Abstract
The anti-inflammatory drug high-dose intravenous immunoglobulin, widely used to suppress inflammation, depends on a specific α-2,6-sialylated glycoform of IgG Fc to induce Interleukin 4 (IL-4) and Signal Transducer and Activator of Transcription 6 (STAT6) signaling for its activity. Here we show that anti-inflammatory activities of IL-4 can be attributed to the direct action of this cytokine on myeloid effector cells, depending on their expression of the IL-4 receptor alpha chain (IL-4Rα/CD124). However, in their basal state, these cells express low levels of IL-4Rα and would not be expected to result in significant signaling compared with other cell populations. This apparent paradox can be explained by the observation that during inflammation, triggered by a variety of stimuli (including autoantibodies, adjuvants, and TLR ligands), IL-4Rα is up-regulated specifically on these cells, priming them for STAT6 signaling. The regulation is mediated by a soluble, proteinase K-sensitive factor, released to the circulation by bone marrow-derived, non-B/non-T cells found in several organs, including the lungs, and fat. We propose that this regulation is part of a homeostatic mechanism to limit excessive inflammation and tissue damage. High-dose intravenous immunoglobulin thus exploits an endogenous feedback loop, general to inflammation, that could be further targeted for therapeutic purposes.
Keywords: neutrophils, monocytes, macrophages
Inflammation is a necessary host response that has evolved to protect the organism after exposure to potentially life-threatening pathogens. Although this response is essential for host survival, unregulated activation of the multiple components of the immune system that characterize this response can result in catastrophic sequelae (1). To balance this potent response, many homeostatic mechanisms exist that limit excessive immune activation. One such example is the up-regulation of surface receptors mediating inhibitory signals, thereby directly or indirectly balancing signaling from activating receptors. Examples of important inhibitory receptors that are up-regulated to balance activating signals are cytotoxic T-lymphocyte antigen 4 (CTLA-4/CD152), the death receptor Fas (CD95), and Fc gamma receptor (FcγR) IIB (2–4). However, despite these mechanisms, excessive, misdirected, and chronic immune activation is the underlying basis for a large group of inflammatory diseases, often with an autoimmune etiology, including rheumatoid arthritis (RA) and systemic lupus erythematosus (5, 6). Deficiencies in many inhibitory receptors, including those listed above, have been linked to increased susceptibility for autoimmune diseases (7–9). Much attention has been given to the development of anti-inflammatory drugs that can attenuate these chronic inflammatory conditions, with the goal of restoring normal homeostatic mechanisms. High-dose intravenous immunoglobulin (IVIG) is a preparation of IgG pooled from thousands of donors and is commonly administered to patients with autoimmune diseases at a high dose (1–2 g/kg) with therapeutic benefit (10). Human and animal studies have identified the active component of the drug to the Fc domain of the IgG molecule (11, 12) and have further identified a minor glycoform of IgG, containing terminal sialic acid on the Asn-297–attached Fc glycan (sialylated Fc; sFc) as responsible for the anti-inflammatory activity of this preparation (13, 14). We recently showed that IVIG- or sFc-mediated protection in a model of RA, the serum transfer K/BxN model, is abrogated in mice lacking IL-4, IL-4Rα, or STAT6 (15). These studies identified IL-4–induced STAT6 signaling as an important component of an sFc-induced homeostatic mechanism functioning to limit excessive inflammation and prompted us to further study this pathway.
Results and Discussion
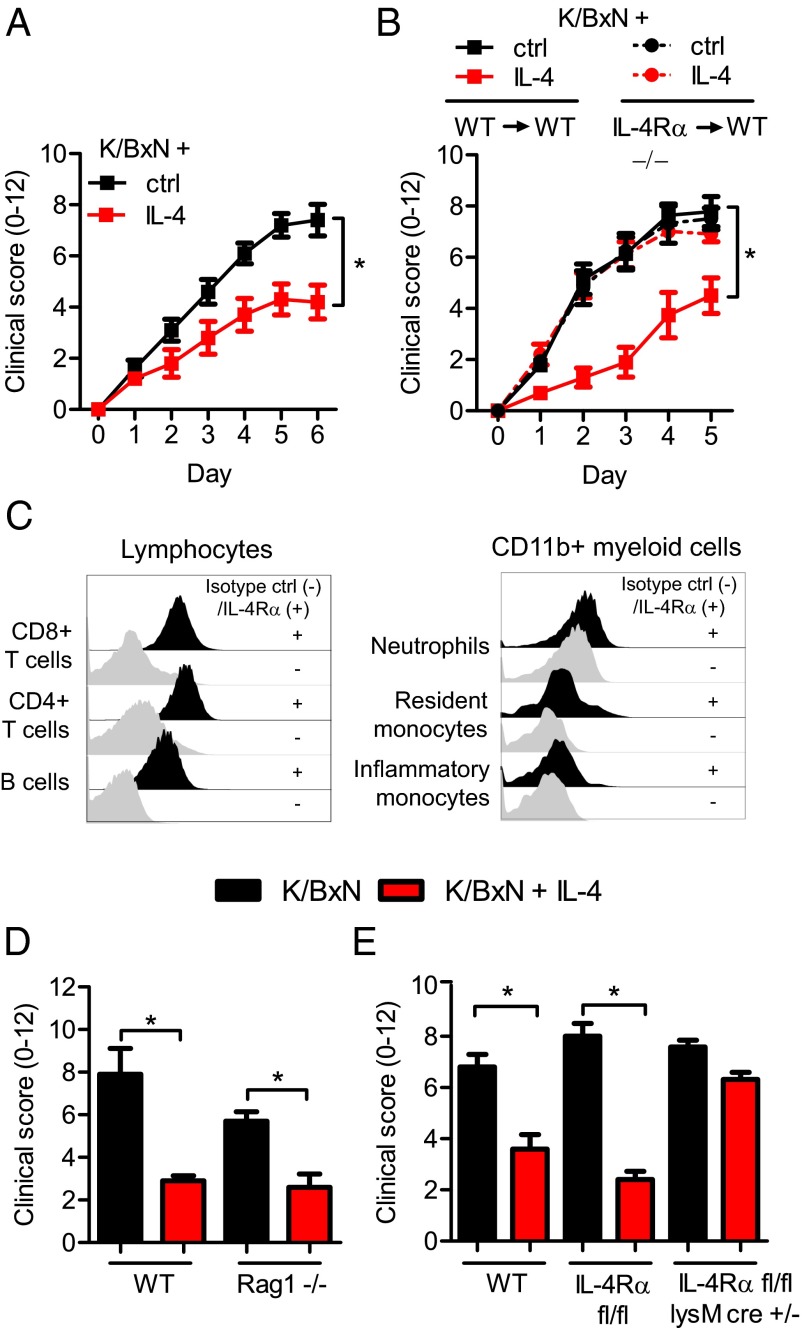
Inflammation triggered by K/BxN serum transfer is mediated by pathogenic antibodies targeting the ubiquitous antigen glucose-6-phosphate isomerase (16). These antibodies form immune complexes in recipient mice that result in swelling and inflammatory infiltrates in the joints. The inflammation requires expression of activating FcγRs on inflammatory myeloid effector cells, such as neutrophils, monocytes, and macrophages (17–19). In the K/BxN model, IL-4 administration protects from joint inflammation (Fig. 1A), which requires IL-4Rα and downstream STAT6 signaling (15). To further dissect the anti-inflammatory properties of IL-4 in the K/BxN model, we investigated the prerequisites for this protection. IL-4Rα is expressed by many cells, including both hematopoietic and nonhematopoietic cell populations (20). To determine the cell populations responsible for this IL-4–mediated protection, we irradiated wild-type (WT) mice and reconstituted them with either WT or IL-4Rα–deficient bone marrow (BM) cells (Fig. S1A). Whereas recipients of WT BM cells were protected from K/BxN inflammation by IL-4, recipients of IL-4Rα–deficient BM cells were not (Fig. 1B and Fig. S1 B–E). This result identified BM-derived cells, i.e., hematopoietic cells, as a primary target for IL-4–mediated suppression of autoantibody-induced joint inflammation. Examining the expression of IL-4Rα on BM-derived hematopoietic cells by flow cytometry, we found that B and T cells are the predominant populations expressing the receptor in naïve mice, whereas CD11b+ myeloid cells express much lower levels (Fig. 1C and Fig. S2A). In agreement with the flow cytometry data, quantitative PCR of sorted cell populations showed that B and T cells express 10-fold more IL-4Rα mRNA compared with neutrophils and monocytes (Fig. S2B), consistent with the original observations of IL-4–induced signaling originating from B and T cells (21, 22). To determine whether these cell types were required in our system, we challenged IL-4–treated B-cell– and T-cell–deficient Rag1−/− mice with K/BxN serum and observed that they were still protected from inflammation by IL-4 (Fig. 1D). In contrast, mice lacking IL-4Rα on CD11b+ myeloid cells (Fig. S2C), mediated by LysMcre-driven excision of floxed IL-4Rα alleles, were not protected by IL-4 (Fig. 1E). We concluded therefore that IL-4Rα expression is required on BM-derived myeloid cells for IL-4–mediated protection from inflammation, despite the low levels of receptor expressed by these cells in their basal state.
Fig. 1.
IL-4 protects from K/BxN-mediated joint inflammation by targeting IL-4Rα on myeloid cells. (A) WT mice were given K/BxN serum and IL-4 (red) or control (black). Joint swelling (0–12) was followed daily for 6 d and presented as mean and SEM; n = 5 per group. (B) WT mice were irradiated and reconstituted with either WT (WT→WT) or IL-4Rα−/− (IL-4Rα−/−→WT) BM cells. Eight weeks after irradiation and BM transfer, mice were injected with K/BxN serum and IL-4 (red) or control (black). Joint swelling over time is presented as mean and SEM; n = 5–8 per group. (C) Flow cytometry analysis of IL-4Rα expression (black histograms) compared with an isotype control (gray histograms) on major blood cell populations in naïve mice. (D) WT and Rag1−/− injected with K/BxN serum and IL-4 (red) or control (black). Data are shown for day 5 as mean and SEM; n = 5 per group. (E) WT, IL-4Rα fl/fl, and IL-4Rα fl/fl LysMcre+/− mice injected with K/BxN serum and IL-4 (red) or control (black). Data are shown for day 5 as mean and SEM; n = 4–8 per group. *P < 0.05 by Mann–Whitney u test comparing IL-4–treated and control-treated mice at day 6 (A) or 5 (B, D, and E).
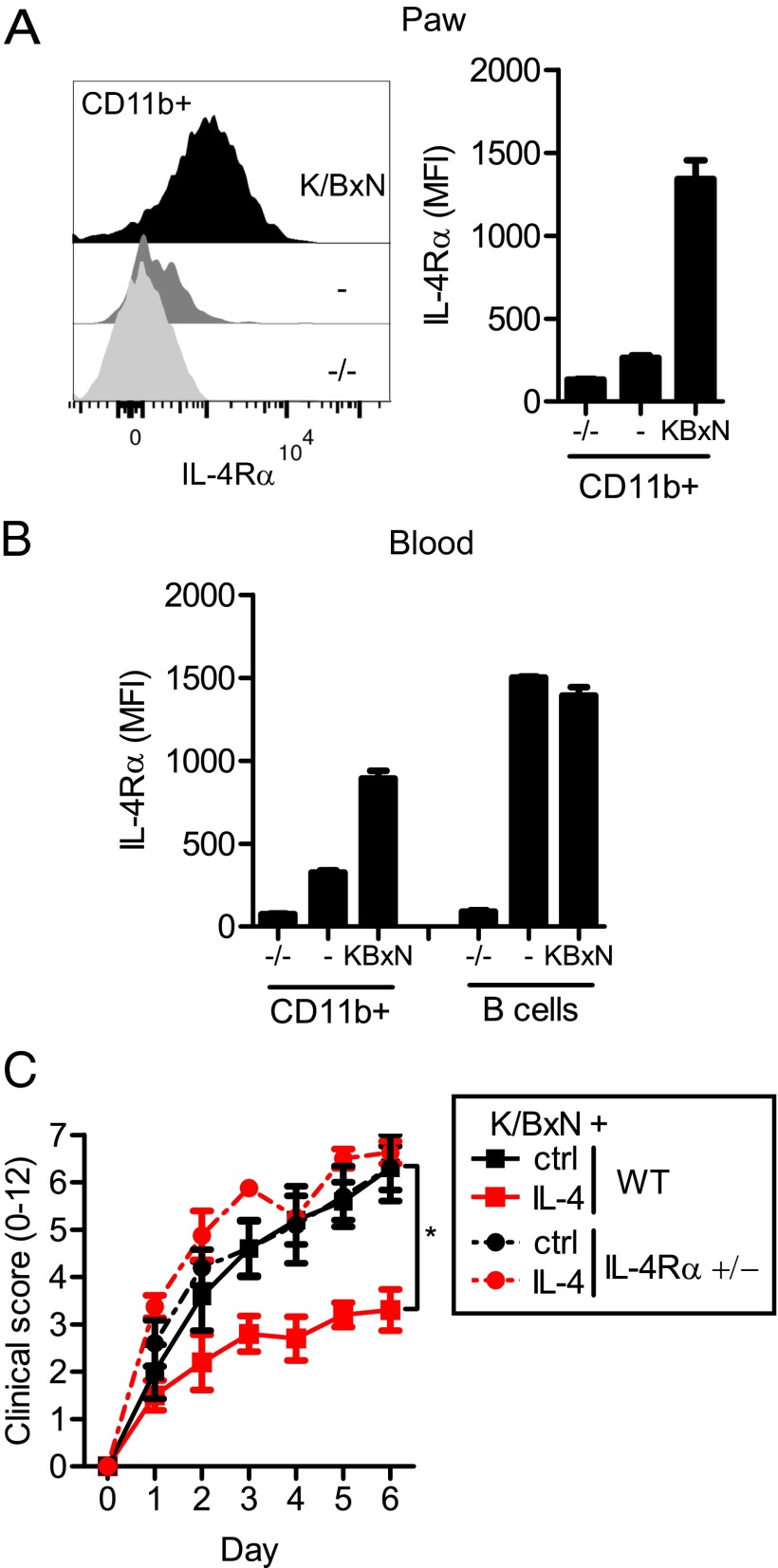
During K/BxN-mediated inflammation, as well as most types of acute inflammation, a high number of CD11b+ myeloid effector cells—predominately neutrophils but also inflammatory monocytes—are recruited from the blood to the inflammatory site (Fig. S3A). Interestingly, we found that these joint-infiltrating myeloid cells express very high IL-4Rα levels compared with these same cells in naïve mice (Fig. 2A). Although this phenotype was especially pronounced on the infiltrating inflammatory cells, it could also be seen on circulating cells isolated from K/BxN-challenged mice (Fig. 2B and Fig. S3B). This induction of IL-4Rα was seen on CD11b+ myeloid cells, with the highest levels of expression found on neutrophils, followed by inflammatory monocytes. In contrast, no up-regulation of IL-4Rα was seen on lymphocytes. We concluded that K/BxN-triggered inflammation induces IL-4Rα on CD11b+ cells and provides the basis for enhanced IL-4–mediated effects on these cells.
Fig. 2.
K/BxN inflammation up-regulates IL-4Rα specifically on myeloid cells. (A) Flow cytometry analysis of IL-4Rα expression on CD11b+ cells isolated on day 5 from paws of IL-4Rα−/− (−/−), WT (−), and K/BxN-injected WT (K/BxN) mice. Data are representative of more than five experiments. (B) IL-4Rα expression on blood CD11b+ cells and B cells as described above. (C) WT and IL-4Rα+/− mice injected with K/BxN serum and IL-4 (red) or control (black). Data are shown as mean and SEM; n = 4–5 per group. *P < 0.05 by Mann–Whitney u test comparing IL-4–treated and control-treated mice at day 6.
To identify the mechanism of this induction, we surveyed the known inducers of IL-4Rα. Several mediators have been shown to up-regulate IL-4Rα on T cells, including IL-2 and -4, dependent on STAT5 and STAT6 signaling, respectively (23, 24). However, both IL-2–deficient and STAT6-deficient mice show equivalent IL-4Rα up-regulation on myeloid cells as WT mice after K/BxN serum administration (Fig. S3 C and D). TNF-α has been shown to up-regulate IL-4Rα on endothelial cells (25). To address whether TNF-α–induced signaling is directly responsible for the observed IL-4Rα up-regulation on myeloid cells following K/BxN-induced inflammation, we used a mixed BM chimeric approach. Irradiated mice were reconstituted with a 1:1 mixture of WT and TNF-α receptor (TNF-αR)−/− BM and then challenged with K/BxN serum. The chimeric mice developed significant inflammation, in contrast to TNF-αR−/− mice, and WT and TNF-αR−/− myeloid cells up-regulated the IL-4Rα to similar levels (Fig. S3F). A similar approach was used to determine whether signaling downstream of FcγRs was responsible for the IL-4Rα up-regulation, because FcγRs are central to antibody-mediated inflammation. However, in WT/FcγR chimeric mice, FcγR-deficient myeloid cells up-regulated IL-4Rα to the same level as WT-derived myeloid cells (Fig. S3G). Together these results demonstrated that IL-4Rα is up-regulated during K/BxN-mediated inflammation on BM-derived myeloid cells and that this up-regulation is not directly related to IL-2, STAT6, TNF-αR, or FcγR signaling.
To determine the relationship between IL-4Rα surface expression and IL-4–mediated protection from inflammation, we generated mice expressing only one functional allele of the IL-4Rα (IL-4Rα+/− mice), which, as expected, expressed 50% of the level of IL-4Rα compared with WT mice (Fig. S4A). These IL-4Rα+/− mice, however, were not protected from K/BxN inflammation by the used dose of IL-4 (Fig. 2C and Fig. S4B), demonstrating that a threshold of IL-4Rα exists for IL-4 stimulation to mediate protection from K/BxN-triggered inflammation.
We next examined whether IL-4Rα up-regulation was specific for the K/BxN model of inflammation or whether the increased IL-4Rα surface expression on myeloid cells is a common feature of inflammation triggered by diverse stimuli. We challenged mice with several different inflammatory stimuli and analyzed blood cells 20 h later. As shown in Table 1 and Fig. S5, myeloid cells up-regulate IL-4Rα as a general response to acute inflammatory stimuli, including TLR ligands (poly[inosinic:cytidylic] acid [Poly(I:C)], LPS, and Zymosan), common adjuvants [Alum, incomplete Freund's adjuvant (IFA), and complete Freund's adjuvant (CFA)], T-cell activation by anti-CD3, and IgG-induced anaphylaxis. Consistent with our previous results, neutrophils displayed the highest levels of IL-4Rα, followed by inflammatory monocytes. In contrast, neither T nor B cells showed any evidence of up-regulation. For stimuli that induced a major up-regulation of IL-4Rα (LPS, Zymosan, Alum, IFA, and CFA), IL-4Rα levels remained elevated for several days before returning to baseline. These results suggested that IL-4Rα regulation may be mediated by a common factor(s), rapidly induced during the inflammatory response, thus behaving like an acute phase reactant.
Table 1.
IL-4Rα up-regulation on CD11b+ blood cells 20 h after injection
| Reagent | Dose | Pathway | IL-4Rα |
| PBS | i.p./i.v. 200 μL | N.A. | — |
| IgGic* | i.v. 75 μg | FcγRs | + |
| Anti-CD3†‡ | i.p. 30 μg | T-cell activation | +/++ |
| Poly(I:C) | i.p. 150 μg | TLR3 | + |
| LPS | i.p. 10 μg | TLR4 | +++ |
| Zymosan | i.p. 1 mg | TLR2 | +++ |
| Alum | i.p. 200 μL | NLRP3? | +++ |
| IFA | i.p. 100 μL | NOD2? | +++ |
| CFA | i.p. 100 μL | Multiple | +++ |
| K/BxN‡ | i.v. 200 μL | FcγRs | ++ |
Sheep IgG (75 μg) + 3 (x75 μg) hybridomas (2E4, 2G4, 3B10) binding unique epitopes on sheep IgG.
Clone 17A2.
Delayed activity, peaking after several days.
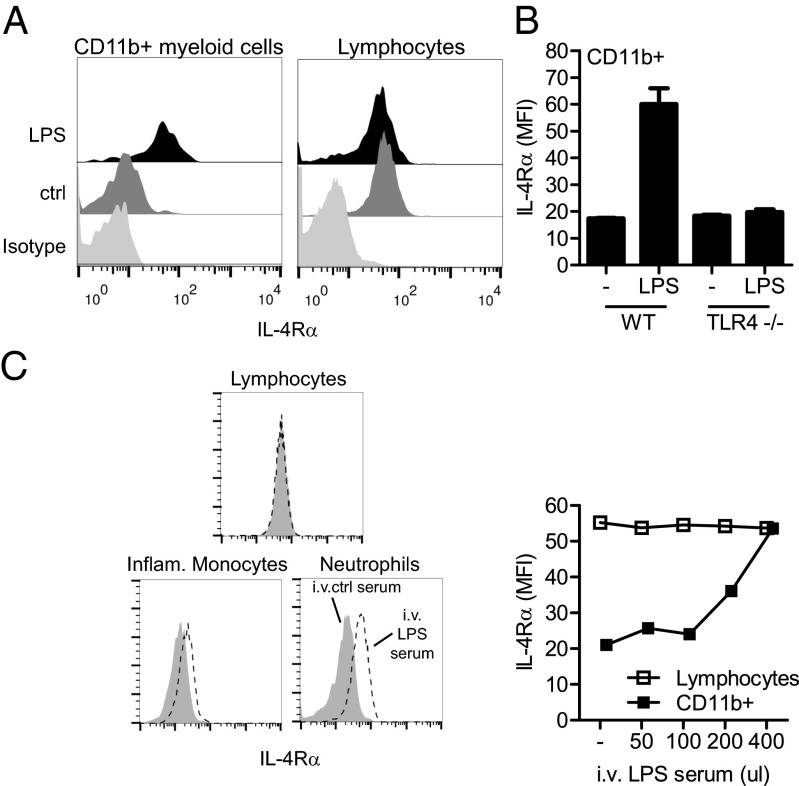
To test this hypothesis, we turned to the TLR4 ligand LPS, which displayed a potent ability to induce myeloid IL-4Rα expression; neutrophils from LPS-injected mice induced IL-4Rα expression, achieving levels equivalent to the resting levels of IL-4Rα on B and T cells in both inbred and outbred strains (Fig. 3A and Fig. S6 A and B). As expected, this activity requires TLR4 signaling, as shown by comparing WT and TLR4−/− mice (Fig. 3B). Using a mixed BM chimeric approach, we determined that the LPS-mediated regulation of IL-4Rα is not directly downstream of TLR4 signaling because WT and TLR4−/− cells in the same chimeric mouse up-regulated IL-4Rα to an equivalent level (Fig. S6C). This finding indicated that, at a minimum, a two-step process occurs after LPS binding to TLR4 to promote IL-4Rα up-regulation on myeloid cells. To test this model, we recovered sera from WT mice injected with LPS (LPS serum) or untreated mice (control serum) that were then transferred to TLR4−/− mice, and IL-4Rα expression on myeloid and lymphoid cells was monitored. Consistent with our previous results, mice that received the LPS serum showed up-regulation of IL-4Rα selectively on myeloid cells, again with the highest response in neutrophils followed by inflammatory monocytes. At a dose of 400 μL of LPS serum, this myeloid-specific up-regulation led to similar expression levels on myeloid cells as on lymphocytes (Fig. 3C).
Fig. 3.
An IL-4Rα–regulating factor is released after LPS injection. (A) Flow cytometry analysis of IL-4Rα expression, compared with an isotype control (light gray), on myeloid cells and lymphocytes 20 h after injection of LPS (black) or control (dark gray). Data are representative of more than five experiments. (B) IL-4Rα expression on blood CD11b+ myeloid cells of WT and TLR4−/− mice injected with LPS 20 h earlier. Data are representative of two experiments. (C) Flow cytometry analysis of IL-4Rα on indicated blood cells 20 h after the injection of different doses of pooled sera from LPS (LPS serum) or control (ctrl serum) into TLR4−/− mice. Data are representative of two experiments.
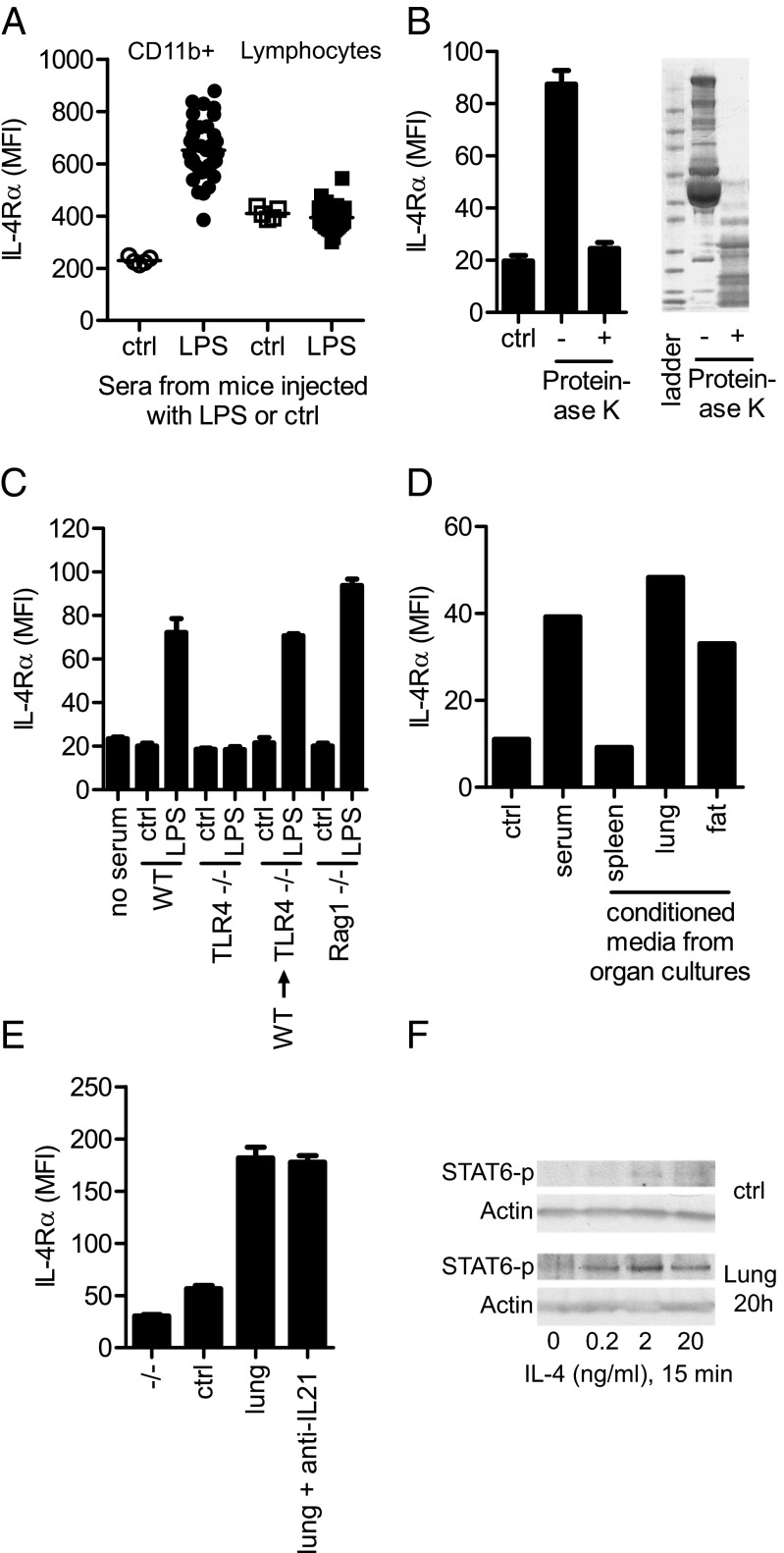
To further define this pathway, we developed an ex vivo system, in which LPS serum was capable of up-regulating IL-4Rα selectively on myeloid cells, but not lymphocytes, derived from TLR4−/− mice (Fig. 4A). To firmly establish that this regulation was not mediated by the LPS found in the LPS serum, circulating cells from mice deficient in the TLR4 signaling molecules MyD88 and TRIF were also used and displayed a clear response to the LPS serum (Fig. S7A). Further, direct addition of LPS to WT-derived circulating cells did not result in the up-regulation of IL-4Rα on myeloid cells (Fig. S7B). To define the nature of the activity in LPS serum, we pretreated the LPS serum with proteinase K or by heating to 90 °C for 45 min. Both of these treatments completely abrogated the IL-4Rα regulatory activity of the LPS serum (Fig. 4B and Fig. S7C). These results support the hypothesis that LPS induces a soluble protein factor(s) that regulates IL-4Rα expression on myeloid effector cells.
Fig. 4.
BM-derived non-B, non-T cells in the lung and fat secrete an IL-4Rα–regulating protein. (A) Flow cytometry analysis of IL-4Rα expression on blood myeloid cells and lymphocytes from TLR4−/− mice cultured ex vivo for 18 h with 0.5% control or LPS serum. Data are shown as mean and individual mice; n = 5 (control); n = 35 (LPS). Data are representative of >10 experiments. (B Left) Flow cytometry analysis of IL-4Rα expression on TLR4−/− myeloid blood cells cultured ex vivo with 0.5% control or LPS serum, pretreated (+) or not (−) with Proteinase K. (Right) SDS/PAGE gel of LPS serum pretreated or not with Proteinase K. Data are representative of three experiments. (C) Flow cytometry analysis of IL-4Rα expression on TLR4−/− myeloid blood cells cultured ex vivo with 0.5% serum from control or LPS-injected WT, TLR4−/−, and Rag1−/− mice, as well as irradiated TLR4−/− mice reconstituted with WT BM cells (WT→TLR4−/−). Data are representative of two experiments. (D) WT mice were injected with LPS, and organs were harvested, cut into pieces, and cultured ex vivo for 8 h before the cell-free supernatant was collected and added to TLR4−/− blood cells. Graph shows flow cytometry analysis of IL-4Rα expression on myeloid cells cultured ex vivo with 0.5% LPS serum or 10% (vol/vol) supernatant from organ cultures. Data are representative of three experiments. (E) Flow cytometry analysis of IL-4Rα expression on TLR4−/− thioglycolate elicited macrophages cultured 20 h with 10% supernatant from lung cultures, as described in D, with control or a neutralizing anti–IL-21 antibody (50 μg/mL). IL-4Rα−/− thioglycolate-elicited macrophages (−/−) defines the background staining. Data are representative of two experiments. (F) Western blot of phospho-STAT6 (STAT6-p) and actin from TLR4−/− thioglycolate-elicited macrophages, pretreated with supernatant from lung or control cultures, pulsed with indicated doses of IL-4 for 15 min. Data are representative of three experiments.
We next set out to determine the signaling events that promote production and release of the IL-4Rα–regulating protein(s). As expected, sera from LPS-injected TLR4−/− mice did not display the IL-4Rα–regulatory activity found in sera from LPS-injected WT mice. However, irradiated TLR4−/− mice reconstituted with WT BM cells, resulting in mice expressing TLR4 only on BM-derived cells, still produced the IL-4Rα–regulating protein(s) after injection of LPS. Similarly, B-cell– and T-cell–deficient Rag1−/− mice readily produced the protein(s) following LPS injection (Fig. 4C). These findings suggested that the protein(s) is released from BM-derived non-B/non-T cells. Next, we isolated organs from LPS-injected mice, cultured them ex vivo for 8 h, and assayed the conditioned supernatant. Interestingly, an IL-4Rα–regulatory activity was clearly found in both supernatants from lung and fat cultures, supposedly released by BM-derived cells residing in these organs. This result was in contrast to supernatants from other organs, including the spleen, which showed low activity (Fig. 4D). In line with this observation, no difference in IL-4Rα–regulating ability was seen in the sera derived from splenectomized or sham-operated mice injected with LPS (Fig. S7D).
To extend our findings, we next turned to another source of myeloid effector cells, (TLR4−/−) thioglycolate-elicited macrophages. These inflammatory macrophages behaved very similarly to circulating myeloid cells when cultured for 20 h with LPS serum or conditioned medium from lung (Fig. 4E). Because macrophages have been shown to up-regulate the IL-4Rα after exposure to IL-21 (26), we investigated whether this cytokine was responsible for the LPS-mediated up-regulation of IL-4Rα on myeloid cells. However, we were unable to abrogate the response of either thioglycolate-elicited macrophages or circulating myeloid cells with an IL-21–neutralizing antibody (anti–IL-21) (Fig. 4E and Fig. S7E). Thioglycolate-elicited macrophages pretreated with lung supernatant to up-regulate the IL-4Rα also responded with increased STAT6 phosphorylation, compared with nontreated cells, when exposed to low levels of IL-4 (Fig. 4F). We concluded that during acute inflammation, IL-4Rα–regulating protein(s) are released into the circulation by BM-derived non-B/non-T cells, residing in lung and fat tissues, priming myeloid effector cells for STAT6 signaling.
An anti-inflammatory activity has long been attributed to IL-4. This notion was originally based on the ability of IL-4 to effectively dampen the production of proinflammatory cytokines from activated human monocytes (27, 28). In murine studies, IL-4–induced signaling has been shown to attenuate RA-like inflammation in the collagen-induced arthritis (CIA) model by using either an adenoviral delivery system or an osmotic pump continuously delivering IL-4 (29, 30). However, other protocols for IL-4 administration have not exhibited similar protection in the CIA model (31). These studies, however, did not directly address the cell population targeted by IL-4. Consistent with our results, Cao et al. showed that IL-4Rα signaling in myeloid cells indeed has a potent anti-inflammatory activity in the proteoglycans-induced arthritis model (32).
The IL-4Rα forms a heterodimer with either the common cytokine receptor gamma chain (γc/CD132) or IL-13Rα1 (CD213a1), making type 1 and 2 IL-4R, respectively. IL-4 interacts with both of these receptors, whereas IL-13 only interacts with the type 2 receptor (20, 33). We have previously shown that exogenous IL-13, like IL-4, protects from K/BxN-mediated inflammation, thus showing that engaging the type 2 IL-4R is sufficient for an anti-inflammatory activity in this model (15). The IL-4Rα belongs to a family of receptors that can interact with γc. This family also includes alpha chains making up cytokine receptors for IL-2, -7, -9, -15, and -21 (34). Interestingly, the IL-2Rα (CD25) is highly up-regulated during T-cell activation, something that has been proposed to enable the cell to respond to low physiological levels of IL-2 (35). This observation, thus, has similarities to the regulation we describe herein for IL-4Rα, suggesting that this type of regulation could be a common feature for this family of receptors. However, whereas the IL-2Rα is responsible for making the heterotrimeric high affinity IL-2R (IL-2Rα/IL-2Rβ/γc) (36), no IL-4R exists in the absence of IL-4Rα.
In conclusion, our studies support a model of a homeostatic mechanism involving the up-regulation of IL-4Rα on myeloid effector cells as a general response to inflammation. This myeloid-specific IL-4Rα up-regulation was seen in response to an array of different stimuli, indicating that a conserved pathway is likely involved. We propose that this common response to inflammatory stimuli plays a role in restoring homeostasis following an inflammatory response and is a significant component of a regulated immune response.
Materials and Methods
Joint inflammation was induced by transferring arthritogenic K/BxN serum (17). IL-4 was administered just before K/BxN as IL-4:anti–IL-4 complexes with prolonged in vivo half-life. IL-4Rα expression was determined by flow cytometry using the anti–IL-4Rα clone M1. Detailed experimental procedures are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Pete Stavropoulos, Pontus Boström, Ajay Chawla, Fred Finkelman, Manish Ponda, Brian T. Chait, Matam Vijay-Kumar, Philipp Scherer, Michelle Lepherd, Ruben Peraza, and Klara Velinzon, and members of the J.V.R. laboratory for providing reagents, technical help, and helpful discussions. F.W. is a Wenner–Gren Fellow supported by the Wenner–Gren Foundations. This work was supported by grants from the National Institutes of Health (to J.V.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312525110/-/DCSupplemental.
References
- 1.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 2.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 4.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 5.Klareskog L, Padyukov L, Lorentzen J, Alfredsson L. Mechanisms of disease: Genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat Clin Pract Rheumatol. 2006;2(8):425–433. doi: 10.1038/ncprheum0249. [DOI] [PubMed] [Google Scholar]
- 6.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 8.Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med. 1996;335(22):1643–1649. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- 9.Su K, et al. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol. 2004;172(11):7186–7191. doi: 10.4049/jimmunol.172.11.7186. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 11.Debré M, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342(8877):945–949. doi: 10.1016/0140-6736(93)92000-j. [DOI] [PubMed] [Google Scholar]
- 12.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18(4):573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 14.Schwab I, Biburger M, Krönke G, Schett G, Nimmerjahn F. IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur J Immunol. 2012;42(4):826–830. doi: 10.1002/eji.201142260. [DOI] [PubMed] [Google Scholar]
- 15.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 17.Monach PA, et al. Neutrophils in a mouse model of autoantibody-mediated arthritis: Critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis Rheum. 2010;62(3):753–764. doi: 10.1002/art.27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon S, Rajasekaran N, Jeisy-Walder E, Snapper SB, Illges H. A crucial role for macrophages in the pathology of K/B x N serum-induced arthritis. Eur J Immunol. 2005;35(10):3064–3073. doi: 10.1002/eji.200526167. [DOI] [PubMed] [Google Scholar]
- 19.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 20.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 21.Howard M, et al. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosmann TR, Bond MW, Coffman RL, Ohara J, Paul WE. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci USA. 1986;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao W, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9(11):1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohara J, Paul WE. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci USA. 1988;85(21):8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugli SM, et al. Tumor necrosis factor alpha enhances the expression of the interleukin (IL)-4 receptor alpha-chain on endothelial cells increasing IL-4 or IL-13-induced Stat6 activation. J Biol Chem. 1997;272(9):5487–5494. doi: 10.1074/jbc.272.9.5487. [DOI] [PubMed] [Google Scholar]
- 26.Pesce J, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116(7):2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.te Velde AA, Klomp JP, Yard BA, de Vries JE, Figdor CG. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988;140(5):1548–1554. [PubMed] [Google Scholar]
- 28.Essner R, Rhoades K, McBride WH, Morton DL, Economou JS. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J Immunol. 1989;142(11):3857–3861. [PubMed] [Google Scholar]
- 29.Kim SH, et al. Gene therapy for established murine collagen-induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res. 2000;2(4):293–302. doi: 10.1186/ar104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horsfall AC, et al. Suppression of collagen-induced arthritis by continuous administration of IL-4. J Immunol. 1997;159(11):5687–5696. [PubMed] [Google Scholar]
- 31.Joosten LA, et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40(2):249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Brombacher F, Tunyogi-Csapo M, Glant TT, Finnegan A. Interleukin-4 regulates proteoglycan-induced arthritis by specifically suppressing the innate immune response. Arthritis Rheum. 2007;56(3):861–870. doi: 10.1002/art.22422. [DOI] [PubMed] [Google Scholar]
- 33.LaPorte SL, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132(2):259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23(5):598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HP, Kelly J, Leonard WJ. The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: Importance of two widely separated IL-2 response elements. Immunity. 2001;15(1):159–172. doi: 10.1016/s1074-7613(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 36.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17(5):349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.