Abstract
Although common in birds, social monogamy, or pair-living, is rare among mammals because internal gestation and lactation in mammals makes it advantageous for males to seek additional mating opportunities. A number of hypotheses have been proposed to explain the evolution of social monogamy among mammals: as a male mate-guarding strategy, because of the benefits of biparental care, or as a defense against infanticidal males. However, comparative analyses have been unable to resolve the root causes of monogamy. Primates are unusual among mammals because monogamy has evolved independently in all of the major clades. Here we combine trait data across 230 primate species with a Bayesian likelihood framework to test for correlated evolution between monogamy and a range of traits to evaluate the competing hypotheses. We find evidence of correlated evolution between social monogamy and both female ranging patterns and biparental care, but the most compelling explanation for the appearance of monogamy is male infanticide. It is only the presence of infanticide that reliably increases the probability of a shift to social monogamy, whereas monogamy allows the secondary adoption of paternal care and is associated with a shift to discrete ranges. The origin of social monogamy in primates is best explained by long lactation periods caused by altriciality, making primate infants particularly vulnerable to infanticidal males. We show that biparental care shortens relative lactation length, thereby reducing infanticide risk and increasing reproductive rates. These phylogenetic analyses support a key role for infanticide in the social evolution of primates, and potentially, humans.
Social monogamy, or pair-living, is much more common among birds (90% of species) (1) than mammals (less than 3% of species) (2). In many bird species, the successful rearing of offspring requires investment of both pair-members in incubation and provisioning, effectively constraining the adults to stay in a pair (3). In mammals, by contrast, female internal gestation and lactation characteristically results in highly skewed parental investment, with males continuing to search for additional reproductive partners after each successful mating. Because of this behavior, the typical mammal mating system can be characterized as polygynandry, whereby multiple males mate with multiple females within a breeding season (4–6). Because social monogamy in mammals is relatively uncommon and appears to require a fundamental change in male reproductive strategy, understanding its evolution has generated a great deal of interest. Additionally, the evolutionary history of social monogamy in mammals may help uncover the selective pressures that led to the formation of long-term pair bonds in humans.
Three separate hypotheses have been proposed to explain the evolution of social monogamy: parental care, mate guarding, and infanticide risk. First, social monogamy may arise where the cost of raising offspring is high, such that a female must rely on the help of others, particularly for carrying infants (2, 7). For example, the females of socially monogamous New World primates (callitrichids and Aotus) typically give birth to twins and cannot cope with infant carrying without the help of a male (2). Although some socially monogamous mammal species are associated with high levels of care (8–10), biparental care has been discounted as a general explanation because it is not ubiquitous in socially monogamous mammals (11). It may be that rather than high-cost offspring giving rise to monogamy, monogamy enables the production of high-cost offspring. Second, social monogamy may arise when females occupy small but discrete ranges, making it difficult for males to monopolize more than one female. Males may choose to form a pair to guard the female from rival males seeking to mate with her (6, 11). It has been argued that this was the route to social monogamy among small ungulates (12), and a similar suggestion has been used to explain monogamy in other mammals, including primates (6, 11). Finally, social monogamy might arise where the risks of infanticide are high and resident males can provide protection against infanticidal males (13–16). Where lactation is longer than gestation, females are expected to avoid suckling two infants of different ages simultaneously by delaying the return to oestrus after parturition. Where oestrus is delayed, it can pay a male, who is not the father, to kill an unweaned infant so that the female returns to oestrus sooner (17). There remains no consensus over which of the above hypotheses best explains monogamy in primates. Some researchers have proposed that a combination of explanations may be plausible (16), but others doubt whether it is possible to test between these hypotheses effectively or to infer the historical origin of social monogamy (18).
Social monogamy is more common in primates than in other mammalian orders, accounting for more than a quarter of species across all of the major primate clades (Dataset S1). Social monogamy in primates evolved directly from polygynandry and appeared relatively late (16 Mya) in primate history (19, 20). Interestingly, social monogamy appears to be a stable state; once monogamy evolves there are few transitions back into polygynous mating systems (19). Primates, therefore, represent an intriguing case for understanding the factors associated with the evolution of social monogamy.
Here we use likelihood-based phylogenetic comparative methods in a Bayesian framework (21) to examine each of the three hypotheses for the origin of social monogamy, testing for correlated evolution between mating systems and a key dichotomized marker trait for each hypothesis: paternal care, female ranging patterns, and male infanticide. Given previous disagreement about potential explanations for social monogamy, we predicted that there would be evidence for correlated evolution between social monogamy and each of the putative markers; however, our temporal discrete analyses can also identify the likely factors driving the switch to monogamy versus the responses following that switch. For discrete traits, we compare the fit of the dependent model of evolution between mating systems and these traits to a model in which the traits are constrained to evolve independently. In addition to correlated evolution, we use ancestral-state reconstructions and model rate parameters to examine whether certain traits preceded monogamy and whether they tended to make the appearance of monogamy more likely. For example, if a trait evolved after the emergence of social monogamy and is more likely to arise in the presence of monogamy, it could be seen as the result of pair-living. Conversely, if a trait is the key functional driver or sufficient condition for the evolution of monogamy, we expect its appearance to be rapidly and regularly followed by the emergence of monogamy.
Results
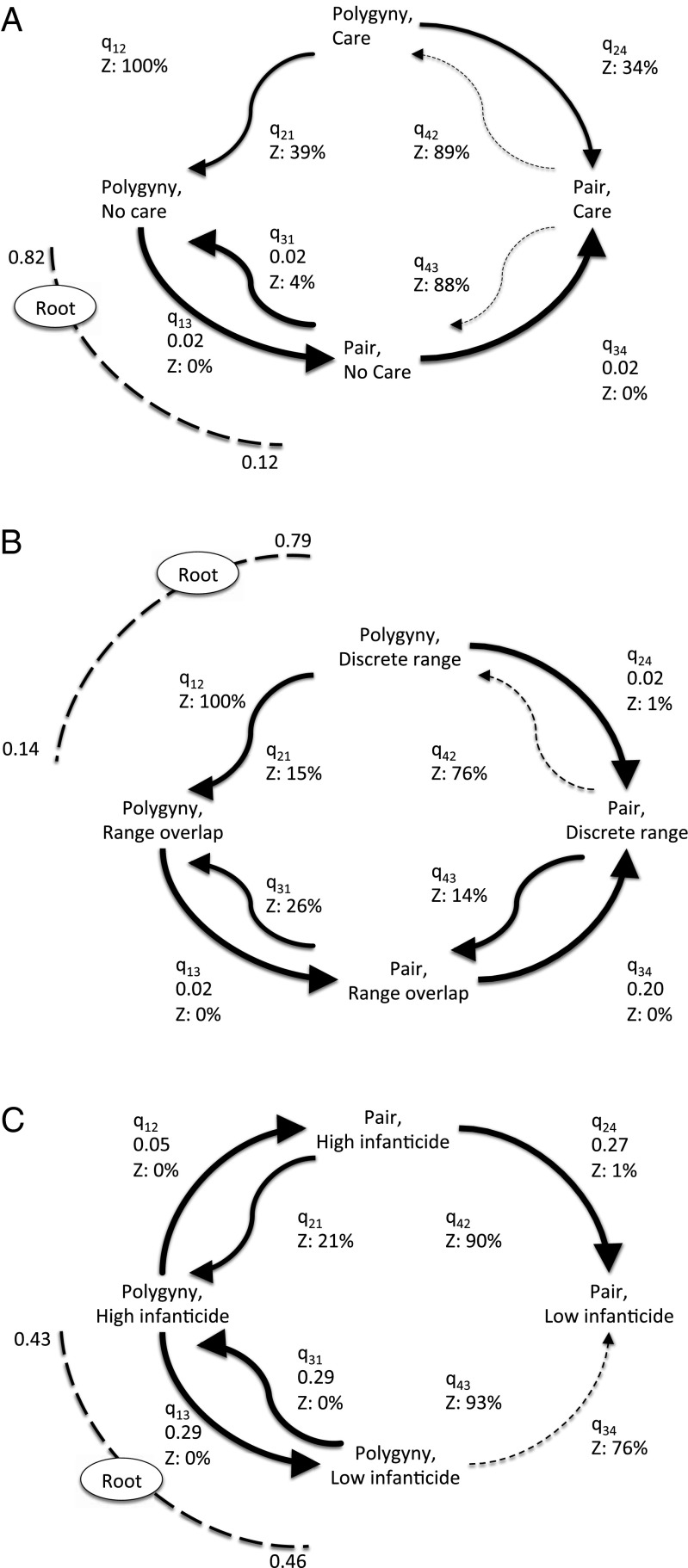
We find decisive support for correlated evolution between social monogamy and paternal care, female ranging patterns, and male infanticide (Table 1). This supports our intuition that these traits represent a suite of social behaviors linked to social monogamy. However, the demonstration of correlation between traits does not identify any direction of causality. The ancestral state reconstructions (SI Appendix and Figs. S4–S7) and model rates (Fig. 1) suggest that only male infanticide precedes the initial shift to social monogamy. Paternal care only evolves after a switch to social monogamy and not in polygynous mating systems (Fig. 1A). Moreover, once paternal care evolves within social monogamy it is unlikely to be lost. This finding suggests that biparental care was not a factor driving the shift to monogamy. Within a few lineages, discrete female ranges arose independently of social monogamy; however, a rapid subsequent switch to discrete ranges following the evolution of social monogamy suggests they might contribute to its maintenance, but were not a causal factor in its appearance (Fig. 1B).
Table 1.
Likelihoods for dependent and independent models of correlated evolution between mating system and other traits
| Coevolution analysis | Dependent model |
Independent model |
Log10 Bayes factor | ||
| Log likelihood | SE | Log likelihood | SE | ||
| Paternal care | −83.10 | ±0.02 | −0.92.82 | ±0.05 | 4.22 |
| Discrete female ranges | −95.60 | ±0.03 | −119.40 | ±0.02 | 10.34 |
| Infanticide | −163.55 | ±0.04 | −174.55 | ±0.04 | 4.78 |
The log10 Bayes factor indicates the relative support for the dependent model over the independent model. Values <1 suggest little support, 1–2 strong support and >2 decisive support for the dependent over the independent model (54).
Fig. 1.
Coevolution between primate mating system and: (A) paternal care, (B) discrete female ranges, and (C) reported infanticide. The ancestral state reconstruction is depicted by dashed lines, which includes the proportion of the posterior distribution for alternative states. Z denotes visits assigned to zero as a proportion of the posterior probability distribution. Thickness of arrows reflects proportion of time transition rate is not assigned to zero, with dashed line >50% zeros and no line ≥90% zeros. Number below rate name (qij) is the mean transition rate where rate distribution has zero or very low z value.
In contrast, as well as strong correlated evolution between male infanticide and mating systems, male infanticide precedes the switch to social monogamy (Fig. 1C and Table 1). First, there is little support for a transition from polygyny to monogamy with low infanticide; social monogamy is inferred to have been far more likely to evolve from polygyny in the presence of high infanticide. Second, once social monogamy evolves there is a high probability of a subsequent reduction in male infanticide and a smaller probability of transitions back to polygyny with infanticide remaining high. Taken together, these data suggest that social monogamy with high infanticide is an unstable state, whereas social monogamy with low infanticide is a very stable one.
In addition, socially monogamous species have lower risk of infanticide, in terms of the proportion of the breeding cycle devoted to lactation (the “weaning proportion”), than do polygynous species (Table 2). This finding hints at one mechanism by which social monogamy may reduce infanticide risk: social monogamy facilitates a shorter lactation period compared with gestation, thereby reducing infanticide risk. There was a strong reduction in the weaning proportion with the emergence of social monogamy in most clades (SI Appendix and Fig. S8). Furthermore, a Markov Chain Monte Carlo (MCMC) phylogenetic t test of paternal care with weaning proportion shows that species without paternal care have a significantly higher weaning proportion than those with paternal care (Table 2).
Table 2.
Phylogenetic t test of mating system and weaning proportion and paternal care and weaning proportion using MCMC methods in BayesTraits
| Model | β | SE β | P value |
| Weaning proportion and mating system | 0.01 | 0.00 | 0.044 |
| Weaning proportion and paternal care | 0.16 | 0.05 | <0.001 |
Discussion
Our results uncover the evolutionary relationship between social monogamy and its hypothesized causes. The evolution of both discrete female ranges and paternal care followed the shift to social monogamy rather than preceded it. Thus, there is little evidence to suggest that discrete female ranges are the cause of social monogamy in primates (contra refs. 6 and 11).
Similarly, although paternal care and social monogamy showed strong correlated evolution, paternal care (as seen in the callitrichids and Aotus) followed the appearance of social monogamy (15, 22). Biparental care in birds also appears to be a secondary adaptation following pair formation (23). In primates, paternal care is associated with a shortening of interbirth intervals and an increase in reproductive rates similar to that seen in birds and other mammals (2, 7, 15, 24). In socially monogamous primate species, such as Aotus and the callitrichids, paternal care shortens the lactation period, presumably because females can increase the resources devoted to lactation when relieved of some of the costs of infant care. Although this behavior can make monogamy more profitable (15), it appears not to be the cause of monogamy.
Of the traits we tested, high male infanticide alone consistently preceded the appearance of social monogamy across primates. Our analyses suggest that socially monogamous species are much more likely to have low male infanticide rates (Fig. 1C), presumably as social monogamy provides an effective counter-strategy. Social monogamy can reduce the incidence of infanticide because one or both pair-members can defend infants (13, 15). Shortened lactation time associated with biparental care may, however, play a particularly important role by hastening oestrus resumption, and so further mitigating infanticide risk (25). Both partners may, therefore, initially benefit from male protection of unweaned infants and in some species there is a secondary benefit of increasing reproductive rates via biparental care (15).
This association between infanticide and social monogamy raises the question of why more primate species are not monogamous. This question becomes particularly relevant when considering the extraordinarily high rates of infanticide in species such as gorillas and langurs, where it has been estimated that infanticide accounts for between 34% (in gorillas, Gorilla gorilla beringei) and 64% (in langurs, Semnopithecus entellus) of all infant deaths (26, 27). One would expect a strong pressure for these species to opt for social monogamy if it is an effective defense against infanticide. However, a switch to social monogamy may only be possible where ecological conditions permit. Other factors play a role in determining optimal grouping patterns: predation pressure drives up group size (28, 29) and resource distribution and habitat use also impact on group size and structure (30, 31). Hanuman langurs and gorillas incur high levels of mortality from infanticide but also live in a habitat with high predation risk. The pressure to maintain cohesive social groups as an antipredator defense may render social monogamy a nonstarter.
Nevertheless, these results could explain why social monogamy is more prevalent among primates than in other mammalian orders. Complex sociality in primates is associated with large brains (32), which in turn is associated with altricial young and long development and lactation periods (25, 33). This extended dependency period increases the time unweaned infants are vulnerable to male infanticide (34). However, social monogamy can help overcome the gray ceiling imposed by the high metabolic and developmental costs of large brains through male care (24), and allow for slow reproductive rates (35) without increased infanticide risk. Encephalization in great apes (and especially humans) has led to very long lactation periods and heightened risk of infanticide (as measured by the weaning proportion). Chimpanzees deal with the infanticide risk imposed by this extended dependency period with a polygynandrous mating system: males defend females and infants within their territory (36), and females ensure paternity confusion through multiple mating with community males (37). Promiscuous mating in orangutans has also been suggested as an anti-infanticide strategy (38). For gorillas, although polygyny ensures paternity certainty for the group male, it also results in the highest infanticide rates seen among apes (37). Human pair bonds may also be a response to the pressure that long infant dependency places on females to find effective protection for their young (39). Indeed, a recent reconstruction of ancestral mating/marriage systems in humans suggests that Australopithecines (40, 41) and early modern humans (42) may have been (at least facultatively) monogamous. The transition to social monogamy in humans has been proposed to depend on females choosing to stay faithful to males, even when of lower quality (43). Once in place these pair-bonds would facilitate paternal care in the form of male protection and provisioning (44, 45). Male infanticide could thus have been the pressure that drove females as well as males to stay in long-term consortships or bonds (35).
Methods
Primate Phylogeny.
Analyses were conducted on a Bayesian posterior distribution of 10,000 phylogenies based on mitochondrial and autosomal genes for 230 primate species [version 2 of the 10kTrees Project (46)]. A large posterior sample allowed us to infer the evolutionary history of traits while accounting for phylogenetic uncertainty (46). To display ancestral character traits, we inferred a maximum-clade credibility tree from the complete 10kTrees sample (46) using TreeAnnotator (47) with nodes dated using median molecular branch lengths and six known fossil calibration points.
Primate Trait Data.
Primate trait data were extracted from the literature and from primary sources (SI Appendix and Dataset S1). Levels of allocare vary widely across primate species; we define paternal care as occurring only where males provide care for at least 30% of infant time (48). We use two indices of infanticide pressure. First, we collated data on actual cases of infanticide for wild populations; we only included cases where the infanticide was substantiated either by direct observation or by the exclusion of other possibilities. It may be that infanticide is affected by sampling effort, such that it is more likely to have been observed in well-studies species, and may have been missed in less well-studied ones. For example, it is only after extensive fieldwork that infanticide has been suggested to take place following male absence in Hylobates lar (49). We accounted for possible sampling issues in several ways. First, we only included species that had at least 20 publications. Second, we classified infanticide rates as low or high, so as to allow for the possibility that low levels of infanticide are occurring in species where it has not been documented. Third, we demonstrate that systematic sampling biases could not account for the observed patterns (SI Appendix). Fourth, we used an independent index of infanticide risk that is not open to such bias (the proportion of the breeding cycle, defined as Gestation + Lactation, devoted to lactation) (17). To avoid the issues of nonnormally distributed data associated with ratios, we converted these data to a measure of the proportion of the breeding cycle taken up by lactation, hereafter termed “weaning proportion” [L/(L+G)], which was arc sine-transformed for analysis. Seasonal breeders with a reproductive “time-out” period were excluded, as a weaning proportion value above 50% would falsely suggest that infanticide would bring a female back into oestrus sooner, and thereby improve mating opportunities for males.
Analyses.
Modeling trait evolution.
We used a likelihood framework and Bayesian inference to model the evolution of traits along the branches of the primate phylogeny. Following Shultz, Opie, and Atkinson (20), analyses were carried out in BayesTraits (50, 51) using an MCMC (52) sampling algorithm together with a reversible jump (RJ) procedure. Rather than fitting a model in which all changes are possible, the RJ procedure searches the posterior distribution of possible models by linking (setting to equal) or removing (setting to zero) transition-rate parameters. Models are then sampled in proportion to their likelihood, accounting for variation in the number of parameters. This process allows us to explore the space of possible models and derive a Bayesian posterior distribution of model log-likelihoods, rate parameters, and inferred ancestral states on the primate phylogeny.
Maximum-likelihood analysis of the data, which gives point estimates of model parameters, indicated a small number of changes per unit of branch length such that the prior distribution on rates could be described by an exponential probability distribution. The prior was seeded from an exponential hyperprior with a mean and variance in the range of 0–2 (50). The rate deviation value, which sets the amount that the rate parameters of the model change in each iteration of the Markov chain, was varied to ensure that acceptance rates were between 15% and 40% (50). Convergence was checked visually by evaluating changes in the log-likelihood in Tracer (53). Each MCMC chain was run five times for 5 million iterations sampled every 100, with the first 50,000 iterations discarded as the burn-in period, to ensure that convergence had been reached. The posterior probabilities for the transition models, rate parameters, log-likelihoods, and states at ancestral nodes from the run with the median likelihood taken from the postconvergence portion of each run are reported.
Ancestral states.
We used an RJ MCMC approach within the Multistate procedure in BayesTraits (50, 51) to infer states at ancestral nodes for each trait (SI Appendix and Figs. S4–S7). Although these results are drawn on the maximum-clade credibility tree, the analysis was performed across the posterior distribution of 10,000 primate trees. The ancestral state probabilities for each node of the tree (the colored pies in SI Appendix and Figs. S4–S7) are the combined posterior probability of each state at that node with the posterior probability that the node itself exists.
Correlated evolution between social monogamy and other traits.
The Discrete option in BayesTraits can be used to test for the correlated evolution of two binary traits over a phylogeny. We coded mating system as a binary variable-polygyny (0) (including both uni-male/multifemale and multimale/multifemale systems) versus social monogamy (1), and tested for evidence of correlated evolution with the other binary traits (Table 1). Evidence for correlated evolution was measured using a Bayes factor (54) comparing model fit between a model in which the traits are allowed to evolve independently (the independent model) to one in which rates of change in one trait are dependent on rates in the other (the dependent model). The independent model can be rejected if there is support for the dependent model, indicating correlated evolution between the mating system and the chosen trait. For comparisons between models we calculated the log10 Bayes factor, generated with Tracer (53). The log10 Bayes factor shows the weight of evidence to support one model over another, from 0–0.5 (insubstantial), to 0.5–1.0 (substantial), to 1.0–2.0 (strong), to >2.0 (decisive) (54). We also tested the relative timing of the evolution of the traits by comparing the transition rates between states (Fig. 1), and the switches inferred from the ancestral state reconstructions (SI Appendix and Figs. S4–S7).
A random-walk MCMC procedure in BayesTraits Continuous (50, 55) was used to infer the ancestral states of the continuous trait weaning proportion (representing infanticide risk) across the primate phylogeny, which was then plotted onto the maximum-clade probability primate tree (SI Appendix and Fig. S8). Phylogenetic t tests were run using Continuous to test for correlated evolution between the binary mating system trait and weaning proportion, and paternal care and weaning proportion, in a Bayesian framework using MCMC methods, where the percentage of the posterior of the regression coefficient that crosses zero indicates the P value (55).
Supplementary Material
Acknowledgments
This work was supported in part by a European Research Council Advanced grant (to C.O.); a Rutherford Discovery Fellowship (to Q.D.A.); a European Research Council Advanced grant (to R.I.M.D.); and a Royal Society University Research Fellowship (to S.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307903110/-/DCSupplemental.
References
- 1.Lack D. Ecological Adaptations for Breeding in Birds. London: Methuen & Co; 1968. [Google Scholar]
- 2.Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 3.Orians GH. On the evolution of mating systems in birds and mammals. Am Nat. 1969;103(934):589. [Google Scholar]
- 4.Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav. 1980;28(4):1140–1162. [Google Scholar]
- 5.Dobson FS. Competition for mates and predominant juvenile male dispersal in mammals. Anim Behav. 1982;30(4):1183–1192. [Google Scholar]
- 6.Brotherton PNM, Komers PE. Mate guarding and the evolution of social monogamy in mammals. In: Reichard UH, Boesch C, editors. Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals. Cambridge, UK: Cambridge Univ Press; 2003. pp. 42–58. [Google Scholar]
- 7.Clutton-Brock TH, Harvey PH. Primate ecology and social organization. J Zool. 1977;183(1):1–39. [Google Scholar]
- 8.Wynne-Edwards KE, Lisk RD. Differential effects of paternal presence on pup survival in two species of dwarf hamster (Phodopus sungorus and Phodopus campbelli) Physiol Behav. 1989;45(3):465–469. doi: 10.1016/0031-9384(89)90059-0. [DOI] [PubMed] [Google Scholar]
- 9.Ribble D. The evolution of social and reproductive monogamy in Peromyscus: Evidence from Peromyscus californicus (the California mouse) In: Reichard UH, Boesch C, editors. Monogamy: Mating Strategies and Partnerships in Birds, Humans, and Other Mammals. Cambridge, UK: Cambridge Univ Press; 2003. pp. 161–166. [Google Scholar]
- 10.Clutton-Brock TH. Mammalian mating systems. Proc R Soc Lond B Biol Sci. 1989;236(1285):339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- 11.Komers PE, Brotherton PNM. Female space use is the best predictor of monogamy in mammals. Proc Biol Sci. 1997;264(1386):1261–1270. doi: 10.1098/rspb.1997.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brotherton PNM, Manser MB. Female dispersion and the evolution of monogamy in the dik-dik. Anim Behav. 1997;54(6):1413–1424. doi: 10.1006/anbe.1997.0551. [DOI] [PubMed] [Google Scholar]
- 13.van Schaik CP, Dunbar RIM. The evolution of monogamy in large primates: A new hypothesis and some crucial tests. Behaviour. 1990;115(1-2):30–61. [Google Scholar]
- 14.van Schaik CP, Kappeler PM. Infanticide risk and the evolution of male-female association in primates. Proc Biol Sci. 1997;264(1388):1687–1694. doi: 10.1098/rspb.1997.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunbar RIM. The mating system of callitrichid primates: I. Conditions for the coevolution of pair bonding and twinning. Anim Behav. 1995;50(4):1057–1070. [Google Scholar]
- 16.Palombit RA. Infanticide and the evolution of pair bonds in nonhuman primates. Evol Anthropol. 1999;7(4):117–129. [Google Scholar]
- 17.van Schaik CP. Social counterstrategies against infanticide by males in primates and other mammals. In: Kappeler P, editor. Primate Males: Causes and Consequences of Variation in Group Composition. Cambridge, UK: Cambridge Univ Press; 2000. pp. 34–52. [Google Scholar]
- 18.van Schaik CP, Kappeler PM. The evolution of social monogamy in primates. In: Reichard UH, Boesch C, editors. Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals. Cambridge, UK: Cambridge Univ Press; 2003. pp. 59–80. [Google Scholar]
- 19.Opie C, Atkinson QD, Shultz S. The evolutionary history of primate mating systems. Commun Integr Biol. 2012;5(5):458–461. doi: 10.4161/cib.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shultz S, Opie C, Atkinson QD. Stepwise evolution of stable sociality in primates. Nature. 2011;479(7372):219–222. doi: 10.1038/nature10601. [DOI] [PubMed] [Google Scholar]
- 21.Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294(5550):2310–2314. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- 22.Dunbar RIM. The mating system of callitrichid primates: II. The impact of helpers. Anim Behav. 1995;50(4):1071–1089. [Google Scholar]
- 23.Shultz S, Dunbar RIM. Social bonds in birds are associated with brain size and contingent on the correlated evolution of life-history and increased parental investment. Biol J Linn Soc Lond. 2010;100(1):111–123. [Google Scholar]
- 24.Isler K, Van Schaik CP. Why are there so few smart mammals (but so many smart birds)? Biol Lett. 2009;5(1):125–129. doi: 10.1098/rsbl.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee PC. Comparative ecology of postnatal growth and weaning among haplorhine primates. In: Lee PC, editor. Comparative Primate Socioecology. Cambridge, UK: Cambridge Univ Press; 1999. pp. 111–139. [Google Scholar]
- 26.Sommer V. Infanticide among the langurs of Jodhpur: Testing the sexual selection hypothesis with a long-term record. In: Parmigiani S, vom Saal F, editors. Infanticide and Parental Care. Reading, UK: Harwood; 1994. pp. 155–198. [Google Scholar]
- 27.Watts DP. Infanticide in mountain gorillas: New cases and a reconsideration of the evidence. Ethology. 1989;81(1):1–18. [Google Scholar]
- 28.Shultz S, Noë R, McGraw WS, Dunbar RIM. A community-level evaluation of the impact of prey behavioural and ecological characteristics on predator diet composition. Proc Biol Sci. 2004;271(1540):725–732. doi: 10.1098/rspb.2003.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Schaik CP. Why are diurnal primates living in groups? Behaviour. 1983;87(1-2):120–144. [Google Scholar]
- 30.Isbell LA. Contest and scramble competition: Patterns of female aggression and ranging behavior among primates. Behav Ecol. 1991;2(2):143–155. [Google Scholar]
- 31.Sterck EHM, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol. 1997;41(5):291–309. [Google Scholar]
- 32.Shultz S, Dunbar RIM. The evolution of the social brain: Anthropoid primates contrast with other vertebrates. Proc Biol Sci. 2007;274(1624):2429–2436. doi: 10.1098/rspb.2007.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evol Anthropol. 1993;1(6):191–194. [Google Scholar]
- 34.van Schaik CP. Vulnerability to infanticide by males: Patterns among mammals. In: van Schaik CP, Janson CH, editors. Infanticide by Males and its Implications. Cambridge, UK: Cambridge Univ Press; 2000. [Google Scholar]
- 35.Dunbar RIM, Shultz S. Understanding primate brain evolution. Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):649–658. doi: 10.1098/rstb.2006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams JM, Oehlert GW, Carlis JV, Pusey AE. Why do male chimpanzees defend a group range? Anim Behav. 2004;68(3):523–532. [Google Scholar]
- 37.Harcourt AH, Greenberg J. Do gorilla females join males to avoid infanticide? A quantitative model. Anim Behav. 2001;62(5):905–915. [Google Scholar]
- 38.Knott CD, Emery Thompson M, Stumpf RM, McIntyre MH. Female reproductive strategies in orangutans, evidence for female choice and counterstrategies to infanticide in a species with frequent sexual coercion. Proc Biol Sci. 2010;277(1678):105–113. doi: 10.1098/rspb.2009.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunbar RIM. The social brain hypothesis and its implications for social evolution. Ann Hum Biol. 2009;36(5):562–572. doi: 10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- 40.Nelson E, Rolian C, Cashmore L, Shultz S. Digit ratios predict polygyny in early apes, Ardipithecus, Neanderthals and early modern humans but not in Australopithecus. Proc Biol Sci. 2011;278(1711):1556–1563. doi: 10.1098/rspb.2010.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reno PL, Meindl RS, McCollum MA, Lovejoy CO. Sexual dimorphism in Australopithecus afarensis was similar to that of modern humans. Proc Natl Acad Sci USA. 2003;100(16):9404–9409. doi: 10.1073/pnas.1133180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker RS, Hill KR, Flinn MV, Ellsworth RM. Evolutionary history of hunter-gatherer marriage practices. PLoS ONE. 2011;6(4):e19066. doi: 10.1371/journal.pone.0019066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavrilets S. Human origins and the transition from promiscuity to pair-bonding. Proc Natl Acad Sci USA. 2012;109(25):9923–9928. doi: 10.1073/pnas.1200717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lovejoy CO. Reexamining human origins in light of Ardipithecus ramidus. Science. 2009;326(5949):e1–e8. [PubMed] [Google Scholar]
- 45.Lovejoy CO. The origin of man. Science. 1981;211(4480):341–350. doi: 10.1126/science.211.4480.341. [DOI] [PubMed] [Google Scholar]
- 46.Arnold C, Matthews LJ, Nunn CL. The 10kTrees website: A new online resource for primate phylogeny. Evol Anthropol. 2010;19(3):114–118. [Google Scholar]
- 47.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7(1):214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross C, MacLarnon A. The evolution of non-maternal care in anthropoid primates: A test of the hypotheses. Folia Primatol (Basel) 2000;71(1-2):93–113. doi: 10.1159/000021733. [DOI] [PubMed] [Google Scholar]
- 49.Borries C, Savini T, Koenig A. Social monogamy and the threat of infanticide in larger mammals. Behav Ecol Sociobiol. 2011;65(4):685–693. [Google Scholar]
- 50.Pagel MD, Meade A, Barker D. Bayesian estimation of ancestral character states on phylogenies. Syst Biol. 2004;53(5):673–684. doi: 10.1080/10635150490522232. [DOI] [PubMed] [Google Scholar]
- 51.Pagel MD, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am Nat. 2006;167(6):808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- 52.Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. J Chem Phys. 1953;21(6):1087. [Google Scholar]
- 53. Rambaut A, Drummond A (2007) Tracer v1.4. Available from http://beast.bio.ed.ac.uk/Tracer. Accessed July 25, 2012.
- 54.Kass RE, Raftery AE. Bayes factors. J Am Stat Assoc. 1995;90(430):773–795. [Google Scholar]
- 55.Organ CL, Shedlock AM, Meade A, Pagel M, Edwards SV. Origin of avian genome size and structure in non-avian dinosaurs. Nature. 2007;446(7132):180–184. doi: 10.1038/nature05621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.