Abstract
Vaccines are among the most effective approaches to prevent and control many infectious diseases. Because of safety and reproducibility concerns, whole-cell vaccines (WCVs), made from live or killed microorganisms and including hundreds of antigenic components, have been mostly replaced by acellular or subunit vaccines composed of well-defined, purified antigen components. The efficacy of acellular vaccines is inferior to that of WCVs, however, for two major reasons: limited antigen diversity and reduced immunogenicity, especially in a lack of activation of antigen-specific T-cell immunity, which plays an important role in protection against mucosal and intracellular pathogens. Here we present the multiple antigen-presenting system (MAPS), which enables the creation of a macromolecular complex that mimics the properties of WCVs by integrating various antigen components, including polysaccharides and proteins, in the same construct and that induces multipronged immune responses, including antibody, Th1, and Th17 responses. Using antigens from various pathogens (Streptococcus pneumoniae, Salmonella typhi, and Mycobacterium tuberculosis), we demonstrate the versatility of the MAPS system and its feasibility for the design of unique defined-structure subunit vaccines to confer comprehensive protection via multiple immune mechanisms. Moreover, MAPS can serve as a tool for structure-activity analysis of cellular immunogens.
Since the advent of smallpox immunization, active vaccination became one of the most effective strategies for the prevention and control of infectious diseases. In modern vaccine development, subunit vaccines are often favored over whole-cell approaches that use attenuated or killed microorganisms as immunogens, such as vaccines against smallpox, pertussis, tuberculosis, and typhoid, owing to several advantages, including well-characterized antigen composition, better-defined immune responses and protective mechanisms, and a reduced risk of side effects (1).
The efficacy of current subunit vaccines rarely matches that of whole-cell vaccines (WCVs), however, due mainly to two important hurdles: limited antigen coverage and the monotonic (generally antibody-based) mechanism of protection. For bacterial pathogens in particular, current approaches have focused on the generation of antibody responses to surface molecules, most commonly the capsular polysaccharides (CPSs). Although this strategy has proven effective against several extracellular bacterial pathogens (e.g., Haemophilus influenzae type b, Neisseria meningitidis, Streptococcus pneumoniae), it has had far more limited success for mucosal and intracellular pathogens [e.g., Staphylococcus aureus, Mycobacterium tuberculosis (TB), parasites, and fungi], against which specific T-cell responses (e.g., Th1 and/or Th17 responses) or combinations of B-cell and T-cell immunity are required to ensure protection (2–6). Furthermore, for a pathogen with many different serotypes [particularly S. pneumoniae, in which more than 94 CPSs or serotypes have been identified (7)], targeting serotype-specific CPSs provides limited coverage and also carries a risk of serotype replacement, as has been observed after introduction of the pneumococcal conjugate vaccine pneumococcal conjugate vaccine (PCV) (7). Similarly, although their prevalence in the rise of pertussis cases remains to be determined, strains lacking pertactin, one of the target antigens composing the acellular vaccine, have been identified in the United States (8) as a potential example of the advent of escape mutants after the introduction of a subunit vaccine (9).
With these issues in mind, we have developed the multiple antigen-presenting system (MAPS) to reproduce the antigenic and immunologic strengths of WCV strategies but in a defined, acellular system. MAPS uses purified PS and proteins as immunogens to provide defined composition/formulation and reduced safety concerns; however, distinct from other subunit approaches, in the MAPS system, the isolated antigen components are specifically reassembled into an integrated macromolecular complex, based on the hypothesis that by mimicking some chemical and physical features of a whole-cell construct, such a complex could lead to the activation of comprehensive B- and T-cell immune responses and thereby provide the multipronged protection characteristic of WCVs.
Results
Creation of Macromolecular MAPS Complexes with Defined Antigenic, Chemical, and Physical Properties.
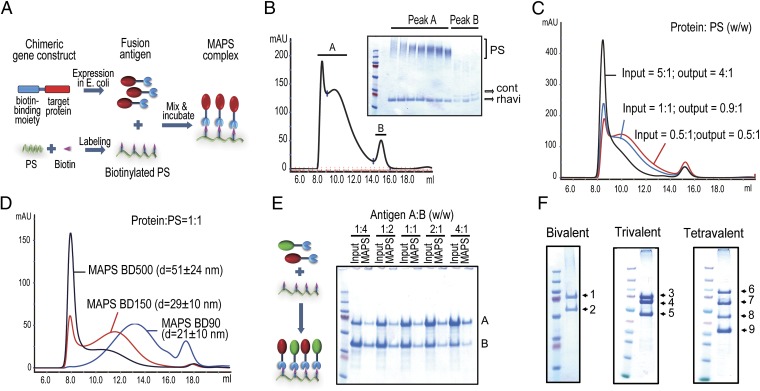
Instead of relying on covalent linking, MAPS uses an affinity-based coupling approach, thereby reducing potential epitope damage and providing flexibility and versatility in the preparation and formulation of MAPS constructs (as discussed below). Fig. 1A shows the construction of MAPS complexes. A biotin-binding protein, rhizavidin (rhavi) (10) is modified and recombinantly expressed in Escherichia coli (Fig. S1A). The target protein antigens are genetically fused to the rhavi moiety (Fig. S1B), and the target PS is biotinylated, at 0.05–1 mg of biotin per 1 mg of PS (Fig. S2), to provide a scaffolding matrix for the complex. Table S1 lists the protein antigens used in this study. After mixing and incubation, the fusion antigens spontaneously attach to the PS backbone via a strong affinity linkage of biotin and rhavi, resulting in a macromolecular construct (Fig. 1A). The assembled MAPS complexes are isolated by size-exclusion chromatography in the early eluents owing to their high molecular weight (Fig. 1B, peak A), whereas the unlinked protein antigens are separated in the latter fractions (Fig. 1B, peak B). Moreover, because the assembly is highly specific, any contaminating proteins (not fused to rhavi) that may be copurified with the target antigens are not incorporated into the complexes and are removed by chromatography (Fig. 1B).
Fig. 1.
Generation of multimolecular MAPS complexes. (A) Schematic diagram of MAPS technology. (B) Separation of MAPS complexes by size-exclusion chromatography. A mixture of rhavi and biotinylated dextran 500 (BD500; 425–500 kDa) was incubated overnight at 4 °C and then applied onto a Superose-6 column. Assembled MAPS complexes were eluted in early fractions (peak A) owing to their high molecular weight, whereas the excess rhavi was separated in the latter fractions (peak B). cont, contaminating proteins. (C) MAPS complexes containing different protein/PS ratios were prepared by incubation of 0.5 mg of rhavi-sp0435 with 0.1, 0.5, or 1 mg of biotinylated dextran 90 (BD90; 60–90 kDa). Input and output ratios of protein and PS antigens were similar. (D) Size of MAPS complexes using rhavi-sp0435 with BD90, BD150 (150 kDa), or BD500 [protein/PS 1:1, (wt/wt)]. (E) Preparation of MAPS complexes containing two different fusion antigens combined at the indicated ratios (wt/wt, input). (F) SDS/PAGE of multivalent MAPS complexes containing between two and four different fusion antigens. 1, rhavi-PdT; 2, rhavi-PsaA; 3, rhavi-PdT; 4, rhavi-SP_0785; 5, rhavi-SP_1500; 6, rhavi-PdT/SP1534; 7, rhavi-SP_0785; 8, rhavi-SP_1500; 9, rhavi-SP_0435.
MAPS complexes can be prepared efficiently even at low concentrations of antigens (0.1–1 mg/mL) (Fig. S3). The association between PS and protein antigens is very stable, resistant to treatment with reducing SDS buffer, and undergo dissociation only on heating to 100 °C, allowing for estimation of assembly efficiency by comparing the SDS/PAGE antigen profiles of unheated and samples heated at 100 °C (Fig. S3). An overnight incubation at 4 °C or 25 °C results in >90% incorporation of the antigens into MAPS complexes (Fig. S3).
Given the high assembling efficiency, we theorized that the protein/PS ratio in the resulting complexes should be closely correlated to the input ratio. As shown in Fig. 1C, this is indeed the case; as the number of protein molecules bound to each PS increases, the complexes with higher protein/PS weight ratios are also larger (Fig. 1C). At a given protein/PS ratio, the size of a MAPS complex is determined by the length/molecular weight of the PS, as demonstrated in dextran preparations of 90–500 kDa (Fig. 1D). Depending on the selected PS, MAPS complexes can display a range of sizes from 20 nm to several hundred nanometers in diameter, using a PS/protein weight ratio of 1:1–1.5. Limitated biotinylation of the PS or, alternatively, addition of excess protein can ensure that no unreacted biotin sites remain in the final construct.
The antigen formulation of MAPS complexes is versatile, allowing for various combinations of biotinylated PS and fusion protein and the inclusion of multiple protein antigens in the same construct. The incorporation of each individual antigen was confirmed by SDS/PAGE of the purified MAPS complex (Fig. 1 E and F). Given that the coupling mediated by rhavi–biotin interaction is independent of the identity/property of the target antigens, the amount of any antigen incorporated into a MAPS complex is determined by its input concentration in the assembling reaction (Fig. 1E). Fig. 1F shows several examples in which two to four different protein antigens were incorporated in MAPS complexes, using biotinylated dextran or serotype 1 pneumococcal CPS as the backbone.
Immunization with MAPS Complexes Elicits Robust IgG Antibody Against PS via a CD4+ T-Cell–Dependent Pathway.
Compared with whole-cell immunogens, purified antigens are much less effective in activating certain immune responses even in the presence of adjuvants, such as aluminum salts. Most purified PSs do not induce immune memory, class switching, or high-titer IgG antibodies when used as immunogens (11). Similarly, little or no antigen-specific Th1 or Th17 responses have been observed after vaccination with purified protein antigens (12). Current strategies to circumvent this problem rely either on chemical modification of antigen molecules, such as covalent conjugation of PS to a protein carrier, or on novel adjuvants and/or delivery vehicles.
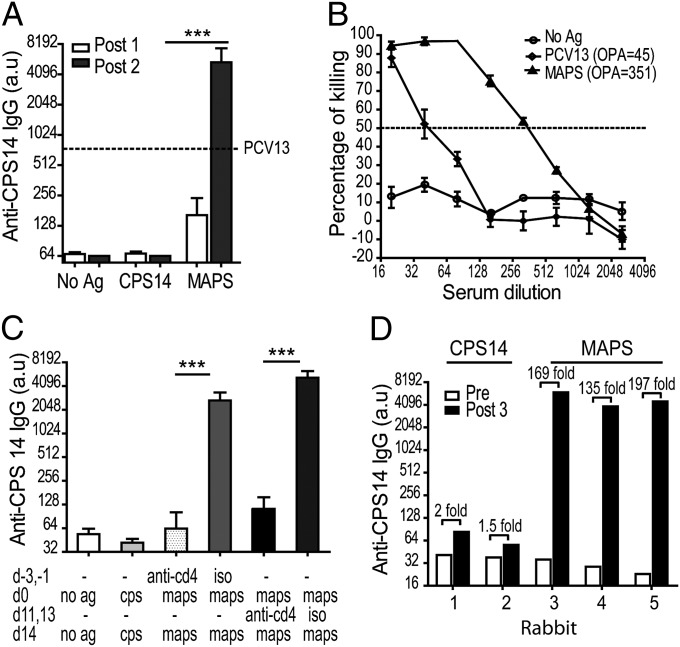
In contrast, assembly of the isolated components into a MAPS complex, a virtual “whole-cell like” construct, significantly increases and broadens the immunogenicity of the incorporated PSs and protein antigens. As shown in Fig. 2A, control mice that were immunized twice with the purified serotype-14 pneumococcal CPS (CPS14) developed no detectable CPS-specific IgG. In contrast, a single immunization with a CPS14 MAPS complex [given with Al(OH)3, as was done with all immunizations in this study] resulted in a detectable rise of anti-CPS14 IgG in the serum (open bars). After two immunizations, the average serum titer of anti-CPS14 IgG in MAPS-immunized mice was more than 80-fold higher than that in mice that received uncoupled CPS14 (black bars) and eightfold higher than that in mice (n = 10) that received two immunizations with an equal amount of CPS14 contained in the licensed PVC Prevnar 13 (PCV13; Pfizer) (positive control; dashed line).
Fig. 2.
Immunization with MAPS complexes induces a robust PS-specific antibody response. (A) Mice (n = 10 per group) were immunized with adjuvant alone (aluminum hydroxide; no Ag), adjuvanted CPS14, or MAPS complex composed of biotinylated CPS14 coupled with rhavi-sp0435. Two s.c. immunizations of 1 µg CPS14 per dose were administered. Serum IgG antibody against CPS14 was measured by ELISA after the first immunization (post 1, open bars) and the second immunization (post 2, black bars) with adjuvant alone or with CPS14 or MAPS complex composed of biotinylated CPS14 and rhavi-sp0435. The dashed line indicates the anti-CPS14 IgG titer in a standard serum from mice that received two immunizations with PCV13. Bars represent mean with SEM. ***P < 0.0001. (B) Opsonophagocytic killing of serotype 14 pneumococcus (strain 1401) mediated by serum from MAPS-immunized mice as described above. (C) Depletion of CD4+ T cells abolished induction and recall of PS-specific IgG antibody. Iso, isotype control. ***P < 0.0001. (D) Anti-CPS14 IgG in rabbits immunized with free CPS14 or MAPS complex composed of biotinylated CPS14 coupled with rhavi-sp0435.
The activity of the antibodies was tested in an opsonophagocytic killing assay (OPA) against serotype-14 pneumococci. Serum from MAPS-immunized mice had an approximately eightfold higher OPA value compared with the PCV13 control serum (Fig. 2B). Depletion of CD4+ T cells significantly abolished the generation of CPS-specific IgG antibodies (Fig. 2C), suggesting that the PS presented in the context of MAPS was processed using a thymus-dependent pathway. Several experiments in which immunization with MAPS induced a robust IgG antibody response against different PSs are shown in Figs. S4, S5, and S6.
The elevated anti-CPS response induced by MAPS complexes was also confirmed in the rabbit model. Intramuscular immunization with CPS14 MAPS complex resulted in an up to 200-fold rise in anti-CPS IgG titer (Fig. 2D, rabbits 3, 4, and 5), compared with an only 1- to 2-fold rise in rabbits that received an equivalent amount of CPS14. Also of note, the affinity interaction between PS and the rhizavidin moiety alone was sufficient to enhance antibody responses to the PS (Fig. S7).
Immunization with MAPS Complex Induces Multipronged Immunity, Including Antibody, Th1, and Th17 Responses to Protein Antigens.
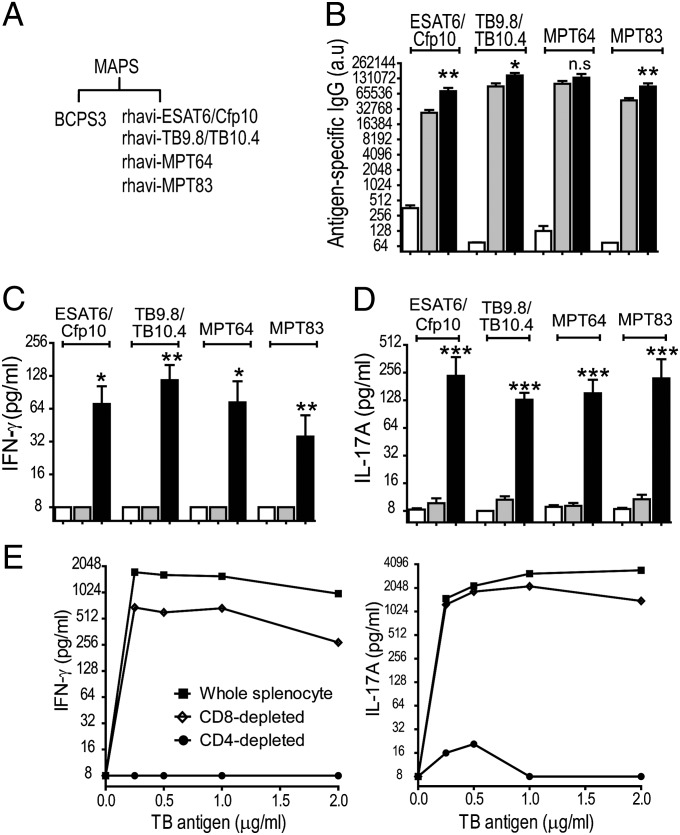
We next evaluated the ability of MAPS complexes to elicit antigen-specific cellular responses, using TB proteins as the target antigens. As shown in Fig. 3, immunization of mice with a mixture of purified TB protein antigens [ESAT6/Cfp10, TB9.8/TB10.4, MPT64, and MPT83 (13)] resulted in rises in serum antibody levels (Fig. 3B, mixture), but no detectable Th1 or Th17 responses to the target antigens (Fig. 3 C and D, mixture). However, when the same protein antigens were presented as a part of a MAPS complex using pneumococcal CPS3 as the backbone, significant activation of both B-cell and T-cell immunity was observed (Fig. 3A). Mice developed higher antibody titers (Fig. 3B; MAPS), as well as specific cellular responses leading to the production of IFN-γ and IL-17A on stimulation of blood samples with the target TB antigens (Fig. 3 C and D, MAPS).
Fig. 3.
Immunization with MAPS complex elicits antibody, Th1, and Th17 responses to protein antigens. (A) Mice were immunized with adjuvant alone, a mixture of CPS3 and TB antigens (mixture), or MAPS complex prepared with biotinylated serotype-3 pneumococcal CPS (BCPS3) and four fusion TB antigens, rhavi-ESAT6/Cfp10, rhavi-TB9.8/TB10.4, rhavi-MPT64, and rhavi-MPT83 [1:1:1:1, (wt/wt)]. (B–D) The MAPS complexes elicited significantly higher antibody titers against target proteins (B) and robust Th1 and Th17 responses from peripheral blood, as measured by production of IFN-γ (C) and IL-17A (D). Bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.0001. (E) Effect of depletion of CD4+ and CD8+ T cells on IFN-γ (Left) and IL-17A production (Right). Open bars represent no Ag; gray bars, mixture; black bars, MAPS.
Depletion of CD4+, but not CD8+, T cells from the isolated splenocytes greatly abrogated the cytokine production that could be recalled by coincubation of cells with TB antigens (Fig. 3E), confirming that the cytokine responses induced by MAPS complexes are derived mainly from CD4+ T cells.
Effect of Antigen Molecule Size on Generation of Th1 and Th17 Responses.
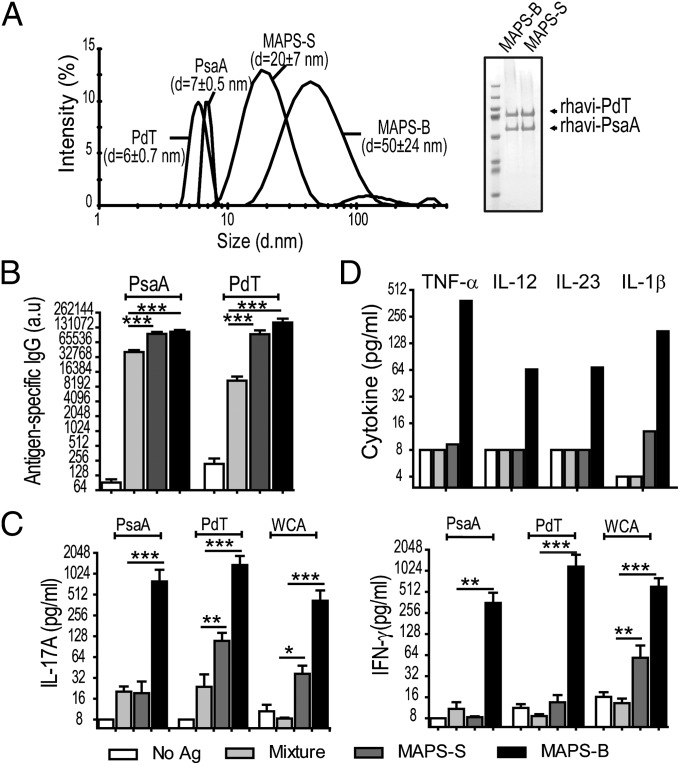
To further understand the impact of the physical properties of antigen molecules on their immunogenicity, we prepared two MAPS complexes containing the same pneumococcal protein antigens, the surface adhesin PsaA (14) and the pneumolysoid PdT (15), but attached to biotinylated dextran of different average molecular weights (90 kDa or 500 kDa), designated MAPS-S and MAPS-B, respectively (Fig. 4A). Using dynamic light scattering, we measured the average diameter of MAPS-S as 20 nm and that of MAPS-B as 50 nm. (The diameter of purified PsaA and PdT is <10 nm.) Mice were immunized with a simple mixture of two pneumococcal proteins with nonbiotinylated dextran (and thus no complex was formed) or with the smaller (MAPS-S) or larger (MAPS-B) complex. As shown in Fig. 4, the antigens presented in the form of larger complexes elicited significantly greater immune responses compared with antigens from smaller complexes or nonassembled antigens. Immunization of mice with the uncoupled protein/PS mixture elicited antibodies to both protein antigens, but no T-cell responses (Fig. 4 B and C, light-gray bars). In contrast, mice immunized with the MAPS-S complex developed significantly higher levels of anti-PsaA and anti-PdT antibodies. In addition, in these mice, low but detectable Th1 and Th17 responses could be recalled by stimulation of peripheral blood cells with purified PsaA, PdT, or pneumococcal whole-cell antigen (Fig. 4 B and C, dark-gray bars). Mice that received MAPS-B exhibited similar levels of antibody response as the MAPS-S group, but associated with significantly greater activation of both Th1 and Th17 cells, as evidenced by more robust production of IFN-γ and IL-17A cytokines on stimulation with the target antigens (Fig. 4 B and C, black bars).
Fig. 4.
Larger MAPS complexes induce significantly greater B- and T-cell immune responses. (A) Small (MAPS-S) and larger MAPS complexes (MAPS-B) were prepared using rhavi-PdT and rhavi-PsaA [in 1:1 ratio (wt/wt)], attached to BD90 or BD500. (Left) The size of MAPS complexes was measured by dynamic light scattering. (Right) SDS/PAGE indicated similar incorporation of both antigens in small and large MAPS complexes. (B) Immunization with small and large MAPS complexes elicited significantly higher antigen-specific antibody responses compared with the uncoupled protein/dextran mixture (mixture). (C) Immunization with larger MAPS complexes, but not small complexes or uncoupled proteins, elicited Th17 (Left) and Th1 (Right) responses against target antigens. Bars represent mean ± SEM. **P < 0.01; ***P < 0.0001. (D) Stimulation of isolated mouse peritoneal macrophages with larger MAPS complex induced proinflammatory cytokines. Macrophages were stimulated with an uncoupled protein/dextran mixture and small or large MAPS complexes at 1 µg/mL in the presence of aluminum hydroxide (1.2 mg/mL). Cells stimulated with aluminum hydroxide alone (no Ag) served as negative controls.
We investigated the immunologic basis of these different T-cell activation responses through in vitro assays using mouse peritoneal macrophages. Consistent with the foregoing in vivo findings, stimulation of cells with MAPS of larger size led to significantly greater secretion of several proinflammatory cytokines, including TNF-α, IL-12, IL-23, and IL-1β (Fig. 4D), all of which have important roles in the development of Th1- and/or Th17-mediated cellular responses (16, 17). To evaluate this further, we designed MAPS constructs that would or would not activate Toll-like receptor (TLR) 2, which enhances Th17 responses (18, 19). We did this through the incorporation of a lipidated version of rhavi to the biotinylated PS (Fig. S8A). Experiments using HEK293 cells confirmed dose-dependent TLR2 activation by a MAPS construct containing the lipidated version of rhavi, which was not observed with a nonlipidated MAPS (Fig. S8A).
We also prepared MAPS constructs by including TB antigens and either the nonlipidated or the lipidated version of rhavi (Fig. S8B). Peripheral blood cells from mice immunized with the lipidated (and TLR2-activating) TB MAPS construct produced significantly higher concentrations of IL-17A after stimulation with TB antigens than cells from mice immunized with MAPS alone, suggesting that the inclusion of a TLR2-activating motif in MAPS significantly enhances T-cell responses (Fig. S8C).
Designing a MAPS-Based Multiplex Subunit Vaccine That Protects Against Pneumococcal Disease via Combined B- and T-Cell–Mediated Immunity.
The foregoing results demonstrate the capacity of the MAPS system to present multiple antigens simultaneously and to generate B- and T-cell immune responses. To evaluate this approach for comprehensive protection, we tested it in two pneumococcal disease models. The pathogenesis of pneumococcal disease involves two different stages, an initial (and necessary) nasopharyngeal (NP) colonization (20) and subsequent bloodstream invasion, leading to invasive diseases such as bacteremia, sepsis, and meningitis. Protection against different pathogenic stages appears to involve different immune mechanisms. Antibodies against CPSs and specific protein antigens [e.g., pneumolysin, pneumococcal surface protein A, other surface proteins (21)] are thought to confer resistance to invasive infections, whereas antigen-specific Th17 responses facilitate the clearance of NP colonization (22).
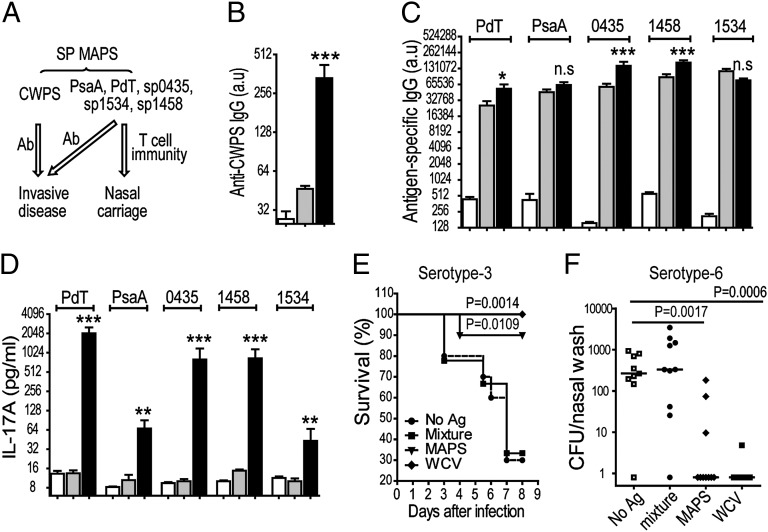
A prototype MAPS-based pneumococcal vaccine was designed to include pneumococcal cell wall PS (CWPS), a putative protective antigen that is antigenically conserved across all pneumococcal strains (23, 24), as the backbone along with five conserved, protective protein antigens (25, 26) (Fig. 5A). Mice that received this MAPS vaccine developed robust antibody responses to CWPS and the five protein antigens (Fig. 5 B and C), as well as antigen-specific Th17 responses (Fig. 5D).
Fig. 5.
Design of a MAPS-based multiplex vaccine for the prevention of invasive infection and NP colonization with virulent pneumococci. (A) A pneumococcal MAPS vaccine with biotinylated CWPS and five protein antigens (rhavi-PdT, rhavi-PsaA, rhavi-0435, rhavi-1458, and rhavi-1534) elicited protection against invasive disease and carriage. (B–D) MAPS elicited antibodies to the CWPS (B), as well as antibodies (C) and Th17 responses (D) to the five incorporated pneumococcal proteins. Bars represent mean ± SEM. Statistical analyses were performed in comparison with the mixture group. *P < 0.05; **P < 0.01. ***P < 0.0001. (E and F) Vaccination with MAPS complex protected against invasive infection (E) and NP colonization (F) with pneumococci. A pneumococcal WCV (100 µg total protein per dose) served as a positive control. Horizontal lines represent the median density of colonization. Statistical analyses were performed in comparison with the no Ag group. Open bars represent no Ag; gray bars, mixture; black bars, MAPS.
We tested protection in both infection and colonization models, using a pneumococcal WCV known to confer protection via combined B- and T-cell–mediated processes (27) as a positive control. As shown in Fig. 5E, in a lethal lung aspiration challenge with a serotype-3 strain, 90% of MAPS-immunized mice survived the infection, compared with only 30% of the mice that received adjuvant alone or a mixture of uncoupled CWPS and protein antigens. Significant protection was also observed when MAPS-immunized mice were challenged in a NP colonization model with a serotype-6B strain; the median density of pneumococcal colonization was over 100-fold lower compared with mice in the control groups (Fig. 5F). In both models, the efficacy of the MAPS vaccine was similar to that of the WCV and, moreover, was independent of capsular antibody.
Discussion
Live attenuated or inactivated WCVs are highly effective in eliciting broad and long-lasting immunity to many pathogens, particularly those for which natural infection confers protection (1). In many other instances, however, these strategies are inadequate, either because natural infection is not protective or attenuation of the organism is insufficient to ensure safety. More recently, public safety concerns with cellular approaches have limited their use in many areas of the developed world (28), leading to a shift to defined subunit vaccines.
Because subunit antigens alone are often poorly immunogenic, the addition of adjuvants, including aluminum salts, oil-in-water emulsions, and saponin-based molecules (29), has been necessary to enhance the immune response. These adjuvants, as well as nanoparticles or microparticles, share a particulate nature, a property with known important adjuvant effects (30, 31). More recently, natural ligands and synthetic agonists for pattern recognition receptors have been evaluated; several are currently in clinical trials, and one (monophosphoryl lipid A, a Toll-like receptor 4 agonist) is currently used with antigens and alum (32).
Here we present an acellular, defined approach to eliciting broad immunity. A major advantage of this approach is that MAPS constructs are highly immunogenic with respect to B- and T-cell responses, likely owing to their particulate nature, with no need for added adjuvants other than alum. In MAPS, immunogenicity depends mainly on the size of the PS scaffold. Although the incorporation of a TLR2 agonist in MAPS enhances T-cell responses (33), it is not required; alum-adjuvanted MAPS constructs elicit potent T- and B-cell immunity, as well as protection against mucosal colonization and disease, as demonstrated with the pneumococcal MAPS vaccine.
Thus, the MAPS approach holds particular promise for the development of alternatives to WCVs involving combined subunit vaccines against pathogens for which a combination of humoral and cellular responses against a diversity of antigen components is advantageous to ensure protection. In this respect, the MAPS system provides a platform that integrates the strengths of defined subunit vaccines and WCVs. The capability to incorporate multiple proteins onto the same PS backbone with MAPS enhances coverage in the same vaccine construct. Furthermore, we demonstrate that the attachment, via affinity interactions, of the purified components into a macromolecular and multimolecular complex significantly enhances the generation of specific B-cell responses to PSs and the Th1/Th17 immune responses to proteins, neither of which could be elicited by the uncoupled components.
As a unique approach to vaccine design, the MAPS technology will need to be carefully evaluated with respect to safety, with particular attention to the immunogenicity of biotin and/or the rhavi moieties. Our studies to date have been reassuring in this regard. In mice, immunization with MAPS complexes formed by the addition of excess protein (saturating all potential biotin sites) did not result in a rise in levels of antibodies to biotin (Fig. S9A) or a decline in serum biotin levels (Fig. S9B). Mice immunized with MAPS develop antibodies to rhavi, but this protein has no known homology to human proteins, and antibodies to rhavi do not cross-react with egg avidin (10) (Fig. S10).
Our data presented here challenge the generally accepted view that covalent linkage of PSs to proteins is required for IgM-to-IgG switching and induction of a booster response or T-cell memory (34). In contrast, we demonstrate that covalent coupling of PSs is not an absolute requirement, because affinity interactions between PSs and proteins are sufficient and highly effective for CD4+ T-cell priming of antibody responses to the PS. At the same time, differences among species in the antibody responses to PSs have been reported (35–37), such that data derived from weanling mice and/or rabbits do not necessarily predict the responses of young children. These issues will be important to keep in mind as clinical applications of the MAPS technology are planned.
In summary, MAPS provides an attractive approach for eliciting comprehensive B-and T-cell immune responses against pathogens, with the advantage of a precisely defined acellular platform. The flexible formulation and readily adjustable chemical/physical properties of MAPS complexes not only are beneficial for vaccine design and optimization, but also provide a useful tool for exploring how different antigenic constructs interact with the immune system and dissecting the chemical, physical, and/or molecular requirements for the activation of various immune responses.
Materials and Methods
Assembly and Purification of MAPS Complexes.
Each MAPS complex was assembled by incubation of biotinylated PS with fusion antigens at 4 °C or room temperature overnight. The assembled complex was isolated by size-exclusion chromatography, using Tris buffer (pH 8.0, 150 mM NaCl) as an eluant. The fractions containing MAPS complex were pooled and concentrated by ultrafiltration. The protein concentration in a MAPS complex was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce), and the PS concentration was determined by an anthrone assay. For MAPS complexes containing more than one protein antigens, the relative amount of each antigen incorporated into the MAPS complex was estimated by SDS/PAGE after heating to 100 °C to dissociate the components.
Immunization of Animals.
For immunization of mice, the MAPS complex, CPS, or protein/CPS mixture was diluted with saline solution (clinical grade) at the indicated concentrations and then mixed with aluminum hydroxide (final concentration 1.2 mg/mL) for overnight adsorption at 4 °C. Subcutaneous immunizations were administered biweekly to C57BL6/J mice (n = 10 per group) beginning at age 4–6 wk, in a total volume of 200 µL. Blood samples were obtained at 2 wk after the first, second, or third immunization, as indicated, for antibody and cytokine analysis.
For immunization of rabbits, the MAPS complex and CPS samples were prepared as described above. Intramuscular immunizations were given to 4-mo-old New Zealand White rabbits (Cocalico Biologicals), in a total volume of 500 µL. Peripheral blood samples were obtained before immunization and after the third immunization for antibody analysis.
Pneumococcal Infection of Mice.
All pneumococcal challenges were performed at 2 wk after the last bleeding. In the NP colonization model, mice were inoculated intranasally with 107 CFU of a serotype-6B strain (strain 0603) in 20 µL of PBS. To quantify NP colonization, an upper respiratory culture was done 10 d later by instilling sterile saline solution retrograde through the transected trachea of the euthanized animal, collecting 0.1 mL from the nostrils, and plating dilutions of samples on blood agar plates containing 2.5 µg/mL gentamicin.
In the aspiration-pneumonia challenge model, immunized mice were gently anesthetized with isoflorane, held supine, and given a 100-µL intranasal inoculation containing 106 CFU of a serotype-3 strain (WU2) as described previously (23). The mice were evaluated for any evidence of illness at least twice daily. A blood culture was obtained from any ill-appearing animal for detection of bacteremia before euthanasia with CO2 inhalation and terminal exsanguination. In all cases, blood cultures obtained from ill-appearing mice confirmed the presence of pneumococcal bacteremia.
Statistical Analysis.
Statistical analyses were performed using PRISM version 5.0 (GraphPad Software). All data on antibody titer, cytokine concentration, and NP colonization densities were analyzed using the Mann–Whitney U test, and differences in survival were analyzed using the Mantel–Cox test. Statistical analyses were performed between animal groups that received protein/PS mixture or MAPS complex, unless indicated otherwise. All bar graphs represent mean ± SEM.
Supplementary Material
Acknowledgments
We thank Christine Hale and Gordon Dougan for providing S. typhimurium strain C5.507; Soushun Szu, Rachel Schneerson, and John Robbins for providing the S. typhi Vi polysaccharide; Robert Husson for helpful discussions and providing M. tuberculosis extracts; and Marc Lipsitch and Simon Dove for input and discussions. R.M. gratefully acknowledges support from the Translational Research Program at Children’s Hospital Boston and the National Institutes of Health (Grant R01 AI067737).
Footnotes
Conflict of interest statement: R.M. has provided consultative services to GlaxoSmithKline and serves on the scientific advisory board at Merck.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307228110/-/DCSupplemental.
References
- 1.Plotkin SA, Plotkin SL. The development of vaccines: How the past led to the future. Nat Rev Microbiol. 2011;9(12):889–893. doi: 10.1038/nrmicro2668. [DOI] [PubMed] [Google Scholar]
- 2.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21(6):443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho JS, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 5.Khader SA, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175(2):788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 6.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19(6):377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Queenan AM, Cassiday PK, Evangelista A. Pertactin-negative variants of Bordetella pertussis in the United States. N Engl J Med. 2013;368(6):583–584. doi: 10.1056/NEJMc1209369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipsitch M, O’Hagan JJ. Patterns of antigenic diversity and the mechanisms that maintain them. J R Soc Interface. 2007;4(16):787–802. doi: 10.1098/rsif.2007.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helppolainen SH, et al. Rhizavidin from Rhizobium etli: The first natural dimer in the avidin protein family. Biochem J. 2007;405(3):397–405. doi: 10.1042/BJ20070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology. 5th Ed. New York: Garland; 2001. [Google Scholar]
- 12.Petrovsky N, Aguilar JC. Vaccine adjuvants: Current state and future trends. Immunol Cell Biol. 2004;82(5):488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 13.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: Where are we and where do we need to go? PLoS Pathog. 2012;8(5):e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry AM, Paton JC. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64(12):5255–5262. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry AM, Ogunniyi AD, Miller DC, Paton JC. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect Immun. 1999;67(2):981–985. doi: 10.1128/iai.67.2.981-985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone, I: Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 17.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 18.Kwok SK, et al. TLR2 ligation induces the production of IL-23/IL-17 via IL-6, STAT3 and NF-kB pathway in patients with primary Sjogren’s syndrome. Arthritis Res Ther. 2012;14(2):R64. doi: 10.1186/ar3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyirenda MH, et al. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187(5):2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 20.Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986;18(Suppl A):35–45. doi: 10.1093/jac/18.supplement_a.35. [DOI] [PubMed] [Google Scholar]
- 21.Moffitt KL, Malley R. Next-eneration pneumococcal vaccines. Curr Opin Immunol. 2011;23(3):407–413. doi: 10.1016/j.coi.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: Implications for vaccine development. J Mol Med (Berl) 2010;88(2):135–142. doi: 10.1007/s00109-009-0579-4. [DOI] [PubMed] [Google Scholar]
- 23.Malley R, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74(4):2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel LS, Waltman WD, 2nd, Gray B, Briles DE. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb Pathog. 1987;3(4):249–260. doi: 10.1016/0882-4010(87)90058-1. [DOI] [PubMed] [Google Scholar]
- 25.Moffitt KL, Malley R, Lu YJ. Identification of protective pneumococcal T(H)17 antigens from the soluble fraction of a killed whole cell vaccine. PLoS ONE. 2012;7(8):e43445. doi: 10.1371/journal.pone.0043445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whaley MJ, et al. Concomitant administration of recombinant PsaA and PCV7 reduces Streptococcus pneumoniae serotype 19A colonization in a murine model. Vaccine. 2010;28(18):3071–3075. doi: 10.1016/j.vaccine.2010.02.086. [DOI] [PubMed] [Google Scholar]
- 27.Lu YJ, et al. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine. 2010;28(47):7468–7475. doi: 10.1016/j.vaccine.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gangarosa EJ, et al. Impact of anti-vaccine movements on pertussis control: The untold story. Lancet. 1998;351(9099):356–361. doi: 10.1016/s0140-6736(97)04334-1. [DOI] [PubMed] [Google Scholar]
- 29.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Ramon G. Diphtheria toxin and anatoxin. Ann Inst Pasteur (Paris) 1924;38:1–10. [Google Scholar]
- 31.Xiang SD, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40(1):1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Casella CR, Mitchell TC. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65(20):3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 34.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 2011;17(12):1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson P, Insel RA. A polysaccharide-protein complex from Haemophilus influenzae type b, I: Activity in weanling rabbits and human T lymphocytes. J Infect Dis. 1981;144(6):509–520. doi: 10.1093/infdis/144.6.509. [DOI] [PubMed] [Google Scholar]
- 36.Pichichero ME, Porcelli S, Treanor J, Anderson P. Serum antibody responses of weanling mice and two-year-old children to pneumococcal-type 6A-protein conjugate vaccines of differing saccharide chain lengths. Vaccine. 1998;16(1):83–91. doi: 10.1016/s0264-410x(97)00146-1. [DOI] [PubMed] [Google Scholar]
- 37.Anderson P, Pichichero M, Insel R, Farsad P, Santosham M. Capsular antigens noncovalently or covalently associated with protein as vaccines to Haemophilus influenzae type b: Comparison in two-year-old children. J Infect Dis. 1985;152(3):634–636. doi: 10.1093/infdis/152.3.634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.