Abstract
Epoxyeicosatrienoic acids (EETs), lipid mediators produced by cytochrome P450 epoxygenases, regulate inflammation, angiogenesis, and vascular tone. Despite pleiotropic effects on cells, the role of these epoxyeicosanoids in normal organ and tissue regeneration remains unknown. EETs are produced predominantly in the endothelium. Normal organ and tissue regeneration require an active paracrine role of the microvascular endothelium, which in turn depends on angiogenic growth factors. Thus, we hypothesize that endothelial cells stimulate organ and tissue regeneration via production of bioactive EETs. To determine whether endothelial-derived EETs affect physiologic tissue growth in vivo, we used genetic and pharmacological tools to manipulate endogenous EET levels. We show that endothelial-derived EETs play a critical role in accelerating tissue growth in vivo, including liver regeneration, kidney compensatory growth, lung compensatory growth, wound healing, corneal neovascularization, and retinal vascularization. Administration of synthetic EETs recapitulated these results, whereas lowering EET levels, either genetically or pharmacologically, delayed tissue regeneration, demonstrating that pharmacological modulation of EETs can affect normal organ and tissue growth. We also show that soluble epoxide hydrolase inhibitors, which elevate endogenous EET levels, promote liver and lung regeneration. Thus, our observations indicate a central role for EETs in organ and tissue regeneration and their contribution to tissue homeostasis.
Keywords: autacoid, organ regeneration, small molecule mediator, angiocrine, VEGF
Endogenously produced lipid autacoids—locally acting lipid mediators—play a central role in the response to tissue injury (1). Products of arachidonic acid metabolism (eicosanoids), including prostaglandins and leukotrienes, are generated by distinct enzymatic systems initiated by cyclooxygenase (COX) and lipoxygenase (LOX) (2, 3). There is, however, another prominent enzymatic pathway for which arachidonic acid is the substrate: the cytochrome P450 (CYP) system (2). This eicosanoid pathway consists of two main branches: ω-hydroxylases, which convert arachidonic acid into hydroxyeicosatetraenoic acids, and epoxygenases, which convert it to epoxyeicosatrienoic acids (EETs) (4, 5). Although the COX- and LOX-derived eicosanoids have been intensely studied in tissue regeneration (6, 7), the investigation of CYP-derived eicosanoids has primarily focused on inflammation and cardiovascular functions (3).
EETs are lipid mediators produced by CYP epoxygenases (CYP2C8 and CYP2J2 in humans) and metabolized by soluble epoxide hydrolase (sEH) to the corresponding dihydroxyeicosatrienoic acids (2). Endothelial cells (ECs) contribute to tissue mass regulation, including the growth of adipose tissue, normal prostate, and bone, by supplying oxygen and nutrients to the growing tissue via neovascularization. However, ECs, which express CYP epoxygenases and sEH, also act through paracrine (trophic) effects on these growing tissues (8–10). In the vasculature, bioactive EETs are produced predominantly in the endothelium (8). ECs play a critical role in the development of a vascular network, tissue growth, and regenerative processes, including wound healing, corneal neovascularization, neonatal retinal vessel formation, and liver and lung regeneration (8, 11). The human liver can generate high EET levels, mostly 14,15- and 11,12-EET, as well as abundant sEH (12). Modulation of liver sEH may therefore permit the increase or decrease of EET levels, as observed in response to inflammatory signals (13). EETs have anti-inflammatory effects and stimulate vasodilation, cellular proliferation, migration, and angiogenesis (8). Nevertheless, the role of these epoxyeicosanoids in normal tissue growth, such as organ regeneration, has not been studied. Normal tissue and organ regeneration require an active microvascular endothelium that provides trophic support (11). For instance, liver or lung regeneration requires angiocrine signals from the sinusoidal or pulmonary endothelium, respectively (14, 15). Although the roles of many growth factors/cytokines have been characterized in liver and lung regeneration (14, 15), EETs have not been implicated in organ regeneration to date. Thus, given that EETs are produced by the microvasculature, we hypothesized that the endothelial-derived EETs can promote tissue and organ regeneration via pleomorphic effects.
Recent studies have shown that EETs stimulate tissue growth in culture and in animal studies. EETs enhance axonal growth in neuronal cell cultures (16). Mice with targeted gene deletion of sEH have increased plasma EETs and exhibit a larger pancreatic islet size (17). Moreover, local administration of EETs accelerates wound epithelialization and neovascularization in a mouse ear-wound model (18). However, the role of endothelial-derived EETs in tissue regeneration remains poorly characterized. To determine whether endothelial-derived EETs play a role in physiologic tissue growth, we used genetic and pharmacological tools to manipulate endogenous EET levels in seven well-characterized animal models: (i) liver regeneration, (ii) kidney compensatory growth, (iii) lung compensatory growth, (iv) angiogenesis Matrigel plug assay, (v) corneal micropocket assay, (vi) wound healing, and (vii) neonatal retinal vessel formation. Here, we report that EETs accelerate tissue growth and organ regeneration. The production of EETs in the endothelium might thus be a critical paracrine mediator, revealing a unique trophic function for the endothelium in stimulating tissue growth.
Results
Endothelial-Derived EETs Promote Liver Regeneration, as Well as Kidney and Lung Compensatory Growth.
To determine whether EET autacoids produced by the endothelium promote tissue growth, we have generated three lines of transgenic mice with high endothelial EET levels: mice with endothelial (Tie2-promoter driven) expression of either human CYP2C8 or human CYP2J2 (Tie2-CYP2C8-Tr and Tie2-CYP2J2-Tr) and mice with global disruption of the gene that encodes sEH (sEH-null) (19). Tie2-CYP2C8-Tr and Tie2-CYP2J2-Tr mice have significantly increased endothelial EETs compared with wild-type (WT) mice, as measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) (19, 20), and sEH-null mice have significantly increased plasma EETs (21). In contrast, COX- and LOX-derived metabolites are unaffected in these mice (20). To characterize the impact of reduced endothelial EETs, we also generated a unique murine model with endothelial expression of human sEH (Tie2-sEH-Tr) (22). ECs isolated from these mice have significantly decreased EET levels relative to ECs isolated from WT mice (20).
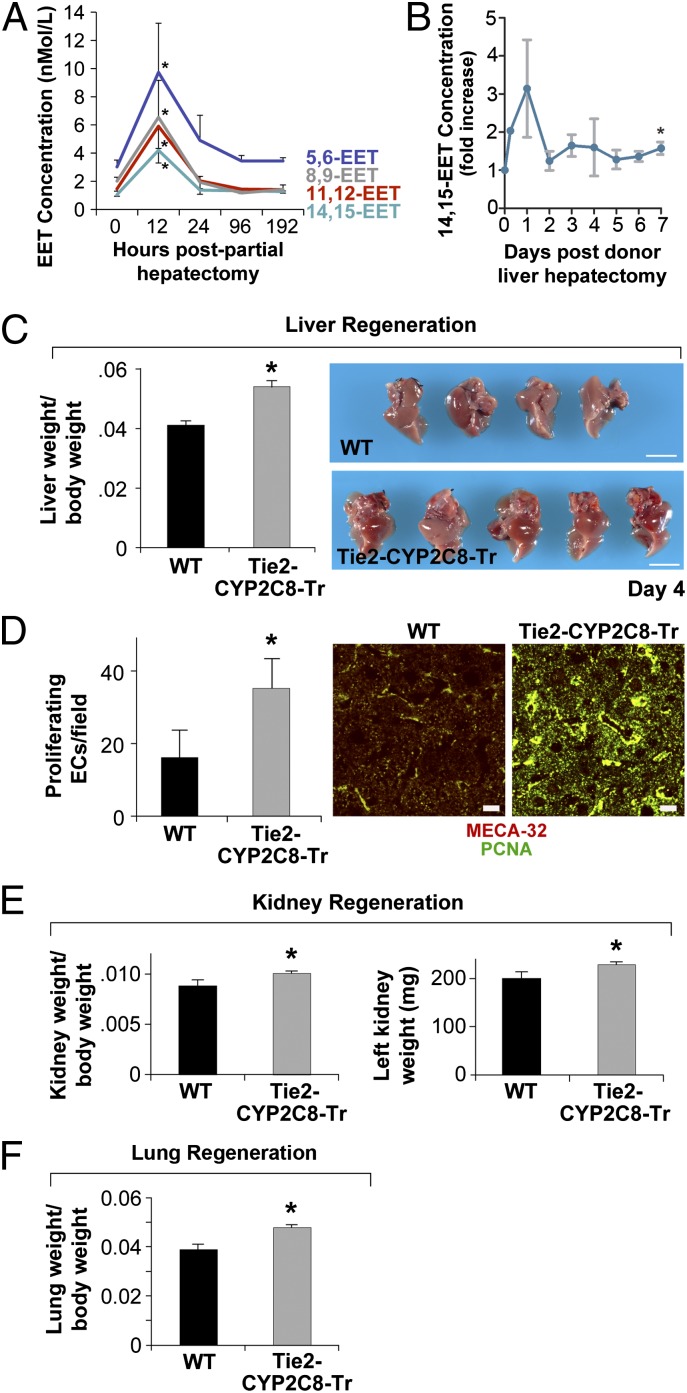
Organ regeneration is controlled, in part, by the microvascular endothelium (11, 14, 15). To determine whether EETs are naturally up-regulated during liver regeneration, we performed LC-MS/MS analysis of plasma from mice undergoing partial hepatectomies (removal of two-thirds of the murine liver). Plasma 14,15-, 11,12-, 8,9-, and 5,6-EET were elevated by 300%, 305%, 225%, and 222%, respectively, when measured 12 h after partial hepatectomy (Fig. 1A). Similarly, human donors undergoing partial liver resections for living-related liver transplantation have a 1.5- to 3-fold increase in 14,15-EET levels 1–7 d after donor liver hepatectomy (Fig. 1B).
Fig. 1.
Endothelial-derived EETs promote liver, kidney, and lung regeneration. (A) Plasma 14,15-, 11,12-, 8,9-, and 5,6-EET are elevated 12 h after partial hepatectomy. *P < 0.05 vs. day 0. (B) The 14,15-EET levels are increased 1–7 d after human donor liver hepatectomy in humans undergoing living-related donor liver resections for recipients undergoing liver transplantation. *P < 0.05 vs. day 0. (C) Liver regeneration is accelerated in Tie2-CYP2C8-Tr mice on day 4 after partial hepatectomy. n = 5 mice per group. *P < 0.05 vs. WT. Representative photos are shown. (Scale bars, 1 cm.) (D) Immunofluorescent double staining for MECA-32 and proliferating cell nuclear antigen (PCNA) shows increased EC proliferation in livers from Tie2-CYP2C8-Tr vs. WT mice on day 4 after partial hepatectomy. Red, MECA-32-stained ECs; green, PCNA-stained proliferating cells. Colocalization of red and green fluorescence (yellow) indicates proliferating ECs. (Scale bars, 20 µm.) (E) Kidney regeneration is significantly increased in Tie2-CYP2C8-Tr mice 21 d after partial nephrectomy. n = 5–8 mice per group. *P < 0.05 vs. WT. (F) Lung regeneration is significantly increased in Tie2-CYP2C8-Tr mice 4 d after left pneumonectomy. n = 5 mice per group. *P < 0.05 vs. WT.
To determine whether endothelial-derived EETs affect liver regeneration, we performed partial hepatectomies in Tie2-CYP2C8-Tr and WT mice. By day 4 after partial hepatectomy, Tie2-CYP2C8-Tr mice exhibited a 32% increase in the liver/body weight ratio compared with WT controls (Fig. 1C). In contrast, there were no significant differences in liver/body weight ratio at baseline or after sham operation. Histological analysis of the Tie2-CYP2C8-Tr livers revealed increased hepatocyte proliferation characterized by thickening of the hepatocyte cords, multinucleation, and increased nuclear size—features that are typical of dividing cells in a regenerating liver (Fig. S1A). EC proliferation was increased in the livers from Tie2-CYP2C8-Tr mice compared with WT mice as determined by immunofluorescent double staining for vessels [anti-mouse panendothelial cell antigen-32 (MECA-32)] and proliferation (Ki-67) (Fig. 1D). Because angiogenesis is necessary for regeneration and EETs have been shown to stimulate angiogenesis via VEGF (20, 23), we next analyzed the livers from Tie2-CYP2C8-Tr and WT mice on day 4 after hepatectomy for VEGF. Livers from Tie2-CYP2C8-Tr mice exhibited an increase in VEGF expression compared with WT mice (Fig. S1B).
Trichrome staining of livers revealed increased collagen deposition in the basement membrane of blood vessels in livers from the Tie2-CYP-Tr mice compared with WT mice (Fig. S1C), consistent with acceleration of liver regeneration with new and functional liver tissue. We next isolated liver sinusoidal ECs and demonstrated that both 11,12-EET (1 μM) and 14,15-EET (1 μM) stimulate liver EC proliferation from 45% to 81%, respectively (Fig. S1D).
To determine whether endothelial-derived EETs stimulate kidney compensatory growth, we performed unilateral nephrectomies in Tie2-CYP2C8-Tr and WT mice. Kidney compensatory growth was significantly increased in Tie2-CYP2C8-Tr mice compared with WT mice on day 21 (Fig. 1E). To determine whether endothelial-derived EETs also stimulate lung compensatory growth, we performed unilateral pneumonectomies and observed a 23% increase in contralateral lung growth on day 4 following left pneumonectomy in Tie2-CYP2C8-Tr compared with WT mice (Fig. 1F).
Pharmacological Manipulation of EETs and Their Action in Organ Regeneration.
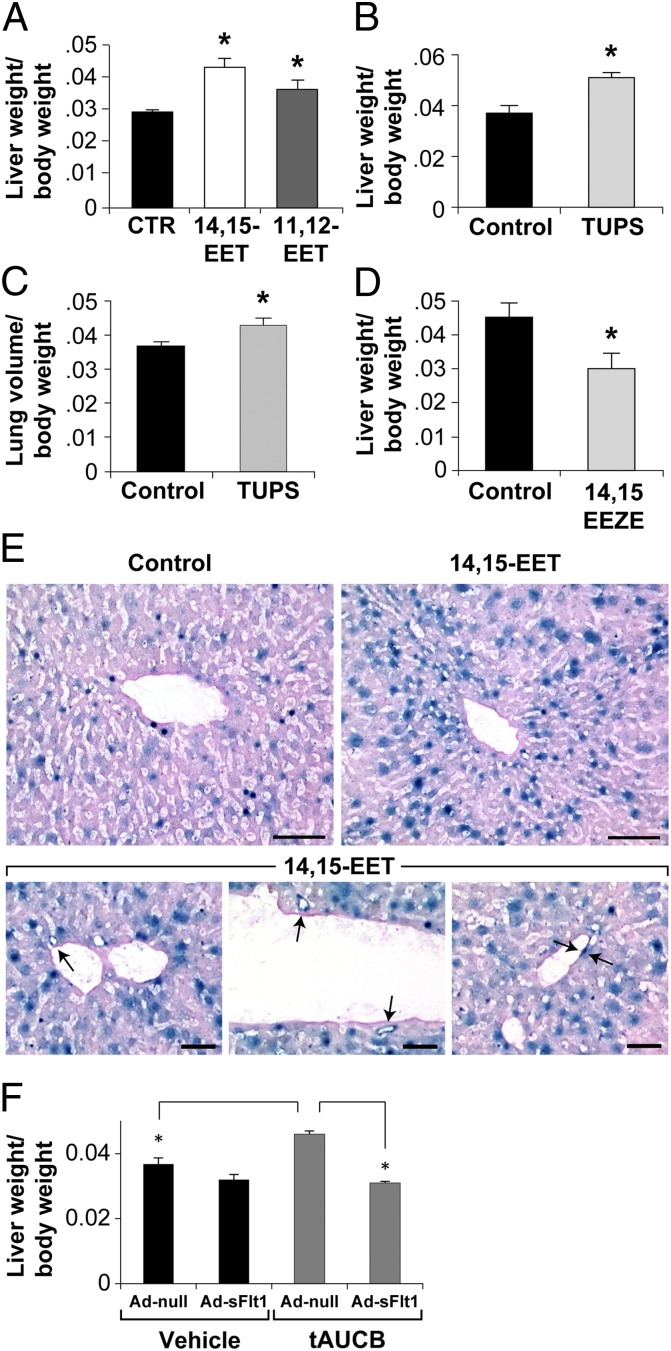
To demonstrate that EETs are sufficient to promote organ regeneration and to unambiguously show that the organ-regenerative effect was specific to EETs, and not mediated by other downstream effects of CYP enzymes, we administered 14,15- or 11,12-EET to mice by osmotic minipump. Consistent with our results in the Tie2-CYP2C8-Tr mice, systemic administration of 14,15-EET significantly increased the liver/body weight ratio by 46% compared with vehicle-treated mice (Fig. 2A). Similarly, systemic administration of 11,12-EET increased liver/body weight ratio by 23% relative to vehicle control (Fig. 2A).
Fig. 2.
Pharmacological manipulation of EETs and their action in organ regeneration. (A) Systemic administration of 14,15- or 11,12-EET (15 μg⋅kg−1⋅d−1) via minipump stimulates liver regeneration on day 4 after partial hepatectomy compared with vehicle-treated mice. n = 5 mice per group. *P < 0.05 vs. vehicle. (B) The sEHi TUPS increases liver regeneration at day 4 after partial hepatectomy. TUPS was given at a dose of 10 mg⋅kg−1⋅d−1. n = 5 mice per group. *P < 0.05 vs. control. (C) The sEHi TUPS increases lung regeneration at day 4 after partial pneumonectomy. TUPS was given at a dose of 10 mg⋅kg−1⋅d−1. n = 5 or 6 mice per group. *P < 0.05 vs. control. (D) Systemic administration of the EET antagonist 14,15-EEZE (0.21 mg per mouse) inhibits liver regeneration on day 7 after partial hepatectomy. n = 5 mice per group. *P < 0.05 vs. control. (E) Regenerating livers in VEGF–LacZ-Tr mice treated with 14,15-EET (15 μg⋅kg−1⋅d−1) show β-galactosidase staining (marker of VEGF production) in sinusoidal and venous ECs (arrows) and hepatocytes. (Scale bars, 100 μm.) (F) VEGF depletion with Ad-sFlt suppresses EET-induced liver regeneration by the sEHi tAUCB but has no effect on vehicle-treated liver regeneration. n = 5 mice per group. *P < 0.05.
To confirm the observed effects of exogenous EETs and to establish the clinical relevance of pharmacological EET modulation, we next characterized the effect of EET modifying compounds in liver regeneration and lung compensatory growth models. We first studied compounds that increase EETs by inhibiting sEH, the main metabolizing enzyme of EETs. Notably, several structurally distinct sEH inhibitors (sEHis) are being evaluated for clinical indications (3, 24, 25). Systemic administration of the sEHi 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (TUPS, UC1709) increased liver regeneration by 38% compared with vehicle-treated mice (Fig. 2B) and also increased lung growth by 23% (Fig. 2C).
To corroborate the specificity of these findings, we next sought to block the action of EETs. Although the receptor(s) for EETs has not yet been identified (3), the EET antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) is the most specific reagent available for pharmacologic suppression of EET activity (26) and has been used to antagonize the biological effects of EETs in other disease models (20, 23). To determine whether an EET antagonist could inhibit organ regeneration, we administered 14,15-EEZE following partial hepatectomy. Mice treated with 14,15-EEZE demonstrated reduced liver regeneration (Fig. 2D). Thus, pharmacological modulation of EET levels recapitulates the results obtained in genetically engineered mice with respect to stimulation and suppression of tissue and organ regeneration. Collectively, these observations demonstrate that tissue regeneration is significantly stimulated by endothelial EETs, an effect that is likely promoted by EET-dependent regulation of angiogenesis.
EETs stimulate VEGF production in livers undergoing liver regeneration (Fig. S1B). To identify the source of VEGF, we analyzed livers from VEGF–LacZ-transgenic mice treated with 14,15-EET or vehicle (in these mice the LacZ reporter is introduced into the VEGF locus) as described (20). In VEGF–LacZ-transgenic mice systemically treated with 14,15-EET, liver sinusoidal, and vein ECs and hepatocytes stained positive for the LacZ product β-galactosidase, whereas in vehicle-treated VEGF–LacZ-transgenic mice, this marker for VEGF production was absent in the liver sinusoidal ECs and reduced in hepatocytes (Fig. 2E). To further determine whether VEGF-stimulated angiogenesis plays a functional role in EET-stimulated liver regeneration, we depleted VEGF by expressing a soluble form of the VEGF receptor 1 (sFlt) using an adenoviral delivery system. In vehicle-treated mice, which have low systemic VEGF levels, VEGF depletion had no significant effect on liver regeneration (Fig. 2F). In contrast, in mice treated with tAUCB, which have elevated plasma 14,15-EET levels by LC-MS/MS analysis (Fig. S2A), VEGF depletion suppressed the accelerated liver regeneration induced by sEHi {trans-4-[4-(3-adamantan-1-ylureido)cyclohexyloxy]-benzoic acid (tAUCB, UC1471)} (Fig. 2F). Thus, EETs required VEGF for liver regeneration. Using VEGF–LacZ-transgenic mice, we show that liver sinusoidal ECs and hepatocytes treated with EETs can generate VEGF and that the proliver regenerative activity of EETs is mediated by VEGF production.
Endothelial-Derived EETs Stimulate Tissue Vascularization and Corneal Neovascularization.
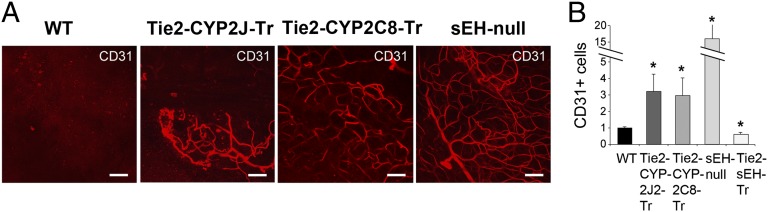
To determine whether the modulation of endothelial-derived EETs directly affected tissue vascularization in vivo, we used two well-characterized animal models: angiogenesis Matrigel plug assay and corneal micropocket assay (27–29). Matrigel plugs implanted into WT mice (negative control) in the absence of exogenous growth factors demonstrated no stimulation of blood vessels. Compared with WT mice, sEH-null, Tie2-CYP2J2-Tr, and Tie2-CYP2C8-Tr mice each exhibited pronounced invasion of ECs into the plug. This result was indicated by a striking increase in CD31-positive microvessels when analyzed by whole-mount immunofluorescent staining (Fig. 3A), consistent with the proangiogenic effects of EETs and the increased vascularization previously observed in sEH-null mice (17, 30). These results also correlate with the plasma 14,15-EET levels that we recently reported in these different transgenic mice strains (20). Flow cytometry quantification of dissociated cells revealed a 4- to 15-fold increase in CD31-positive cells in the plugs from Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and sEH-null mice compared with WT mice (Fig. 3B). Conversely, in plugs from Tie2-sEH-Tr mice, there was a nearly 40% decrease in the number of infiltrating ECs compared with WT mice (Fig. 3B). Thus, mice genetically engineered to have reduced endothelial EETs showed reduced angiogenesis in this assay.
Fig. 3.
Endothelial-derived EETs stimulate tissue vascularization. (A) Whole-mount immunofluorescent CD31 staining of Matrigel plugs with no growth factors added shows increased vascularization in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and sEH-null mice vs. WT mice. (Scale bars, 100 µm.) (B) Matrigel plug angiogenesis, quantified by flow cytometry, showing relative fraction of CD31+/CD45− ECs infiltrated into the plug. EC numbers (relative to WT) are increased in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and sEH-null mice and decreased in Tie2-sEH-Tr mice relative to WT mice. n = 6–8 plugs per group. *P < 0.05 vs. WT.
We recently demonstrated that VEGF signaling is required for EET-induced tumor angiogenesis (20). To determine whether VEGF or fibroblast growth factor 2 (FGF-2) contributed to EETs’ stimulated angiogenesis in regeneration, we performed corneal micropocket assays in Tie2-CYP2C8-Tr and WT mice. Implantation of FGF-2 or VEGF-containing pellets stimulated corneal neovascularization over 6 d in WT animals, as we and others have reported (29). The vessel length and area of neovascularization induced by FGF-2 in Tie2-CYP2C8-Tr mice was unchanged relative to WT mice (Fig. S2B). In contrast, VEGF-stimulated angiogenesis in Tie2-CYP2C8-Tr mice was increased by ∼60% compared with WT mice, reflected by increased vessel length and neovascularization area (Fig. S2C).
Endothelial-Derived EETs Accelerate Wound Healing and Stimulate Neonatal Retinal Vessel Formation.
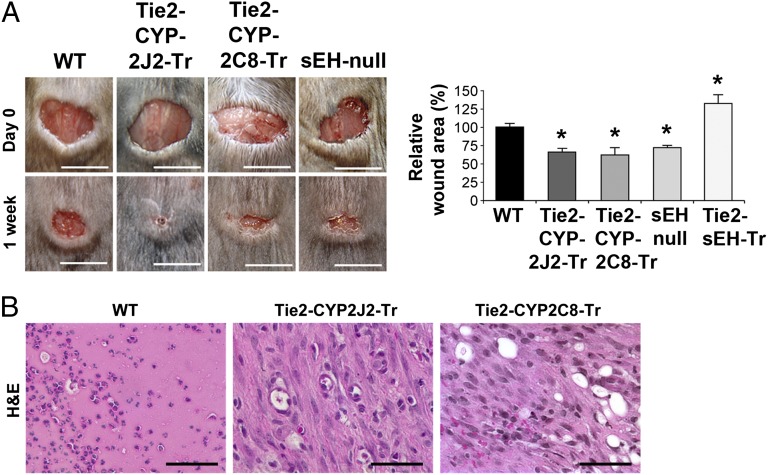
Because the endothelium and VEGF both play a pivotal role in wound healing (31), we next examined the role of the endothelial-derived EETs in wound healing. By manipulating EET levels in the tissue, as opposed to local administration in the wound in methylcellulose discs [as reported (18)], we examined the systemic role of EETs in wound healing. Wound healing was accelerated in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and sEH-null mice compared with WT mice 1 wk after tissue injury (Fig. 4A). Immunohistochemical analysis revealed more mature wounds in the genetically altered mice with granulation tissue that was progressively more organized (i.e., healing) and increased vascularization compared with WT mice (Fig. 4B and Fig. S3A). By contrast, wound healing in Tie2-sEH-Tr mice (reduced endothelial EETs) was suppressed (Fig. 4A). Analysis of the time course revealed that there was a delay in wound healing in the Tie2-sEH-Tr mice (Fig. S3B). To determine whether systemic administration of EETs would accelerate wound healing, we administered EETs (14,15- or 11,12-EET) via miniosmotic pump. Systemic administration of either 14,15- or 11,12-EET stimulated wound healing (Fig. S3C). Sander et al. recently demonstrated that local application of EETs and the sEHi tAUCB accelerates epithelialization and neovascularization in healing wounds (18). Consistent with these results, we now show that topical application of sEHi TUPS, as a cream or systemic administration, accelerated wound healing (Fig. S3D).
Fig. 4.
Endothelial-derived EETs accelerate wound healing and stimulate neonatal retinal vessel formation. (A) Wound healing is accelerated in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and sEH-null mice, and suppressed in Tie2-sEH-Tr mice, relative to WT as quantified by wound area after 7 d. *P < 0.05 vs. WT. n = 10–12 wounds per group. (Scale bars, 1 cm.) (B) Hematoxylin and eosin (H&E)-stained sections of wounds in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice on day 7 reveals more mature wounds with collagen deposition with granulation compared with WT mice. (Scale bars, 100 µm.)
To further study the role of endothelial-derived EETs on tissue vascularization, we examined a physiological model of neonatal retinal vessel formation. Consistent with EETs’ stimulating corneal neovascularization and wound healing, the total area of newly formed microvessels in the neonatal retina, which is also tightly regulated by VEGF and its receptors (32), was significantly increased in Tie2-CYP2C8-Tr compared with WT mice on postnatal day 5 (Fig. S3E).
Discussion
Using genetic and pharmacological means to manipulate endogenous EET levels, we show that these lipid autacoids play a critical role in accelerating tissue growth, including neonatal retinal vessel formation, corneal neovascularization, liver regeneration, kidney and lung compensatory growth, and wound healing. We demonstrate that tissue regeneration, including organ regeneration and wound healing, is dependent on endothelial-derived EETs. Production of EETs in the endothelium might thus be a critical regulator of tissue growth. Although we show that the endothelium, acting as a source of local EETs, is a major regulator of tissue and organ regeneration, our studies with exogenously administrated 14,15- and 11,12-EET indicate that high levels of systemic EETs can also stimulate tissue growth.
Tissue repair and regeneration are partially mediated by angiogenesis (11, 33, 34). Here we show that the role of the endothelium extends beyond neovascularization to include the endothelial paracrine effect of EETs on tissue regeneration. The endothelium (and the supporting stroma) also secrete angiogenic factors (14, 35). This process establishes a positive feedback loop ensuring sustained trophic support by endothelium-derived factors (14, 35). For example, VEGF and FGFs induce angiogenesis in wounds and skin regeneration (36). Our studies show that VEGF-mediated neovascularization is enhanced in mice with high endothelial-derived EETs, consistent with the results obtained by others (23). Local administration of EETs was recently shown to accelerate wound epithelialization and neovascularization in a mouse ear-wound model (18). We confirmed this finding using topical application of the sEHi TUPS as well as by systemic administration of 14,15- and 11,12-EET by miniosmotic pump. These strategies should be investigated to improve wound healing in humans. Recent advances in tissue engineering and drug delivery, such as scaffolds (37), to deliver growth factors for vascularized tissue-engineering therapies might be optimized to improve prolonged delivery of EETs, which have a short half-life (3), for tissue and organ regeneration.
Like wound healing, organ regeneration is a tissue repair response (38). However, the precise signals and mechanisms of organ regeneration remain elusive. Lipid autacoids such as COX-derived prostaglandin E2 have been implicated in wound healing, organ regeneration, and the repair of gastrointestinal ulcers in animal models (6). Accumulating experimental evidence suggests an essential role of angiogenesis for organ development and regeneration. Most studies have focused on the role of stimulating organ regeneration with VEGF and other angiogenic growth factor and cytokine-related mechanisms. After hepatectomy, hepatocyte regeneration is stimulated by VEGF (39). VEGF induces liver sinusoidal ECs to secrete growth and survival factors such as hepatocyte growth factor and IL-6 that result in hepatocyte proliferation (35), again underscoring the trophic function of the endothelium. Our studies suggest that EETs are an important paracrine growth and survival factor in liver regeneration.
The role of such angiocrine growth factors is not unique to liver regeneration. VEGF, but not FGF-2, accelerates compensatory lung growth after unilateral pneumonectomy (40). However, two selective VEGF receptor inhibitors had no effect on lung regeneration, suggesting that VEGF is not the only regulator of tissue regeneration (40). Our data suggest that EETs also play an important role in lung tissue repair. Impaired lung regeneration is present in life-threatening diseases such as bronchopulmonary dysplasia and congenital diaphragmatic hernia in premature infants (40). Several studies support the notion that ECs within the lungs actively promote normal alveolar growth and lung regeneration (15). Conversely, genetic or pharmacological antagonism of the VEGF nitric oxide pathway can lead to decreased lung angiogenesis and poor lung regeneration (41). Similar to the liver and lung regeneration discussed above, kidney blood vessels appear to have a role in kidney regeneration (42). Consistent with our findings in liver regeneration and lung compensatory growth, EETs stimulated kidney compensatory growth.
Although angiogenesis is an important target of EETs, we show that the endothelium acts as a source for angiocrine trophic factors and that EETs appear to play a prevalent role in this process. EETs may have direct trophic effects on the stem cell compartment to account for their stimulatory actions on tissue regeneration. For instance, chemokines, such as stromal cell-derived factor 1 (SDF-1), have been shown to play a role in mesenchymal stem cell (MSC) homing in therapeutic angiogenesis, wound healing, and liver regeneration, as well as neuronal regeneration (43). Because EETs were recently shown to up-regulate SDF-1α and VEGF in healing wounds (18), it will be important to determine whether EETs alter other factors that influence wound repair. Furthermore, sEHis have been shown to stimulate axonal growth in neuronal cell cultures (16). An EET agonist was recently reported to stimulate MSC-derived adipocyte proliferation and differentiation (44), suggesting a role in adipose regeneration.
Our results offer a mechanistic rationale for evaluating sEHis as novel therapeutics for a number of human diseases, such as hepatic insufficiency after liver damage or surgical resection in various liver disorders including trauma or infection. In addition, sEHis could have therapeutic efficacy in diseases characterized by immature lung development such as bronchopulmonary dysplasia. Topical sEHis could also be useful in accelerating wound healing in both diabetic and nondiabetic populations. The pharmacologically accessible EET autacoid system offers an entirely unique approach to enhance normal tissue growth, thus accelerating critical life-saving processes, such as organ regeneration and wound healing. However, in seeking to increase the level or activity of EETs for therapeutic indications, it will be important to balance these beneficial effects with our finding that direct administration of exogenous EETs can also stimulate cancer growth in preclinical models (20).
Materials and Methods
Generation of Tie2-CYP2J2, Tie2-CYP2C8, and Tie2-sEH Transgenic Mice.
Reagents.
The 14,15- and 11,12-EET were obtained from Cayman Chemical. The 14,15-, 11,12-EET or vehicle were administered intraperitoneally via osmotic minipump (Alzet) at a dose of 15 μg⋅kg−1⋅d−1. TUPS was synthesized as described (24, 25), and TUPS was completely dissolved in PEG 400 at a concentration of 10 mg/mL and mixed into Vanicream to obtain a 0.1% (wt:vol) formulated cream. The sEHi (TUPS) was administered orally by gavage in an aqueous solution of 10% (vol/vol) DMSO in 0.5% methylcellulose (10 mg⋅kg−1⋅d−1) or as a 0.1% cream applied topically; control mice received vehicle. The EET antagonist 14,15-EEZE (0.21 mg per mouse) was administered as we recently described (20).
Tissue Homeostasis and Angiogenesis Assays.
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Boston Children's Hospital. Genetically modified mice with high EET (Tie2-CYP2C8-Tr, Tie2-CYP2J2-Tr, and sEH-null) or low EET (Tie2-sEH-Tr) levels were compared with WT littermate control mice. Six-month-old male mice were used. In vivo Matrigel plug whole-mount staining of CD31 was performed as described (27). Briefly, Matrigel (Becton-Dickinson) (400 μL) was injected on each side of the ventral midline with sphingosine-1-phosphate (1 μM). Matrigel plugs were collected on day 7. Fluorescent staining (CD31) of Matrigel plug sections was performed as described (27). Quantification of ECs in the Matrigel plugs was performed by FACS following enzymatic digestion of the Matrigel plugs as described (28). Flow cytometry was performed by using FACS Calibur and CellQuest software (BD Biosciences). ECs were defined as CD31+/CD45− cells. Corneal neovascularization assays (80 ng of FGF2 or 160 ng of VEGF) were performed as described (29). For wound-healing studies, two 8-mm dermal punch biopsy wounds were performed per mouse. Wound area was quantified via computerized analysis with IP-LAB software (Scanalytics). Partial hepatectomy and unilateral pneumonectomy were performed as we described (33, 45). For the unilateral nephrectomies, the kidney was isolated, renal pedicle was ligated, and the kidney was excised. For systemic administration of 14,15- and 11,12-EET, male C57BL/6J mice (Jackson Laboratories) were used. For in vivo VEGF depletion, Ad-null and Ad-sFlt were administered systemically as described (20). Mouse liver ECs were isolated from 8- to 10-wk old nude mice. Excised mouse liver tissues were processed to make single cell suspension. The cells were then incubated with anti-mouse CD31 antibody (eBioscience), and liver ECs were isolated by MACS (Miltenyi Biotec) according to the manufacturer’s protocol, using anti-rat IgG microbeads (Miltenyi Biotec). They were plated onto 1.5% gelatin-coated culture plates and grown in microvascular endothelial cell growth medium 2 (EGM-2MV) (Lonza). To improve the purity of liver ECs, magnetic sorting was performed by using two MACS columns set up in series. For mouse liver EC proliferation, mouse liver ECs were seeded at 2 × 105 cells into a plate with different concentrations of EET. Cell number was counted every day for 3 d. The data are presented as the average of three different well counts ± SEM for each group. The experiment was repeated three times.
Immunohistochemistry.
Wound and liver samples were processed and immunohistochemical stainings were performed as we described (46). For rat platelet endothelial cell adhesion molecule (PECAM-1; CD31), sections were treated with 40 μg/mL proteinase K (Roche Diagnostics) for 25 min at 37 °C as described (47). Rat anti-mouse PECAM-1 (Pharmingen; 1:250), MECA-32 rat anti-mouse panendothelial cell antigen (Pharmingen; 1:250), and proliferating cell nuclear antigen (PCNA; Vector; 1:50), and VEGF rabbit (Thermo Scientific; 1:100) were incubated at 4 °C overnight. MECA-32 and PCNA staining was completed as we described (29). β-Galactosidase staining was performed as described (20).
LC-MS/MS Analysis.
ECs were isolated from WT, Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and Tie2-sEH-Tr mice, and LC-MS/MS analysis was performed as described (19, 20, 22). All human studies were reviewed and approved by the Institutional Review Board at Lahey Clinic Medical Center. Informed consent was obtained from the human donors. Plasma samples (n = 53) were obtained from five human donors undergoing partial liver resections for living-related liver transplantation.
Statistical Analysis.
The Student’s two-sided unpaired t test was used to analyze the difference between the two groups. P < 0.05 were considered statistically significant. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Kristin Johnson for photography and preparation of the figures; J. Alyce Bradbury, Joan P. Graves, Laura M. DeGraff, and Ricky Sanchez for excellent technical assistance; and Sung Hee Hwang for sEHi. This work was supported by National Cancer Institute Grant RO1CA148633-01A4 (to D.P.); the Stop and Shop Pediatric Brain Tumor Fund (M.W.K.); the C. J. Buckley Pediatric Brain Tumor Fund (M.W.K.); the Joshua Ryan Rappaport Fellowship (H.D.L.); the Children’s Hospital Boston Surgical Foundation and the Vascular Biology Program (M.P. and H.D.L.); a Howard Hughes Medical Institute Research Fellowship (to B.T.K.); Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) (Z01 025034 to D.C.Z.; Z01 050167 to K.B.T.); NIH Grant R01 GM088199 (to C.R.L.); NIH Grant GM31278 and Robert A. Welch Foundation Grant GL625910 (to J.R.F.); NIEHS Superfund Basic Research Program NIH Grant P42 ES004699 and NIH Grants R01 ES002710 and R01 ES013933 (to B.D.H.); and NIH Grant CA045548 (to D.E.I.). B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311565110/-/DCSupplemental.
References
- 1.Gronert K. Lipid autacoids in inflammation and injury responses: A matter of privilege. Mol Interv. 2008;8(1):28–35. doi: 10.1124/mi.8.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 3.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30(3-4):525–540. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene ER, Huang S, Serhan CN, Panigrahy D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011;96(1-4):27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronert K, et al. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280(15):15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 8.Fleming I. Vascular cytochrome p450 enzymes: Physiology and pathophysiology. Trends Cardiovasc Med. 2008;18(1):20–25. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Rupnick MA, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99(16):10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Street J, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 12.Kaspera R, Totah RA. Epoxyeicosatrienoic acids: Formation, metabolism and potential role in tissue physiology and pathophysiology. Expert Opin Drug Metab Toxicol. 2009;5(7):757–771. doi: 10.1517/17425250902932923. [DOI] [PubMed] [Google Scholar]
- 13.Fife KL, et al. Inhibition of soluble epoxide hydrolase does not protect against endotoxin-mediated hepatic inflammation. J Pharmacol Exp Ther. 2008;327(3):707–715. doi: 10.1124/jpet.108.142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdu E, et al. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2011;117(4):632–642. doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luria A, et al. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108(22):9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander AL, et al. Cytochrome P450-derived epoxyeicosatrienoic acids accelerate wound epithelialization and neovascularization in the hairless mouse ear wound model. Langenbecks Arch Surg. 2011;396(8):1245–1253. doi: 10.1007/s00423-011-0838-z. [DOI] [PubMed] [Google Scholar]
- 19.Lee CR, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24(10):3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panigrahy D, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122(1):178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seubert JM, et al. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99(4):442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edin ML, et al. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25(10):3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webler AC, et al. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol. 2008;295(5):C1292–C1301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50(16):3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai HJ, et al. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur J Pharm Sci. 2010;40(3):222–238. doi: 10.1016/j.ejps.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauthier KM, et al. 14,15-Epoxyeicosa-5(Z)-enoic acid: A selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90(9):1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Claesson-Welsh L, Shibuya M. VEGF receptor signal transduction. Methods Enzymol. 2008;443:261–284. doi: 10.1016/S0076-6879(08)02013-2. [DOI] [PubMed] [Google Scholar]
- 28.Adini A, et al. Matrigel cytometry: A novel method for quantifying angiogenesis in vivo. J Immunol Methods. 2009;342(1-2):78–81. doi: 10.1016/j.jim.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Panigrahy D, et al. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest. 2002;110(7):923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pozzi A, et al. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280(29):27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 31.Bao P, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Amore PA. Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci. 1994;35(12):3974–3979. [PubMed] [Google Scholar]
- 33.Greene AK, et al. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg. 2003;237(4):530–535. doi: 10.1097/01.SLA.0000059986.96051.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drixler TA, et al. Liver regeneration is an angiogenesis-associated phenomenon. Ann Surg. 2002;236(6):703–711, discussion 711–712. doi: 10.1097/00000658-200212000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: Role of VEGFR-1. Science. 2003;299(5608):890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 36.Tassi E, et al. Impact of fibroblast growth factor-binding protein-1 expression on angiogenesis and wound healing. Am J Pathol. 2011;179(5):2220–2232. doi: 10.1016/j.ajpath.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17(8):424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Simpson DG. Dermal templates and the wound-healing paradigm: The promise of tissue regeneration. Expert Rev Med Devices. 2006;3(4):471–484. doi: 10.1586/17434440.3.4.471. [DOI] [PubMed] [Google Scholar]
- 39.Dogrul AB, et al. Antiangiogenic response after 70% hepatectomy and its relationship with hepatic regeneration and angiogenesis in rats. Surgery. 2010;147(2):288–294. doi: 10.1016/j.surg.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai MK, et al. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L742–L747. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 41.Galambos C, et al. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol. 2002;27(2):194–203. doi: 10.1165/ajrcmb.27.2.4703. [DOI] [PubMed] [Google Scholar]
- 42.Harari-Steinberg O, Pleniceanu O, Dekel B. Selecting the optimal cell for kidney regeneration: Fetal, adult or reprogrammed stem cells. Organogenesis. 2011;7(2):123–134. doi: 10.4161/org.7.2.15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11(2):189–197. doi: 10.1517/14712598.2011.546338. [DOI] [PubMed] [Google Scholar]
- 44.Vanella L, et al. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96(1-4):54–62. doi: 10.1016/j.prostaglandins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakurai MK, Greene AK, Wilson J, Fauza D, Puder M. Pneumonectomy in the mouse: Technique and perioperative management. J Invest Surg. 2005;18(4):201–205. doi: 10.1080/08941930591004485. [DOI] [PubMed] [Google Scholar]
- 46.Kaipainen A, et al. PPARalpha deficiency in inflammatory cells suppresses tumor growth. PLoS ONE. 2007;2(2):e260. doi: 10.1371/journal.pone.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panigrahy D, et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA. 2008;105(3):985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.