Abstract
Obesity is recognized as a chronic low grade and systemic inflammatory disease. Angiogenesis is critical for adipose tissue expansion. Several evidences have demonstrated that angiogenesis sustains inflammation by preparing oxygen and nutrients for inflammatory cells and inflammation in turn can cause insulin resistance, type 2 diabetes, and cardiovascular disease. The understanding of mechanisms of obesity especially main roles of inflammation and angiogenesis in fat mass expansion can lead to therapeutic approaches in growing field of obesity and its related disorders. In this review, we studied the relationship between obesity, angiogenesis, and inflammation.
Keywords: Obesity, Angiogenesis, Inflammation
Introduction
Inflammation is an intricate network of chemical signals, cell types and factor interactions in response to tissue damage against pathogenic, traumatic or toxic injury. The inflammatory process is the result of balance between pro- and anti-inflammatory molecules1-3 such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), leptin, resistin, monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP) and anti-inflammatory molecule of Adiponectin.4 These molecules involve in regulation of cell chemotaxis, migration and proliferation. Although inflammation participates in healing process but imbalance between pro- and anti-inflammatory molecules lead to sustained inflammation (chronic inflammation) and in turn it mediates a wide spectrum of diseases such as psoriasis, rheumatoid arthritis, osteoarthritis, metabolic syndrome-associated disorders including type 2 diabetes and atherosclerosis, ocular disorders, Crohn disease, and cancer.5,6
Obesity is a complex metabolic disorder that is one of the most prevalent public health problems. Based on the World Health Organization (WHO) definition, obesity is considered as body mass index (BMI) of 30 kg/m2 or higher. Obesity is associated with the development of most common and severe human diseases such as type 2 diabetes mellitus, coronary heart disease (CHD), hypertension, increased incidence of certain cancers, respiratory complications (obstructive sleep apnea), and osteoarthritis.7,8
Angiogenesis is a process of growth and remodeling of an initial vascular system which is modified to create an intricate branching network and matured vasculature.9 It is a multistep process that consists of extracellular matrix (ECM) degradation, endothelial cell proliferation and migration, tubal formation and anastomosis.10,11 Physiological angiogenesis is essential for the wound healing, growth and action of female reproductive organs, such as ovary and endometrium during menstrual cycle and pregnancy.12 In addition, disturbance of the mechanisms involving in physiological angiogenesis can lead to a growing list of diseases either in the form of overproliferation of blood vessels such as cancer, psoriasis, arthritis, retinopathies, obesity, diabetes, asthma, and atherosclerosis or inadequate angiogenesis including heart and brain ischemia, neurodegeneration, hypertension, osteoporosis, respiratory distress, preeclampsia, endometriosis, postpartum cardiomyopathy, and ovarian hyper stimulation syndrome.13-16
Chronic low grade inflammation during obesity
Obesity was identified as a chronic low grade inflammation especially in white adipose tissue (WAT) nearly a decade ago when increase in the cytokine (TNF-α) and progress of insulin resistance with diet-induced obesity were demonstrated in rodents.17 Numerous studies have demonstrated the increase in plasma levels of MCP-1 after 4 weeks high-fat diet.18
In relation to the origin of the inflammatory response that leads to insulin resistance in obesity, an initial mechanism is the inhibition of signaling downstream of the insulin receptor. For example, exposure of cells with TNF-α leads to inhibitory phosphorylation of serine residues of insulin receptor substrate-1 (IRS-1).19,20 Another mechanism is that nutrient overload in adipocytes induces endoplasmic reticulum (ER) stress that leads to activation of inflammatory cascades.21 ER is an organelle specialized in the cell for protein folding, maturation, storage and transport of nutrients. Following nutrient surplus, misfold or unfolded proteins aggregate in the ER and create unfolded protein response.22 It leads to increased activity of c-Jun NH2-terminal kinase C (JNK), IκB Kinase (IKK-ß), IRS-1 and nuclear factor kappa-B (NF-κB) pathways that in turn cause to increase expression of pro-inflammatory cytokines such as TNF-α, IL-6, CRP, and disruption of insulin signaling.23,24 Moreover, rapid and dynamic expansion of fat tissue in obesity leads to adipose tissue hypoxia.25,26 Finally, the ER responds to this cellular stress in the form of increased activation of inflammatory pathways.27,28
There are at least two different states of macrophage infiltrations in obese adipose tissue. M1 or inflammatory macrophages that are induced by pro-inflammatory mediators such as lipopolysaccharide (LPS) and interferon (IFN-γ) and anti-inflammatory M2 macrophages normally exist in adipose tissue that are produced by cytokines such as IL-4, IL-13, and IL-10.29,30 Several evidences have shown that high fat diet changes phenotype of macrophages in adipose tissue from M2 to M1 that in turn accelerate adipose tissue inflammation.31
Furthermore, surplus glucose metabolism also can cause to increase in mitochondrial production of reactive oxygen species (ROS).32,33 ROS production in obesity causes activation of inflammatory pathways and in turn participates in inhibition of insulin signaling including JNK, IKK (NF-κB kinase)34 and the activation of these kinases in obesity show the overlap of metabolic and immune pathways. The IKKB, one of the inflammatory kinases, can affect insulin signaling by at least two ways;35 first, direct phosphorylation of IRS-1 on serine residues and the second is the phosphorylation of inhibitor of NF-κB (IκB). Thus, activation of transcription factor can stimulate generation of several inflammatory mediators such as TNF-α and IL-6.36 In addition, other mechanism that participates in inflammation-induced insulin resistance is at least 3 members of the suppressor of cytokine signaling (SOCS) family37 with inhibition of insulin signaling through IRS-1 and IRS-2 tyrosine phosphorylation or by targeting IRS-1, IRS-2 proteosomal degradation.38
Adipose tissue expansion and angiogenesis
Adipose tissue has a large vascularity and each adipocyte is enclosed by one or more capillaries.39 Reciprocal interplay between endothelial cells and adipocytes is nicely shown, as dysfunction of each compartment can has a substantial effect on the other system.40 Functional vascular system is critical for adipose tissue expansion by several mechanisms including, supplying oxygen and nutrients, waste products removal, and trigger of growth and survival signals for physiological function of adipocytes by growth factors and cytokines available in plasma.41 During expansion of adipose tissue, hypoxia is an important factor for vascular growth and remodeling.42 Likewise, increased adipocyte size leads to over longer distance for diffusion of oxygen and nutrients to adipocytes and results in a decrease of partial oxygen pressure to 20 mmHg against 40 mmHg in obese versus lean mice, respectively. Adipose tissue in response to hypoxia generates hypoxia inducible factor 1-α (HIF1-α),27,43 a heterodimer consisting of the oxygen regulated HIF1-α subunit and the constitutively expressed HIF1-β.44 In normoxic condition, HIF1-α is degraded by an oxygen-dependent hydroxylation of two proline residues. In contrast, in hypoxic conditions the level of prolyl hydroxylation decreases and protein translocates into the nucleus, and binds to hypoxia response elements (HRE) of target genes such as VEGF-A and angiopoietin-2 and thus, induces an angiogenic response that can participate in better oxygenated and nutritionally-enriched microenvironment of tumors.45
Adipose tissue generates several angiogenic and angiostatic factors including placental growth factor (PLGF), FGF2, angiopoietin-2, angiostatin, endostatin, leptin, thrombospondin (TSP-1), resistin, IGF (insulin growth factor), HGF, etc.,46,47 but most of the angiogenic activity is attributed to vascular endothelial growth factor (VEGF/VEGFR).48 The plasticity of adipose vasculature is the result of a net balance between angiogenic factors and inhibitors. Adipose tissue produces multiple angiogenic inhibitors such as adiponectin49 that significantly decrease in obese animals and humans and their blood levels have inverse correlation with their BMI. In addition, a number of studies have demonstrated that it inhibits angiogenesis in mouse corneal, CAM in vivo model and tumor angiogenesis.50,51
Accumulating these evidences demonstrated that the relationship between capillary endothelial cells and adipocytes can be created through paracrine signaling pathways and direct cell-cell interactions.52,53 For example, expression of αvβ3 integrin and plasminogen activator inhibitor 1 in human pre-adipocytes and capillary endothelial cells direct preadipocyte migration toward developing networks to coordination of the development of both tissues at the same location.54
Relationship between obesity, inflammation and angiogenesis
Angiogenesis maintains inflammation by supplying oxygen and nutrients for the cells of inflammatory region. In chronic inflammatory condition such as obesity, nitric oxide (NO), an inflammatory cytokine, is produced by inducible NO synthase (iNOS). NO dilates vessels and stimulates its permeability that leads to immune cell extravasation. Adhesion of immune cells to the endothelium is one of the most important steps in inflammatory process.55 Adhesion molecules such as E-selectin play a communication role in inflammatory process and can have a role in immune cell extravasation.56 There are similar roles for adhesion molecules, intracellular adhesion molecule (ICAM-1), vascular cellular adhesion molecule (VCAM-1), and integrins.57
Inflammatory cells also release angiogenic factors including: VEGF, Angiopoietins, fibroblast growth factor (bFGF), Hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), transforming growth factor (TGF-ß), etc that have mitogenic and migratory effects on endothelium.58-60 A common stimulus for both chronic inflammation and angiogenesis is hypoxia61 that induces HIFS expression; it in turn increases transcription of angiogenic genes such as, VEGF and Ang-2.5,62
Activation of transcription factor NF-κB is considered as the primary event in inflammation.63,64 Several studies have demonstrated interplay between NF-κB and Ang-Tie2 signaling pathway.65 Ang-2 is an angiogenic factor that upregulates several pro-inflammatory pathways that can be led to leukocyte recruitment and infiltration through NF-κB signaling interaction.66
Adipose tissue remodeling is the changes in turnover of the adipocytes, angiogenesis and rebuild of the extra cellular matrix in response to factors such as growth factors and expansion of adipose tissue, variations of hormonal concentrations, aging and diseases such as cancer and cachexia.67 Some studies have shown that high fat diet (60% calories of fat) approximately stimulates complete remodeling of epididymal fat tissue of male mice.68
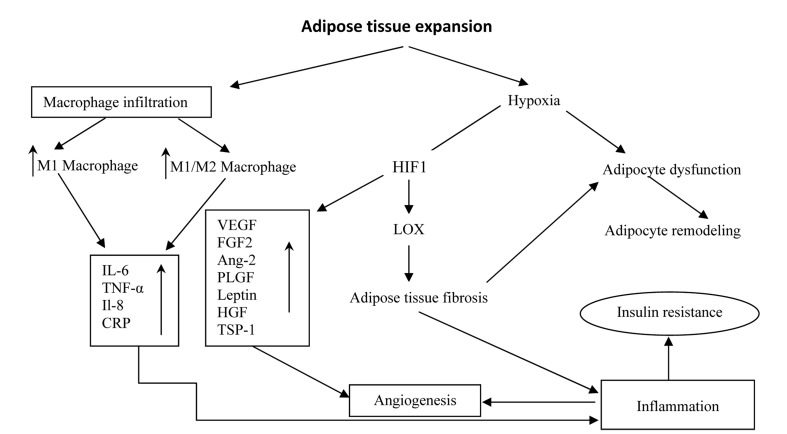
During adipose tissue expansion, blood flow per unit adipocyte surface and delivery rate of oxygen and nutrients to the adipocyte becomes suboptimal.69 On the other hand, PO2 is inversely correlated with pro-inflammatory markers. Therefore, hypoxic condition of adipose tissue during obesity leads to inflammation, fibrosis and adipocyte dysfunction.70,71 Adipocyte dysfunction may participate in the cycle of adipose tissue remodeling.72 Hypoxia in adipose tissue leads to increase of transcription factor of HIF that in turn causes angiogenic response by binding to the HRE of target genes such as VEGFA, angiopoietin-273,74 and lysyl oxidase (LOX), one enzyme with cross-link between elastins and collagens in the extracellular matrix that increases extracellular tensile strength and leads to HIF-1α mediated fibrosis.75 Furthermore, adipose tissue macrophages in obesity shift to an M1 inflammatory phenotype and the increase in the number of M1 macrophages and the M1/M2 ratio can lead to systemic inflammation and induction of insulin resistance that have been shown in figure 1.76,77
Figure 1.
Relationship between adipose tissue expansion, angiogenesis and inflammation
In conclusion, obesity is identified as a chronic low grade inflammation state and macrophages possibly play a critical role in inflammation. Hypoxia is considered as a common stimulus for inflammation and angiogenesis. Thus, there is a reciprocal interplay between inflammation and angiogenesis and both are involved in pathophysiology of obesity. Close relationship between inflammatory cells, angiogenesis and adipose tissue expansion should be considered in prevention and/or treatment of obesity.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Benelli R, Lorusso G, Albini A, Noonan DM. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr Pharm Des. 2006;12(24):3101–15. doi: 10.2174/138161206777947461. [DOI] [PubMed] [Google Scholar]
- 2.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 3.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95(9):858–66. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 4.Odrowaz-Sypniewska G. Markers of pro-inflammatory and pro-thrombotic state in the diagnosis of metabolic syndrome. Adv Med Sci. 2007;52:246–50. [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wubben DP, Adams AK. Metabolic syndrome: what's in a name? WMJ. 2006;105(5):17–20. [PubMed] [Google Scholar]
- 7.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–8. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwandt P. Can we slow down the global increase of adiposity? Int J Prev Med. 2011;2(3):115–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 11.Salehi E, Amjadi FS, Khazaei M. Angiogenesis in health and disease: role of vascular endothelial growth factor (VEGF). J Isfahan Med Sch. 2011;29(132):312–26. [Google Scholar]
- 12.Groothuis PG. Angiogenesis and vascular remodelling in female reproductive organs. Angiogenesis. 2005;8(2):87–8. doi: 10.1007/s10456-005-0391-x. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 14.Clapp C, Thebault S, Jeziorski MC, Martinez DLE. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89(4):1177–215. doi: 10.1152/physrev.00024.2009. [DOI] [PubMed] [Google Scholar]
- 15.Zarei M, Khazaei M, Sharifi MR, Pourshanazari AA. Coronary angiogenesis during experimental hypertension: is it reversible? J Res Med Sci. 2011;16(3):269–75. [PMC free article] [PubMed] [Google Scholar]
- 16.Khazaei M, Fallahzadeh AR, Sharifi MR, Afsharmoghaddam N, Javanmard SH, Salehi E. Effects of diabetes on myocardial capillary density and serum angiogenesis biomarkers in male rats. Clinics (Sao Paulo) 2011;66(8):1419–24. doi: 10.1590/S1807-59322011000800019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14(12):1225–30. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 18.Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13(8):1311–20. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- 19.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser (307). J Biol Chem. 2000;275(12):9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 20.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 22.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 23.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24(23):10161–8. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48(9):1905–14. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 26.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118(9):2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 28.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293(4):E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 29.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7(6):485–95. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280(6):4617–26. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zick Y. Role of Ser/Thr kinases in the uncoupling of insulin signaling. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S56. doi: 10.1038/sj.ijo.0802503. [DOI] [PubMed] [Google Scholar]
- 35.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277(50):48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 36.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 37.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277(44):42394–8. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 38.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24(12):5434–46. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9(2):107–15. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 40.Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. 2007;262(2):173–83. doi: 10.1111/j.1365-2796.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 41.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109(15):5874–9. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho CH, Koh YJ, Han J, Sung HK, Jong LH, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100(4):e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 43.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32(3):451–63. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 44.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12(19-20):853–9. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47(3):149–61. [PubMed] [Google Scholar]
- 47.Tahergorabi Z, Khazaei M. The effect of high-fat diet on serum angiogenic biomarkers and their correlation with serum leptin level in mic. Bratisl Med J. 2013 doi: 10.4149/bll_2014_065. [DOI] [PubMed] [Google Scholar]
- 48.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 50.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101(8):2476–81. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 52.Bouloumie A, Lolmede K, Sengenes C, Galitzky J, Lafontan M. Angiogenesis in adipose tissue. Ann Endocrinol (Paris) 2002;63(2 Pt 1):91–5. [PubMed] [Google Scholar]
- 53.Khazaei M, Tahergorabi Z. Systemic ghrelin administration alters serum biomarkers of angiogenesis in diet-induced obese mice. Int J Pept. 2013;2013:249565. doi: 10.1155/2013/249565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crandall DL, Busler DE, McHendry-Rinde B, Groeling TM, Kral JG. Autocrine regulation of human preadipocyte migration by plasminogen activator inhibitor-1. J Clin Endocrinol Metab. 2000;85(7):2609–14. doi: 10.1210/jcem.85.7.6678. [DOI] [PubMed] [Google Scholar]
- 55.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10(3):149–66. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 56.Danese S, Sans M, de la, Graziani C, West G, Phillips MH, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130(7):2060–73. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 57.Ley K. Pathways and bottlenecks in the web of inflammatory adhesion molecules and chemoattractants. Immunol Res. 2001;24(1):87–95. doi: 10.1385/IR:24:1:87. [DOI] [PubMed] [Google Scholar]
- 58.Gong R, Rifai A, Dworkin LD. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J Am Soc Nephrol. 2006;17(9):2464–73. doi: 10.1681/ASN.2006020185. [DOI] [PubMed] [Google Scholar]
- 59.Funa K, Uramoto H. Regulatory mechanisms for the expression and activity of platelet-derived growth factor receptor. Acta Biochim Pol. 2003;50(3):647–58. [PubMed] [Google Scholar]
- 60.Lee YC. The involvement of VEGF in endothelial permeability: a target for anti-inflammatory therapy. Curr Opin Investig Drugs. 2005;6(11):1124–30. [PubMed] [Google Scholar]
- 61.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175(10):6257–63. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 62.Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4(1):3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 63.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 64.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 65.Pacifico F, Leonardi A. NF-kappaB in solid tumors. Biochem Pharmacol. 2006;72(9):1142–52. doi: 10.1016/j.bcp.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 66.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–9. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 67.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 69.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118(2):710–21. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–20. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13(4):371–6. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med (Berl) 2007;85(12):1301–7. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 74.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 75.Oleggini R, Gastaldo N, Di DA. Regulation of elastin promoter by lysyl oxidase and growth factors: cross control of lysyl oxidase on TGF-beta1 effects. Matrix Biol. 2007;26(6):494–505. doi: 10.1016/j.matbio.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–82. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59(5):1171–81. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]