Abstract
Since the days of Elton, population cycles have challenged ecologists and resource managers. Although the underlying mechanisms remain debated, theory holds that both density-dependent and density-independent processes shape the dynamics. One striking example is the large-scale fluctuations of sardine and anchovy observed across the major upwelling areas of the world. Despite a long history of research, the causes of these fluctuations remain unresolved and heavily debated, with significant implications for fisheries management. We here model the underlying causes of these fluctuations, using the California Current Ecosystem as a case study, and show that the dynamics, accurately reproduced since A.D. 1661 onward, are explained by interacting density-dependent processes (i.e., through species-specific life-history traits) and climate forcing. Furthermore, we demonstrate how fishing modifies the dynamics and show that the sardine collapse of the 1950s was largely unavoidable given poor recruitment conditions. Our approach provides unique insight into the origin of sardine–anchovy fluctuations and a knowledge base for sustainable fisheries management in the California Current Ecosystem and beyond.
Keywords: species replacement, population modeling, climate change, ecosystem-based management
Marine fish typically show multidecadal fluctuations in abundance, mainly attributed to overexploitation (1), climate (2), or a combination of both (3). Furthermore, such low-frequency variability may arise from density-dependent processes (i.e., cohort-resonance) potentially masking external or anthropogenic effects (4), whereas fishing-induced demographic changes increase short-term variability (5–7). To understand these fluctuations, it is essential to disentangle anthropogenic forcing from internal processes, as well as understanding the way they interact (7). One of the most striking examples of population fluctuations is the alternating regimes of sardine and anchovy observed across the major upwelling areas of the world (8, 9). Although ocean–atmosphere forcing is considered the main underlying driver (8–11), no generally accepted theory regarding sardine–anchovy fluctuations presently exists (12). Nevertheless, insight accumulated over a long history of research highlights that the key to understanding population cycles in general, and the sardine–anchovy puzzle in particular, lies not in identifying a single factor but a combination of interacting factors (12–14).
In the California Current Ecosystem (CCE), the Pacific sardine (Sardinops sagax) and northern anchovy (Engraulis mordax) exhibit pronounced fluctuations, spanning several orders of magnitude in terms of biomass (15, 16). These fluctuations, occurring with a dominant periodicity of ∼60 y (15, 16), have been linked to changes in the strength and position of the Aleutian Low, as reflected by the Pacific Decadal Oscillation (PDO) index (17, 18), and correlated with patterns of flow, upwelling, and physical/biotic conditions (10, 12, 19). Furthermore, fishing has been suggested to affect the dynamics [e.g., the rate of collapse of the Pacific sardine in the early 1950s (20)], whereas interspecific competition is considered unsubstantiated (12), owing to the lack of negative correlation between sardine and anchovy in sediment records (15, 16), no significant causal association between sardine and anchovy landings (11), and marginal niche overlap in terms of habitat and prey preferences (19, 21). Without accounting for potential synergies of external and anthropogenic factors or how internal processes modify the response to climate and fishing, our understanding of sardine–anchovy fluctuations, as well as prospects for sustainable management in the face of climate change (22), are limited. To address these issues, we here investigate the underlying causes of the sardine–anchovy fluctuations in the CCE using a model setup (Fig. 1A and SI Appendix) consisting of age-structured population models (23, 24) based on numbers (sardine) or biomass (anchovy), environmentally sensitive stock-recruitment (S-R) models (25–27), and a climate module capable of simulating the natural (red-shifted) variability of the marine environment (28, 29).
Fig. 1.
Model setup, validation, and simulation. (A) A schematic description of model setup, consisting of cohort models using numbers (N) or biomass (B), S-R models with resampled noise (ε), and a climate module simulating SST. (B) Model validation by hindcasting sardine (black) and anchovy (gray) SSB from 1935 onward, as well as from 1661 onward (C), compared with SDRs (circles). Correlations between estimated SSB and observed SDR: r = 0.52, P = 0.004 (sardine) and r = 0.85, P < 0.001 (anchovy). Solid and dashed lines indicate simulations with 95% confidence intervals and circles observations. (D) A single run forced by simulated SST (red; 5-y running mean).
Results and Discussion
According to previous knowledge of recruitment dynamics (25–27), the fitted S-R models demonstrate a high degree of explained variance (77.2% and 70.9% for sardine and anchovy, respectively) (SI Appendix, Tables S3 and S4). In addition, a cross-validation routine (SI Appendix) shows high R2 values when fitted to a random subset of the data and accuracy in predicting the remaining data (SI Appendix, Fig. S1). Furthermore, model residuals were normally distributed and temporally uncorrelated (SI Appendix, Fig. S2).The functional relationships between recruitment (R) and spawning stock biomass (SSB) were represented by dome-shaped curves (SI Appendix, Fig. S3 A and B), illustrating density-dependent regulation due to resource competition at high stock levels (25–27). The climate effect showed a positive (curvilinear) and negative (linear) relationship (SI Appendix, Fig. S3 C and D), reflecting opposite responses of sardine and anchovy recruitment to sea surface temperature (SST) (8–11). In the case of sardine recruitment, the slight nonlinear relationship may represent a dome-shaped response (30), but with a downward slope at much higher temperatures than observed in the SST time-series used during model fitting. Although temperature influences key physiological processes (e.g., growth and maturation), the opposite response to SST is likely mediated by changes in thermal stratification, mixing, upwelling, or associated alterations in habitat availability and zooplankton composition (12, 19, 21). The final models recreate the long-term and interannual variability in recruitment, with the observed estimates well within the confidence intervals (SI Appendix, Fig. S3 E and F). As illustrated by a number of validation exercises (SI Appendix), our model setup was able to accurately hindcast the population dynamics throughout the 20th century (Fig. 1B), including the collapse and subsequent recovery of the sardine (during the 1950s and 1980s), as well as periods covered by stock assessments (SI Appendix, Fig. S4). More strikingly, the model was able to recreate the sardine–anchovy fluctuations over the past ∼350 y (Fig. 1C), as inferred from fish scale deposition rates (SDRs) (15, 16), when forced by a paleo-climatic reconstruction of the PDO from A.D. 1661 onward (31) (SI Appendix). Furthermore, a frequency analysis comparing the Fourier spectra of simulations with observed time scales of variability from SDRs (15) confirms the occurrence of 50- to 100-y cycles for both species, with dominant periodicities of ∼60 and ∼80 y for anchovy and sardine, respectively (SI Appendix, Fig. S5).
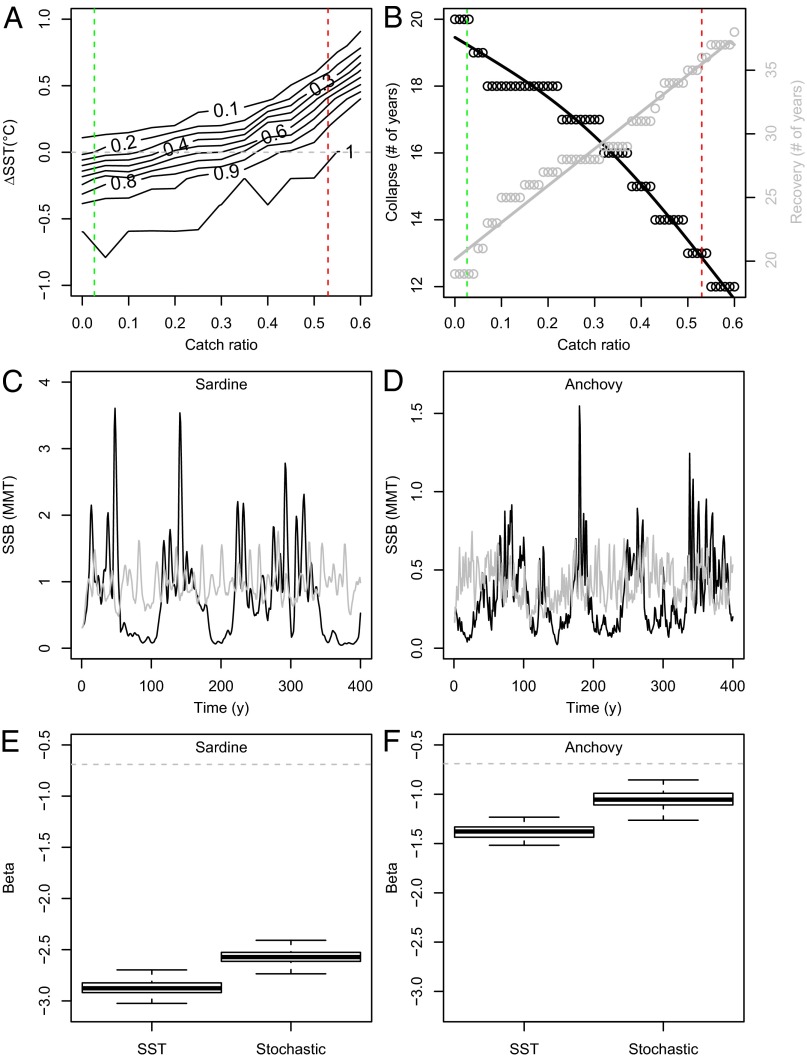
To examine the relative importance and potential synergy of external and internal processes on population regulation and provide insight into the ongoing debate of climate vs. fishing effects, particularly regarding the Pacific sardine (32, 33), the validated model was used to explore whether, given the present knowledge on climate forcing and density-dependent regulation, the sardine stock could in hindsight have been managed to avoid the drastic collapse in the early 1950s. We performed multiple stochastic simulations and estimated the probability of collapse as the percentage of simulations in which SSB falls below 0.09 million metric tons (MMT) for each combination of SST (i.e., ±1 °C of observed SST) and exploitation (i.e., annual catch ratios ranging from 0 to 60% of SSB). This threshold was based on the probability density function of sardine SSB after multiple long-term simulations without fishing (e.g., Fig. 1D) and defined as the peak of the lower mode of the bimodal distribution (SI Appendix, Fig. S6). Hence, the threshold represents the predominant minimum SSB in the absence of fishing below which the stock is considered as collapsed. The simulations were initialized at the mean SSB before the collapse (1942–1947), run for 20 y, and replicated 1,000 times for each combination of climate and fishing. Our results show that the stock collapse was largely unavoidable regardless of exploitation level, but especially given extreme catch ratios (e.g., 54% occurred before the collapse), resulting in a ∼100% risk of decline below the biomass limit (Fig. 2A). Although lower catch ratios would not eliminate the probability of collapse (i.e., ∼20% risk at zero catch), reducing exploitation would markedly affect the rate of decline (20) (i.e., delay the stock collapse and accelerate its subsequent recovery; Fig. 2B).
Fig. 2.
Climate, fishing, and density-dependent effects. (A) Probability of sardine collapse and (B) the mean number of years until collapse and subsequent recovery above 0.09 MMT for each combination of SST (±1 °C change relative to observed SST; Fig. 1B) and catch ratios. Dashed lines show the observed SST (gray), maximum catch ratios (red) before the collapse, and mean catch ratios in the 1980s (green). Single runs of SSB and boxplots of β (after 1,000 runs) for sardine (C and E) and anchovy (D and F) when forced by SST (black) or by Gaussian noise alone (gray). Gray dashed lines indicate β of the input SST time series.
Note that if competition is erroneously introduced into the model [i.e., by allowing growth and juvenile mortality to depend on the SSB of the competing species (SI Appendix)], the previously proposed reduction fishery on anchovy (34) would have proved a misguided management strategy (12), not promoting sardine recovery but causing a marked decline of anchovy as well (SI Appendix, Fig. S7). Such a decline would potentially have impaired the main pathway of energy flow to higher trophic levels (e.g., tunas, marine mammals, and birds) and induced drastic changes in the structure and functioning of this wasp-waist ecosystem (35). Hence, the success of ecosystem-based management strategies (36) hinges on a thorough understanding of the complex interactions of multiple external and internal processes impacting ecosystems at large, and population dynamics in particular.
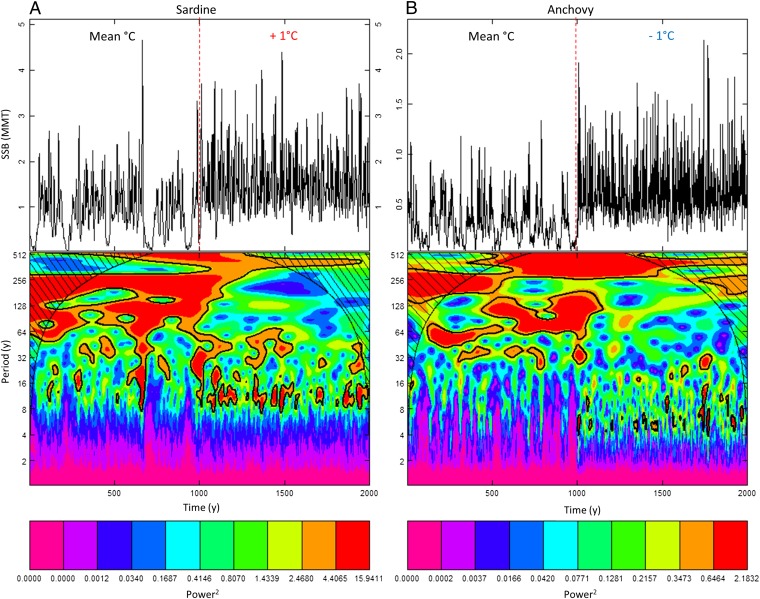
We further disentangled density-dependent and density-independent effects regulating the sardine–anchovy dynamics by performing multiple simulations forced either by climate (SST) or Gaussian noise alone (resampled from the residuals of the S-R models) and without fishing. As a measure of population variability we estimated the spectral exponent (β), the slope of the power spectrum of SSB in log coordinates (7, 27), for each species and run. Our results show pronounced low-frequency variability, whether forced by red-shifted (climate) or white noise, caused by cohort-resonance of the recruitment signal (Fig. 2 C and D). The longer fluctuations of sardine (increasingly negative β) compared with anchovy (Fig. 2 E and F) are due to differences in life-history traits (e.g., slower growth, later maturation, lower natural mortality; SI Appendix), as well as the degree of density-dependence in the S-R relationship (SI Appendix, Fig. S3 A and B). To visualize the effect of density-dependence, we simulated long-term population dynamics under mean and favorable SST regimes for recruitment (i.e., +1 °C for sardine and −1 °C for anchovy; Fig. 3). These simulations illustrate that during mean SST lower-frequency climate-induced variability dominates, whereas during favorable SST conditions (and high SSB) shorter periods of ∼10 and ∼5 y are induced through density-dependent feedbacks, corresponding to the life span of sardine and anchovy, respectively. Note that shorter-term variability is superimposed on the longer-term cycles, not only in our simulations (e.g., Fig. 1D) but in the observed population dynamics throughout the 20th century (e.g., the high sardine SSBs during the 1930s and 2000s; Fig. 1B). This highlights how the interplay and shifting importance of different sources of variability across time scales [i.e., stochastic recruitment processes (interannual), cohort-resonance (mainly decadal), and red-shifted climate forcing (multidecadal)] combine to create the sardine–anchovy fluctuations.
Fig. 3.
Long-term population dynamics in response to temperature changes. A wavelet plot of simulated sardine (A) and anchovy (B) dynamics forced by mean and favorable SST regimes (i.e., an increase and decrease by 1 °C for sardine and anchovy, respectively, during the last 1,000 y). Black contour lines indicate areas of significance, as well as the cone of influence (dashed) within which edge effects are present and estimated periodicities uncertain.
Finally, we illustrate potential management implications by investigating how synergistic effects of climate and fishing influence population variability (5–7). We estimated the mean value of β (from SSB) for each combination of SST (i.e., ±2 °C of mean SST, reflecting the range of SST) and exploitation [i.e., annual fishing mortalities (F) ranging from 0 to 1] after 1,000 stochastic runs. As a proxy for the strength of density-dependence and the degree of demographic truncation, we derived the mean correlation coefficient between SSB and productivity [i.e., ln(R/SSB)] and the (weighted) mean age-of-spawner, respectively. We show that both climate and fishing alter the variability of sardine and anchovy dynamics (Fig. 4 A and B), but through different internal processes. Climate primarily induce long-term variability (e.g., Fig. 2 C and D) but may induce short-term fluctuations through increasing density-dependent feedbacks at high stock size, illustrated by increasingly negative correlation between SSB and productivity during favorable SST regimes for sardine (warm) and anchovy (cold) (Fig. 4 C and D). Fishing-induced demographic changes, illustrated by decreasing mean age-of-spawner (Fig. 4 E and F), may not only reduce the harvestable stock but induce high-frequency (stochastic) variability, generally considered as undesirable from both a management and conservation viewpoint (7, 37).
Fig. 4.
Causes of variability. (A and B) The population variability (β), (C and D) correlation (r) between SSB and ln(R/SSB), and (E and F) weighted mean age-of-spawner (Am) for sardine (Left) and anchovy (Right) in response to climate (i.e., increase and decrease in mean SST by 2 °C) and exploitation [fishing mortality (F) from 0 to 1].
We have shown how climate variability and density-dependent processes, mediated through species-specific life-history traits, interact and give rise to the sardine–anchovy fluctuations in the CCE, and how fishing may modify the range and rate of population change. Hence, our approach provides unique insight into the synergy and underlying factors of the sardine–anchovy puzzle and a knowledge base for sustainable management of these important marine resources. We suggest that our approach, accurately reproducing the dynamics in the CCE over the past ∼350 y, combined with complementary modeling (11) and meta-analytical techniques (12), could provide an appropriate framework to further unravel the origin of the apparent synchrony and teleconnections among sardine and anchovy populations worldwide (38).
Materials and Methods
To investigate and simulate the population dynamics of Pacific sardine and northern anchovy, we developed and applied a model setup (Fig. 1A and SI Appendix) consisting of (i) age-structured cohort models (23, 24) based on numbers-at-age (sardine) or biomass-at-age (anchovy); (ii) environmentally sensitive S-R models (25–27); and (iii) a 1/f β climate module (28, 29) capable of generating surrogate climate time series accurately resembling the natural (red-shifted) variability of the observed SST time series used during S-R model fitting. The population dynamic simulations were performed by estimating R (i.e., based on the S-R models) given by the values of SST and SSB. In addition, a stochastic element was included by adding Gaussian noise (ε; resampled randomly from the residuals of the S-R models) to account for unexplained sources of recruitment variability. After having accounted for intrinsic processes (i.e., growth, maturation, and natural mortality), as well as external factors (i.e., fishing mortality) in the age-structured cohort models, the forward simulation loop is reiterated by estimating R in the following year. We performed a number of validation exercises by hindcasting the population dynamics of sardine and anchovy over multiple time scales (SI Appendix). To assess the uncertainty of the simulations, 1,000 replications were run with Gaussian noise (i.e., resampled randomly from the residuals of the S-R models) added at each time step. Mean values and the 95% confidence interval of the hindcasted dynamics were computed. All statistical analyses were conducted using R software version 2.12.1 (www.r-project.org).
Supplementary Material
Acknowledgments
We thank L. Jacobson, K. Hill, and S. McClatchie at the National Oceanic and Atmospheric Administration for providing data and comments regarding S-R modeling, as well as former and present colleagues for their efforts understanding sardine–anchovy biology in the CCE and beyond. M.L. was supported by a Scripps Institution of Oceanography Postdoctoral Fellowship and by National Science Foundation Grant OCE-0928425.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 13240.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305733110/-/DCSupplemental.
References
- 1.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293(5530):629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 2.Beaugrand G, Brander KM, Alistair Lindley J, Souissi S, Reid PC. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426(6967):661–664. doi: 10.1038/nature02164. [DOI] [PubMed] [Google Scholar]
- 3.Lindegren M, Möllmann C, Nielsen A, Stenseth NC. Preventing the collapse of the Baltic cod stock through an ecosystem-based management approach. Proc Natl Acad Sci USA. 2009;106(34):14722–14727. doi: 10.1073/pnas.0906620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornstad ON, Nisbet RM, Fromentin JM. Trends and cohort resonant effects in age-structured populations. J Anim Ecol. 2004;73:1157–1167. [Google Scholar]
- 5.Hsieh CH, et al. Fishing elevates variability in the abundance of exploited species. Nature. 2006;443(7113):859–862. doi: 10.1038/nature05232. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CNK, et al. Why fishing magnifies fluctuations in fish abundance. Nature. 2008;452(7189):835–839. doi: 10.1038/nature06851. [DOI] [PubMed] [Google Scholar]
- 7.Rouyer T, Sadykov A, Ohlberger J, Stenseth NC. Does increasing mortality change the response of fish populations to environmental fluctuations? Ecol Lett. 2012;15(7):658–665. doi: 10.1111/j.1461-0248.2012.01781.x. [DOI] [PubMed] [Google Scholar]
- 8.Lluch-Belda D, et al. Worldwide fluctuations of sardine and anchovy stocks—the regime problem. S Afr J Mar Sci. 1989;8(1):195–205. [Google Scholar]
- 9.Schwartzlose RA, et al. Worldwide large-scale fluctuations of sardine and anchovy populations. S Afr J Mar Sci. 1999;21(1):289–347. [Google Scholar]
- 10.Chavez FP, Ryan J, Lluch-Cota SE, Niquen C M. From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science. 2003;299(5604):217–221. doi: 10.1126/science.1075880. [DOI] [PubMed] [Google Scholar]
- 11.Sugihara G, et al. Detecting causality in complex ecosystems. Science. 2012;338(6106):496–500. doi: 10.1126/science.1227079. [DOI] [PubMed] [Google Scholar]
- 12.MacCall AD. In: Climate Change and Small Pelagic Fish. Checkley DM, Alheit J, Oozeki Y, editors. Cambridge, UK: Cambridge Univ Press; 2009. pp. 285–299. [Google Scholar]
- 13.Turchin P. Population regulation: A synthetic view. Oikos. 1999;84(1):153–159. [Google Scholar]
- 14.Stenseth NC, et al. Common dynamic structure of canada lynx populations within three climatic regions. Science. 1999;285(5430):1071–1073. doi: 10.1126/science.285.5430.1071. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner T, Soutar A, Ferreira-bartrina V. Reconstruction of the history of Pacific sardine and northern anchovy populations over the past 2 millennia from sediments of the Santa-Barbara Basin. CCOFI Rep. 1992;33:24–40. [Google Scholar]
- 16.Field DB, et al. In: Climate Change and Small Pelagic Fish. Checkley DM, Alheit J, Oozeki Y, editors. Cambridge, UK: Cambridge Univ Press; 2009. pp. 45–63. [Google Scholar]
- 17.Mantua N, Hare S, Zhang Y, Wallace J, Francis RA. Pacific interdecadal climate oscillation with impacts on salmon production. Bull Am Meteorol Soc. 1997;78(6):1069–1079. [Google Scholar]
- 18.Minobe S. A 50-70 year climatic oscillation over the North Pacific and North America. Geophys Res Lett. 1997;24(6):683–686. [Google Scholar]
- 19.Rykaczewski RR, Checkley DM., Jr Influence of ocean winds on the pelagic ecosystem in upwelling regions. Proc Natl Acad Sci USA. 2008;105(6):1965–1970. doi: 10.1073/pnas.0711777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith P, Moser H. Long-term trends and variability in the larvae of Pacific sardine and associated fish species of the California Current region. Deep Sea Res Part II Top Stud Oceanogr. 2003;50(14-16):2519–2536. [Google Scholar]
- 21.van der Lingen CD, Hutchings L, Field JG. Comparative trophodynamics of anchovy Engraulis encrasicolus and sardine Sardinops sagax in the southern Benguela: Are species alternations between small pelagic fish trophodynamically mediated? Afr J Mar Sci. 2006;28(3-4):465–477. [Google Scholar]
- 22.Lindegren M, et al. Ecological forecasting under climate change: The case of Baltic cod. Proc Biol Sci. 2010;277(1691):2121–2130. doi: 10.1098/rspb.2010.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill K, Lo N, Macewicz B, Crone PR, Felix-Uraga R. Assessment of the Pacific Sardine Resource in 2010 for U.S. Management in 2011. La Jolla, CA: US Department of Commerce; 2010. [Google Scholar]
- 24.Jacobson L, Lo N, Barnes J. A biomass-based assessment model for northern anchovy, Engraulis mordax. Fish Bull. 1994;92(4):711–724. [Google Scholar]
- 25.Jacobson L, MacCall A. Stock-recruitment models for Pacific sardine (Sardinops sagax) Can J Fish Aquat Sci. 1995;52(3):566–577. [Google Scholar]
- 26.Lindegren M, Checkley DM. Temperature dependence of Pacific sardine (Sardinops sagax) recruitment in the California Current revisited and revised. Can J Fish Aquat Sci. 2013;70(2):245–252. [Google Scholar]
- 27.Fissel BE, Lo NCH, Herrick SF. Daily egg production, spawning biomass and recruitment for the central subpopulation of northern anchovy 1981-2009. CCOFI Rep. 2011;52:116–135. [Google Scholar]
- 28.Vasseur D, Yodzis P. The color of environmental noise. Ecology. 2004;85(4):1146–1152. [Google Scholar]
- 29.Rouyer T, Fromentin J, Stenseth NC, Cazelles B. Analysing multiple time series and extending significance testing in wavelet analysis. Mar Ecol Prog Ser. 2008;359:11–23. [Google Scholar]
- 30.Takasuka A, Oozeki Y, Kubota H, Lluch-Cota SE. Contrasting spawning temperature optima: Why are anchovy and sardine regime shifts synchronous across the North Pacific? Prog Oceanogr. 2008;77(2-3):225–232. [Google Scholar]
- 31.Biondi F, Gershunov A, Cayan D. North Pacific decadal climate variability since 1661. J Clim. 2001;14(1):5–10. [Google Scholar]
- 32.Zwolinski JP, Demer DA. A cold oceanographic regime with high exploitation rates in the Northeast Pacific forecasts a collapse of the sardine stock. Proc Natl Acad Sci USA. 2012;109(11):4175–4180. doi: 10.1073/pnas.1113806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacCall AD, Hill KT, Crone P, Emmett R. Weak evidence for sardine collapse. Proc Natl Acad Sci USA. 2012;109(19):E1131–, author reply E1132–E1133. doi: 10.1073/pnas.1203526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEvoy AF. The Fisherman’s Problem. Ecology and Law in the California Fisheries 1850-1980. New York: Cambridge Univ Press; 1986. [Google Scholar]
- 35.Cury P, et al. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J Mar Sci. 2000;57(3):603–618. [Google Scholar]
- 36.McLeod K, Leslie H. Ecosystem-Based Management for the Oceans. Washington, DC: Island; 2009. [Google Scholar]
- 37.Botsford LW, Holland MD, Samhouri JF, White JW, Hastings A. Importance of age structure in models of the response of upper trophic levels to fishing and climate change. ICES J Mar Sci. 2011;68(6):1270–1283. [Google Scholar]
- 38.Alheit J, Bakun A. Population synchronies within and between ocean basins: Apparent teleconnections and implications as to physical-biological linkage mechanisms. J Mar Syst. 2010;79(3-4):267–285. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.