Abstract
Primordial cells presumably combined RNAs, which functioned as catalysts and carriers of genetic information, with an encapsulating membrane of aggregated amphiphilic molecules. Major questions regarding this hypothesis include how the four bases and the sugar in RNA were selected from a mixture of prebiotic compounds and colocalized with such membranes, and how the membranes were stabilized against flocculation in salt water. To address these questions, we explored the possibility that aggregates of decanoic acid, a prebiotic amphiphile, interact with the bases and sugar found in RNA. We found that these bases, as well as some but not all related bases, bind to decanoic acid aggregates. Moreover, both the bases and ribose inhibit flocculation of decanoic acid by salt. The extent of inhibition by the bases correlates with the extent of their binding, and ribose inhibits to a greater extent than three similar sugars. Finally, the stabilizing effects of a base and ribose are additive. Thus, aggregates of a prebiotic amphiphile bind certain heterocyclic bases and sugars, including those found in RNA, and this binding stabilizes the aggregates against salt. These mutually reinforcing mechanisms might have driven the emergence of protocells.
Keywords: origin of life, nucleosides, vesicles, fatty acids, micelles
The origin of RNA (1) and how it became associated with amphiphilic membranes in primordial cells are unclear. RNA is a polymer of units containing the sugar ribose covalently bound to one of four nucleobases; amphiphiles are molecules that possess both a hydrophobic and a hydrophilic moiety and therefore can aggregate into membranes in water. We know that two of the four units of RNA can be synthesized under simulated prebiotic conditions (2), that simple amphiphiles such as fatty acids spontaneously aggregate into vesicles in an aqueous environment (3), and that such vesicles can encapsulate nucleic acid and its building blocks (4, 5). Fundamental questions remain, however, regarding how the bases and sugar in RNA were selected from a heterogeneous mixture of prebiotic organic compounds, concentrated sufficiently to react, and colocalized with vesicles. It also is unclear how the first membranes were stabilized in seawater, given that fatty acids precipitate at high salt concentrations (6).
Previous lines of research suggest possible answers to these questions. Prebiotic chemical processes might have preferentially generated at least two of the four nucleotides (consisting of a base bound to ribose and phosphate) from simple organic precursors (2). These building blocks, if appropriately activated, then might have polymerized on mineral surfaces (7), which also stimulate fatty acid vesicle formation (8). Finally, the incorporation of alcohols and glycerol monoesters in fatty acid membranes might have increased their stability in seawater (4, 9–11).
We hypothesize a simpler, more integrated scenario that complements these mechanisms. In this scenario, aggregates of amphiphiles preceded RNA and facilitated its synthesis by binding and concentrating the bases and sugar of which it is composed. The observation that the assembly of amphiphilic aggregates proceeds spontaneously, whereas the synthesis of RNA requires energy, supports this scenario. Moreover, the planar structure of the bases and the hydrogen-bonding potential of sugars suggest mechanisms by which these compounds could interact with fatty acid aggregates. We further hypothesize a functional consequence of the binding: stabilization of the amphiphilic aggregates in the presence of salt. The mechanisms we hypothesize are mutually reinforcing and, under prebiotic conditions, could drive the emergence of vesicles enriched in components of RNA.
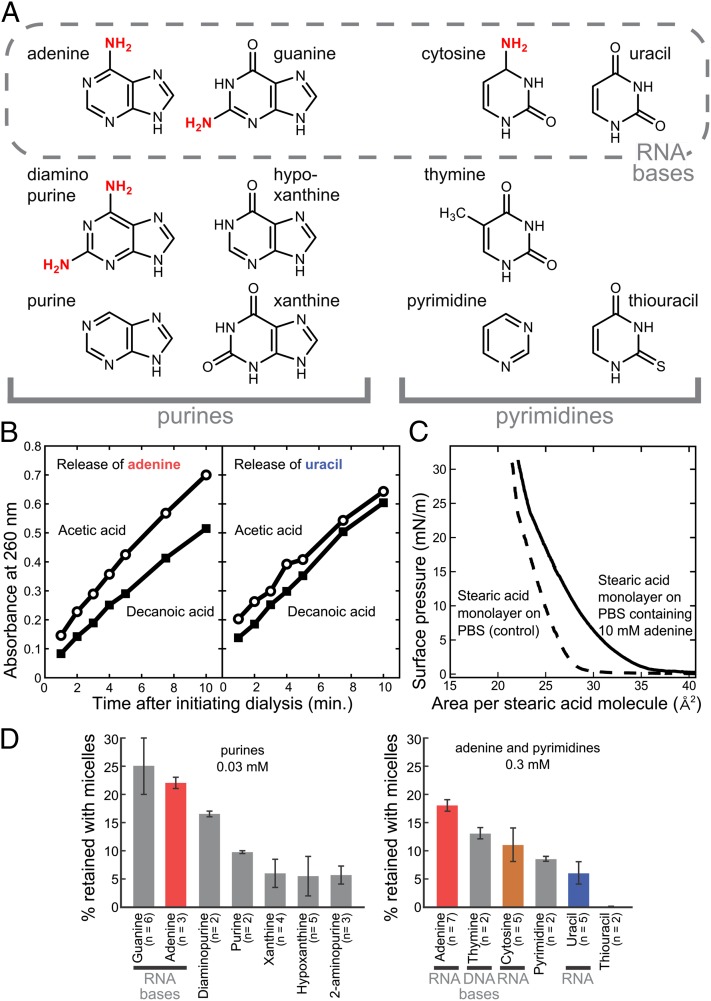
The array of bases we investigated is shown in Fig. 1A, including the nucleobases found in RNA: adenine, guanine, cytosine, and uracil. We primarily used decanoic acid (a carboxyl group attached to a chain of nine additional carbons) as our amphiphile because it is synthesized under prebiotic conditions (12) and is long enough to self-assemble into vesicles (13). (We use the term “decanoic acid” to refer to both the protonated and unprotonated forms of the molecule.) Vesicles enclose an aqueous volume, as a cell does, in contrast to smaller aggregates, such as micelles, that have no aqueous core (Fig. S1). Above pH 8, decanoic acid forms only micelles. Vesicles typically start to form as the proton concentration becomes sufficient, below pH 8, to bridge carboxyl groups by hydrogen bonding, thereby reducing surface charge (13). Because of the sensitivity of decanoic acid aggregates to pH, this parameter must be controlled tightly, and our procedures for doing so are described in SI Materials and Methods.
Fig. 1.
Decanoic acid aggregates selectively bind heterocyclic nitrogenous bases. (A) Structures of purines and pyrimidines tested for interactions with decanoic acid aggregates. Diaminopurine contains an amine at the 2-position in addition to the 6-position as in adenine; 2-aminopurine, also tested in some experiments, has an amine only at the 2-position. Amine substituents are indicated in red. (B) Adenine dialyzes more slowly from a decanoic acid solution than from an acetic acid solution. (Left) Results of a representative experiment in which adenine, at 15 mM, diffused from either 180 mM decanoic acid or from 180 mM acetic acid. Aliquots of dialysis buffer were collected at indicated times and assayed for adenine by measuring absorbance at 260 nm. The rate of release was 24 ± 5% lower from decanoic acid (P < 0.05). (Right) Results of a corresponding representative control experiment with uracil in place of adenine. The rate of release was 8 ± 7% greater, not lower, from decanoic acid (P > 0.05). (C) The presence of 10 mM adenine in a subphase of PBS increases the surface pressure of a Langmuir monolayer of stearic acid. Measurement uncertainty is ±1 mN/m. Stearic acid (18 carbons) was used instead of decanoic acid because the latter does not form a stable Langmuir monolayer. (D) Nucleobases are retained with decanoic acid micelles during ultrafiltration. A solution of 180 mM decanoic acid and each base at 0.03 mM (for purines) or 0.3 mM (for pyrimidines) was partially centrifuged through a 3-kDa–cutoff filter. These concentrations optimize both the percentage of base retained by micelles and the detection of base by absorbance; adenine was evaluated at both 0.3 and 0.03 mM to enable comparison of all the bases. Values are averages, and error bars represent average deviations. (The difference between the means for cytosine and uracil is significant based on Student t test: P = 0.028 by a one-tailed test and 0.056 by a two-tailed test.)
Results
In a series of preliminary experiments (Figs. S2 and S3), we found that nucleobases and ribose interact with decanoic acid strongly enough to alter the pH at which vesicles form within a solution of micelles (results are summarized in Table 1). Among the nucleobases tested, the magnitude of the pH shift was in the order of adenine > cytosine > uracil. (Guanine was not sufficiently soluble to test.) Between the sugars, ribose had a greater effect than glucose. The differences in the magnitudes of these effects suggest they are the result of direct interaction of the compounds with the decanoic acid aggregates rather than of a change in nonspecific parameters of the solution, such as ionic strength or viscosity.
Table 1.
The rank order of effects of nucleobases and sugars is consistent across several tests for interaction with decanoic acid aggregates
| Test for interaction | Bases | Sugars |
| Altered pH of vesicle transition | A > C > U (Figs. S2 and S3) | Ribose > glucose (Figs. S2 and S3) |
| Retention during dialysis | A > U (Fig. 1B) | |
| Retention during ultrafiltration | A ∼ G > C > U (Fig. 1D) | |
| Reduction in flocculation | A > C > U (Fig. 2C) | Ribose > glucose (Fig. 2E) |
The tests are described in the text and figure legends.
Binding of Nucleobases to Aggregates of Fatty Acids.
To confirm direct interaction between the bases and the aggregates, as well as to better quantify the strength of interaction, we used three independent assays for binding. In these experiments, we focused on fatty acid micelles and monolayers rather than vesicles to differentiate between adsorption and encapsulation.
First, we determined that adenine dialyzes more slowly from decanoic than from acetic acid (21 ± 7% slower averaged over six experiments, P ∼ 0.003; Fig. 1B). This result suggests that adenine binds to micelles, because acetic acid has the same hydrophilic moiety as decanoic acid but a hydrophobic tail too short (one carbon) to support micelle formation. As controls, we tested two compounds, uracil and thiouracil, that show weak or no interaction with decanoic acid aggregates by other measures (Figs. S2 and S3 and Fig. 1D). We found that the rates of uracil dialysis from decanoic and acetic acids are indistinguishable within experimental uncertainty (3 ± 10% faster, not slower, from decanoic acid, n = 2, P > 0.05; Fig. 1B), and the difference in rates of thiouracil dialysis also is insignificant (6 ± 4% faster, not slower, from decanoic acid, n = 2, P > 0.05). These results suggest that the slower dialysis of adenine from decanoic vs. acetic acid is the result of its binding to micelles rather than to a nonspecific property of the solution, such as viscosity.
In a second test for interaction between adenine and long-chain fatty acids, we found that the base interacts with a fatty acid monolayer in a Langmuir trough. In these experiments, a fatty acid is dispersed over the surface of an aqueous solution, altering the surface tension at the air–solution interface. The change in surface tension is expressed as surface pressure, defined for Langmuir monolayers as the surface tension of pure water minus the surface tension of the system under study. Decreasing the surface area, by moving a barrier, concentrates the fatty acid molecules and increases the surface pressure. We found that the presence of adenine in solution below a stearic acid monolayer increases the surface pressure observed at a given surface area (Fig. 1C). This result suggests that adenine adsorbs to or inserts in the monolayer of fatty acid molecules. In the absence of a stearic acid monolayer, surface pressures of an adenine solution and of a buffer-only solution are indistinguishable, indicating that adenine alone does not partition to the air–solution interface enough to affect surface pressure measurably.
We used ultrafiltration as our third binding assay. Samples were centrifuged through a 3-kDa–cutoff filter, which retains decanoic acid micelles and, presumably, any bases associated with them. We found that RNA bases are retained with decanoic acid micelles, and the extent of their retention differs, with adenine ∼ guanine > cytosine > uracil (Fig. 1D and Table 1). Moreover, adenine and guanine are retained to a greater extent than all five other purines tested, and the three pyrimidines in RNA or DNA are retained to a greater extent than thiouracil (Fig. 1D).
We conclude from these three diverse binding assays that (a) nucleobases bind to fatty acid aggregates, (b) the strength of nucleobase binding to fatty acid aggregates correlates well with the magnitude of the pH shifts that they induce in micelle–vesicle transitions (Table 1), and (c) structurally related bases exhibit substantial variation in binding.
We quantitatively assessed the affinity of adenine binding to decanoic acid micelles by repeating the filtration assay over a range of adenine concentrations, 0.01–3 mM. Scatchard analysis of the results suggests two modes of binding, one with a Kd of about 11 μM and one, with much lower affinity, that is not saturated at the highest adenine concentration tested (Fig. S4). In contrast, 2-aminopurine appears to lack a high-affinity binding mode; whereas the percentage of adenine retained with micelles increases from 18 ± 1% at 0.3 mM to 22 ± 1% at 0.03 mM (Fig. 1D), retention of 2-aminopurine declines over this concentration range from 9.3 ± 0.8% (n = 3) to 5.7 ± 1.6% (n = 3). The relatively low absorbance of 2-aminopurine and the other purines besides adenine precluded testing them at the low concentrations required to further evaluate for high-affinity binding.
We found that the mechanism by which bases bind to decanoic acid micelles is not simply related to hydrophobicity. Including 0.4 M NaCl in the filtration assay with 0.03 mM adenine increased the amount of the base retained with micelles, by 68 ± 2% (average of duplicates), suggesting a hydrophobic interaction is involved. However, we found no strong correlation between the extent of binding and the hydrophobicity of the bases, as measured by their partitioning into octanol vs. water (R2 = 0.2 and 0.04 for binding measured at 0.3 and 0.03 mM, respectively) (Fig. S5).
Inhibition of Decanoic Acid Flocculation by Nucleobases and Ribose.
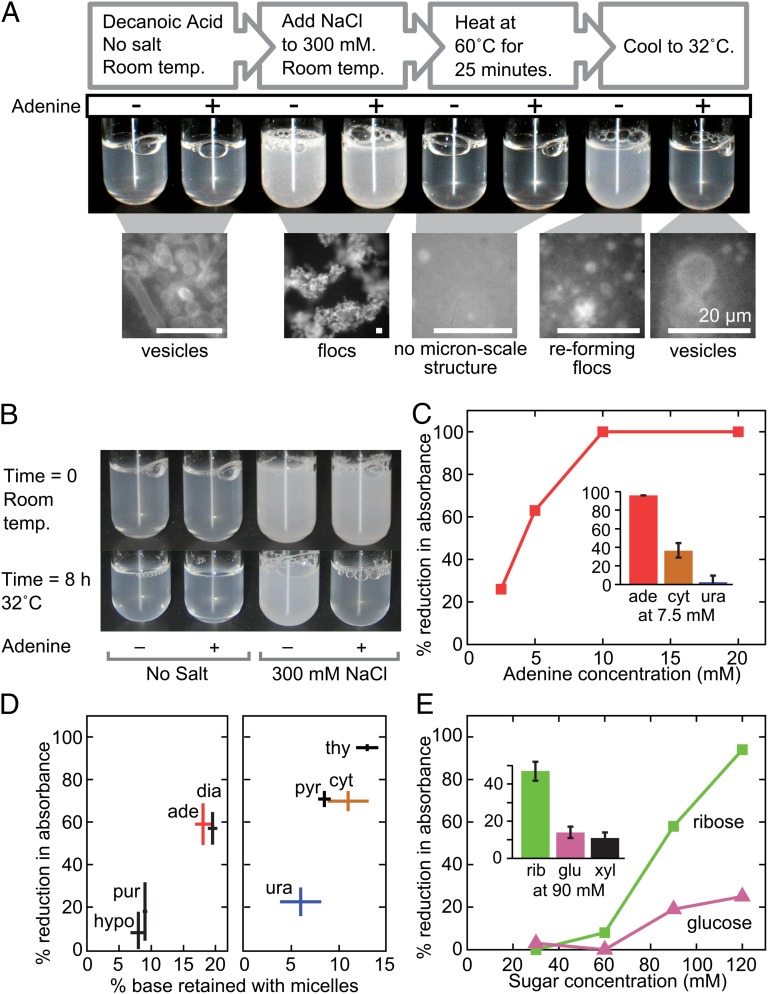
Having established the plausibility of a scenario in which aggregates of amphiphiles might have facilitated RNA synthesis by binding its components, we next tested the functional element of our hypothesis, that these components might have stabilized the aggregates against precipitation by salt. Salt concentrations in ancient oceans likely were at least as high as those in modern oceans (14), and decanoic acid flocculates in the presence of even modest concentrations of NaCl (Fig. 2A) (a phenomenon previously reported as precipitation in ref. 6). We began our investigation with adenine because it exhibits strong interaction with fatty acid aggregates in all our assays (Table 1).
Fig. 2.
Nucleobases and sugars inhibit flocculation of decanoic acid induced by salt. (A) Adenine reduces reflocculation of decanoic acid, and enables vesicle formation, after dissolution of flocs by heat. Test tube solutions of 80 mM decanoic acid/pH 7.65, without and with 30 mM adenine, were treated as indicated. Corresponding samples for microscopy contained 10 μM rhodamine 6G as a dye and were heated and cooled to the indicated temperatures on the microscope stage. Scale bars are 20 μm. (B) Incubation with adenine at 32 °C reduces preexisting flocs. Shown is 80 mM decanoic acid/pH 7.6, with and without 25 mM adenine and 300 mM NaCl as indicated, before and after incubation at 32 °C for 8 h. (The larger volume used for the preincubation set was chosen arbitrarily.) To quantitate the effect, aliquots were incubated in a 96-well plate in parallel, and turbidity was measured after 8 h; the presence of adenine reduced absorbance at 490 nm by 74 ± 1% (average of duplicates). (C) Nucleobases inhibit salt-induced flocculation of decanoic acid. The main panel shows the percent reduction in absorbance of a solution of 80 mM decanoic acid/300 mM NaCl (compared with controls with no base added) vs. the concentration of adenine, in the plate-based assay for flocculation described in the text; results are representative of three experiments. (Inset) Percent reduction in absorbance of a solution of 80 mM decanoic acid/300 mM NaCl containing 7.5 mM adenine, cytosine, or uracil (compared with controls with no base added); error bars represent average deviations of duplicate samples. (D) The inhibition of salt-induced flocculation of decanoic acid by nitrogenous bases correlates with their binding to decanoic acid micelles. The purines (Left) were tested in the plate-based assay for flocculation at 2.5 mM, and the pyrimidines (Right) were tested at 10 mM; samples were run in duplicate, and error bars represent average deviations. Values for percent retained with micelles are from the filtration assay run with bases at 0.3 mM. (E) Sugars inhibit salt-induced flocculation of decanoic acid. The main panel shows the percent reduction in absorbance of a solution of 80 mM decanoic acid/300 mM NaCl (compared with controls with no sugar added) vs. the concentration of sugar, in the plate-based assay for flocculation; the results are representative of three experiments. (Inset) Percent reduction with 90 mM sugar; error bars represent average deviations of duplicate samples.
We found that adenine inhibits salt-induced decanoic acid flocculation, thereby preserving vesicles. Salt-induced flocs in a decanoic acid solution dissolve upon heating, and in the absence of adenine, they begin to reform as the temperature falls to about 32 °C (Fig. 2A). With the inclusion of adenine, however, the solution remains relatively clear at this temperature, and epifluorescence microscopy shows that instead of flocs, vesicles as large as ∼10 μm form (Fig. 2A). Moreover, we found that in addition to inhibiting reflocculation upon cooling, adenine at 32 °C substantially eliminates preexisting flocs (Fig. 2B). Adenine’s inhibition of flocculation persists to temperatures as low as 30 °C; at room temperature, the base has no apparent effect (Fig. 2A). Stabilization of vesicles might account for this shift in equilibrium between decanoic acid vesicles and flocs; this explanation is consistent with our finding (in the absence of salt) that vesicles extruded to about 100 nm in diameter grow faster in the presence of adenine than in the presence of the nonbinding base thiouracil (Fig. S6).
To determine the concentration dependence and specificity of adenine’s effect on flocculation temperature, we established the following high-throughput assay using a 96-well plate: Decanoic acid solutions are flocculated by the addition of salt and then heated to 60 °C, which dissolves the flocs and renders the solutions virtually clear. Solution turbidity then is measured as the solutions cool and flocs reform. At 32 °C and below, the turbidity of decanoic acid solutions containing 300 mM NaCl is primarily caused by flocs (Fig. 2A), so turbidity may be used as a measure of flocculation.
We found that as little as 2.5 mM adenine inhibits NaCl-induced flocculation (Fig. 2C). The other nucleobases tested also inhibit flocculation, in the order adenine > cytosine > uracil (Fig. 2C, Inset). This is the same order seen in the extent of their binding to fatty acid aggregates (Fig. 1D and Table 1), suggesting that the inhibition of flocculation is related to binding as we hypothesized. Moreover, the correlation between inhibition of flocculation and binding is generalizable to a large group of bases (Fig. 2D).
Several sugars, too, inhibit flocculation of decanoic acid caused by NaCl, and ribose does so more effectively than glucose or xylose (Fig. 2E). This order is noteworthy for three reasons. (a) Ribose is the sugar found in RNA and DNA. (b) For sugars, as with bases, the extent of inhibition of flocculation correlates with the shift they cause in the pH dependence of vesicle formation (Table 1). (c) Diastereomers are not equally effective, because xylose is less inhibitory than ribose. Ribose is indistinguishable from arabinose in the flocculation assay, and xylose is indistinguishable from lyxose (Table S1). The downward orientation of the C3 hydroxyl group (in standard projections) common to ring structures of ribose and arabinose, but not present in xylose or lyxose, might cause the difference in efficacy, if hydroxyl groups of sugars are involved in the binding to fatty acid aggregates.
Finally, we found that the inhibitory effects of adenine and ribose on salt-induced flocculation are approximately additive, at least when adenine alone inhibits by less than 50% and ribose alone inhibits by over 50% (n = 5). In one such experiment, for example, 3 mM adenine alone inhibited by 26 ± 11%, 90 mM ribose alone inhibited by 64 ± 5%, and the combination inhibited by 86 ± 2% (uncertainties expressed as average deviation of duplicate samples). The additivity of the adenine and ribose effects suggests the two compounds can bind to decanoic acid aggregates simultaneously.
Discussion
Taken together, our observations support a scenario in which the bases and sugar required for RNA were selected and concentrated by binding to aggregates of prebiotic amphiphiles. Further, the resulting stabilization of the aggregates against salt might have created a positive feedback loop in which vesicles that bound bases and sugar resisted flocculation, thereby preserving more surface area to bind additional bases and sugar, further enhancing stability.
The prebiotic presence of these components at significant concentrations is plausible. Long-chain fatty acids are found in meteorites (15) and may be formed by natural processes on Earth (16, 17). Nucleobases also have been found in meteorites (18) and are produced by plausible Earth-based prebiotic reactions (19). Recent work describes how ribose might have been generated prebiotically (20, 21). Under prebiotic conditions, organic matter might have been relatively long-lived, and processes such as adsorption might have concentrated these components (22). We suggest that the binding of bases and sugars to amphiphilic aggregates was one of these processes. Because we have shown that multiple compounds bind to these aggregates and that their stabilizing effects are additive, bases and sugars need not have reached concentrations at which they alone would have stabilized vesicles.
Mechanisms by which bases and sugars bind to fatty acid aggregates might involve several variables, including planarity, hydrophobicity, and hydrogen bonding. All the bases are planar, and planarity may facilitate insertion in a lipid membrane. The increase in adenine binding in the presence of 0.4 M salt suggests that charge is not a major factor and implicates hydrophobic interactions, despite the lack of a simple correlation between binding and hydrophobicity (Fig. S5). Amines on the bases could hydrogen bond with the carboxyl groups of fatty acids, and most of the bases retained at higher fractions with micelles have amine groups. Sugars may interact with a fatty acid aggregate through hydrogen bonding between the carboxyl groups and hydroxyl groups of the sugar, as has been suggested for the hydroxyl groups in glycerol monoesters (11). The unique configuration of hydroxyl groups in ribose was noted previously to explain its exceptionally rapid permeation of protocells (23–24).
Following the colocalization of the nucleobases and ribose, the next logical step in the emergence of life is the formation of the glycosidic bond, which may be facilitated by orientation of the base and sugar on amphiphilic aggregates.
Materials and Methods
Decanoic acid was dissolved, with heating, in 190 mM NaOH to yield a 180-mM solution. For the flocculation experiments, it then was diluted to 80 mM, and for the plate-based assay, 100 mM bicine was included. pH was adjusted with 0.5–1 M HCl or NaOH. Further experimental procedures are summarized in the text and figure legends. A detailed description of materials and methods is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank D. Castner for arrangements at the University of Washington that enabled this research, H. Shen and her laboratory for the use of equipment, O. Einarsdottir and her laboratory for space and equipment at the project outset, E. Bowman for assistance with the Langmuir trough study, S. Pun and her laboratory for use of and assistance with the dynamic light scattering apparatus, J. Doedens and D. Virca for critical reading of the manuscript, and N. Nathanson for advice on Scatchard analysis. The Langmuir trough studies were supported by National Science Foundation (NSF) CHE 1040126, and the fluorescence microscopy studies were supported by NSF MCB 0744852.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300963110/-/DCSupplemental.
References
- 1. Robertson MP, Joyce GF (2012) The origins of the RNA world. Cold Spring Harbor Perspectives in Biology, eds Atkins JF, Gesteland RF, Cech TR (Cold Spring Harbor Lab Press, Cold Spring Harbor, NY), Vol 4, p a003608. [DOI] [PMC free article] [PubMed]
- 2.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459(7244):239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 3.Deamer D, Dworkin JP, Sandford SA, Bernstein MP, Allamandola LJ. The first cell membranes. Astrobiology. 2002;2(4):371–381. doi: 10.1089/153110702762470482. [DOI] [PubMed] [Google Scholar]
- 4.Apel CL, Deamer DW, Mautner MN. Self-assembled vesicles of monocarboxylic acids and alcohols: Conditions for stability and for the encapsulation of biopolymers. Biochim Biophys Acta. 2002;1559(1):1–9. doi: 10.1016/s0005-2736(01)00400-x. [DOI] [PubMed] [Google Scholar]
- 5.Mansy SS, et al. Template-directed synthesis of a genetic polymer in a model protocell. Nature. 2008;454(7200):122–125. doi: 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monnard P-A, Apel CL, Kanavarioti A, Deamer DW. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology. 2002;2(2):139–152. doi: 10.1089/15311070260192237. [DOI] [PubMed] [Google Scholar]
- 7.Ferris JP, Hill AR, Jr, Liu R, Orgel LE. Synthesis of long prebiotic oligomers on mineral surfaces. Nature. 1996;381(6577):59–61. doi: 10.1038/381059a0. [DOI] [PubMed] [Google Scholar]
- 8.Hanczyc MM, Fujikawa SM, Szostak JW. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science. 2003;302(5645):618–622. doi: 10.1126/science.1089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen IA, Salehi-Ashtiani K, Szostak JW. RNA catalysis in model protocell vesicles. J Am Chem Soc. 2005;127(38):13213–13219. doi: 10.1021/ja051784p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansy SS, Szostak JW. Thermostability of model protocell membranes. Proc Natl Acad Sci USA. 2008;105(36):13351–13355. doi: 10.1073/pnas.0805086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer SE, Deamer DW, Boncella JM, Monnard P-A. Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology. 2009;9(10):979–987. doi: 10.1089/ast.2009.0384. [DOI] [PubMed] [Google Scholar]
- 12.Naraoka H, Shimoyama A, Harada K. Molecular distribution of monocarboxylic acids in Asuka carbonaceous chondrites from Antarctica. Orig Life Evol Biosph. 1999;29(2):187–201. doi: 10.1023/a:1006547127028. [DOI] [PubMed] [Google Scholar]
- 13.Namani T, Walde P. From decanoate micelles to decanoic acid/dodecylbenzenesulfonate vesicles. Langmuir. 2005;21(14):6210–6219. doi: 10.1021/la047028z. [DOI] [PubMed] [Google Scholar]
- 14.Gornitz V, editor. Encyclopedia of Paleoclimatology and Ancient Environments. New York: Springer; 2008. [Google Scholar]
- 15.Lawless J, Yuen G. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature. 1979;282:396–398. [Google Scholar]
- 16.McCollom TM, Ritter G, Simoneit BRT. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig Life Evol Biosph. 1999;29(2):153–166. doi: 10.1023/a:1006592502746. [DOI] [PubMed] [Google Scholar]
- 17.Proskurowski G, et al. Abiogenic hydrocarbon production at Lost City hydrothermal field. Science. 2008;319(5863):604–607. doi: 10.1126/science.1151194. [DOI] [PubMed] [Google Scholar]
- 18.Callahan MP, et al. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci USA. 2011;108(34):13995–13998. doi: 10.1073/pnas.1106493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luisi PL. The Emergence of Life. Cambridge, UK: Cambridge Univ Press; 2006. [Google Scholar]
- 20.Ricardo A, Carrigan MA, Olcott AN, Benner SA. Borate minerals stabilize ribose. Science. 2004;303(5655):196. doi: 10.1126/science.1092464. [DOI] [PubMed] [Google Scholar]
- 21.Lambert JB, Gurusamy-Thangavelu SA, Ma K. The silicate-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science. 2010;327(5968):984–986. doi: 10.1126/science.1182669. [DOI] [PubMed] [Google Scholar]
- 22.Stüeken EE, et al. Did life originate from a global chemical reactor? Geobiology. 2013;11(2):101–126. doi: 10.1111/gbi.12025. [DOI] [PubMed] [Google Scholar]
- 23.Sacerdote MG, Szostak JW. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc Natl Acad Sci USA. 2005;102(17):6004–6008. doi: 10.1073/pnas.0408440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei C, Pohorille A. Permeation of aldopentoses and nucleosides through fatty acid and phospholipid membranes: Implications to the origins of life. Astrobiology. 2013;13(2):177–188. doi: 10.1089/ast.2012.0901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.