Abstract
The inhibitory reversible oxidation of protein tyrosine phosphatases (PTPs) is an important regulatory mechanism in growth factor signaling. Studies on PTP oxidation have focused on pathways that increase or decrease reactive oxygen species levels and thereby affect PTP oxidation. The processes involved in reactivation of oxidized PTPs remain largely unknown. Here the role of the thioredoxin (Trx) system in reactivation of oxidized PTPs was analyzed using a combination of in vitro and cell-based assays. Cells lacking the major Trx reductase TrxR1 (Txnrd1−/−) displayed increased oxidation of PTP1B, whereas SHP2 oxidation was unchanged. Furthermore, in vivo-oxidized PTP1B was reduced by exogenously added Trx system components, whereas SHP2 oxidation remained unchanged. Trx1 reduced oxidized PTP1B in vitro but failed to reactivate oxidized SHP2. Interestingly, the alternative TrxR1 substrate TRP14 also reactivated oxidized PTP1B, but not SHP2. Txnrd1-depleted cells displayed increased phosphorylation of PDGF-β receptor, and an enhanced mitogenic response, after PDGF-BB stimulation. The TrxR inhibitor auranofin also increased PDGF-β receptor phosphorylation. This effect was not observed in cells specifically lacking PTP1B. Together these results demonstrate that the Trx system, including both Trx1 and TRP14, impacts differentially on the oxidation of individual PTPs, with a preference of PTP1B over SHP2 activation. The studies demonstrate a previously unrecognized pathway for selective redox-regulated control of receptor tyrosine kinase signaling.
Keywords: redox regulation, cell signaling
Classical protein tyrosine phosphatases (PTPs) display a conserved active site HC(X5)R signature that renders them specific for phosphotyrosine (1–3). PTPs act as modifiers of growth factor-induced signaling and have important roles during development and in pathological conditions, such as cancer, diabetes, and inflammation (1–3).
The active sites of PTPs typically display a lowered pKa of the catalytic cysteine residue (4–7), which therefore is predominantly present as a reactive thiolate (-S−) that promotes the dephosphorylating reaction. However, the reactive cysteine thiolate in the active site also makes PTPs highly susceptible to inhibitory oxidation. Multiple molecular variants of oxidized PTPs have been described, including a sulfenic acid derivative (-SOH) or a sulfenylamide form involving the main chain nitrogen of the serine residue juxtaposed to the active site cysteine (8, 9). Other oxidized versions of PTPs include glutathionylated, nitrosylated, and sulfhydrated forms, or variants with internal disulfides or irreversibly oxidized sulfinic (-SO2) or sulfonic (-SO3) derivatives (8–13).
Growth factors like EGF, PDGF, and insulin, that act through tyrosine kinase receptors, induce reactive oxygen species (ROS) and oxidation of a selected number of PTPs (14–17). PTP oxidation has also been demonstrated after activation of other classes of cell surface receptors, such as integrins, G protein coupled receptors (GPCRs), and B- and T-cell receptors (18–22). The mechanisms involved still remain incompletely characterized but include cell surface receptor-induced activation of NADPH oxidases (NOXs) and spatially restricted inhibitory tyrosine phosphorylation of peroxiredoxins (Prxs) (20, 23–26). Recent studies have also implied ROS derived from the mitochondrial respiratory chain as regulators of PTP oxidation (18, 27). As for the molecular identity of the relevant oxidants, most studies have focused on hydrogen peroxide, but peroxidized lipids and oxidized glutathione have also been identified as mediators of PTP oxidation (28, 29).
Studies in which ROS producers and scavengers have been overexpressed or deleted have demonstrated that PTP oxidation represents a general mechanism for cross-talk between redox- and growth factor signaling (reviewed in ref. 30). Some key findings in this context involve the modulation of growth factor signaling, occurring together with altered PTP oxidation, after manipulation of the expression levels or activities of members of the Prx-, glutathione peroxidase (GPx)-, NOX, and superoxide dismutase protein families (21, 23, 31–33).
Physiologically relevant PTP oxidation is a reversible process. This suggests that reactivation of oxidized PTPs represents an additional potential control mechanism impacting on PTP oxidation and growth factor signaling. However, this aspect of regulation of PTP activity remains largely unexplored. The major reducing systems of cells, which should be plausible candidates for reactivation of reversibly oxidized PTPs, are the thioredoxin (Trx) and glutathione (GSH) systems. Both are NADPH-dependent, oxidoreductase-dependent systems with a large range of cellular functions, often functionally overlapping with each other.
In the Trx system, NADPH is used by isoenzymes of selenium-dependent thioredoxin reductase (TrxR) that maintain the active site motif of Trx isoenzymes in the reduced dithiol state, which in turn reduce protein targets to maintain, for example, antioxidant defense, DNA proliferation, transcription factor regulation, and apoptosis regulation (34, 35). The most prominent and ubiquitously expressed components of the Trx system are the cytosolic TrxR1 (encoded by the Txnrd1 gene) and its main substrate Trx1 (34, 35). Another less characterized substrate of TrxR1 is TRP14 (36), discovered as a TrxR1-dependent oxidoreductase affecting TNF-α–induced NF-κB activation, which displays a more restricted substrate specificity than Trx1 (37, 38).
In the GSH system, NADPH is used by glutathione reductase that reduces glutathione disulfide to GSH, which is used by isoenzymes of the GST and glutaredoxin (Grx) family of proteins. These protect cells from xenobiotics (39) or maintain protein targets in a reduced state, essentially analogously to the Trx system (40), although there are also clear differences in some functions and targets between Trx and Grx systems (41).
A few earlier studies have described a capacity of selected reducing proteins to reactivate oxidized PTPs (12, 14, 42). These studies have mostly been done in cell-free systems and have not explored the potential impact of cellular reducing pathways on growth factor signaling.
In this study the potential role of the Trx system in regulation of PTP oxidation was analyzed, with a focus on its effects on the oxidation status of two selected PTPs; PTP1B and SHP2. A combination of cell- and in vitro-based experiments demonstrated that TrxR1, via either of its two substrates Trx1 and TRP14, can regulate the oxidation of PTP1B, but not SHP2, in a manner that affects growth factor and receptor tyrosine kinase signaling.
Results
Oxidation of PTP1B, but Not SHP2, Is Increased in Txnrd1−/− Cells.
To analyze the importance of the cytosolic Trx system for regulation of PTP oxidation, the oxidation states of PTP1B and SHP2 were monitored in mouse embryonic fibroblasts (MEFs) with WT or Txnrd1 knockout (Txnrd1−/−) genotypes (43).
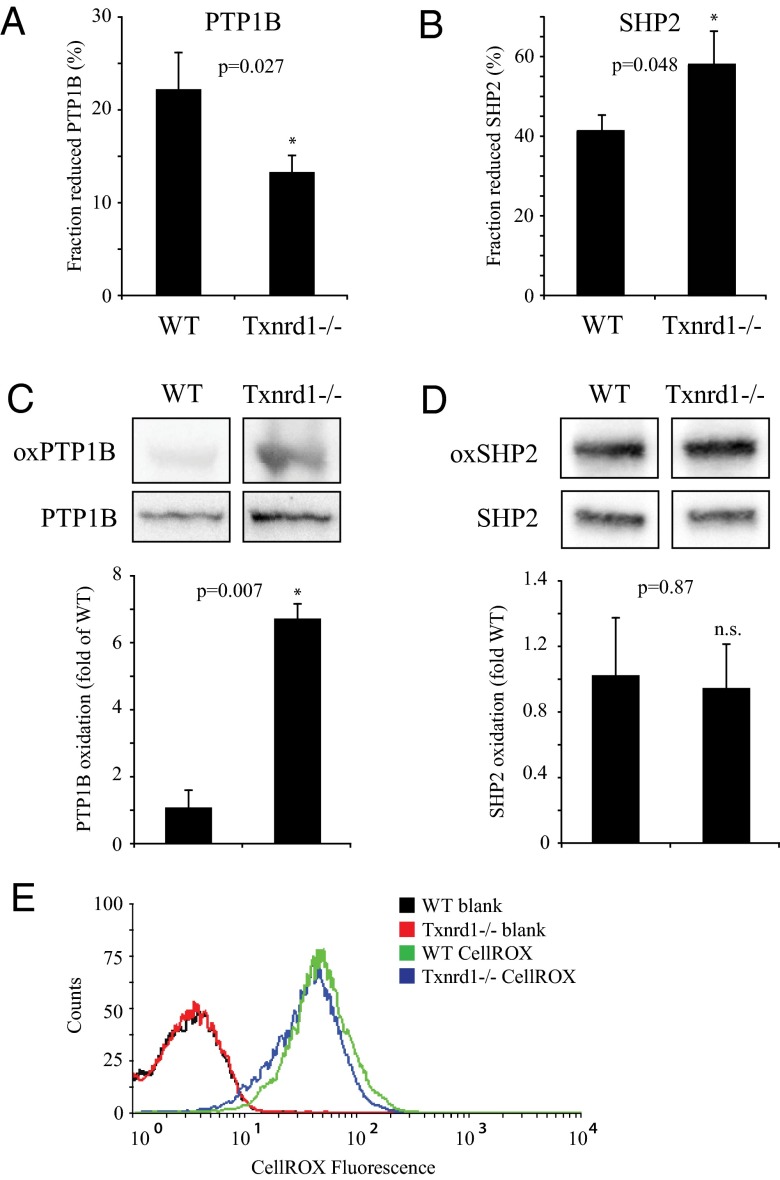
Analyses of immunoprecipitated PTP1B showed increased oxidation of PTP1B in Txnrd1−/− cells compared with WT cells (Fig. 1A). Corresponding analyses of SHP2 showed the reverse effect (Fig. 1B). Independent evidence for differential effects on the oxidation status of PTP1B and SHP2 by Txnrd1 deletion was obtained in experiments on the same cells using a cysteinyl-labeling assay (44). In this assay the difference in PTP1B oxidation between the cells became even more evident (Fig. 1C), whereas SHP2 oxidation showed no significant difference (Fig. 1D).
Fig. 1.
Deletion of Txnrd1 leads to increased oxidation of PTP1B but not SHP2. Immunoprecipitated PTP1B (A) and SHP2 (B) from immortalized WT and Txnrd1−/− MEFs were assayed for activity under reducing (including DTT treatment) and nonreducing conditions. Bars represent the fraction of reduced PTPs (n = 4, ±SEM). (C and D) The basal oxidation status of PTP1B and SHP2 was monitored in WT and Txnrd1−/− MEFs using a cysteinyl-labeling assay. Bars show oxidation status after normalization to expression levels (n = 3, ±SEM). (E) Basal ROS levels in WT and Txnrd1−/− cells were determined by measuring CellROX fluorescence (n = 3).
No differences in overall cellular ROS levels were detected when comparing WT and Txnrd1−/− cells (Fig. 1E), in agreement with previous findings showing compensatory up-regulation of the GSH system in TrxR1 null cells (45).
Taken together these experiments demonstrated that deletion of Txnrd1 in MEF cells led to an increased oxidation state of PTP1B, which occurred in the absence of major effects on SHP2 oxidation and which was not related to overall cellular levels of ROS. The experiments thus suggest that different PTPs display a differential dependency on TrxR1 for their oxidation state not related to the generalized cellular oxidation state.
Exogenous Trx System Components Reactivate in Vivo-Oxidized PTP1B but Not SHP2.
The effects of addition of components of the Trx system to cell lysates were subsequently analyzed in relation to PTP oxidation status.
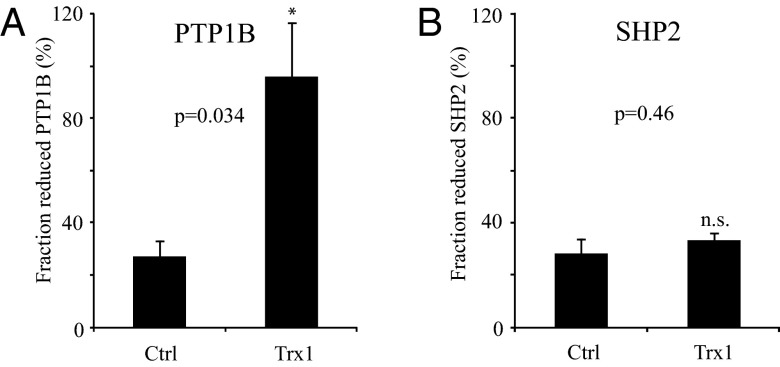
To induce oxidation of PTPs, cultured NIH 3T3 cells were initially treated with 400 μM H2O2 for 5 min. The activities of PTP1B and SHP2 were subsequently analyzed in control cell lysates, as well as in lysates incubated with the major components of the cytosolic Trx system (Trx1, TrxR1, and NADPH) and in lysates treated with the strong artificial reducing agent DTT. The activities with DTT were assumed to represent those of fully reduced PTP species. As shown in Fig. 2A, the Trx system components added to cell lysates significantly reactivated PTP1B, whereas the activity and thus oxidation state of SHP2 remained unaffected.
Fig. 2.
Exogenous Trx system components reactivate in vivo oxidized PTP1B but not SHP2. NIH 3T3 cells were treated with H2O2 to oxidize endogenous PTPs. Immunoprecipitated PTP1B (A) (n = 3, ± SEM) and SHP2 (B) (n = 4, ± SEM) were left untreated (Ctrl) or treated with thioredoxin system components (Trx) or DTT and assayed for PTP activity. Bars represent the fraction of reduced PTPs.
These experiments thus provided independent evidence for a role of the Trx system in controlling the oxidation state and activity of PTP1B.
The Trx System Reduces Oxidized Recombinant PTP1B in Vitro but Not SHP2.
To further characterize the effects of Trx1 on PTP oxidation, a set of experiments were performed to analyze the ability of Trx1 to reactivate in vitro-oxidized recombinant PTP1B and SHP2.
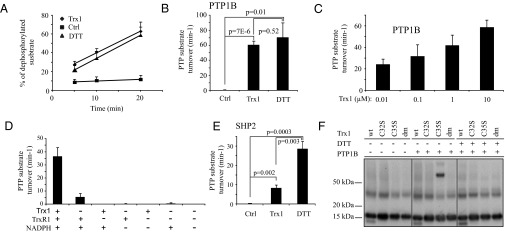
Analyses of oxidized recombinant PTP1B demonstrated the induction of a linear time-dependent peptide dephosphorylation activity upon incubation with DTT. A combination of Trx1, TrxR1, and NADPH (Trx1/TrxR1/NADPH) showed a reactivating capacity for PTP1B, which in this assay was similar to that of 5 mM DTT (Fig. 3 A and B). Additional experiments demonstrated a clear dose dependency in the ability of Trx1 to activate oxidized PTP1B (Fig. 3C). Further analyses showed that TrxR1 alone together with NADPH could induce a modest activation of PTP1B but that the full combination of NADPH, TrxR1, and Trx1 provided the most potent reactivation capacity (Fig. 3D). In contrast, the complete Trx system could only to a minor extent reactivate oxidized recombinant SHP2 (Fig. 3E). Finally, substrate-trapping experiments with active site mutants of Trx1 clearly revealed the formation of a DTT-sensitive intermolecular disulfide-linked complex between oxidized PTP1B and a C35S variant of Trx1, whereas no complex could be formed together with either WT Trx1, the C32S variant, or the active site double mutant of Trx1 (Fig. 3F). Because Cys32 of Trx1 provides the nucleophilic attack on substrates with Cys35 being the resolving residue (46), this finding strongly suggests that PTP1B is reduced by Trx1 through formation of a transient intermolecular disulfide intermediate, with Cys32 acting as the attacking nucleophile.
Fig. 3.
The Trx system reduces oxidized recombinant PTP1B but not SHP2. Recombinant oxidized PTP1B (A and B) (n = 3–8, ±SEM) and SHP2 (E) (n = 4–6, ±SEM) were left untreated (Ctrl) or treated with the Trx system (Trx) or DTT. Oxidized recombinant PTP1B was also treated with increasing concentrations of Trx1 in the presence of TrxR1 and NADPH (C) (n = 3, ±SEM) and treated with combinations of individual Trx system components (D) (n = 3, ±SEM). PTP activity was measured over time and turnover rates calculated using the slope from the generated graphs. Substrate trapping of PTP1B was analyzed by immunoblotting with antibodies for Trx1 using samples of different variants of Trx mixed with oxidized PTP1B and analyzed on SDS/PAGE in the presence or absence of DTT, as indicated in F.
Together these experiments provide independent evidence for a selective effect of the Trx system on reactivation of oxidized PTPs.
Deletion of Txnrd1 in MEFs Leads to an Increase in PDGF-β Receptor Phosphorylation.
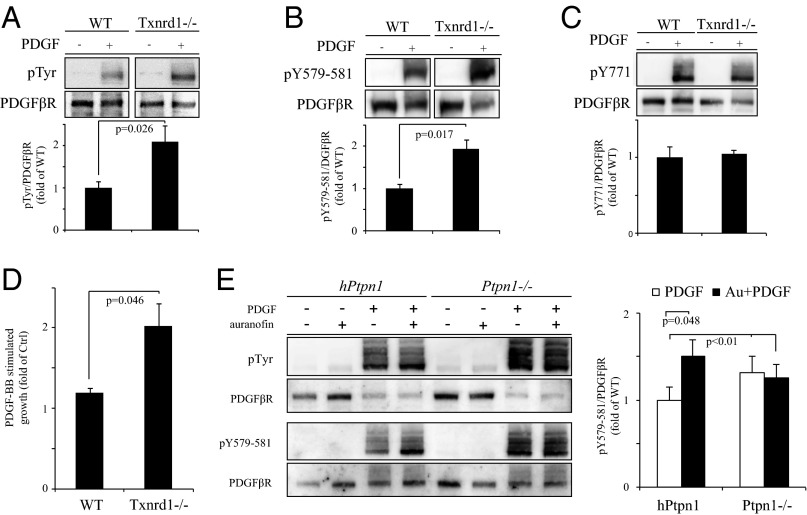
Activation of PDGF-β receptor (PDGFβR) is associated with phosphorylation of specific tyrosine residues, required for downstream signaling. PTPs, including PTP1B, regulate this receptor phosphorylation in a site-specific manner (47–49). Because PTP1B showed a decreased activity and higher oxidation state in Txnrd1−/− cells, experiments were performed to analyze whether PDGFβR phosphorylation was affected by Txnrd1 deletion.
Upon activation of PDGFβR with PDGF-BB an increased receptor tyrosine phosphorylation was observed in Txnrd1−/− cells, compared with WT cells (Fig. 4A). Furthermore, the PDGFβR site pY579-581, known to be regulated by PTP1B (47–49), also showed an increase in phosphorylation levels in Txnrd1−/− cells upon ligand stimulation (Fig. 4B). In contrast, no changes were seen at the SHP2-regulated PDGFβR site pY771 (Fig. 4C). The increased PDGFβR tyrosine phosphorylation in Txnrd1−/− cells was paralleled by an enhanced growth-stimulatory response to PDGF-BB, compared with WT fibroblasts (Fig. 4D).
Fig. 4.
Deletion or inhibition of Txnrd1 leads to changes in PDGF-BB responses that are partially dependent on PTP1B. WT and Txnrd1−/− MEFs (A–D), or PTP1B knockout MEFs (Ptpn1−/−) and Ptpn1−/− MEFs reconstituted with human PTP1B (hPtpn1) (E), were stimulated with or without PDGF-BB and analyzed for overall PDGFβR phosphotyrosine levels (A and E), phosphorylation of pY579-581 (B and E) and pY771 (C), the predominant target site of PTP1B and SHP2, respectively, and mitogenic response (D; n = 3, ±SEM). In the case of Ptpn1−/− and hPtpn1 treatments with or without PDGF-BB were performed in the absence or presence of pretreatment for 60 min with 1 µM auranofin to block TrxR1 (E). Receptor phosphorylation was determined after immunoblotting with antibodies against PDGFβR (A–C and E), phosphotyrosine (A and E), pY579-581 (B and E), or pY771 PDGFβR phosphorylation site (C). Bar diagrams (A–C and E) demonstrate quantification of relative total PDGFβR phosphotyrosine, Y579-581 phosphorylation, and Y771 phosphorylation (n = 3, ±SEM).
These experiments thus identified a cross-talk of the cellular TrxR1-dependent Trx system with growth factor and receptor tyrosine kinase signaling.
PTP1B Is Involved in Trx1-Dependent Modulation of PDGFβR Phosphorylation.
Next, a set of experiments was performed to investigate whether the increased phosphorylation of the pY579-581 residues of PDGFβR in Txnrd1−/− cells could be related to increased oxidation and thus decreased activity of PTP1B. For this purpose the effects on PDGFβR phosphorylation of the TrxR1 inhibitor auranofin were analyzed in cells deficient in PTP1B (Ptpn1−/−) as well as in PTP1B-reconstituted Ptpn1−/− cells (hPtpn1).
In agreement with previous studies (47), the absence of PTP1B was associated with an increased total tyrosine phosphorylation of PDGFβR and in the phosphorylation of the pY579-581 sites (Fig. 4E). Importantly, treatment of cells with the TrxR1 inhibitor auranofin increased phosphorylation levels at the pY579-581 in the PTP1B-expressing cells, but this effect was clearly diminished in Ptpn1−/− cells (Fig. 4E).
These experiments thus strongly imply the TrxR1-dependent regulation of PTP1B as one of the critical intermediates in the cross-talk between the Trx system and PDGFβR signaling.
TRP14 Is Also Capable of Reactivating Oxidized PTP1B.
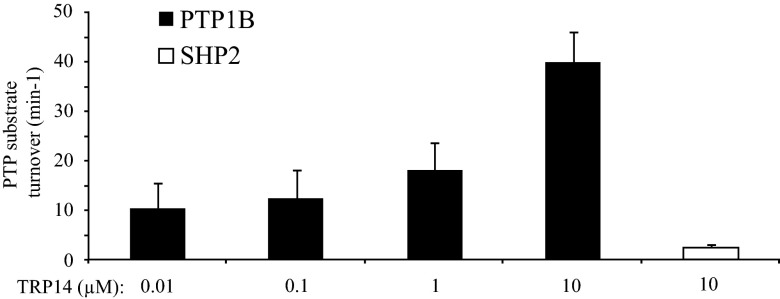
Although the experiments described above demonstrated that Trx1 can mediate the dependence of PTP1B upon TrxR1 for its reactivation, it cannot be ruled out that other substrates of TrxR1 can also contribute to the observed effects. Experiments were thus performed to analyze whether the recently characterized alternative TrxR1 substrate TRP14 (38) could also reduce oxidized PTP1B. Experiments designed as in Fig. 3 were thus performed with TRP14 in place of Trx1. This revealed that TRP14, together with TrxR1 and NADPH, indeed demonstrated a dose-dependent effect on PTP1B activity, whereas oxidized SHP2 could not be reactivated (Fig. 5).
Fig. 5.
TRP14 reduces oxidized PTP1B but not SHP2. Oxidized recombinant PTP1B was treated with increasing concentrations of TRP14 in the presence of TrxR1 and NADPH, whereas SHP2 was treated with TrxR1, NADPH, and the highest concentration of TRP14. PTP activity was measured over time and turnover rates calculated using the slope from the generated graphs (n = 4, ±SEM).
This experiment showed that TRP14 is another redox active protein that, in addition to Trx1, can mediate the TrxR1-dependent activation of oxidized PTP1B.
Discussion
This study identifies previously unrecognized selective effects of the Trx system on the oxidation state of PTP1B, associated with modulation of biochemical and cellular PDGF responsiveness. This pathway for control of PDGF signaling may also be relevant for other growth factors acting through tyrosine kinase receptors. Interestingly, the Trx system did not seem to be important for the control of SHP2 oxidation, suggesting an earlier neglected level of specificity in the processes of reactivation of oxidized PTPs. Finally, TRP14 was identified as an additional TrxR1-dependent regulator of the oxidation of PTP1B but not SHP2.
Previous work addressing reactivation of oxidized PTPs, using recombinant PTPs in vitro, showed an ability of Trx1 to reactivate oxidized PTP1B with a potency similar to that of DTT (12, 14), which is in line with this work. In another study, which characterized the role of oxidized “backdoor cysteines” of SHP2, reactivating ability was demonstrated by DTT and GSH, whereas no effect was seen by Trx system components (42). Interestingly, the latter study demonstrated that the catalytic domain of SHP2 was sensitive to Trx system component reduction (42), indicating that determinants controlling the differential effects of the Trx system and GSH reside in structure(s) outside the catalytic domain.
The fact that TrxR1-dependent reductive pathways efficiently activate PTP1B but not as efficiently SHP2 (Fig. 1–3) has interesting implications in view of the role(s) of the Trx system in signaling pathways and metabolic homeostasis. Whole-body knockout mouse models for either TrxR1 or Trx1 are embryonically lethal (50, 51). It is interesting to note that liver-specific knockouts for both Ptpn1 and Txnrd1 have been made (52–54). It will be an interesting topic for future studies to investigate similarities and differences of these mice related to receptor-dependent signaling pathways.
The phenotype of a TRP14 knockout in animal models is not known. The finding that TRP14 is another TrxR1-dependent activator of oxidized PTP1B is nonetheless most interesting (Fig. 5). TRP14 was originally described as a dynein light chain LC8-interacting protein that inhibits NF-κB signaling upon TNF-α stimulation of HeLa cells (37). Although TRP14 shares many structural features with Trx1, this redoxin cannot reduce classic Trx1 protein disulfide substrates, such as ribonucleotide reductase, peroxiredoxins, or methionine sulfoxide reductases (36, 55). The efficient reactivation of PTP1B by TRP14 identifies TRP14 as a TrxR1 substrate with specific functions in modulation of cellular signaling pathways. The relative importance of TRP14 vs. Trx1 in regulation of PTP1B oxidation remains to be determined in future studies.
The finding of a PTP1B-dependent, auranofin-mediated effect on PDGFβR phosphorylation was also notable. On the basis of the inactivation of PTP1B in Txnrd1−/− cells, the stimulatory effects of auranofin on PDGFβR phosphorylation are interpreted to be a consequence of effects of auranofin on TrxR1 (56). It should be noted that auranofin has also been shown to act as a direct PTP inhibitor (57). However, direct inhibition of PTP1B by auranofin was inefficient and showed an IC50 of >400 µM (57), whereas auranofin inhibits TrxR1 at stoichiometric submicromolar concentrations (56). Thus, our findings collectively lead to the conclusion that auranofin in the present study affects PDGFβR phosphorylation through inhibitory effects on TrxR1, rather than by direct effects on PTP1B. It should be noted, however, that other PTPs such as PTPN12 seem to be more sensitive to direct inhibition by auranofin (57).
The present study demonstrates striking differences between PTP1B and SHP2 with regard to their susceptibility to reactivation by Trx1 and TRP14. The experiments do not identify the molecular basis for this differential sensitivity, but a number of possibilities can be envisioned. First, it is possible that yet-to-be-defined sequence-specific interactions occur between Trx1 and TRP14 within the active-site region of PTP1B that facilitate Trx1- and TRP14-mediated reactivation of PTP1B. Second, the type of oxidative modification(s) of the two PTPs may differ. The oxidized version described for PTP1B are sulfenic, sulfinic, and sulfonic acid derivatives, as well as forms with an intramolecular sulfenylamide, and variants containing S-Nitrosylated and glutathionylated cysteines. As for SHP2, sulfenic acid forms and species with inter- and intramolecular disulphide bonds have been observed (9, 11, 13, 42, 58–61). Future studies should address to what extent these different types of oxidized variants display biologically relevant differential sensitivity to the major cellular reducing systems. It is also noted that SHP2 sensitivity to various reductants could vary depending on it being in a “closed” or “open” conformation. However, on the basis of the absence of Trx1-dependent alterations in the phosphorylation of the PDGF receptor SHP2 target site in vivo (Fig. 4C), this study suggests that the “open” conformation is not regulated by Trx1.
A number of additional topics for continued analyses are suggested by the findings of this study. It remains to obtain a complete picture of how conditional Trx1, or TRP14, deletion affects the oxidation state of the whole “PTPome” in different cell types, and downstream effects on signaling events. One interesting finding to further explore is the fact that TrxR1 knockout mice display early embryonic lethality (50, 51), although the enzyme is dispensable in several conditional knockouts (52–54). Future studies should investigate whether this is related to aberrant signaling pathways related to inactivation of specific PTPs. Recent mass spectrometry-based methods for analyses of global PTP oxidation in different tissues and cells should be highly useful in this context (62). Our present findings should also stimulate further analyses of whether Trx system components are subject to direct control by tyrosine kinase signaling, as has been described for PTP oxidation-inducing NOX enzymes (20, 23, 24).
On the basis of the identification of TrxR1-mediated control of growth factor signaling, it will be highly interesting to investigate whether alterations in TrxR1 levels or activity, as observed for example in an animal model of liver cancer (54) and arrhythmia (63), are associated with etiologically relevant alterations in growth factor and receptor tyrosine kinase signaling.
Materials and Methods
Cell Culture and Reagents.
The MEFs used in this study have been described before in the context of TrxR1 (43) and PTP1B (48) and were cultured in DMEM with 10% (vol/vol) FBS, 2 mM l-glutamine, and 20 nM sodium selenite with penicillin and streptomycin. Details about antibodies and other reagents are given in SI Materials and Methods.
Assays of PTP Oxidation.
The cysteinyl-labeling assay of PTP oxidation was performed as described in ref. 64. The activity-based assay followed procedures as described in ref. 27.
Crystal Violet Assay to Analyze Cell Growth.
WT and Txnrd1−/− MEFs were seeded at 2 × 103 per well of a 96-well plate. The next day medium was replaced by low serum-containing medium (1% FBS) with or without 50 ng/mL PDGF-BB. After 3 d culture cells were fixed using 2% (wt/vol) formaldehyde. Then cells were stained with 0.04% crystal violet/1% EtOH, and the plate was dried thoroughly. The relative number of cells per well was determined by resolving crystal violet in 1% SDS and measuring the absorbance at 590 nm.
ROS Measurement.
Cells were grown overnight to 70% confluency and then starved for 24 h in medium containing 1% FCS. Cells were incubated for 30 min with 5 µM CellROX (Life Technologies), washed three times with PBS, trypsinized and collected, and kept on ice before analysis on a Becton Dickinson FACSCalibur, and data were analyzed using Cyflogic software.
Analyses of PDGFβR Phosphorylation.
PDGFβR phosphorylation was analyzed from starved cells after 5 min of 50 ng/mL PDGF-BB stimulation. Where indicated, cells received a 1-h treatment with 1 µM auranofin before stimulation. A detailed description is provided in SI Materials and Methods.
Treatment of Cell Lysates with Trx System Components.
NIH 3T3 cells were treated with 400 μM H2O2 for 5 min. After lysis cleared cell lysates were treated with a combination of Trx system components (Trx1 10 μM, TrxR1 0.5 μM, and NADPH 1 mM) or 50 mM DTT for 30 min. Activity assays of PTP1B and SHP2 was performed as described above. A detailed description is provided in SI Materials and Methods.
In Vitro Treatments of Recombinant PTP1B and SHP2 with Trx System Components.
Recombinant PTP1B and SHP2 were incubated with Trx system components (Trx1 or TRP14 10 μM, TrxR1 0.5 μM, and NADPH 1 mM) in 50 mM Tris and 2 mM EDTA for 30 min before analysis with PTP activity assay. A detailed description is provided in SI Materials and Methods.
Substrate Trapping in Vitro of PTP1B by Trx1 Mutant Variants.
Recombinant variants of N-terminally his-tagged human Trx1 of 14.6 kDa (WT, C32S, C35S, and the active site double mutant C32SC35S) were prereduced with 20-fold molar excess of DTT, desalted, and subsequently incubated with equal amounts of oxidized PTP1B (500 nM each) for 20 min at room temperature and subsequently analyzed by SDS/PAGE, with or without DTT, followed by Western blot using antibodies against Trx. A detailed description is provided in SI Materials and Methods.
Statistical Analysis.
All statistical analysis was performed using unpaired two-tailed Student’s t test.
Supplementary Material
Acknowledgments
We thank members of A.Ö. group for helpful discussions throughout the project. hPtpn1 and Ptpn1−/− cells were kindly provided by Benjamin G. Neel. A.Ö. was supported by the Swedish Research Council, the European Union-sponsored PTPNET, and the Swedish Cancer Society (Cancerfonden). E.S.J.A. acknowledges support for these studies from the Swedish Research Council (Medicine), the Swedish Cancer Society, and Karolinska Institutet.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302891110/-/DCSupplemental.
References
- 1.Tonks NK. Protein tyrosine phosphatases: From genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7(11):833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117(6):699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JN, et al. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21(21):7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters GH, Frimurer TM, Olsen OH. Electrostatic evaluation of the signature motif (H/V)CX5R(S/T) in protein-tyrosine phosphatases. Biochemistry. 1998;37(16):5383–5393. doi: 10.1021/bi971187i. [DOI] [PubMed] [Google Scholar]
- 5.Jackson MD, Denu JM. Molecular reactions of protein phosphatases—insights from structure and chemistry. Chem Rev. 2001;101(8):2313–2340. doi: 10.1021/cr000247e. [DOI] [PubMed] [Google Scholar]
- 6.Lohse DL, Denu JM, Santoro N, Dixon JE. Roles of aspartic acid-181 and serine-222 in intermediate formation and hydrolysis of the mammalian protein-tyrosine-phosphatase PTP1. Biochemistry. 1997;36(15):4568–4575. doi: 10.1021/bi963094r. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZY. Chemical and mechanistic approaches to the study of protein tyrosine phosphatases. Acc Chem Res. 2003;36(6):385–392. doi: 10.1021/ar020122r. [DOI] [PubMed] [Google Scholar]
- 8.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423(6941):773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 9.Salmeen A, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423(6941):769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 10.Huyer G, et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272(2):843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 11.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37(16):5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal. 2011;4(203):ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YY, et al. Cysteine S-nitrosylation protects protein-tyrosine phosphatase 1B against oxidation-induced permanent inactivation. J Biol Chem. 2008;283(50):35265–35272. doi: 10.1074/jbc.M805287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273(25):15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 15.Persson C, et al. Preferential oxidation of the second phosphatase domain of receptor-like PTP-alpha revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2004;101(7):1886–1891. doi: 10.1073/pnas.0304403101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279(36):37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 17.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270(5234):296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 18.Taddei ML, et al. Integrin-mediated cell adhesion and spreading engage different sources of reactive oxygen species. Antioxid Redox Signal. 2007;9(4):469–481. doi: 10.1089/ars.2006.1392. [DOI] [PubMed] [Google Scholar]
- 19.Chiarugi P, et al. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161(5):933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, et al. Redox regulation of interleukin-4 signaling. Immunity. 2008;29(4):551–564. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon J, et al. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 2005;24(13):2331–2341. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabet F, et al. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res. 2008;103(2):149–158. doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181(7):1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu RF, et al. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171(5):893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae YS, et al. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275(14):10527–10531. doi: 10.1074/jbc.275.14.10527. [DOI] [PubMed] [Google Scholar]
- 26.Woo HA, et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140(4):517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Sandin A, et al. Hypoxia followed by re-oxygenation induces oxidation of tyrosine phosphatases. Cell Signal. 2011;23(5):820–826. doi: 10.1016/j.cellsig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Conrad M, et al. 12/15-lipoxygenase-derived lipid peroxides control receptor tyrosine kinase signaling through oxidation of protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2010;107(36):15774–15779. doi: 10.1073/pnas.1007909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett WC, et al. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38(20):6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 30.Ostman A, Frijhoff J, Sandin A, Böhmer FD. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. 2011;150(4):345–356. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- 31.Choi MH, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435(7040):347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 32.Loh K, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10(4):260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juarez JC, et al. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci USA. 2008;105(20):7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnér ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267(20):6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 35.Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 36.Woo JR, et al. Structural basis of cellular redox regulation by human TRP14. J Biol Chem. 2004;279(46):48120–48125. doi: 10.1074/jbc.M407079200. [DOI] [PubMed] [Google Scholar]
- 37.Jeong W, Chang TS, Boja ES, Fales HM, Rhee SG. Roles of TRP14, a thioredoxin-related protein in tumor necrosis factor-alpha signaling pathways. J Biol Chem. 2004;279(5):3151–3159. doi: 10.1074/jbc.M307959200. [DOI] [PubMed] [Google Scholar]
- 38.Jeong W, Jung Y, Kim H, Park SJ, Rhee SG. Thioredoxin-related protein 14, a new member of the thioredoxin family with disulfide reductase activity: Implication in the redox regulation of TNF-alpha signaling. Free Radic Biol Med. 2009;47(9):1294–1303. doi: 10.1016/j.freeradbiomed.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 39.Salinas AE, Wong MG. Glutathione S-transferases—a review. Curr Med Chem. 1999;6(4):279–309. [PubMed] [Google Scholar]
- 40.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780(11):1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6(1):63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 42.Chen CY, Willard D, Rudolph J. Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry. 2009;48(6):1399–1409. doi: 10.1021/bi801973z. [DOI] [PubMed] [Google Scholar]
- 43.Mandal PK, et al. System x(c)- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J Biol Chem. 2010;285(29):22244–22253. doi: 10.1074/jbc.M110.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boivin B, Yang M, Tonks NK. Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Sci Signal. 2010;3(137):pl2. doi: 10.1126/scisignal.3137pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandal PK, et al. Loss of thioredoxin reductase 1 renders tumors highly susceptible to pharmacologic glutathione deprivation. Cancer Res. 2010;70(22):9505–9514. doi: 10.1158/0008-5472.CAN-10-1509. [DOI] [PubMed] [Google Scholar]
- 46.Holmgren A. Thioredoxin structure and mechanism: Conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure. 1995;3(3):239–243. doi: 10.1016/s0969-2126(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 47.Persson C, et al. Site-selective regulation of platelet-derived growth factor beta receptor tyrosine phosphorylation by T-cell protein tyrosine phosphatase. Mol Cell Biol. 2004;24(5):2190–2201. doi: 10.1128/MCB.24.5.2190-2201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278(2):739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- 49.Klinghoffer RA, Kazlauskas A. Identification of a putative Syp substrate, the PDGF beta receptor. J Biol Chem. 1995;270(38):22208–22217. doi: 10.1074/jbc.270.38.22208. [DOI] [PubMed] [Google Scholar]
- 50.Jakupoglu C, et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25(5):1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui M, et al. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178(1):179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 52.Suvorova ES, et al. Cytoprotective Nrf2 pathway is induced in chronically txnrd 1-deficient hepatocytes. PLoS ONE. 2009;4(7):e6158. doi: 10.1371/journal.pone.0006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delibegovic M, et al. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58(3):590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlson BA, et al. Thioredoxin reductase 1 protects against chemically induced hepatocarcinogenesis via control of cellular redox homeostasis. Carcinogenesis. 2012;33(9):1806–1813. doi: 10.1093/carcin/bgs230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong W, Yoon HW, Lee SR, Rhee SG. Identification and characterization of TRP14, a thioredoxin-related protein of 14 kDa. New insights into the specificity of thioredoxin function. J Biol Chem. 2004;279(5):3142–3150. doi: 10.1074/jbc.M307932200. [DOI] [PubMed] [Google Scholar]
- 56.Gromer S, Arscott LD, Williams CH, Jr, Schirmer RH, Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J Biol Chem. 1998;273(32):20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- 57.Krishnamurthy D, et al. Gold(I)-mediated inhibition of protein tyrosine phosphatases: A detailed in vitro and cellular study. J Med Chem. 2008;51(15):4790–4795. doi: 10.1021/jm800101w. [DOI] [PubMed] [Google Scholar]
- 58.Rinna A, Torres M, Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med. 2006;41(1):86–91. doi: 10.1016/j.freeradbiomed.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lou YW, et al. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 2008;275(1):69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 60.Nardozza AP, et al. Reactive oxygen species and epidermal growth factor are antagonistic cues controlling SHP-2 dimerization. Mol Cell Biol. 2012;32(10):1998–2009. doi: 10.1128/MCB.06674-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paulsen CE, et al. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat Chem Biol. 2012;8(1):57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karisch R, et al. Global proteomic assessment of the classical protein-tyrosine phosphatome and “Redoxome”. Cell. 2011;146(5):826–840. doi: 10.1016/j.cell.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aota M, et al. Protection against reperfusion-induced arrhythmias by human thioredoxin. J Cardiovasc Pharmacol. 1996;27(5):727–732. doi: 10.1097/00005344-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 64.Boivin B, Tonks NK. Analysis of the redox regulation of protein tyrosine phosphatase superfamily members utilizing a cysteinyl-labeling assay. Methods Enzymol. 2010;474:35–50. doi: 10.1016/S0076-6879(10)74003-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.