Abstract
Sister chromatid cohesion depends on Sororin, a protein that stabilizes acetylated cohesin complexes on DNA by antagonizing the cohesin release factor Wings-apart like protein (Wapl). Cohesion is essential for chromosome biorientation but has to be dissolved to enable sister chromatid separation. To achieve this, the majority of cohesin is removed from chromosome arms in prophase and prometaphase in a manner that depends on Wapl and phosphorylation of cohesin’s subunit stromal antigen 2 (SA2), whereas centromeric cohesin is cleaved in metaphase by the protease separase. Here we show that the mitotic kinases Aurora B and Cyclin-dependent kinase 1 (Cdk1) destabilize interactions between Sororin and the cohesin subunit precocious dissociation of sisters protein 5 (Pds5) by phosphorylating Sororin, leading to release of acetylated cohesin from chromosome arms and loss of cohesion. At centromeres, the cohesin protector shugoshin (Sgo1)-protein phosphatase 2A (PP2A) antagonizes Aurora B and Cdk1 partly by dephosphorylating Sororin and thus maintains cohesion until metaphase. We propose that the stepwise loss of cohesion between chromosome arms and centromeres is caused by local regulation of Wapl activity, which is controlled by the phosphorylation state of Sororin.
Keywords: cell cycle, chromosome segregation, prophase pathway
Replicated DNA molecules (sister chromatids) remain physically connected with each other from S phase until metaphase to enable biorientation of chromosomes on the mitotic spindle. This association, called sister chromatid cohesion, is mediated by cohesin, whose core subunits Structural maintenance of chromosomes 1 (Smc1), Smc3, Sister chromatid cohesion 1 (Scc1), and Scc3/stromal antigen (SA/STAG) form a molecular ring. This structure is crucial for cohesion, presumably because cohesin topologically embraces the two sister chromatids (reviewed in ref. 1). The core subunits, in turn, recruit the cohesin-binding proteins Pds5, Wapl, and Sororin (1–3).
Cohesion is established during DNA replication in S phase and depends on Smc3 acetylation (4–6) by the acetyltransferases Esco1 and Esco2 (2). This modification leads to recruitment of Sororin, which stabilizes cohesin on DNA by inactivating the cohesin release factor Wapl (2, 7–11). Wapl binds to Pds5 proteins (8) (Pds5A/B in vertebrates) (12) and, in the absence of Sororin, the resulting Wapl–Pds5 heterodimers dissociate cohesin from chromatin (3). Sororin directly associates with Pds5 proteins and thereby dissociates Wapl from Pds5 in vitro (2), implying that Sororin antagonizes Wapl by changing its interaction with Pds5.
In higher eukaryotes, cohesin is released from mitotic chromosomes in two steps (13). Most cohesin complexes are removed from chromosome arms in prophase and prometaphase via the so-called “prophase pathway” (13, 14). The remaining cohesin, predominantly located at centromeres, is cleaved by the protease separase in metaphase, leading to loss of cohesion and anaphase onset (15). The prophase pathway depends on Wapl (7, 8) and the mitotic kinases Polo-like kinase (Plk1) and Aurora B (16–19). A critical substrate of Plk1 in the prophase pathway is SA2, one of several SA/STAG paralogs, as nonphosphorylatable SA212A reduces cohesin dissociation from chromosome arms in prometaphase (20). At centromeres, cohesin is protected from the prophase pathway by the MEI-S332/complex (21–24), which dephosphorylates SA2 in vitro (21). SA212A suppresses the Sgo1-depletion phenotype (25), suggesting that one key function of Sgo1–PP2A is to protect SA2 from phosphorylation.
Previous work had shown that Sororin is phosphorylated in mitosis (10), that this phosphorylation prevents binding of Sororin to Pds5A in vitro (2), that the phosphorylation of Sororin on putative Cdk1 sites is required for its dissociation from chromatin and partial loss of cohesion (26), and that alanine mutation of these sites bypasses the requirement for Sgo1 in cohesion (27). It has also been proposed that Sororin phosphorylation by Cdk1 enables Plk1 binding to Sororin, leading to SA2 phosphorylation by Plk1 (28). However, it remains unknown on which sites Sororin is phosphorylated in vivo, by which mechanism Sororin phosphorylation enables the dissociation of cohesin from mitotic chromosomes, and if Sororin and SA2 phosphorylation contribute to cohesin dissociation through the same mechanism.
Here, we report the in vivo phosphosites on Sororin in human mitotic cells, and show that both Aurora B and Cdk1 are required for the generation of these sites, for dissociation of Sororin from cohesin and chromatin by releasing Sororin from Pds5, and for release of acetylated “cohesive” cohesin from mitotic chromosomes. We further show that Sgo1–PP2A protects cohesin at centromeres in part by maintaining Sororin in a dephosphorylated state. In addition, we provide evidence that SA2 phosphorylation by Plk1 and Sororin phosphorylation by Aurora B and Cdk1 contribute to cohesin dissociation through distinct mechanisms.
Results
Identification of Mitotic Phosphorylation Sites on Sororin.
Sororin can be phosphorylated by Cdk1 in vitro but the sites on which Sororin is phosphorylated in vivo have so far only been inferred from Sororin phosphopeptides found in large-scale proteomic studies and from bioinformatic predictions of Cdk1 sites (26, 28). We, therefore, performed a systematic and unbiased mass spectrometric analysis of Sororin’s phosphosites. We used HeLa cells stably expressing mouse Sororin with a localization and affinity purification (LAP) tag at its carboxy teminus. In these “Sororin-LAP cells,” Sororin is expressed at near-endogenous levels from its own promoter and is regulated in a cell-cycle–dependent manner like endogenous Sororin (2). These cells were synchronized in prometaphase with nocodazole, or in S/G2 phase by thymidine release, and Sororin isolated by affinity purification was analyzed by mass spectrometry (MS). We identified 17 phosphosites in mouse Sororin, of which 15 were mitosis specific. Of these, 11 and 9 sites are conserved in human and Xenopus Sororin, respectively (Fig. S1A). Only 5 of the 15 sites identified in our experiments were among the 9 putative Cdk1 sites that had been mutated in previous studies (26, 27).
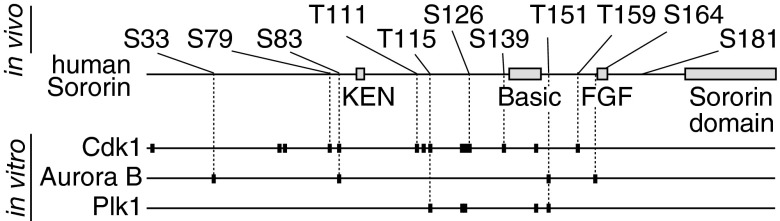
Next we tested if these phosphosites could be generated by Cdk1, Aurora B, or Plk1. Human Sororin expressed in Escherichia coli was incubated with ATP plus each of these kinases and subsequently analyzed by MS. We found that all of the 11 residues conserved between mouse and human Sororin, except Ser181, could be phosphorylated by either Cdk1 or Aurora B (Fig. 1). Plk1 also phosphorylated several sites on Sororin, but each of these could also be generated by Cdk1 or Aurora B (Fig. 1), indicating redundancy in substrate targeting by these kinases, and that Cdk1 and Aurora B might be sufficient for mitotic Sororin phosphorylation.
Fig. 1.
Identification of mitosis-specific phosphorylation sites in Sororin. In vivo and in vitro phosphorylation sites in human Sororin. Mitosis-specific in vivo phosphorylation sites conserved in human Sororin are shown (Upper). Phosphorylation sites identified by MS in human Sororin phosphorylated using either Cdk1, Aurora B, or Plk1 in vitro are shown as small black boxes.
The Wapl Removal Activity of Sororin Is Attenuated by Aurora B- and Cdk1-Dependent Phosphorylation.
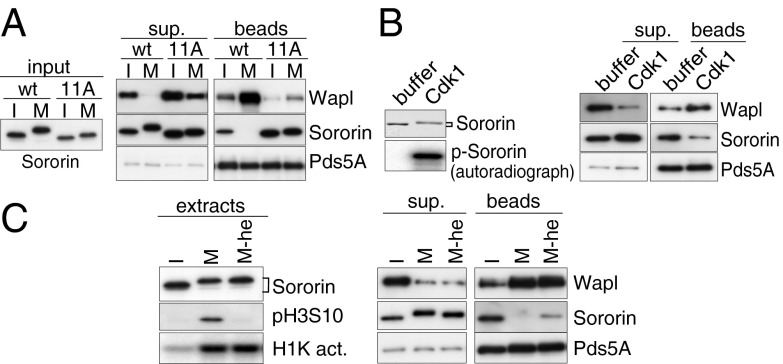
We showed previously that binding of Sororin to Pds5A dissociates Wapl from Pds5A in vitro, and that this Wapl removal activity is reduced by phosphorylation of Sororin (2). To test if the mitotic phosphosites identified here are responsible for the attenuation of Sororin’s activity, we generated a Sororin mutant in which nine phosphosites conserved among vertebrates (Fig. S1A) were mutated to alanine. In addition, we mutated two serine residues directly adjacent to Ser126 (Ser124 and Ser125) to exclude the possibility that these sites become phosphorylated instead of Ser126, because MS had shown that these could also be phosphorylated by Cdk1 in vitro (Fig. 1). The resulting Sororin11A mutant was tested for its Wapl removal activity. As previously reported (2), when Wapl–Pds5A heterodimer bound to Pds5A antibody beads was mixed with wild-type Sororin (SororinWT) preincubated in interphase Xenopus egg extract, SororinWT associated with Pds5A and displaced some Wapl from Pds5A. In contrast, when SororinWT preincubated in mitotic extract was mixed with Wapl–Pds5A, SororinWT did not bind to Pds5A, and Wapl was not displaced. However, Sororin11A associated with Pds5A and displaced Wapl even after preincubation in mitotic extract (Fig. 2A), indicating that phosphorylation of Sororin prevents its binding to Pds5A in mitosis. Interestingly, however, when Sororin11A was incubated in mitotic egg extract, most endogenous Xenopus Pds5A dissociated from Sororin11A (Fig. S1B), implying that mitotic phosphorylation of Pds5A (29) might also contribute to the dissociation of Pds5 from Sororin.
Fig. 2.
Aurora B and Cdk1 attenuate Wapl removal activity of Sororin. (A) Recombinant SororinWT or Sororin11A proteins were preincubated in either interphase (I) or mitotic (M) egg extracts (Left). Anti-Pds5A antibody beads bound to Wapl–Pds5A were incubated with the I- or M-SororinWT/11A. Bead-bound proteins (beads) were separated from the supernatant (sup.) (Right). (B) In vitro Cdk1-dependent phosphorylation is sufficient to inactivate the Wapl removal activity of Sororin. SororinWT protein was incubated with either buffer or Cdk1 and purified (Left), and then mixed with anti-Pds5A antibody beads bound to Wapl–Pds5A. Bead-bound proteins (Right) were separated from the supernatant (Center). (C) Aurora B-dependent phosphorylation weakens the binding affinity of Sororin to Pds5. SororinWT protein was incubated with either I- or M extracts in the presence or absence of Hesperadin (he). The extracts were analyzed by immunoblotting and histone H1 kinase activity assay (H1K act.). Subsequently, Sororin was purified from I- or M extracts, then mixed with anti-Pds5A antibody beads bound to Wapl–Pds5A. Bead-bound proteins were separated from the supernatant (Right).
Next we asked which kinase is required for the inactivation of Sororin. Because all mutated residues in Sororin11A are phosphorylated by Cdk1 or Aurora B in vitro (Fig. 1), we first tested if Cdk1 or Aurora B have a direct role in the inactivation of Sororin, using a Wapl removal assay. Recombinant Sororin was first phosphorylated by Cdk1 in vitro (Fig. 2B, Left, p-Sororin), purified by immunoprecipitation and peptide elution, and subsequently mixed with Wapl–Pds5A. The level of Sororin bound to Pds5A was reduced when Sororin was preincubated with Cdk1, compared with Sororin treated with buffer only; concomitantly, the amount of Wapl dissociated from Pds5A was also reduced (Fig. 2B). Thus, Cdk1 is sufficient for the inactivation of Sororin’s Wapl removal activity. In contrast, Aurora B did not detectably reduce Sororin’s ability to dissociate Wapl from Pds5A, although Aurora B could phosphorylate Sororin (Fig. 1). This might be because (i) purified Aurora B was not fully active as it was not copurified with inner centromere protein (INCENP), which is required for full activation of Aurora B or (ii) Aurora B is not sufficient for the inactivation of Sororin.
We therefore analyzed if Aurora B is required for the inactivation of Sororin. To test this, Sororin was preincubated in mitotic egg extract treated with Hesperadin, an inhibitor of Aurora B (30), and mixed with Wapl–Pds5A. As shown in Fig. 2C, although Hesperadin did not reduce the Wapl removal activity of Sororin, the amount of Sororin bound to Pds5A was slightly but reproducibly increased compared with that preincubated in mitotic extract without Hesperadin. Hesperadin was effective in this assay because it abolished the phosphorylation on Ser10 of histone H3 (pH3), a known target of Aurora B, whereas it did not inhibit Cdk1 activity as represented by histone H1 kinase activity (Fig. 2C, Left). Because Hesperadin increased the binding of SororinWT but not of Sororin11A to Pds5A (Fig. S1B), Aurora B-dependent phosphorylation of Sororin rather than that of Pds5A weakens the association between Sororin and Pds5. These observations indicate that Aurora B contributes to the inactivation of Sororin in mitosis.
Aurora B Activity Is Required for the Dissociation of Sororin from Cohesin and Chromosomes in Mitosis.
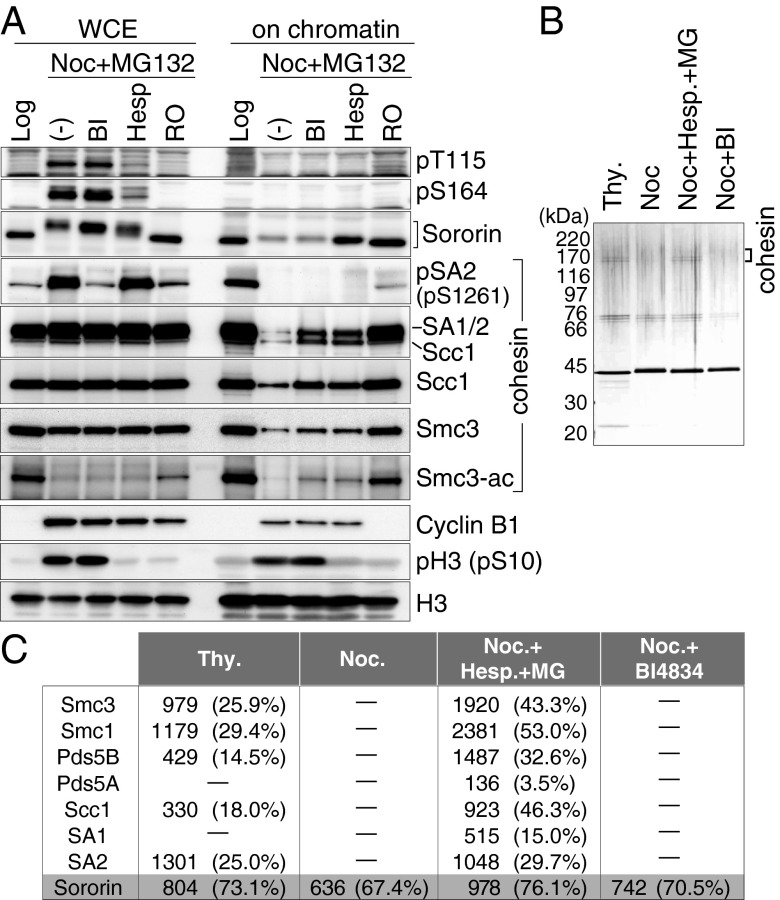
The dissociation of Sororin from chromosomes in mitosis has been shown to depend on Cdk1 (26, 27), but our results indicated that not only Cdk1 but also Aurora B reduces Sororin’s ability to bind to Pds5A. If Sororin’s dissociation from mitotic chromosomes is mediated by releasing Sororin from Pds5, our results predict that both Cdk1 and Aurora B contribute to the dissociation of Sororin from mitotic chromosomes. To test this possibility, HeLa cells arrested in prometaphase by nocodazole were treated with small-molecule inhibitors of Aurora B (Hesperadin), Plk1 (BI 4834), or Cdk1 (RO3306) in the presence of MG132, a proteasome inhibitor that was added to avoid mitotic exit caused by Hesperadin (30). We then analyzed by immunoblotting if Sororin remained associated with chromatin under these conditions (Fig. 3A). Most Sororin dissociated from chromatin in nocodazole-arrested cells. However, when mitotic cells were treated with Hesperadin or RO3360, as much Sororin was detected on chromatin as on chromatin from logarithmically proliferating cells. In contrast, Sororin dissociation was not reduced by BI 4834, although Plk1 was efficiently inhibited because the phosphorylation of SA2 on Ser1261, a residue known to be phosphorylated by Plk1 (20, 29), was inhibited to the extent observed in logarithmically proliferating cells (Fig. 3A, WCE, whole-cell extract), and because cohesin dissociation from mitotic chromatin was reduced (Fig. 3A, on chromatin). RO3306 also diminished the pH3 and pSA2 signals (Fig. 3A), presumably because Cdk1 inhibition led to exit from the mitotic state. These observations indicate that both Cdk1 and Aurora B are required for Sororin’s dissociation from mitotic chromosomes, whereas Plk1 is dispensable for this process.
Fig. 3.
Aurora B is essential for dissociation of Sororin from chromatin and cohesin. (A) DMSO (Log)- or nocodazole (Noc)-treated HeLa cells were additionally treated with DMSO (−), BI 4834 (BI), Hesperadin (Hesp), or RO3306 (RO) in the presence of MG132. Whole-cell extracts (WCE) and chromatin-bound fractions were analyzed by immunoblotting. (B) Silver-stained gel of a Sororin-LAP tandem affinity purification. Sororin-LAP cells were treated with thymidine (Thy), Noc, Noc+Hesp+MG132 (MG), or Noc+BI 4834 (BI). Sororin-LAP was purified from cell extracts by tandem affinity purification. (C) MS analysis of proteins bound to Sororin-LAP purified as in B. Mascot protein scores and sequence coverages in parentheses are shown.
To be able to analyze the phosphorylation of Sororin directly in mitotic cells, we generated phospho-specific antibodies against the pThr115 and pSer164 sites of Sororin, which are phosphorylated by Cdk1 and/or Plk1 and Aurora B in vitro, respectively (Fig. 1). Both sites were phosphorylated specifically in mitosis in HeLa cells (Fig. 3A and Fig. S2A) and the immunosignals generated by these antibodies were reduced by Sororin RNAi (Fig. S2B). It is noteworthy that both pThr115 and pSer164 immunoblot signals were diminished following treatment with Hesperadin as well as RO3306 (Fig. 3A), despite the fact that Thr115 is not phosphorylated by Aurora B in vitro (Fig. 1). Phosphorylation of Thr115 in vivo may, therefore, depend on both Aurora B and Cdk1. We were not able to detect either pThr115 or pSer164 on mitotic chromosomes, even in Wapl-depleted cells where more Sororin remains bound to chromosomes (Fig. S2C). In contrast, pSer1261 on SA2 was strongly detected on mitotic chromosomes in Wapl-depleted cells (Fig. S2C) (8). Thus, unlike SA2, chromatin-bound Sororin may not be phosphorylated, consistent with the hypothesis that phosphorylation releases Sororin from chromatin.
Interestingly, cohesin behaved differently than Sororin in these experiments. Whereas Sororin dissociation was reduced by Hesperadin and RO3306, but not by BI 4834, the level of Scc1 on chromatin was increased by all three inhibitors compared with the levels of Scc1 in nocodazole-treated cells (Fig. 3A). The observation that BI 4834 reduces the dissociation of cohesin from mitotic chromosomes without preventing either the phosphorylation of Sororin or its dissociation from chromosomes suggests that the function of Plk1 in releasing cohesin from mitotic chromosomes is independent of Sororin inactivation. This notion is further supported by the finding that the phosphorylation of Ser1261 on SA2 by Plk1 and Thr115 and Ser164 on Sororin by Aurora B are independent of each other (Fig. 3A). These results indicate that Plk1 and Aurora B independently regulate the dissociation of cohesin by phosphorylating different proteins, SA2, and Sororin, respectively.
We also analyzed whether inhibition of Aurora B affects Sororin binding to cohesin. Sororin-LAP was immunoprecipitated from cells arrested in S phase by thymidine or in mitosis by nocodazole in the presence or absence of BI 4834 or Hesperadin. Immunuprocipitates from each condition (Fig. 3B) were analyzed by MS. Whereas cohesin was copurified with Sororin in S phase, no cohesin was detected in the nocodazole sample or in the sample from BI 4834-treated mitotic cells (Fig. 3 B and C). However, all cohesin subunits were identified in Sororin samples from Hesperadin-treated mitotic cells. This result supports the hypothesis that Sororin binds to cohesin in S phase, but is released upon entry to mitosis in an Aurora B-dependent manner.
Phosphorylation of Sororin Is Specifically Required for the Dissociation of Acetylated Cohesin from Mitotic Chromosomes.
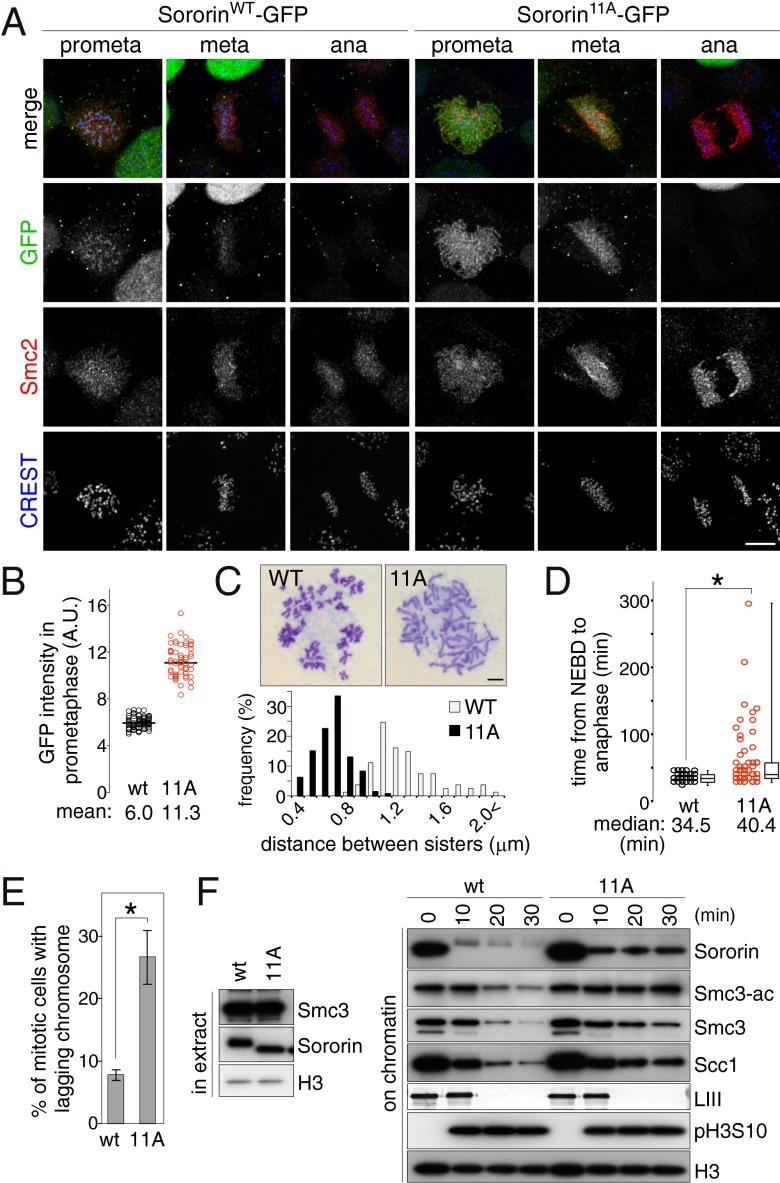
Sororin mutated in putative Cdk1 sites has been shown to persist on mitotic chromosomes and to increase cohesion (26). To analyze the function of the phosphosites that we identified on Sororin in vivo, we generated HeLa cells stably expressing green fluorescent protein (GFP)-tagged SororinWT or Sororin11A at levels similar to those of endogenous Sororin (Fig. S3). Immunofluorescence microscopy after preextraction of soluble proteins showed that in prometaphase only a faint SororinWT-GFP signal was detected between a pair of kinetochores marked by CREST, whereas Sororin11A-GFP was detected along chromosome arms (Fig. 4 A and B and Fig. S4A). Chromosome spreads showed that the distances between chromosome arms were closer to each other in Sororin11A than in SororinWT cells (Fig. 4C). We could not detect Sororin11A on anaphase chromosomes (Fig. 4A), indicating that Sororin11A associates stably with chromosomes before anaphase onset, and then dissociates in anaphase, possibly because of cohesin cleavage by separase. These results indicate that the in vivo phosphosites identified here are essential for the prophase pathway of cohesin dissociation and for loss of arm cohesion.
Fig. 4.
Phosphorylation of Sororin is required for removal of acetylated cohesin. (A) SororinWT- or Sororin11A-GFP cells were preextracted, fixed, and stained for Sororin-GFP (GFP), condensin (Smc2), and kinetochores (CREST). (Scale bar, 10 μm.) (B) Quantification of chromatin-bound Sororin-GFP signal in prometaphase. The fluorescence intensities of Sororin-GFP in the cells described in A are plotted. (n ≥ 57). (C) Sororin11A causes an overcohesion phenotype. SororinWT- or Sororin11A-GFP cells in mitosis were spread and chromosomes were stained with Giemsa. Representative pictures of the most prominent phenotype for each case are shown (Upper). (Scale bar, 10 μm.) Distances between the arms of sister chromatid pairs measured in >80 chromosomes are shown in a histogram (Lower). (D) Time from NEBD to anaphase of the H2B-mCherry expressing SororinWT or Sororin11A cells (n ≥ 60; *P < 0.05). (E) Quantification of lagging chromosome frequency in cells described in D, shown as mean and SEM of five experiments (n ≥ 10 per experiment; *P < 0.05). (F) Sororin11A specifically protects acetylated cohesin from its dissociation in entry into mitosis. Recombinant SororinWT or Sororin11A protein was added to interphase Xenopus egg extracts supplemented with demembranated sperm nuclei and incubated for 90 min to allow DNA replication and the association of Sororin with chromatin. Subsequently, nondegradable cyclin B (Δ90CycB) was added to induce mitosis. At the indicated time points after addition of Δ90CycB, chromatin was isolated and its binding proteins were analyzed by immunoblotting. The disappearance of lamin LIII (LIII) indicates NEBD.
Although anaphase occurred apparently normally in Sororin11A cells, we noticed by time-lapse microscopy that Sororin11A cells took significantly longer to progress from nuclear envelope breakdown (NEBD) to anaphase, compared with SororinWT cells (Fig. 4D), and lagging chromosomes were frequently observed in Sororin11A cells (Fig. 4E). Therefore, higher levels of Sororin on chromosomes delay chromosome segregation, suggesting that Sororin phosphorylation is required for normal progression through mitosis.
We next tested if Sororin phosphorylation is required to release acetylated cohesin from chromosomes, as Smc3 acetylation is required to recruit Sororin to chromatin (2, 9). For this purpose, we used Xenopus egg extracts where the dissociation of cohesin from chromosomes during mitotic entry can be followed with high cell cycle synchrony. SororinWT or Sororin11A proteins were added to interphase extract with demembranated sperm nuclei as a source of chromatin, DNA replication was allowed to take place, mitosis was induced by addition of nondegradable cyclin B (Δ90CycB), and at different time points, chromatin was analyzed by immunoblotting (Fig. 4F). Similar to the results in HeLa cells, significantly more Sororin11A than SororinWT remained on mitotic chromosomes, and sister chromatids were more tightly connected in the presence of Sororin11A (Fig. S4 B and C). Interestingly, Smc3 and Scc1 gradually dissociated from chromatin even in the presence of Sororin11A, but the levels of acetylated Smc3 remained unchanged (Fig. 4F), suggesting that Sororin11A specifically protects acetylated cohesin. These results indicate that Sororin phosphorylation is not essential for dissociating cohesin from chromatin per se, but is specifically needed to release acetylated cohesive cohesin from chromatin and, thereby, to dissolve cohesion. In contrast to Sororin11A, the “phosphomimicking” mutant Sororin12E showed no difference in chromatin-bound levels compared with SororinWT (Fig. S5A). However, less Sororin12E bound to chromatin than SororinWT in higher salt conditions (Fig. S5B), consistent with the notion that multisite phosphorylation weakens the association of Sororin with chromatin.
Sgo1-PP2A Protects Acetylated Cohesin at Centromeres by Antagonizing Sororin Phosphorylation.
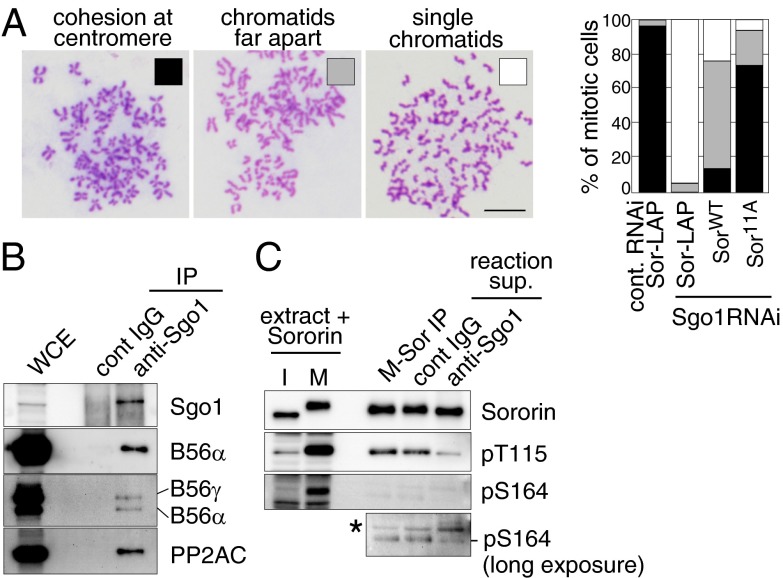
If the phosphorylation of Sororin leads to dissociation of acetylated cohesin and thus to loss of cohesion, Sororin phosphorylation has to be prevented at centromeres because cohesion has to be maintained at this site to enable the biorientation of chromosomes. Indeed, Sororin isolated from prometaphase chromosomes migrated much faster in SDS/polyacrylamide gel electrophoresis (PAGE) than Sororin in whole-cell extracts from the same cell population, implying that centromeric Sororin is less phosphorylated than cytoplasmic Sororin (Fig. 3A). At centromeres, MEI-S332/Shugoshin (Sgo) associated with PP2A protects cohesin from the prophase pathway. We, therefore, analyzed whether Sororin is a target of Sgo–PP2A. To test this, we performed a “rescue” experiment in which Sgo1 (a mammalian ortholog of Sgo) was depleted in the presence of SororinWT or Sororin11A (Fig. S6A). Chromosome spreads showed that Sgo1 depletion caused a cohesion defect in the presence of exogenous SororinWT, whereas Sororin11A largely reverted this defect (Fig. 5A). Sororin11A also overcame the mitotic arrest in Sgo1-depleted cells (Fig. S6B), indicating that nonphosphorylatable Sororin alleviates the requirement for Sgo1 in mitosis, consistent with the possibility that Sororin is a key substrate of Sgo1–PP2A. To test this, we immunoprecipitated Sgo1–PP2A complexes from HeLa cells using anti-Sgo1 antibody beads (Fig. 5B) and mixed these with SororinWT, which had been preincubated in mitotic Xenopus extract to allow Sororin phosphorylation. As in HeLa cells, Sororin phosphorylation on Thr115 and Ser164 occurred almost exclusively in mitotic Xenopus extracts (Fig. 5C), but the phosphorylation of these sites was significantly reduced in the presence of Sgo1 immunoprecipitates (Fig. 5C). This indicates that Sgo1-associated phosphatase activity dephosphorylates Sororin, at least on Thr115 and Ser164. However, Sororin was only dephosphorylated partially under these conditions, as Sororin mixed with Sgo1–PP2A migrated more slowly than interphase Sororin in SDS/PAGE (Fig. 5C). Possible reasons for this are that the activity of immunoprecipitated Sgo1–PP2A was not sufficient to completely dephosphorylate Sororin in the time frame of the assay, or that Sgo1–PP2A only dephosphorylates specific sites on Sororin.
Fig. 5.
Sororin11A overcomes the cohesion defect in Sgo1-depleted cells. (A) Sororin11A rescues cohesion in Sgo1-depleted cells. Sororin-LAP, SororinWT-, or Sororin11A-GFP stably expressing cells were transfected with either control or Sgo1 siRNAs and nocodazole-arrested mitotic cells were spread and stained with Giemsa. More than 130 cells per condition were classified into three categories (Right). Representative images of each category are shown (Left). (Scale bar, 10 μm.) (B and C) Sgo1-associated phosphatase activity dephosphorylates Sororin. HeLa whole-cell extracts (WCE) were mixed with either control IgG or anti-Sgo1 antibody beads. The bead-bound proteins were analyzed (B). The immunoprecipitates were mixed with Sororin preincubated in mitotic Xenopus egg extracts (M-Sor), and the reactions were analyzed (C). The asterisk indicates a nonspecific band.
We further tested if Sgo1 is required for Sororin dephosphorylation in vivo. Because Sororin is released from chromosome by Sgo1 RNAi (2), we codepleted Wapl to retain Sororin on chromosomes. As shown in Fig. S6, pS164 signals, but no pT115 staining, was detected at centromeres after Sgo1 depletion, confirming that Sgo1 is required to dephosphorylate centromeric Sororin, at least on S164.
Sororin and SA2 Phosphorylation Contribute Independently to the Prophase Pathway of Cohesin Dissociation.
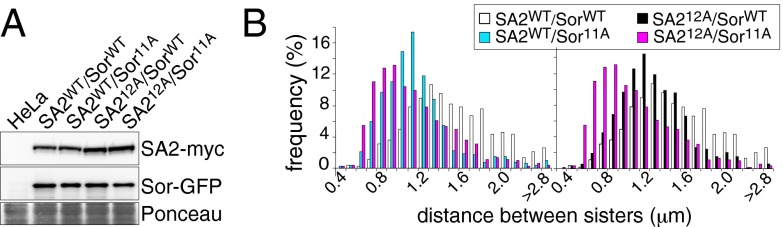
SA2 has also been proposed to be a key substrate of Sgo1–PP2A, as Sgo1-associated phosphatase activity can dephosphorylate SA2 in vitro (21) and a nonphosphorylatable SA2 mutant rescues cohesion defects caused by Sgo1 depletion (25). We, therefore, further investigated the relationship between Sororin and SA2 phosphorylation. As shown above (Fig. 3A), phosphorylation of SA2 and Sororin occurs independently of each other, and inhibition of Plk1 reduces cohesin dissociation from mitotic chromosomes without preventing the dissociation of Sororin from cohesin and chromatin, implying that SA2 and Sororin phosphorylation contribute to the prophase pathway independently. To further test this, we used HeLa cells expressing wild-type SA2 (SA2WT) or nonphosphorylatable SA2 (SA212A) under the control of a doxycycline-regulated promoter (20) and asked if coexpression of SororinWT or Sororin11A had additional effects on cohesion. SororinWT or Sororin11A cDNAs were transiently transfected into these cells and their expression was allowed without induction of SA2WT/12A. SA2WT/12A expression was then induced (Fig. 6A) and mitotic chromosomes were analyzed by chromosome spreads (Fig. 6B). Chromosomes in cells expressing either SA212A or Sororin11A exhibited smaller interchromatid distances than chromosomes in cells expressing both SA2WT and SororinWT. However, in the presence of both SA212A and Sororin11A sister chromatids were even more tightly connected than in the presence of SA212A or Sororin11A alone. These results further support the notion that Sororin phosphorylation contributes to the prophase pathway independently of the Plk1-dependent phosphorylation of SA2.
Fig. 6.
The phosphorylation of SA2 and of Sororin act cooperatively in the prophase pathway. (A) SororinWT- or Sororin11A-GFP was transiently expressed in SA2WT-myc– or SA212A-myc–inducible cells. After induction of SA2WT/12A-myc, whole-cell lysates were analyzed. (B) Cells in A were treated with nocodazole and mitotic chromosomes were spread and stained with Giemsa. Interchromatid distances were shown in histograms (n = 525).
Discussion
Here we provide insight into how the cohesin release factor Wapl becomes activated to enable the resolution of chromosome arms in prophase and prometaphase: Our results indicate that Aurora B and Cdk1 phosphorylate multiple sites on the Wapl antagonist Sororin, that these modifications lead to the dissociation of Sororin from its binding partner Pds5, and that cohesin complexes liberated from Sororin can subsequently be released from chromatin by Wapl.
These results extend the previous findings that phosphorylation of Sororin on putative Cdk1 sites causes dissociation of Sororin from chromatin (26). Our results indicate that in vivo Sororin is phosphorylated both on Cdk1 and Aurora B sites (Fig. 1) and that Aurora B activity is essential for the release of Sororin from chromatin and cohesin (Fig. 3), implying that Cdk1 is not sufficient for releasing Sororin from cohesin and that instead Aurora B is also required for this process. This reveals Sororin as a substrate of Aurora B in the prophase pathway, which has been known for a long time to depend on this kinase (16, 18). Importantly, our results also show that Sororin phosphorylation is not essential for the release of all cohesin from chromosomes, but instead is specifically required for the dissociation of cohesin complexes whose Smc3 has been acetylated (Fig. 4F)—the subpopulation of cohesin that is thought to be cohesive. These results also support the hypothesis that Sororin maintains cohesion only on acetylated cohesin by antagonizing Wapl (2).
Previous work has shown that also phosphorylation of SA2 by Plk1 is required for the dissociation of cohesin from chromosome arms (16–19). However, SA2 phosphorylation is not sufficient for this process because Wapl depletion decreases cohesin dissociation without preventing SA2 phosphorylation (8), consistent with the possibility that SA2 phosphorylation facilitates Wapl-mediated opening of the cohesin ring. It has been proposed that phosphorylation of Sororin on Thr159 by Cdk1 leads to binding of Plk1 to Sororin and thus enables Plk1 to phosphorylate SA2 (28). However, we observed that the expression of nonphosphorylatable mutants of Sororin and SA2 decreases cohesin dissociation in an additive manner (Fig. 6), which is not what one would expect if the only function of Sororin phosphorylation was to enable SA2 phosphorylation. Furthermore, expression of a Sororin4A mutant, in which Thr159 was replaced by Ala, did not decrease the dissociation of Sororin or cohesin from mitotic chromosomes (Fig. S7), implying that Sororin phosphorylation contributes to the release of cohesin not or not only by mediating the phosphorylation of SA2.
If Sororin phosphorylation leads to release of cohesin, Sororin phosphorylation has to be prevented at least at some chromosomal loci until anaphase onset. Our observation that Sororin11A alleviates the requirement for Sgo1 in protecting cohesion (Fig. 5) implies that PP2A bound to Sgo1 might antagonize Sororin phosphorylation. Indeed, Sgo1–PP2A can at least partially dephosphorylate Sororin in vitro (Fig. 5C) and in vivo (Fig. S6C). During preparation of this manuscript, similar observations were reported by Liu et al. (27). Expression of nonphosphorylatable SA2 mutants also restores cohesion and mitotic progression in Sgo1-depleted cells (25), indicating that both phosphorylation of Sororin and SA2 are essential for the removal of cohesin from mitotic chromosomes (Fig. S8). The synergistic action of these two phosphorylation events, possibly together with modifications on Pds5 and Wapl (29), may lead to opening of cohesin’s exit gate (31–33), enabling release of cohesin from DNA. Sgo1–PP2A might inhibit this process at centromeres but also at some chromosomal arm sites.
Materials and Methods
Plasmids and Proteins.
cDNAs for full-length human SororinWT (2) or Sororin11A were subcloned into pET28a (Novagen) or pEGFP-N1 (Clontech). SororinWT or Sororin11A in pET28a were used to transform E. coli BL21(DE3) cells. Fusion proteins were expressed, purified according to the manufacturer’s protocols, and dialyzed against buffer A (25 mM Tris⋅HCl pH 8.0, 100 mM NaCl, 10% glycerol). Recombinant proteins of human Wapl, human Pds5A, and sea urchin Δ90CycB were expressed and purified as described (2).
Preparation of Xenopus Egg Extracts.
Mitotic and interphase egg extracts were prepared as described (2). In brief, mitotic extracts were prepared by crushing unfertilized Xenopus laevis eggs in Mitosis Extraction Buffer (100 mM KCl, 0.1 mM CaCl2, 5 mM MgCl2, 20 mM Hepes-KOH pH 7.5). For interphase extracts, 0.4 mM CaCl2 was added to mitotic extracts with 50 μg/mL cycloheximide to arrest the extract in interphase. To induce mitosis, nondegradable Δ90CycB was added to interphase extracts at 150 nM.
Supplementary Material
Acknowledgments
We thank O. Hudecz, J. Schmitz, V. Bhaskara, I. Poser, and A. Tomioka for assistance and reagents; N. Kraut for BI 4834 and Hesperadin; Y. Watanabe, K. Ohsumi, and A. Losada for antibodies; A. Tedeschi and J. Hutchins for critical comments on the manuscript; and members of the J.-M.P. laboratory for discussions and assistance. This study is supported by a Grant-in-Aid for Young Scientists from Ministry of Education, Culture, Sports, Science, and Technology (MEXT) and Precursory Research for Embryonic Science and Technology (PRESTO) from Japan Science and Technology Agency (JST) (to T.N.), Boehringer Ingelheim, the Vienna Science and Technology Fund (WWTF LS09-13; to J.-M.P.), the Austrian Science Fund (FWF Special Research Program SFB F34 Chromosome Dynamics, Wittgenstein Award Z196-B20 (to J.-M.P.), SFB F3402 and TRP 308-N15 (to K.M.), the European Commission Seventh Framework Programme (FP7/2007-2013) under Grant Agreements 241548 (MitoSys; to K.M. and J.-M.P.) and 262067 (PRIME-XS; to K.M.), and the Austrian GEN-AU initiative financed by the Bundesminsterium für Bildung und Wissenschaft (to J.-M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305020110/-/DCSupplemental.
References
- 1.Nasmyth K, Haering CH. Cohesin: Its roles and mechanisms. Annu Rev Genet. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama T, et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell. 2010;143(5):737–749. doi: 10.1016/j.cell.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Shintomi K, Hirano T. Releasing cohesin from chromosome arms in early mitosis: Opposing actions of Wapl-Pds5 and Sgo1. Genes Dev. 2009;23(18):2224–2236. doi: 10.1101/gad.1844309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolef Ben-Shahar T, et al. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science. 2008;321(5888):563–566. doi: 10.1126/science.1157774. [DOI] [PubMed] [Google Scholar]
- 5.Unal E, et al. A molecular determinant for the establishment of sister chromatid cohesion. Science. 2008;321(5888):566–569. doi: 10.1126/science.1157880. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, et al. Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell. 2008;31(1):143–151. doi: 10.1016/j.molcel.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi R, Gillespie PJ, Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 2006;16(24):2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kueng S, et al. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127(5):955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Lafont AL, Song J, Rankin S. Sororin cooperates with the acetyltransferase Eco2 to ensure DNA replication-dependent sister chromatid cohesion. Proc Natl Acad Sci USA. 2010;107(47):20364–20369. doi: 10.1073/pnas.1011069107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18(2):185–200. doi: 10.1016/j.molcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz J, Watrin E, Lénárt P, Mechtler K, Peters JM. Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol. 2007;17(7):630–636. doi: 10.1016/j.cub.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Losada A, Yokochi T, Hirano T. Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci. 2005;118(Pt 10):2133–2141. doi: 10.1242/jcs.02355. [DOI] [PubMed] [Google Scholar]
- 13.Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103(3):399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 14.Losada A, Hirano M, Hirano T. Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev. 1998;12(13):1986–1997. doi: 10.1101/gad.12.13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293(5533):1320–1323. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- 16.Giménez-Abián JF, et al. Regulation of sister chromatid cohesion between chromosome arms. Curr Biol. 2004;14(13):1187–1193. doi: 10.1016/j.cub.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 17.Lénárt P, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17(4):304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Losada A, Hirano M, Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 2002;16(23):3004–3016. doi: 10.1101/gad.249202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumara I, et al. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9(3):515–525. doi: 10.1016/s1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 20.Hauf S, et al. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 2005;3(3):e69. doi: 10.1371/journal.pbio.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitajima TS, et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441(7089):46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Dej KJ, Lopez JM, Orr-Weaver TL. Control of centromere localization of the MEI-S332 cohesion protection protein. Curr Biol. 2004;14(14):1277–1283. doi: 10.1016/j.cub.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Riedel CG, et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 2006;441(7089):53–61. doi: 10.1038/nature04664. [DOI] [PubMed] [Google Scholar]
- 24.Tang Z, et al. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell. 2006;10(5):575–585. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 25.McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 2005;3(3):e86. doi: 10.1371/journal.pbio.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreier MR, Bekier ME, 2nd, Taylor WR. Regulation of sororin by Cdk1-mediated phosphorylation. J Cell Sci. 2011;124(Pt 17):2976–2987. doi: 10.1242/jcs.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Rankin S, Yu H. Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat Cell Biol. 2013;15(1):40–49. doi: 10.1038/ncb2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang N, Panigrahi AK, Mao Q, Pati D. Interaction of Sororin protein with polo-like kinase 1 mediates resolution of chromosomal arm cohesion. J Biol Chem. 2011;286(48):41826–41837. doi: 10.1074/jbc.M111.305888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegemann B, et al. Systematic phosphorylation analysis of human mitotic protein complexes. Sci Signal. 2011;4(198):rs12. doi: 10.1126/scisignal.2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauf S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161(2):281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buheitel J, Stemmann O. Prophase pathway-dependent removal of cohesin from human chromosomes requires opening of the Smc3-Scc1 gate. EMBO J. 2013;32(5):666–676. doi: 10.1038/emboj.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan KL, et al. Cohesin’s DNA exit gate is distinct from its entrance gate and is regulated by acetylation. Cell. 2012;150(5):961–974. doi: 10.1016/j.cell.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eichinger CS, Kurze A, Oliveira RA, Nasmyth K (2013) Disengaging the Smc3/kleisin interface releases cohesin from Drosophila chromosomes during interphase and mitosis. EMBO J 32(5):656–665. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.