Abstract
Intracellular trafficking between organelles is achieved by coat protein complexes, coat protomers, that bud vesicles from bilayer membranes. Lipid droplets are protected by a monolayer and thus seem unsuitable targets for coatomers. Unexpectedly, coat protein complex I (COPI) is required for lipid droplet targeting of some proteins, suggesting a possible direct interaction between COPI and lipid droplets. Here, we find that COPI coat components can bud 60-nm triacylglycerol nanodroplets from artificial lipid droplet (LD) interfaces. This budding decreases phospholipid packing of the monolayer decorating the mother LD. As a result, hydrophobic triacylglycerol molecules become more exposed to the aqueous environment, increasing LD surface tension. In vivo, this surface tension increase may prime lipid droplets for reactions with neighboring proteins or membranes. It provides a mechanism fundamentally different from transport vesicle formation by COPI, likely responsible for the diverse lipid droplet phenotypes associated with depletion of COPI subunits.
Keywords: regulator, membrane tension, lipid droplet targetting, buffer-in-oil drops
The dynamic behavior of cells requires a constant trafficking between organelles, which is largely achieved by vesicles. On bilayer-bound organelles, protein coats drive budding of transport vesicles (1). The coat protein complexes II (COPII) and I (COPI) generate vesicles from the endoplasmic reticulum and Golgi apparatus, respectively, whereas clathrin coats use various adaptor complexes to generate vesicles from the trans-Golgi network, endosomes, and the plasma membrane. Coatomer is a cytosolic complex that forms the building blocks of the COPI coat. At the Golgi apparatus, coatomer is recruited en bloc to the bilayer by Arf1 in a GTP-dependent manner (1–3). All known coat proteins act on phospholipid bilayer membranes.
Thus, it is surprising that COPI depletion affects lipid droplets (LDs) that are bounded by a single monolayer of phospholipids coating an organic phase of neutral lipids such as triacylglycerols (TAGs) (4–6). LDs expand and shrink during times of energy excess or scarcity (7). LD-bound proteins, including lipases and neutral lipid synthesis enzymes (8–12), mediate these processes. For instance, COPI depletion leads to mistargeting of adipose triglyceride lipase (ATGL), the enzyme catalyzing the first step of TAG lipolysis, to LDs, which results in TAG overstorage in cells (5, 6).
How COPI mediates its effect on the targeting of LD proteins is unknown, but evidence from proteomic and microscopy experiments suggests COPI might act directly on LDs (4–6, 13–15). Interaction of COPI with a monolayer membrane has never been shown. Here we demonstrate that COPI machinery directly assembles at the TAG surface and propose a simple mechanism by which this machinery may regulate protein targeting to LDs. We show that Arf1 and COPI can associate directly with the monolayer of an artificial mother TAG LD and that this association induces the formation of 60-nm “nano” LDs from the mother LD. This budding process increases the surface tension, which makes the mother LD more reactive with its environment, such as soluble enzymes or membranes, and thereby can explain how COPI is involved in the targeting of enzymes to a natural LD surface.
Results
Arf1 Binds TAG/Buffer Interface in a GTP-Dependent Manner.
On lipid bilayer membranes, COPI assembles in two steps: binding of Arf1 to the membrane in a GTP-dependent manner, followed by en bloc recruitment of coatomer by Arf1–GTP (16, 17). We investigate the possibility of this stepwise assembly on artificial LD surfaces.
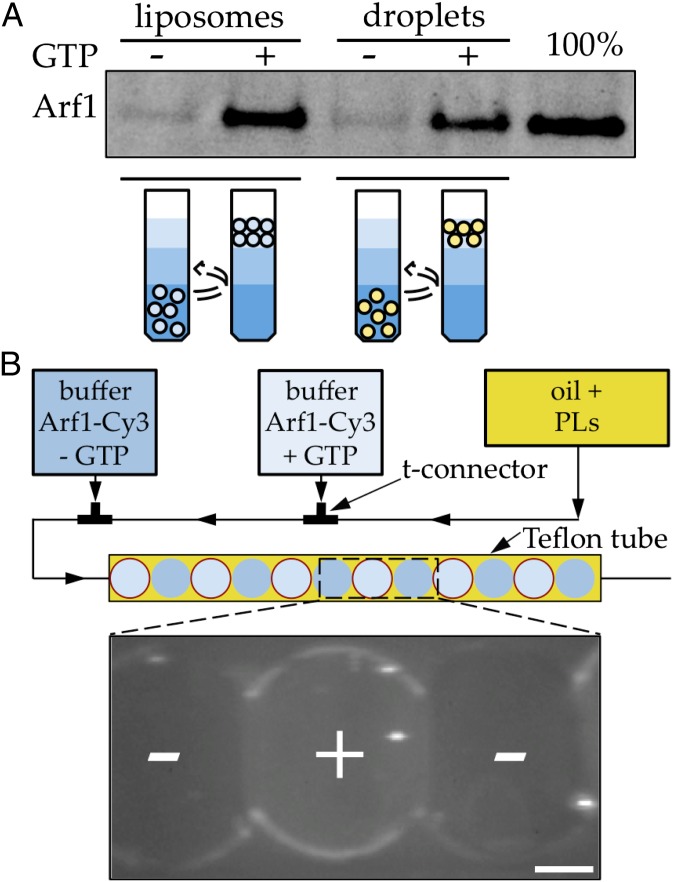
We tested Arf1 binding to LDs with two complementary approaches: flotation assay and microfluidics. We prepared TAG droplets that were surrounded by a monolayer of a phospholipid mixture (PL) of the same composition as that used to prepare control liposomes (PL composition is similar to that of natural LDs) (18). Arf1 binds to such droplets in a GTP-dependent manner and with a similar efficiency as on liposomes (Fig. 1A). We confirmed Arf1 binding to buffer/TAG interfaces using a microfluidic setup allowing direct visualization of protein interactions. We produced micrometric buffer drops in a stream of oil containing the phospholipids. The buffer/TAG interface is then coated with a monolayer of PL, as attested by the change in surface tension (see Fig. 4). In each buffer drop, biochemical reactions taking place at the buffer/TAG interface can be observed by fluorescence microscopy. The small buffer volume minimizes the amount of coatomer and Arf1 required, a decisive advantage compared with the inverse geometry where oil droplets are produced in a stream of buffer. Fig. 1B shows images of buffer droplets containing Cy3-labeled Arf1 and, alternatively, GDP or GTP. In agreement with the biochemical assay, Cy3–Arf1 accumulates in a GTP-dependent manner at the TAG/buffer interface decorated with a monolayer of PL, confirming that Arf1 is able to bind to the LD lipid monolayer surface.
Fig. 1.
GTP-specific binding of Arf1 to LDs. (A) Arf1 binds LDs or liposomes with the same efficiency and in a GTP-dependent manner. Extruded liposomes and TAG droplets containing the same amount of exposed PL (0.5 mM) were incubated during 30 min with Arf1–GDP (500 nM) and, when indicated, with EDTA (2 mM) and GTP (100 µM) to promote activation of Arf1. After separation of the free proteins from the liposomes or TAG droplets in a sucrose gradient, the amount of Arf1 bound to the membrane was revealed by SDS-PAGE gel stained with SYPRO Orange staining. The amount of Arf1 bound to liposomes and TAG droplets is similar (lanes 2 and 4). No binding is observed in the absence of GTP (lanes 1 and 3). Lane 5 represents the amount of Arf1 input in the solutions. (B) Specific binding of Arf1 to TAG/buffer interface in buffer drops watched in epifluorescence imaging. In a microfluidics channel (Upper), micrometric buffer droplets are produced in a stream of oil containing the phospholipids. Consecutive buffer drops containing fluorescent Arf1–Cy3 (30 nM), ARNO (200 nM), and alternately GTP (50 µM) or nucleotide-free control are produced with this setup. Arf1 only labels the aqueous/TAG interface when GTP is present (center buffer drop). Limited labeling of the contour is observed in the control drops (outer buffer drops). The Arf1–Cy3 signal looks lower at the interface between buffer drops because they are adherent and the interface is not vertical, which lowers the integrated intensity. (Scale bar, 50 µm.)
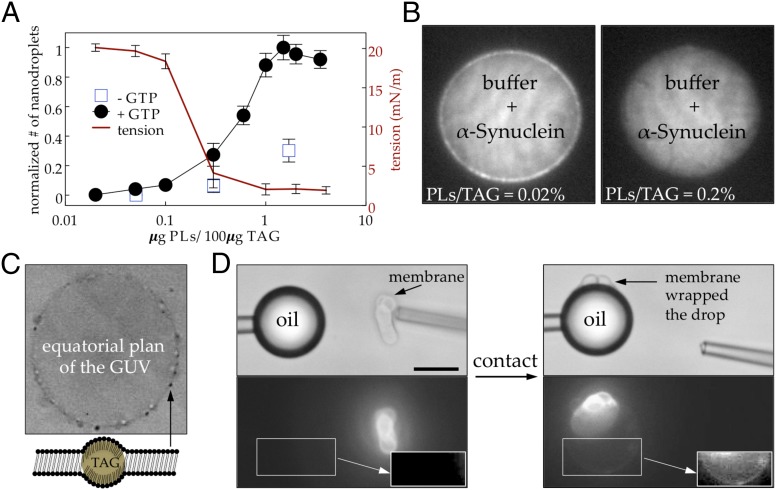
Fig. 4.
Consequence of the tension clamp model: LDs become hyperreactive. (A) Relative number of nanodroplets and surface tension for LDs with various PL concentrations. After separation from buffer drops, the nanodroplets were counted for various PL/TAG ratios (Fig. S3). The result was normalized to the maximum value at PL/TAG = 2% (left y axis). The surface tensions of the LDs were also measured by micropipette aspiration (right y axis, Fig. S5). COPI efficiency is optimal at vanishing surface tension (fully packed phospholipid monolayer) and very limited when the surface tension increases over a few millinewtons per meter (low PL packing). (B) Using the microfluidic setup developed in Fig. S1, we form buffer drops containing only GFP-labeled α-synuclein (11 nM). (Left) the oil contains 0.02% (wt/wt) PLs or (Right) 0.2% PLs. α-Synuclein binds to the interface for lower PL concentration (<0.03), whereas its binding is abolished for higher PL concentration. (C) Micrometer-scale LDs having low phospholipid packing fuse with bilayer membranes. Optical microscopy picture of LDs incubated with a GUV at PL/TAG ratios below 0.3% shows that LDs efficiently fuse with GUVs and are trapped between the two leaflets of the bilayer (Movie S2). The GUV presented here is 35 µm in diameter. When the PL/TAG ratios are larger than 1%, LDs do not fuse with the GUV membrane. (D) A giant artificial LD (30 µm in diameter, Left) with a PL/TAG ratio close to 0.3% and a fluorescent membrane structure mimicking a neighboring organelle (various curvatures, low surface tension) are brought into contact. Before contact, the LD was not fluorescent. As soon as the membrane touches the LD, they fuse and the membrane spreads around the LD surface (Right), demonstrating the hyperreactivity of the LD. (Insets) The giant LD with an increased contrast to better see the fluorescence. This process is shown in Movie S3. LDs with a PL/TAG ratio larger than 1% did not fuse with the membrane and both entities remained intact after contact for 5 min (Movie S4). (Scale bar, 20 µm.)
COPI Machinery Buds Particles from TAG/Buffer Interface.
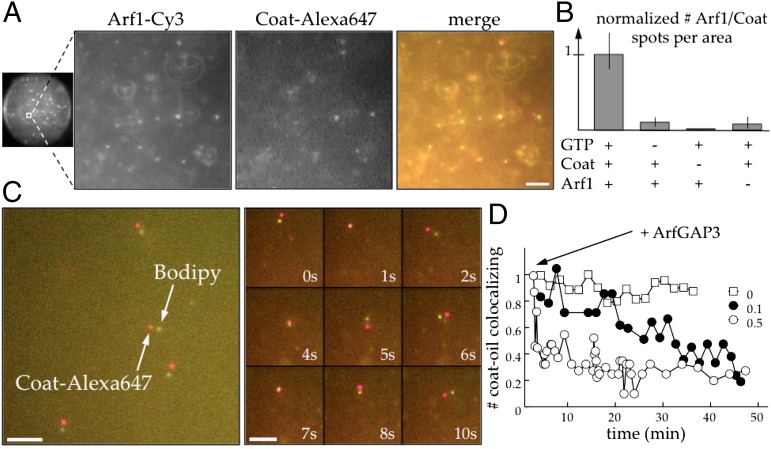
Next, we tested the ability of coatomer to be recruited to Arf1-decorated LDs. We added Alexa 647-labeled coatomer to Cy3–Arf1 and GTP to the buffer-in-oil drops. Under these conditions, the fluorescent proteins did not only cover the TAG/buffer interface. Instead, Arf1 and coatomer formed mobile spots in the aqueous volume and at the buffer/TAG interface (Fig. 2A and Movie S1) in a GTP-dependent manner (Fig. 2B). To determine the content of these spots, we performed the experiment with unlabeled Arf1, labeled coatomer, and oil containing a fluorescent dye (Bodipy). Again, colocalized spots were observed (Fig. 2C), suggesting a budding process in which small oil droplets are detached from the buffer/TAG interface by the COPI coat. After addition of ArfGAP3, which promotes GTP hydrolysis of Arf1 and reverses the coating process (19), the coat dissociated from these spot particles (Fig. 2D).
Fig. 2.
COPI produces nanodroplets from LDs. (A) Particles containing Arf1 and coatomer appear in the buffer drops in the presence of the COPI machinery (Left is a full image of a buffer drop; the other three panels are large magnifications to better see the particles). Less than 2 min after making the buffer drops containing Arf1–Cy3 (30 nM), coatomer (15 nM) labeled with Alexa 647, GTP (50 µM), and ARNO (200 nM), homogenous Arf1–Cy3 and coat–Alexa 647 spots appear in the aqueous volume and at the buffer/TAG interface. Arf1 (green) and coat (red) spots are colocalized, moving together in the buffer drop (Movie S1). The spots are slightly separated because of the time delay to switch laser in the setup. (Scale bar, 5 µm.) (B) The formation of particles is GTP-dependent. In the controls without GTP, coatomer or Arf1, the amount of spots per area is significantly reduced compared with the experiment with 50 µM GTP, 30 nM Arf1, and 15 nM coatomer (Left). (C) The particles are TAG nanodroplets. Same experiment as in A with unlabeled Arf1 (100 nM) and Bodipy dye (1% wt/wt) in the TAG. After collection of the buffer drops as indicated in Fig. S1, colocalized Bodipy/Alexa 647 spots are observed. (Scale bars, 10 µm.) (D) Loss of colocalization over time after ArfGAP3 addition. The sample, recovered as shown in Fig. S1, is split in three vials. The amount of particles is quantified as described in Fig. S3. ArfGAP3 is added in two of the samples at different concentrations (50 and 10 nM) corresponding to fractions equal to 0.5 and 0.1 of the Arf1 concentration. Colocalization of coat–Alexa 647 and TAG–Bodipy is lost over time compared with the control.
Budded Particles Are 60-nm COPI-Coated LDs.
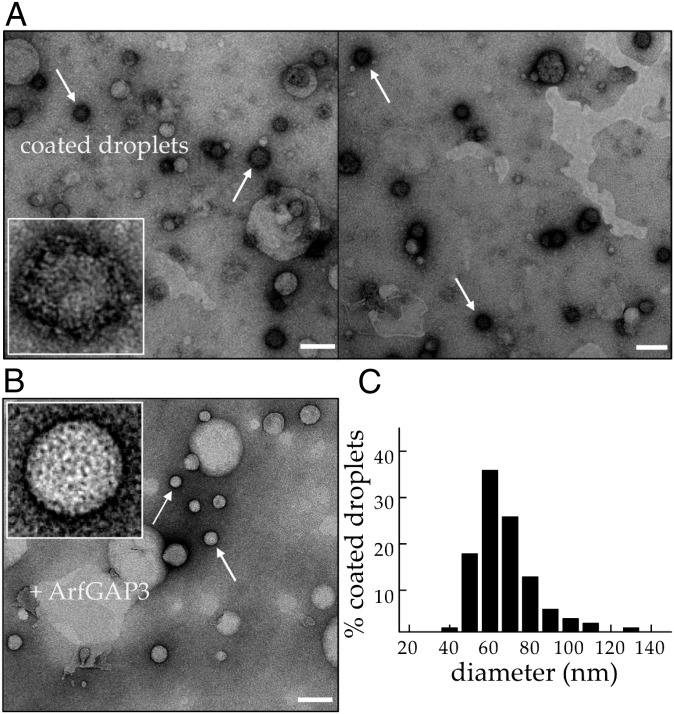
To determine the characteristics of the newly formed particles, we isolated them from the buffer drops (Fig. S1) and observed them by EM. The spots can clearly be identified as nano LDs with coat polymer visible at their surface (Fig. 3A, white arrows and Inset). The coat polymer disappears after treatment with ArfGAP3 (Fig. 3B). The size distribution of these nanodroplets shows that they are monodisperse with a typical diameter of 60 ± 15 nm (Fig. 3C). This distribution is consistent with the estimated size obtained from the diffusion coefficients measured by fluorescence cross-correlation spectroscopy (∼90 nm, Fig. S2A) and by independent direct tracking of the particles collected from the buffer drops (∼100 nm, Fig. S2B). Taken together, these results show that the COPI machinery is able to function on LDs in the same manner as on lipid bilayers by inducing the budding and fission of 60-nm TAG nanodroplets very close to the size of COPI vesicles (2, 3).
Fig. 3.
COPI-induced nanodroplets are 60 nm in diameter. (A) TAG nanodroplets with COPI coat observed by negative staining EM. Particles were extracted from buffer drops containing unlabeled Arf1 (100 nM), coatomer molecules (15 nM), GTP (50 µM), and ARNO (200 nM) in a stream of TAG. Budded TAG nanodroplets can be identified because they are surrounded by a layer of dark coats (COPI, white arrows). Two different fields are shown. (Inset) Magnification of one budded nanodroplet surrounded by a structure shaped as a coat assembly. Large TAG drops (Left) that are sometimes extracted during the process (probably by shear) are not surrounded by a coat. (Scale bar, 100 nm.) (B) The sample recovered under the same conditions as in A was treated with a large amount of ArfGAP3 (ArfGAP3/Arf1 = 1) for 10 min. Sixty-nanometer TAG nanodroplets can be identified but are no longer surrounded by a layer of coat as in A. (Inset) Magnification view of a droplet without coat. (Scale bar, 100 nm.) (C) Size distribution of 278 nanodroplets from 20 EM images similar to that in A. The average lipid nanodroplet size measured by EM is 60 ± 15 nm.
COPI Budding Exclusively Occurs at Interfaces with Low Tension.
Because natural LDs undergo shrinking and growth phases, we decided to probe the effect of the state of the LD surface on the efficiency of the COPI-induced budding process. We performed microfluidic experiments with increasing amounts of PL (Fig. S3). As shown in Fig. 4A, the number of COPI-induced nanodroplets formed in the buffer drops dramatically increases between 0.1% and 1% PL per TAG (wt/wt), suggesting that the COPI–coat machinery acts preferentially on a packed PL monolayer. When covering the interface, PLs, thanks to their amphiphilic nature, decrease the surface tension by shielding TAG molecules from the aqueous buffer. Because an interface with a low surface tension is more deformable, a packed PL monolayer should facilitate the budding of COPI nano LDs (20). Following this hypothesis, we used a micromanipulation approach (Fig. S4) to measure the surface tension of LDs at various PL concentrations. Strikingly, the surface tension decreased sharply from ∼20 mN/m to a vanishing surface tension (below 0.5 mN/m, the detection limit of the technique) exactly in the range of PL concentration (0.1–1% wt/wt PL/TAG) at which COPI reaches its optimum budding efficiency (Fig. 4A). The COPI machinery acts mainly at low surface tension, below a threshold of 2 mN/m.
Budding Nanodroplets Increases the Surface Tension.
The main result of nanodroplet formation should be a decrease in PL packing on the mother LD as nanodroplets “capture” relatively more surface than volume from the mother LD (Fig. S5). Put differently, nanodroplet budding consumes the PL monolayer covering the surface of the mother LD, inducing a decrease in the PL/TAG ratio. Energetically, this consumption will increase the surface tension of the mother LD. With a Langmuir trough we measured the variation of PL packing with the surface tension (and therefore PL/TAG ratio, Fig. S6). Remarkably, a 10% decrease in the PL packing from the maximum pressure (corresponding to maximum lipid packing for which the surface tension is below the detection, 0.5 mN/m) is sufficient to raise the surface tension above the 2 mN/m threshold for COPI budding of nanodroplets. A 10% decrease in the PL packing from the maximum density is achieved by budding off approximately five nanodroplets from a 500-nm mother LD (Fig. S7), a typical size of physiological LDs. Hence, a very limited action of COPI is sufficient to induce a substantial change in surface tension.
LDs with High Surface Tension Can Sense Their Environment.
Upon surface tension increase induced by COPI, a mother LD will probably become more prone to react with its environment (e.g., with soluble proteins or membranes). First, we used α-synuclein as a model of soluble proteins, because it is known to bind natural LDs (21) and is able to sense phospholipid packing on bilayers (22). We found that binding of α-synuclein to LDs is highly dependent on the PL packing at the interface. When the monolayer is not fully packed α-synuclein binds to the LD surface, whereas at full PL coverage no binding occurs (Fig. 4B). Second, we tested the ability of LDs of various PL compositions to fuse with neighboring bilayer membranes by incubating them with model membranes [giant unilamellar vesicles (GUVs), Fig. 4C, or tensionless micrometer-scale membranes resembling organelles with various curvatures, Fig. 4D]. At vanishing surface tension [PL/TAG ratio larger than 1% (wt/wt)], LDs remained intact and did not interact with any of the bilayer membranes. In contrast, at nonzero surface tension [PL/TAG ratio below 0.3% (wt/wt)], the LDs quickly got imbedded in the GUV membrane to form visible lenses between the leaflets of the bilayer (Fig. 4C and Movie S2) or fuse with the tensionless membranes that quickly form a monolayer around them (Fig. 4D and Movie S3). This demonstrates that LDs with lower PL surface packing induced, for instance, by COPI will become hyperreactive and will interact with neighboring membranes or amphiphilic molecules, leading to a remodeling of the composition of the LD surface through the relocation of new molecules.
Discussion
The fact that COPI machinery is able to work on monolayers and bud oil droplets is a unique and apparently an intrinsic capability of the machinery. Because of the specificity of a monolayer compared with a bilayer, we propose that the COPI machinery performs a previously unrecognized function, clamping the surface tension of the monolayer at a buffer/oil interface by preventing it from dropping down to low tensions, below 2 mN/m. At the molecular level, this action of COPI prevents the LD surface from being fully covered by PLs and provides more accessibility to TAG for binding/reacting with other components. Consistent with the specificity of LD phenotypes associated with depletion of COPI subunits (4, 6), this is likely the only protein coat that can perform this function: COPII depends on a transmembrane guanine nucleotide exchange factor, Sec12, whereas ArfGEFs are peripheral proteins that might directly bind to LDs; clathrin coats assemble on membranes rich in anionic lipids, whereas the surface of LDs is very poor in such lipids (18).
Hyperreactivity of LDs after COPI action could have several physiological implications. Cytosolic proteins may directly bind to the mother LD surface. Alternatively, LDs might fuse with surrounding organelles, such as other LDs or bilayer membranes. This mechanism may explain the COPI dependence of targeting ATGL-related LD proteins (Fig. S8). For instance, COPI-induced budding of nanodroplets and the resulting decrease in PL packing might lead to the formation of connections between LD and other bilayer membranes, typically endoplasmic reticulum, through which proteins can exchange. These connections have been suggested in various systems by fluorescence recovery after photobleaching and EM experiments (10, 23, 24). Such a mechanism would probably entail a regulation of the action of COPI so that it is active only whenever the targeting of a specific enzyme is required.
Materials and Methods
Proteins.
Fluorescently labeled Arf1 was generated by using an Arf1 variant, where the single cysteine residue of Arf1 was replaced with serine, and the C-terminal lysine was replaced with cysteine (Arf1-C159S-K181C) (25). Human Arf1-C159S-K181C and yeast N-myristoyltransferase were coexpressed in Escherichia coli supplied with BSA-loaded myristate. Cell lysates were subjected to 35% (vol/vol) ammonium sulfate, and the precipitate, enriched in myristoylated Arf1, was further purified by DEAE-ion exchange. Eluted fractions of interest were concentrated in spin-column filters with a 10-kDa cutoff (Millipore) and fluorescently labeled using Cy3-maleimide (GE Healthcare) according to the manufacturer’s protocol. To remove excess dye, samples were purified by gel filtration using a Superdex 75 column (GE Healthcare).
Recombinant coatomer protein was expressed and purified as described (26). Sf9 insect cells were infected with baculovirus encoding for heptameric coatomer. Coatomer complexes were isolated from the soluble protein fraction by nickel-affinity purification, concentrated in spin-column filters with a 250-kDa cutoff (Millipore), and fluorescently labeled using Alexa-Fluor-647-NHS (Molecular Probes) according to the manufacturer’s protocol. Excess imidazole and dye was removed by gel filtration using a Superose 6 column (GE Healthcare).
PL Mixture Composition.
We chose a lipid composition close to that of natural LDs (18): dioleoylphsphatidylcholine (DOPC):dioleoylphsphatidylethanolamine (DOPE):cholesterol:lyso-phosphatidylinositol:lyso-phosphatidylethanolamine:lyso-phosphatidylcholine (50:20:12:10:5:3).
Unless specified, PL/TAG is fixed at 0.5% (wt/wt).
Buffer.
Unless otherwise indicated, experiments were performed in HKM buffer: 50 mM Hepes, 120 mM Kacetate, and 1 mM MgCl2 (in Milli-Q water).
Preparation of Synthetic Liposomes and Droplets for Flotation Experiments.
For synthetic liposomes, a chloroform solution containing 1 µmol egg PC and 1.6 nmol Rhodamine-PE was dried under argon gas in a glass tube. The lipid film was resuspended in 1 mL HKM buffer. After five cycles of freezing and thawing, the liposome suspension was extruded 19 times through a 0.4-µm polycarbonate filter.
For synthetic droplets, 70 µL TAG was mixed in a glass tube with 0.5 µmol egg PC and 1.6 nmol Rhodamine-PE from stock solutions in chloroform. The solvent was removed using a stream of argon gas and then 0.93 mL of HKM buffer was added to the TAG/PL mixture. An emulsion was obtained by vortex and extruded nine times through a 1-µm polycarbonate filter. After extrusion, the emulsion becomes extremely turbid. Examination of the suspension under a microscope shows micrometer-size LDs that were stable for many hours.
Buffer Drops Preparation.
Two syringes were filled, one with oil and the other with buffer and proteins. Using a syringe pump, streams from both syringes were allowed to flow into a high-pressure T connector with a 250-µm inside diameter constructed of fluorinated ethylene propylene (27) (Fig. S1). Because of the wetting properties of the oil to the T connector and the tube, and the ratio of the flow rates (oil rate/buffer rate = 5), 250-µm buffer droplets are generated at the outlet of the T connector (28) and circulate in a transparent Teflon tube where observations were made. The time of reaction represents the time spent by each microreator in the tube; the length of the tube and the flow rate controls it. The flow rate was 1,250 µL/h and the length of the tube was 2 m. The diameter of the tube was 250 µm, so the reaction time was ∼15 min.
GUVs and Micrometer-Scale LDs.
GUVs were prepared using an electroformation technique (29). One microliter of PL mixture in chloroform at 0.3 mM was dried on an indium tin oxide (ITO)-coated glass plate. The lipid film was desiccated for 1 h. The chamber was sealed with another ITO-coated glass plate. The lipids were then rehydrated with a sucrose solution (300 mOsm). The alternative (8 Hz) voltage between the two glass plates was increased by steps every 6 min: 100 mV, 200 mV, 300 mV, 500 mV, 700 mV, 900 mV, and 1.1 V. The last voltage was maintained for at least 1 h. GUVs were either stored in the chamber at 4 °C overnight or directly collected with a Pasteur pipette.
The LDs used in experiments with GUVs (Fig. 4C) were prepared by first drying PLs and solubilizing them afterward into TAG to obtain the required PL/TAG ratio. A mixture of 5 µL of this PL/TAG solution and 95 µL buffer was first vortexed and then sonicated using a Branson 2510 sonicator working at 40 kHz for 20 s. The diameter of the resulting droplets is a few hundred nanometers.
GUVs and generated LDs were incubated together in buffer for 10 min under gentle shaking and subsequently observed under an optical microscope (Fig. 4C is an example of a GUV incubated with LDs at a 0.3% PL/TAG ratio).
LDs and Membranes Mimicking Organelles.
To obtain membranes resembling organelles in terms of shape (curvatures) and tension (low tension), 5 µL PL lipid in chloroform (3 mM) was dried on a coverslip and placed in a desiccator for 1 h. The lipid film was rehydrated in 20 µL of buffer for 10 min. The solution was collected and injected into a Petri dish.
To prepare giant LDs, PL mixtures were dried and dissolved into TAG at different concentrations (wt/wt, typically from 0.2 to 5%). Then 5 µL of the oil solution was added to 95 µL of buffer and the mixture was vortexed 10 s using a Fischer Vortex Genie 2 at maximum power.
During the experiment, the LD and the membrane were manipulated and brought into contact through two pipettes via aspiration and observed under a microscope (bright field and fluorescence). Note that LDs were injected last into the Petri dish because they tend to float at the buffer/air interface, where they often spread.
Flotation Assay.
Liposomes (120 µL, 1 mM phospholipid) or LDs (120 µL, 0.5 mM phospholipid; TAG/buffer 7/93 vol/vol) were mixed with myristoylated Arf1GDP (0.5 µM) in a final volume of 125 µL. When indicated, the suspension was supplemented with 100 µM GTP and 2 mM EDTA to promote GDP to GTP exchange on Arf1. After incubation for 30 min at room temperature, the sample was adjusted to 30% (wt/vol) sucrose and covered with two cushions of 25% (wt/vol) sucrose (200 µL) and 0% sucrose (50 µL), respectively. The samples were centrifuged for 80 min at 30,000 rpm in a SW60 rotor (Beckman). The top (100 µL), medium (200 µL), and bottom (250 µL) fractions were collected and analyzed by SDS/PAGE using SYPRO Orange staining.
EM.
Nano LDs were collected as indicated in Fig. S1. Samples of 5 µL were absorbed to continuous carbon-coated grids (glow discharged) at room temperature for 1 min, rinsed briefly with HKM buffer, and stained with 1% (vol/vol) uranyl acetate for 20 s. Negatively stained samples were imaged under low-dose conditions in an FEI Tecnai12 microscope (120 kV). Micrographs were collected at 26,000× magnification, giving an unbinned pixel size of 4.2 Å. The diameters of nano LDs were manually measured directly from the micrographs.
Fluorescence Cross-Correlation Spectroscopy.
Oil was labeled with 1% vol/vol Bodipy (excited at 488 nm). Coatomer was labeled with Alexa 647 (excited at 632 nm). The product of reaction was recovered as described in Fig. S1 and analyzed with the FCS setup, Confocor2, on the Zeiss microscope LSM510. The emission and excitation spectra of the two dyes are separated enough to not cause any cross-talk or FRET. For each channel, autocorrelation curves were recorded simultaneously within 30 s for many runs of different samples (representing 10 different experiments). Similarly, a cross-correlation curve was simultaneously generated in a third channel. For the cross-correlated signals, we fit the far-red and green autocorrelation curves, G(τ), with a theoretical model comprising three components, giving, therefore, three diffusion times (30). The choice of such a number of fitting parameters is driven by the fact we may have signals from the free dye, the budded particles, and probable aggregates or just large, polluting particles.
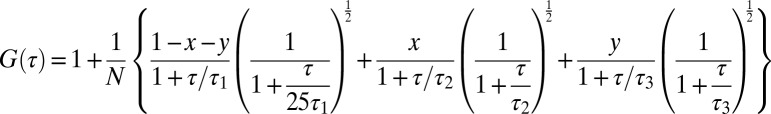 |
The fraction of each particle is given by (x,y) and N yields the total concentration of particles. The diffusion times are τi. For each fitted curve, the access of τi determines the size of the particles by the Stoke–Einstein law,
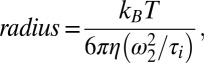 |
where kB stands for the Boltzmann constant, T the temperature (room temperature), η the viscosity of the buffer (1 cSt), and ω2 the width of the focal volume (115 nm at 488 excitation and 155 nm at 647 excitation).
Measurement of the Interfacial Tension of LDs with Micropipettes.
Phospholipids were mixed at the ratio 70% DOPC: 30% DOPE (mol/mol), dried under vacuum for 1 h, and resuspended in TAG. Ten microliters of this solution was vortexed with 200 µL HKM buffer for 30 s. Then 50 µL of this emulsion was injected into a 1-mL HKM buffer drop deposited on a coverslip and observed with optical microscopy.
The interfacial tension (IT) of the droplets was measured using a micromanipulation technique. The device was made up of a micromanipulator and a pipette holder (Narishige). Pipettes were incubated in a 5% (wt/vol) BSA before use to prevent droplets from adhering to the glass. As shown in Fig. S4, micromanipulation of a single droplet enables the IT to be determined through measurement of pipette diameter, droplet diameters, and minimal pressure at which the droplet was drawn into the pipette (31):
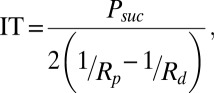 |
where Psuc, Rp, and Rd represent the suction pressure, pipette radius, and droplet radius, respectively. The sizes of pipette and droplet were obtained by image analysis (ImageJ). The suction was carried out using a syringe. The resulting pressure was measured with a pressure transducer (DP103; Validyne Engineering Corp.), the output voltage being monitored with a digital voltmeter. The pressure transducer (range 55 kPa) was calibrated before experiments.
Compression Isotherm of PL Monolayer at the TAG/Buffer Interface.
The lipid mixture isotherm was carried out using a Teflon Langmuir trough (Minimicro; KSV) equipped with hydrophilic barriers made of polyoxymethylene (Derlin). The Whilhelmy pressure sensor (KSV) was coupled with a paper plate. Dimensions of the trough were 165 × 51 mm. Room temperature during the experiments was 20.5 ± 0.5 °C. The trough took place on an antivibration table in a closed box containing a water-saturated atmosphere to prevent evaporation. Lipid mixture was spread on HKM buffer before gently depositing droplets of TAG solution in chloroform (1% vol/vol). The amount of deposited TAG (0.12 µL) was at least fivefold higher than the amount of TAG required for the interface to be saturated. The so formed mixed TAG–lipids monolayer, at the interface between the very thin TAG film and HKM subphase, relaxed during 5 h before compression. The compression rate was held constant (0.5Å2⋅min–1⋅mol–1). The pressure was measured with an accuracy of 0.5 mN/m and the molecular area was controlled with 5% accuracy. The monolayer collapse was observed for 48Å2/mol molecular area and 36.6 mN/m pressure values. The pressure owing to pure oil spreading was determined to be 15.2 mN/m. We carried out independent measurements of the surface pressure as a function of lipid mixture spread at the TAG–HKM interface formed in a 50-mm-diameter vessel (glass and Teflon). Even if similar observations have previously been reported in the literature (32), it is worth noting that the results were consistent within the experimental errors with those obtained with the trough regardless of the size of the oil reservoir (from oil monolayer up to macroscopic amounts of oil).
Supplementary Material
Acknowledgments
We thank T. Melia, C. Burd, and J. Bibette for many helpful discussions. This work was supported by a grant from the Marie Curie Budding and Fusion of Lipid Droplets International Outgoing Fellowship within the Seventh European Community Framework Program (to A.R.T.), a Partner University Funds exchange grant between the Yale University and Ecole Normale Supérieure laboratories, European Research Council Grant 268888 (to B.A.) and National Institutes of Health Grant R01GM097194 (to T.C.W.). F.W. is a fellow of the Boehringer Ingelheim Fonds.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307685110/-/DCSupplemental.
References
- 1.Faini M, Beck R, Wieland FT, Briggs JA. Vesicle coats: Structure, function, and general principles of assembly. Trends Cell Biol. 2013;23(6):279–288. doi: 10.1016/j.tcb.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Serafini T, et al. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: A novel role for a GTP-binding protein. Cell. 1991;67(2):239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89(14):6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453(7195):657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soni KG, et al. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122(Pt 11):1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beller M, et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6(11):e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139(5):855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50(1):3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.McFie PJ, Banman SL, Kary S, Stone SJ. Murine diacylglycerol acyltransferase-2 (DGAT2) can catalyze triacylglycerol synthesis and promote lipid droplet formation independent of its localization to the endoplasmic reticulum. J Biol Chem. 2011;286(32):28235–28246. doi: 10.1074/jbc.M111.256008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilfling F, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24(4):384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49(11):2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penno A, Hackenbroich G, Thiele C. Phospholipids and lipid droplets. Biochim Biophys Acta. 2013;1831(3):589–594. doi: 10.1016/j.bbalip.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Hommel A, et al. The ARF-like GTPase ARFRP1 is essential for lipid droplet growth and is involved in the regulation of lipolysis. Mol Cell Biol. 2010;30(5):1231–1242. doi: 10.1128/MCB.01269-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellong EN, et al. Interaction between the triglyceride lipase ATGL and the Arf1 activator GBF1. PLoS ONE. 2011;6(7):e21889. doi: 10.1371/journal.pone.0021889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura N, Banno Y, Tamiya-Koizumi K. Arf1-dependent PLD1 is localized to oleic acid-induced lipid droplets in NIH3T3 cells. Biochem Biophys Res Commun. 2005;335(1):117–123. doi: 10.1016/j.bbrc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA. 1998;95(19):11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremser M, et al. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96(4):495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 18.Bartz R, et al. Dynamic activity of lipid droplets: Protein phosphorylation and GTP-mediated protein translocation. J Proteome Res. 2007;6(8):3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- 19.Weimer C, et al. Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking. J Cell Biol. 2008;183(4):725–735. doi: 10.1083/jcb.200806140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foret L, Sens P. Kinetic regulation of coated vesicle secretion. Proc Natl Acad Sci USA. 2008;105(39):14763–14768. doi: 10.1073/pnas.0801173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole NB, et al. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J Biol Chem. 2002;277(8):6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 22.Pranke IM, et al. α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J Cell Biol. 2011;194(1):89–103. doi: 10.1083/jcb.201011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquier N, et al. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci. 2011;124(Pt 14):2424–2437. doi: 10.1242/jcs.076836. [DOI] [PubMed] [Google Scholar]
- 24.Ohsaki Y, et al. Biogenesis of cytoplasmic lipid droplets: From the lipid ester globule in the membrane to the visible structure. Biochim Biophys Acta. 2009;1791(6):399–407. doi: 10.1016/j.bbalip.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Manneville JB, et al. COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proc Natl Acad Sci USA. 2008;105(44):16946–16951. doi: 10.1073/pnas.0807102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahlmüller MC, et al. Recombinant heptameric coatomer complexes: Novel tools to study isoform-specific functions. Traffic. 2011;12(6):682–692. doi: 10.1111/j.1600-0854.2011.01177.x. [DOI] [PubMed] [Google Scholar]
- 27.Engl W, Backov R, Panizza P. Controlled production of emulsions and particles by milli- and microfluidic techniques. Curr Opin Colloid Interface Sci. 2008;13(4):206–216. [Google Scholar]
- 28.Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu Rev Fluid Mech. 2004;36:381–411. [Google Scholar]
- 29.Mathivet L, Cribier S, Devaux PF. Shape change and physical properties of giant phospholipid vesicles prepared in the presence of an AC electric field. Biophys J. 1996;70(3):1112–1121. doi: 10.1016/S0006-3495(96)79693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengupta P, Garai K, Balaji J, Periasamy N, Maiti S. Measuring size distribution in highly heterogeneous systems with fluorescence correlation spectroscopy. Biophys J. 2003;84(3):1977–1984. doi: 10.1016/S0006-3495(03)75006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeung A, Dabros T, Masliyah J, Czarnecki J (2000) Micropipette: A new technique in emulsion research. Colloids Surfaces A Physicochem Eng Aspects 174(1–2):169–181.
- 32.Thoma M, Pfohl T, Mohwald H. Thermodynamic relation of an insoluble monolayer at the oil/water interface and at the air/water interface in contact with oil. Langmuir. 1995;11(8):2881–2888. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.