (open triangles) and 8 heterozygote (solid triangles) mice; r = −0.533; P = 0.016; R2 = 0.244). Lcn2 and GFR data are expressed as percentage of the mean value for heterozygote mice on RD. Data are means ± SEM. ****P < 0.0001 (Kruskal–Wallis test).
Treatment with Antioxidants Ameliorates the Renal Dysfunction Induced by the HP Diet.
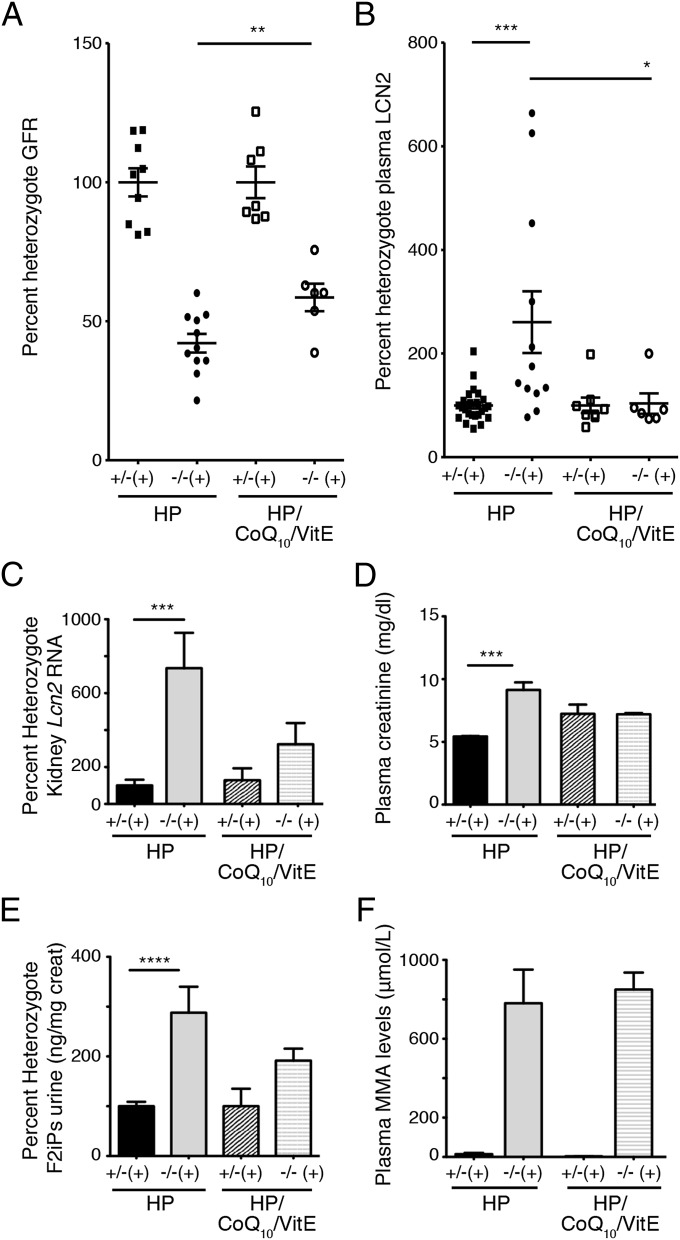
To assess the role of oxidative stress in the deterioration of renal function in the Mut−/−;TgINS-Alb-Mut mice, we included 0.5% wt/wt ubiquinone, a highly bioavailable form of CoQ10, and 0.2% wt/wt VitE in the HP mouse chow. This regimen has been used previously with success to treat an isolated case of MMA with optic nerve atrophy (19), whereas the dose and formulation of CoQ10 was identical to that shown to significantly decrease oxidative stress and increase survival in a mouse model of Huntington disease (20). While Mut−/−;TgINS-Alb-Mut mice fed a HP diet lost >25% of their weight after 3 d, those that consumed the same diet plus antioxidants showed a trend toward increased survival and decreased weight loss (Fig. S5 C and D; P = NS and 0.013, respectively). Moreover, the CoQ10/VitE-treated mice (n = 6) showed a significantly improved GFR (215.08 ± 57.01 μL/min or 58.6 ± 4.94% heterozygote GFR from 151.22 ± 37.6 μL/min or 42.1 ± 3.35% on HP diet, which represents a 42.2% improvement in absolute numbers, or 16.5% improvement in percent heterozygote GFR) vs. mice on HP diet (n = 11; P = 0.0128; Fig. 5A). The improvement in the measured GFR was accompanied by a total abrogation of increased plasma Lcn2 levels and a reduction in Lcn2 mRNA expression in the kidney of the CoQ10/VitE-treated Mut−/−;TgINS-Alb-Mut mice (Fig. 5 B and C), as well as by the normalization of the increased plasma creatinine concentrations seen in males (Fig. 5D). The decrease in Lcn2 was paralleled by a similar decrease in urinary F2iPs (Fig. 5E). On the other hand, plasma MMA concentrations were identical between the Mut−/−;TgINS-Alb-Mut mice receiving either diet (Fig. 5F).
Fig. 5.
Dietary supplementation with CoQ10 (0.5% wt/wt) and VitE (0.2% wt/wt) ameliorated the loss of GFR and was predicted by plasma Lcn2 levels in Mut−/−;TgINS-Alb-Mut mice. (A) Mut−/−;TgINS-Alb-Mut mice on the HP plus antioxidants diet [n = 6 (2 male, 4 female)] showed a significantly improved GFR compared with Mut−/−;TgINS-Alb-Mut mice fed solely HP diet [n = 11 (3 male, 8 female); P = 0.0104]. (B and C) Antioxidants reduced Lcn2 in the plasma (B) and in the kidney tissue (C) of Mut−/−;TgINS-Alb-Mut mice (P = NS compared with treated heterozygote mice). (D–F) Plasma creatinine normalized in the treated male mice (D), and urine F2iPs improved after antioxidant therapy (P = NS compared with treated heterozygote mice) (E), despite the persistence of elevated serum MMA levels (P < 0.0001 between mutant and heterozygote mice on either diet) (F). Data are presented as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (Kruskal–Wallis test).
Discussion
Similar to MMA patients who have received orthotopic liver transplantation, Mut−/−;TgINS-Alb-Mut animals demonstrated significant elevations of plasma methylmalonic acid, CTIN, and diminished GFR following HP-diet administration. Our observation that transgenesis corrected the severe hepatic ultrastructural mitochondrial abnormalities seen in Mut−/− mice (11) in the face of >1,000× elevated plasma methylmalonic acid concentrations suggests that the cellular phenotype could be rescued by tissue-specific expression of Mut (Fig. S2B). Moreover, a significant functional improvement in the GFR was observed in the CoQ10/VitE treated Mut−/−;TgINS-Alb-Mut mice despite the continuous exposure of the kidneys to a HP diet and elevated circulating metabolites (Fig. 5 A and F), whereas daily i.p. administered methylmalonic acid for 2 mo did not have significant nephrotoxic effects in wild-type mice (Fig. S4A). Together, these findings suggest that cell and/or mitochondrial autonomous effects, rather than elevations in methylmalonic acid per se, are responsible for the hepatic mitochondrial changes and the GFR decrease associated with proximal tubular mitochondrial dysfunction. This challenges previous theories about methylmalonate as a toxic agent (21, 22) and further supports observations from gene therapy studies showing that the long-term correction of Mut−/− mice is mediated by a small number of stably transduced cells (13, 23). The concept that a kidney with normal Mut activity may be protected from MMA nephrotoxicity is reinforced by a recent study that proposed providing renal allografts as a form of “cellular” therapy for MMA, despite the implicit knowledge that the allograft will be exposed to very high metabolite concentrations in the recipient patient (24). Further support of a cell-autonomous effect was demonstrated by the local production of methylmalonic acid in the brain tissue on HP diet (Fig. S3D), as well as by the focal decrease in COX in a subset of the Mut−/−;TgINS-Alb-Mut tubules (Fig. 3E), with no obvious impairment in respiratory-chain complex activities analyzed in whole kidney extracts, similar to what is observed in the skeletal muscle of patients with mitochondrial myopathies (25). Given that HP seems necessary for the progression of chronic renal disease in our model (Fig. S4 C and D), we suggest that both mitochondrial dysfunction and toxic metabolite accumulation play a role in disease pathophysiology; hence, conditional cell-autonomy more accurately reflects the current findings.
Gene-expression profiling on whole kidneys collected 2 mo after initiating a HP diet revealed significant enrichment in several inflammatory, signaling, and metabolic pathways. A number of gene-targets of the retinoid X receptor/peroxisome proliferator-activated receptor α (PPARα), including Cyp4a12a and -b, Fabp1, and Hmgcs2, were up-regulated, consistent with the pronounced number of mitochondria in the tubules of patients and mice. Biomarkers of acute and chronic kidney injury, Havcr1 (KIM-1) and Lcn2 (neutrophil gelatinase-associated lipocalin), were up-regulated in the Mut−/−;TgINS-Alb-Mut kidneys. Lcn2, originally identified as a 25-kDa protein associated with neutrophil gelatinase (26), is largely produced by the tubular epithelium of the distal nephron after cellular damage and is associated with acute kidney injury (15), as well as chronic kidney disease progression (27). The murine and patient studies presented here demonstrate a strong correlation of Lcn2 with markers of oxidative stress. Moreover, Lcn2 mRNA expression in the tissue and its resulting plasma concentrations were reduced after the administration of antioxidants, supporting a link between oxidative stress and Lcn2 in the renal disease of MMA and potentially other forms of tubulointerstitial nephritis associated with mitochondrial dysfunction.
The proximal tubular dysfunction was further validated by micropuncture studies of single Mut−/−;TgINS-Alb-Mut nephrons (Fig. 3 B–D) that demonstrated aberrant ammonium excretion in the presence of hyperchloremic metabolic acidosis (Table S2). The diminished GFR can thus be viewed as protective, because it reduces the absorptive load upon the functionally impaired proximal tubule cells. Although it is unclear which exact mechanism(s) underlies the drastic reduction of GFR, single-nephron measurements documented a functional impairment well before any overt structural or ultrastructural changes in the tubules or the podocytes and glomerular architecture were detected. Tubuloglomerular feedback, the response of GFR to an increased delivery of NaCl to the macula densa region, might play a role, although in our approach, all fluid is withdrawn at the end of the proximal tubule, thus normalizing distal delivery to zero in both wild-type and mutant mice. Therefore, it is likely that the marked reduction of GFR in Mut−/−;TgINS-Alb-Mut mice reflects a functional process initiated by the proximal tubule mitochondrial dysfunction, possibly mediated by altered vascular tone at the afferent or efferent arteriole. That increased ROS and induction of genes involved in the generation of metabolites of arachidonic acid, such as 20-HETE, were identified in Mut−/−;TgINS-Alb-Mut kidneys supports the concept that small-molecule mediators may play an important role in the kidney disease of MMA (18). Mut−/−;TgINS-Alb-Mut mice, therefore, represent a unique model to explore mechanisms of tubuloglomerular feedback in the context of mitochondrial dysfunction in the tubules.
The normal levels of renal CoQ9 indicate that impaired CoQ biosynthesis is not a primary determinant of the kidney disease of MMA (Table S4) and is consistent with the distinct renal pathology exhibited by the Mut−/−;TgINS-Alb-Mut animals compared with a model of defective CoQ biosynthesis, the Pdss2kd/kd mice (28). However, the decrease in renal CoQ10 in the Mut−/−;TgINS-Alb-Mut mouse kidney extracts paralleled a previous report of reduced CoQ10 concentrations in MMA patient fibroblasts (29) and further suggested that markers of oxidant stress be explored in both animals and patients. Indeed, dietary stress caused urine F2iPs to increase in the Mut−/−;TgINS-Alb-Mut mice compared with their diet-matched littermates. Furthermore, oxidative markers, including urine F2iPs and plasma oxidized LDL, were elevated in MMA patients with advanced kidney disease (Fig. S6). These findings align with previous reports (30) and suggest a possible set of biomarkers to follow disease severity and/or progression.
Because the renal disease of MMA correlated with markers of oxidant injury in mice and patients, we devised a treatment protocol with two well-established antioxidants, CoQ10 and VitE (Fig. 5). Both compounds are orally administered, display an excellent safety profile, even at high doses, and are given in combination to increase antioxidant capacity (20, 31). We found that treated Mut−/−;TgINS-Alb-Mut mice experienced a significant amelioration in the loss of GFR and a normalization of plasma Lcn2 levels compared with untreated animals fed a HP diet, despite exhibiting the same magnitude of elevation of serum metabolites. Furthermore, a decrease in the GFR was accompanied by an increase in plasma Lcn2, even when the creatinine concentration was normal, establishing plasma Lcn2 as a sensitive biomarker to monitor therapies aimed at the renal disease of MMA. Our findings demonstrate that readily available antioxidants can significantly affect the rate of decline of renal function in a mouse model that replicates the kidney disease of MMA, a relentless disease with limited therapeutic options other than solid-organ transplantation.
Materials and Methods
Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited specific pathogen-free facility and were approved by the Institutional Animal Care and Use Committee of the National Human Genome Research Institute (NHGRI), National Institutes of Health (NIH). The human studies were approved by the NHGRI institutional review board as part of a NIH protocol (ClinicalTrials.gov identifier: NCT00078078) and were performed in compliance with the Helsinki Declaration. Experimental details are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank all patients and their families; Shelley Hoogstraten-Miller for veterinary support; Irene Ginty, Cherry Yang, Tina Aroyo, Theresa Ferrine, and Lemlem Alemu for mouse maintenance; and Isa Bernardini and Roxanne Fischer for processing patient samples. This work was supported by the Intramural Research Program of the National Human Genome Research Institute (I.M., J.R.S., C.W., N.S.T., J.C., J.L.S., R.J.C., A.G.E., and C.P.V.), the National Institute of Diabetes and Digestive and Kidney Diseases (L.L., P.H., and J.S.), the National Cancer Institute (M.A.-A. and M.T.), and the Office of the Director (P.M.Z. and V.H.) of the National Institutes of Health. J.R.S. and C.W. were also supported by the Angels for Alyssa (MMA Research Fund). P.H. was supported by the Philippe Foundation, the Oak Ridge Institute for Science and Education, and Paris Descartes University, France.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE41044).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302764110/-/DCSupplemental.
References
- 1.Walter JH, et al. Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr. 1989;148(4):344–348. doi: 10.1007/BF00444131. [DOI] [PubMed] [Google Scholar]
- 2.Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983;308(15):857–861. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- 3. Manoli I, Venditti CP (2005) Methylmalonic acidemia. GeneReviews, eds Pagon RA, et al. (University of Washington, Seattle, WA) [PubMed]
- 4.D’Angio CT, Dillon MJ, Leonard JV. Renal tubular dysfunction in methylmalonic acidaemia. Eur J Pediatr. 1991;150(4):259–263. doi: 10.1007/BF01955526. [DOI] [PubMed] [Google Scholar]
- 5.Hörster F, et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB) Pediatr Res. 2007;62(2):225–230. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- 6.Oberholzer VG, Levin B, Burgess EA, Young WF. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child. 1967;42(225):492–504. doi: 10.1136/adc.42.225.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauser NS, Manoli I, Graf JC, Sloan J, Venditti CP. Variable dietary management of methylmalonic acidemia: Metabolic and energetic correlations. Am J Clin Nutr. 2011;93(1):47–56. doi: 10.3945/ajcn.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyhan WL, Gargus JJ, Boyle K, Selby R, Koch R. Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur J Pediatr. 2002;161(7):377–379. doi: 10.1007/s00431-002-0970-4. [DOI] [PubMed] [Google Scholar]
- 9.Chandler RJ, et al. Metabolic phenotype of methylmalonic acidemia in mice and humans: The role of skeletal muscle. BMC Med Genet. 2007;8:64. doi: 10.1186/1471-2350-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters H, et al. A knock-out mouse model for methylmalonic aciduria resulting in neonatal lethality. J Biol Chem. 2003;278(52):52909–52913. doi: 10.1074/jbc.M310533200. [DOI] [PubMed] [Google Scholar]
- 11.Chandler RJ, et al. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2009;23(4):1252–1261. doi: 10.1096/fj.08-121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandler RJ, Venditti CP. Adenovirus-mediated gene delivery rescues a neonatal lethal murine model of mut(0) methylmalonic acidemia. Hum Gene Ther. 2008;19(1):53–60. doi: 10.1089/hum.2007.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandler RJ, Venditti CP. Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy. Mol Ther. 2010;18(1):11–16. doi: 10.1038/mt.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenes LG, Sanchez MI. Impaired urinary ammonium excretion in patients with isolated proximal renal tubular acidosis. J Am Soc Nephrol. 1993;4(4):1073–1078. doi: 10.1681/ASN.V441073. [DOI] [PubMed] [Google Scholar]
- 15.Mishra J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 16.Kamijo A, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165(4):1243–1255. doi: 10.1016/S0002-9440(10)63384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroetz DL, Xu F. Regulation and inhibition of arachidonic acid omega-hydroxylases and 20-HETE formation. Annu Rev Pharmacol Toxicol. 2005;45:413–438. doi: 10.1146/annurev.pharmtox.45.120403.100045. [DOI] [PubMed] [Google Scholar]
- 19.Pinar-Sueiro S, Martínez-Fernández R, Lage-Medina S, Aldamiz-Echevarria L, Vecino E. Optic neuropathy in methylmalonic acidemia: The role of neuroprotection. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9084-8. [DOI] [PubMed] [Google Scholar]
- 20.Smith KM, et al. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington’s disease mice. Biochim Biophys Acta. 2006;1762(6):616–626. doi: 10.1016/j.bbadis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Wolff JA, et al. Proximal renal tubular acidosis in methylmalonic acidemia. J Neurogenet. 1985;2(1):31–39. doi: 10.3109/01677068509100141. [DOI] [PubMed] [Google Scholar]
- 22.Kashtan CE, et al. Chronic administration of methylmalonic acid (MMA) to rats causes proteinuria and renal tubular injury. Pediatr Res. 1998;43:309. [Google Scholar]
- 23.Carrillo-Carrasco N, Chandler RJ, Chandrasekaran S, Venditti CP. Liver-directed recombinant adeno-associated viral gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Hum Gene Ther. 2010;21(9):1147–1154. doi: 10.1089/hum.2010.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brassier A, et al. Renal transplantation in 4 patients with methylmalonic aciduria: A cell therapy for metabolic disease. Mol Genet Metab, 10.1016/j.ymgme.2013.05.001. 2013 doi: 10.1016/j.ymgme.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 25.DiMauro S, Tanji K, Bonilla E, Pallotti F, Schon EA. Mitochondrial abnormalities in muscle and other aging cells: Classification, causes, and effects. Muscle Nerve. 2002;26(5):597–607. doi: 10.1002/mus.10194. [DOI] [PubMed] [Google Scholar]
- 26.Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268(14):10425–10432. [PubMed] [Google Scholar]
- 27.Viau A, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120(11):4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng M, et al. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4(4):e1000061. doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas D, et al. Coenzyme Q(10) is decreased in fibroblasts of patients with methylmalonic aciduria but not in mevalonic aciduria. J Inherit Metab Dis. 2009;32(4):570–575. doi: 10.1007/s10545-009-1150-8. [DOI] [PubMed] [Google Scholar]
- 30.Atkuri KR, et al. Inherited disorders affecting mitochondrial function are associated with glutathione deficiency and hypocitrullinemia. Proc Natl Acad Sci USA. 2009;106(10):3941–3945. doi: 10.1073/pnas.0813409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas SR, et al. Dietary cosupplementation with vitamin E and coenzyme Q(10) inhibits atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc Biol. 2001;21(4):585–593. doi: 10.1161/01.atv.21.4.585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.