Abstract
Aims
Animal studies show that transforming growth factor-β1 (TGF-β1) is an important mediator of atrial fibrosis and atrial fibrillation (AF). This study investigated the role of TGF-β1 in human AF and the mechanism of atrial-selective fibrosis.
Methods and results
Atrial specimens from 17 open heart surgery patients and left atrial and ventricular specimens from 17 explanted hearts were collected to assess the relationship between TGF-β1, AF, and differential atrial vs. ventricular TGF-β1 levels. A transgenic mouse model overexpressing active TGF-β1 was used to study the mechanisms underlying the resultant atrial-selective fibrosis. Higher right atrial total TGF-β1 levels (2.58 ± 0.16-fold, P < 0.0001) and active TGF-β1 (3.7 ± 0.7-fold, P = 0.013) were observed in those that developed post-operative AF. Although no ventricular differences were observed, 11 explanted heart failure hearts exhibited higher atrial TGF-β1 levels than 6 non-failing hearts (2.30 ± 0.87 fold higher, P < 0.001). In the transgenic mouse, TGF-β1 receptor-1 kinase blockade resulted in decreased atrial expression of fibrosis-related genes. By RNA microarray analyses in that model, 80 genes in the atria and only 2 genes in the ventricle were differentially expressed. Although these mice atria, but not the ventricles, exhibited increased expression of fibrosis-related genes and phosphorylation of Smad2, there were no differences in TGF-β1 receptor levels or Smads in the atria compared with the ventricles.
Conclusions
TGF-β1 mediates selective atrial fibrosis in AF that occurs via TGF-β Receptor 1/2 and the classical Smad pathway. The differential atrial vs. ventricular fibrotic response occurs at the level of TGF-β1 receptor binding or phosphorylation.
Keywords: Transforming growth factor-beta1, Atrial fibrillation, Heart failure, Cardiac fibrosis, Gene expression
1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia, is the most common cause of stroke, substantially reduces quality of life, and nearly doubles mortality.1 While risk factors for the disease have been well established, the underlying aetiology remains poorly understood.2 Atrial fibrosis is known to be an important substrate for AF3–9; but the underlying mechanisms have not yet been fully elucidated. Understanding the pathways responsible for this fibrotic substrate may lead to a new paradigm for preventing and treating AF.
While TGF-β1 is elevated in animal models of AF,5,10 it is not known whether this elevation is causative or a result of AF. Studies of serum levels of TGF-β1 in human AF have been conflicting, likely due to the complex relationship between tissue activity and release of the protein into the blood stream.11–14 Although fibrosis has been observed in the atria of AF patients,7,9,15 the role of TGF-β1 in human AF has not previously been studied directly at the source, atrial tissue. We have shown that a transgenic mouse overexpressing constitutively active TGF-β1 develops atrial fibrosis without any ventricular fibrosis and AF,16 but the molecular basis for this selective fibrosis remains unknown. We sought to determine whether TGF-β1 activity is associated with AF in humans and then exploited the alpha-MHC-cys33ser TGF-β transgenic mouse model6,17 to investigate the molecular basis for atrial susceptibility to TGF-β1-induced fibrosis.
2. Methods
2.1. Human samples
Right atrial samples were obtained from 17 patients undergoing open heart surgery for coronary artery bypass grafting (CABG) and were immediately flash-frozen in liquid nitrogen. Patients with a recent myocardial infarction or other inflammatory diseases as well as those who were immunosuppressed were excluded. Past medical history was determined by chart review and patient interviews. Pre-existing AF was determined by chart review and confirmed by interpretation of 12-lead electrocardiograms by a board-certified cardiologist. Post-operative AF (lasting at least 30 s) was detected by continuous telemetry monitoring and tracings were confirmed by a board-certified cardiologist.
To study the effects of human heart failure on TGF-β1 levels in atria and ventricles, failing hearts removed from heart transplant recipients and flash frozen in liquid nitrogen were used. For a control comparison group, similarly harvested hearts from healthy patients who died of non-cardiac causes were collected through the California Transplant Donor Network. All tissue was collected while the patient was haemodynamically stable utilizing standards for organ procurement.
All work was approved by the UCSF Committee on Human Research (IRB Number: 10-04430) and followed the principles of the Declaration of Helsinki.
2.2. Animals
For studies of RNA and protein, MHC-TGFcys33ser mice were generated and bred as previously described.17 Experiments were performed in 15 transgenic (Tx) mice and 15 wild-type (Wt) littermates controls at 16 weeks of age. All studies were approved and monitored by the UCSF Laboratory Animal Resource Centerand conformed to the ‘Guide for the Care and Use of Laboratory Animals” (National Institutes of Health publication, 8th Edition, 2011).
2.3. Immunoblotting and histology
Twenty-five micrograms of protein were electrophoresed on 12% Tris-Glycine-polyacrylamide gels and transferred onto polyvinylidene difluoride membrane. For human tissue, membranes were incubated with mouse monoclonal antibody (Chemicon International) against the proteins. For mouse tissue, membranes were incubated with rabbit polyclonal antibody against the proteins shown in Supplementary material online, Table S4. Goat polyclonal anti-β actin antibody at 1:5000 dilutions was used as a loading control. Membranes were then incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (human tissue) or goat anti-rabbit antibody (mouse tissue) for 1 h, and then washed with Tris-buffered saline/Tween. Blots were immersed for 2 min in enhanced chemiluminescence detection reagent, exposed to film, and quantified by densitometry (standardized to the β-actin band).
Histology and fibrosis quantification was performed as previously described.6 Whole hearts were sectioned, fixed, and stained with Sirius red/fast green. Fibrosis was quantified by measuring red pixel content of digitized photomicrographs relative to total tissue area.
2.4. Mink lung epithelial cell assay of TGF-β1 activity
A mink lung epithelial cell (MLEC) assay was used to measure active TGF-β1. A stable line of MLEC transfected with p800LUC vector containing −799 to +71 of the 5′ end of the human PAI-1 gene followed by the firefly luciferase gene was used.18 These cells were plated at 5 × 105 per well and cultured for 8 h and then treated with either rhTGF-β1 (5 ng/mL), 0.01 g/well of tissue, and in the presence or absence of anti-TGF-β1 antibody for 15 h. Cell lysates were assayed for luciferase as described previously.19 Results are expressed as percentage changes of hPAI-1-LUC promoter activity in the absence of TGF-β1 or tissues, normalized for protein level.
2.5. In vivo inhibition of TGF-β RI
In order to determine that the atrial fibrosis was due to TGF-β1 overexpression and not a non-specific effect of the model, five Tx mice were treated with the TGF-β1 Receptor 1 kinase inhibitor, Ki26894 (Kirin Pharma; 50 mg/kg; twice/day via IP injections).20 Five additional untreated Tx mice and five untreated Wt mice were also used for comparison. Hearts were harvested after 5 days of treatment and total RNA was extracted separately from the atria and ventricles of all three groups.
2.6. RNA preparation and hybridization to microarray
Total RNA was extracted separately from the atria (A) and ventricles (V) of 10 mice (5 Tx and 5 Wt) with RNeasy (QIAGEN) and then treated with DNase I. RNA integrity was confirmed using an Agilent Bioanalyzer. Sample preparation, labelling, and array hybridizations were performed according to the standard protocols from the UCSF shared microarray core facilities (http://arrays.ucsf.edu/protocols). We used Mouse Exonic Evidence Based Oligonucleotide (MEEBO) microarrays containing 70-mer oligonucleotide probes for >25 000 transcripts (http://arrays.ucsf.edu/archive/meebo.html). Four two-way comparisons groups were made between TxA and WtA, TxV and WtV, TxA and TxV, and WtA and WtV. The data have been deposited in the National Center for Bio-technology Information's Gene Expression (accession number GSE27133).
2.7. qRT-PCR analysis
Total RNA isolated from atria and ventricles of both Wt (5) and Tx (5) mice were analysed by qRT-PCR to validate findings of the microarray of selected mRNA. One microgram of RNA was used to reverse transcribe cDNA (Clontech, Palo Alto, CA, USA). Agarose gel electrophoresis was used to assure near equivalent levels of cDNA synthesis. The targeting genes and GAPDH were synthesized by Applied Biosystems, Inc. Real-time PCR was performed using Taqman master mix (Applied Biosystems, Inc.) with an ABI 7300 (Applied Biosystems, Inc.). Negative controls without input cDNA were used to assess signal specificity. All gene transcript levels were quantified and normalized for GAPDH transcript levels in each sample. Equivalent amounts (0.5 µL) of cDNA were used for measurement of targeting genes and GAPDH gene transcripts. All samples were amplified in triplicate. Relative quantification was achieved by the comparative: 2–ΔΔCt method .21 The relative increase/decrease (fold-induction/repression) of mRNA of target x in the experimental group was calculated using the control group as the calibrator: 2–Ct, where cycle threshold (Ct) is: {Ct.x[Tx] – Ct.GAPDH[Tx]} – {Ct.x[Wt] – Ct.GAPDH[Wt]}.
2.8. Mouse electrophysiologic studies
AF inducibility studies were performed in intact animals as previously described.16 Mice were anaesthetized by intraperitoneal injection of urethane (2 g/kg) and surface ECGs (standard limb leads) were recorded. A 4 French quadripolar catheter was advanced through the oesophagus for atrial pacing. Inducibility of atrial arrhythmias was tested by transoesophageal burst pacing, for 2 s, with a cycle length ranging from 40 to 20 ms with a 2 ms decrement. AF was defined as a period of rapid irregular atrial rhythm lasting at least 2 s. At the end of the AF inducibility pacing studies, the mice were given heparin and hearts were removed and randomized to either the histology group or immediately used for optical mapping.
Optical mapping was performed using Langendorff perfused hearts as previously described16,22 After the heart was rapidly excised, it was arrested by immersion in cold cardioplegia solution. The aorta was cannulated and retrogradely perfused for retrograde perfusion at a pressure of 60–100 mmHg with modified Krebs–Henseleit solution. The pacing electrodes were sutured to each of the right atrium, left atrium, and left ventricle apex. The cannulated heart was then placed in 37°C Tyrode solution in a temperature-controlled optical recording chamber (maintained at 37°C) and optical mapping was modified from previous described.22 Ten thousand simultaneous optical action potentials (APs) were recorded with a 100 × 100 CMOS camera (Ultima, SciMedia) within a 6 × 6 mm mapping field for atria and 10 × 10 mm for left ventricles after perfusion with 5 μL of 4 mM stock solution of the voltage-sensitive dye Di-4_ANEPPS and 4 µmol/L blebbistatin to eliminate motion artefacts. The tissue was excited using light from a 1000-W tungsten-halogen light source through an excitation filter of 530 nm and transmitted light collected via the CMOS through an emission long-pass filter of >630 nm. Fluorescent optical maps were acquired at 2000 Hz during programmed electrical stimulation. Mapping was recorded during pacing drives of 150, 120, 90, 80, and 60 ms, as well as during S1–S2 pacing using a pacing cycle length of 100 ms (atrium) or 150 ms (ventricle) and maximum S2 of 50 ms decremented by 1 ms. The effective refractory period (ERP) was determined in the LA and LV with a 2 s basic conditioning drive, followed by a single extrastimulus, progressively decremented by 1 ms intervals until loss of capture. Optical mapping data were analysed as previous described16,22 using OMproCCD software (courtesy of Bum-rak Choi, Providence, RI, USA) and custom made Matlab software. Conduction was analysed using conduction vectors and isochronal activation.16,22,23 The conduction velocities were summarized at 50th percentile (P50), the 5th and 95th percentiles (P5 and P95, respectively) of the distribution. Conduction heterogeneity index (P95-P5)/P50 was used to evaluate heterogeneity of CV, as previously published.16,23,24 AP duration was evaluation at 10, 50, 80, and 90% recovery as well as the dF/dt using the optically derived APs.
2.9. Statistical analysis
Normally distributed continuous variables are expressed as means ± SD and compared using t-tests. Proportions were compared using the χ2 test. For the analysis of RNA microarray data, we employed 2 × 2 ANOVA models to identify differentially expressed genes between Tx and Wt, and between atrial and ventricular. Pair-wise comparisons of treatment groups were performed by forming specific contrasts of interest. Fold differences were calculated from the estimated log ratios. We computed moderated t-statistics,25 log-odds ratios of differential expression (based on empirical Bayes shrinkage of the standard errors towards a common value), and adjusted P-values (obtained using the Bonferroni correction) using functions in the limma library of the Bioconductor software package.25 Differentially expressed genes (DE genes) were selected using adjusted P-values ≤ 0.05.
Two-tailed probability values < 0.05 were considered statistically significant.
3. Results
3.1. TGF-β1 in human atrial tissue
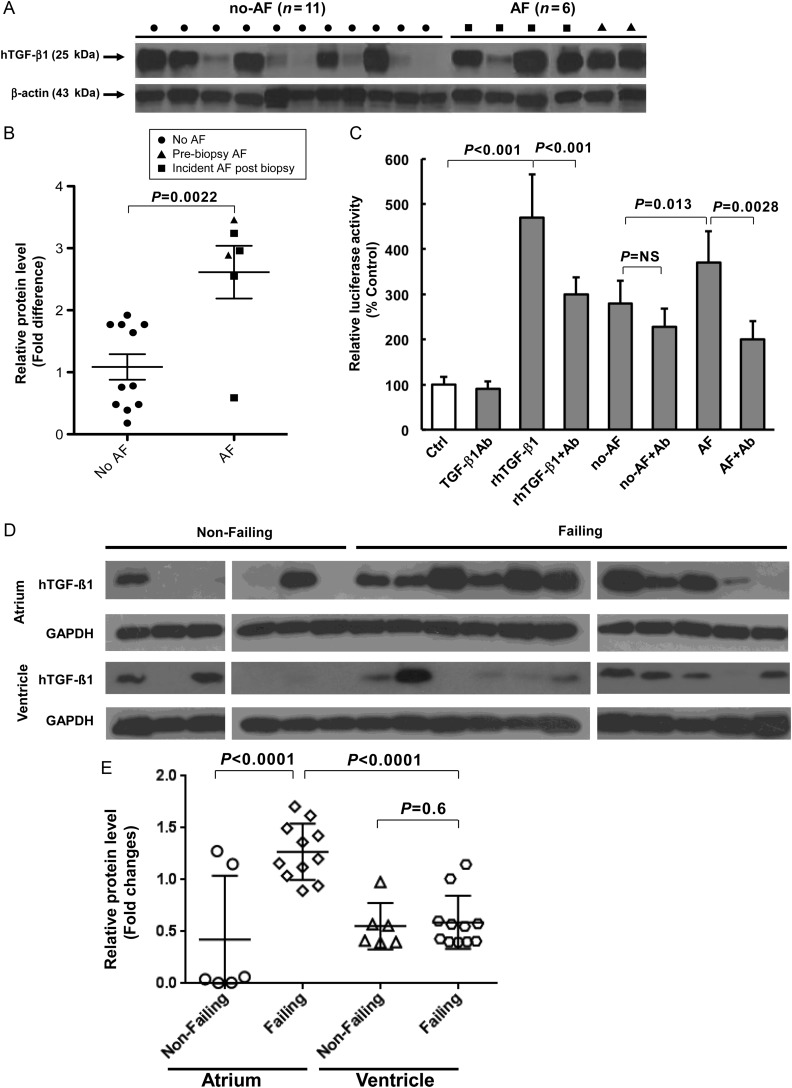
Of the 17 patients undergoing open heart surgery, 2 had previous AF, and 4 developed subsequent AF. Right atrial appendage TGF-β1 protein content was significantly greater in AF patients (Figure 1A and B), whether comparing all AF patients (2.62 ± 1.04-fold, P = 0.0022) or only those who later developed AF (2.34 ± 1.20-fold, P < 0.033) to those without AF (1.09 fold ± 0.68). The MLEC assay revealed that atria from AF patients exhibited significantly more active TGF-β1 (3.7 ± 0.7-fold control compared with 2.8 ± 0.5-fold controls in those without AF, P = 0.013; Figure 1C). Atrial TGF-β1 activity in those that later developed AF (4.1 ± 0.5 fold control) was by itself significantly larger than that observed in those without any AF (2.8 ± 0.5-fold control, P = 0.0004).
Figure 1.
TGF-β1 in human atria. (A) Western blots for TGF-β1 protein in human RA biopsy specimens. (B) Scatter plots of normalized densities with superimposed mean and SD of pooled data from the western blots are shown. (C) MLEC assay of TGF-β1 activity. (D) Western blots for TGF-β1 protein in human left atria and ventricle from patients with heart failure (Failing) undergoing a heart transplant or those without heart failure (Non-Failing) from normal patients donating other organs. (E) Scatter plots of normalized density with superimposed mean and SD of pooled data from the western blots are shown. AF denotes atrial fibrillation and NO-AF denotes control/no atrial fibrillation. Ab denotes antibody to TGF-β1; rh denotes recombinant human.
To determine the effects of heart failure—known to cause atrial remodelling predisposing to AF—on TGF-β1 expression in the atria and ventricle, we evaluated 11 failing hearts and 6 non-failing hearts. Atrial TGF-β1 levels were significantly elevated in the failing hearts compared with the non-failing hearts (2.99 ± 0.44-fold higher, P < 0.0001; Figure 1D and E), whereas ventricular TGF-β1 levels were not significantly different between the two groups. Atrial TGF-β1 levels were significantly higher than ventricular levels (P < 0.0001) only in the failing hearts. In addition, atrial expression levels of TGF-β1 signalling-related genes, including pSmad2, Smad6, and AngII, were significantly increased in failing hearts relative to non-failing hearts (shown in Supplementary material online, Figure S4). It is noteworthy that similar findings were observed in the Tx mice (see below).
3.2. Mouse studies
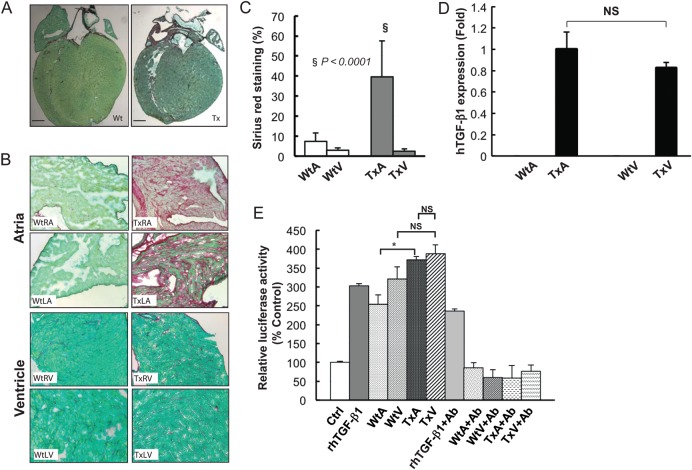
The histologic and atrial electrophysiologic characteristics of the transgenic mouse model overexpressing constitutively active human TGF-β1 have been published.6 To ensure a similar phenotype here and to study the phenotypic differences in the atria and ventricles, Sirius red stains of sections from age- and generation-matched transgenic (Tx) and littermate wild-type (Wt) mice demonstrated significant fibrosis in the transgenic atria (TxA) (Figure 2A–C), without any fibrosis or morphologic abnormalities in the transgenic ventricles (TxV).
Figure 2.
Histology and transgene expression of the TGF-β1 transgenic mouse model. (A) Sirius red staining of whole heart from wild type (Wt)—left panel—and transgenic (Tx) mice—right panel. Scale bars represent 1 mm. (B) Magnified view of Wt RA (left upper panel), Wt LA (left lower panel) and a Tx RA (right upper panel), Tx LA (right lower panel). (C) Quantification of fibrosis. (D) mRNA level of MHC-TGFcys33ser hTGF-β1 transgene determined by qRT-PCR, demonstrating no difference in expression between transgenic atria (TxA) and ventricle (TxV) and no detectable transgene in the wild-type atria (WtA) or ventricles (WtV). (E) MLEC assay of TGF-β1 activity using a stable line of MLECs transfected with a PAI-1 luciferase reporter. Results are expressed as percentage change (mean ± SD) of PAI-1 promoter activity (detected by luciferase) from control conditions (no TGF-β1 or tissue).
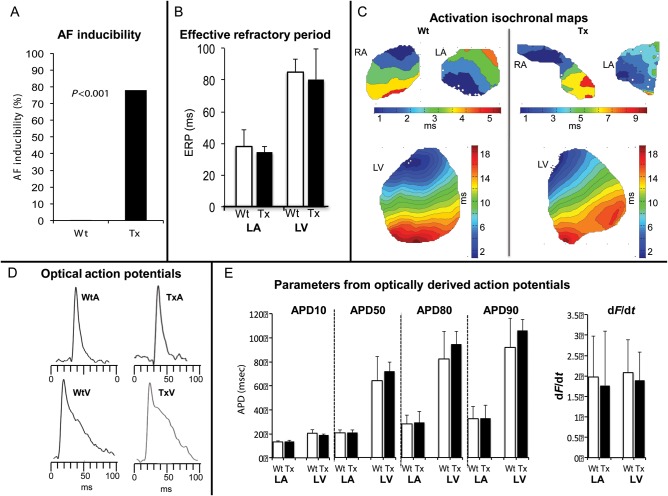
The electrophysiology studies revealed similar phenotypic differences in the atria of Wt and Tx as previously reported6 and AF was inducible in 78% of Tx and 0% of Wt mice (P < 0.001) (Figure 3A). As shown in Figure 3, conduction velocities determined by optical mapping were slower in the atria of Tx mice (0.35 ± 0.08 m/s in TxRA compared with 0.45 ± 0.06 m/s in WtRA, P = 0.019; 0.33 ± 0.08 m/s in TxLA compared with 0.43 ± 0.06 m/s in WtLA, P = 0.029). There was also greater heterogeneity of conduction velocity in the atria of Tx mice (1.68 ± 0.52 in TxRA and 1.69 ± 0.45 in TxLA) compared those from Wt controls (1.17 ± 0.20 WtRA, P = 0.035 and 1.23 ± 0.23 WtLA, P = 0.045). In contrast, there were no differences in ventricular conduction velocity or pattern. There were no differences in ERP, AP morphology, or AP duration between Tx atria and Wt atria or between Tx ventricle and Wt ventricle (Figure 3). Therefore, TGF-β1 overexpression in these mice produced atrial conduction abnormalities (without cellular electrophysiologic abnormalities) and a vulnerable substrate for AF, with no effect on ventricular morphology, histology, or electrophysiology (Figure 3). There were no electrophysiologic differences between TxV and WtV.
Figure 3.
Electrophysiologic properties of the TGF-β1 transgenic mouse. (A) Inducibility of AF in intact anesthetized mice was assessed in each age group. In intact Tx mice, the inducibility of AF was significantly higher in Tx mice. No AF was induced in Wt mice (P < 0.001 vs. all Tx mice). (B) Effective refractory period measured in the atria and ventricles of Wt and Tx mice. (C) Isochronal activation maps of RA, LA, and LV from wild type (Wt) and TGF-β1 overexpression (Tx) mice, respectively. Each isochrone line represents 1 ms. Colour time scales are shown for each chamber. The isochrome maps of the RA and LA from Tx mice show crowding and heterogeneous conduction pattern compared with Wt mice. Isochrome maps of LV of the groups show rapid, smooth conduction with no substantial differences between the two groups. (D) Examples of optically derived APs from the LA and LV of the Wt and Tx mice obtained during optical mapping. (E) Electrophysiologic parameters (APD and dF/dt) from each chamber are shown comparing Wt to Tx. There are no differences in any of the measured parameters between Wt and Tx in either the atria or ventricle. ERP, effective refractory period. APD, action potential duration. dF/dt, change in fluorescent detected voltage of change in time.
3.3. TGF-β1 levels and activity in TGF-β1 mice
Quantitative RT-PCR revealed that transgene (human cis33ser TGF-β1) expression was similar in the TxA and TxV, but absent in WtV and WtA (Figure 2D). Active TGF-β1levels, as assessed by the MLEC assay, was greater in the Tx mice than Wt mice, but were the same in TxA and TxV (Figure 2E).
After 5 days of treatment with Ki26894, a specific TGF-β receptor-I kinase blocker, fibrosis-related gene expression decreased in the atria of Tx mice to levels seen in atria of Wt mice (Supplementary material online, Figure S1A–D).
3.4. Chamber-specific differences in gene expression
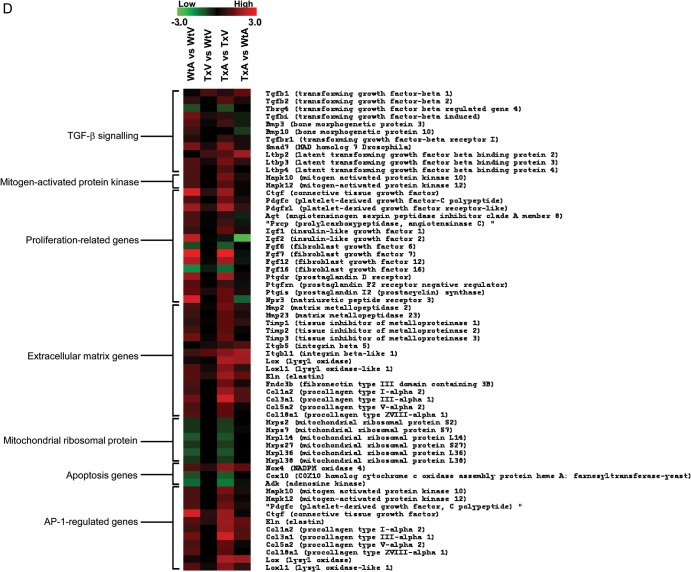
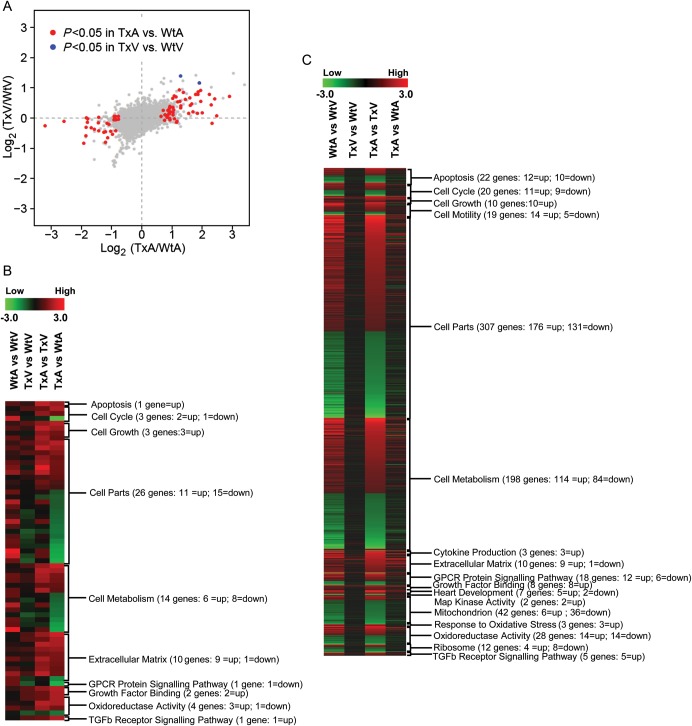
Using the Bonferroni correction, reporters for 80 genes (of approximately 30 000 transcripts that were analysed) were statistically different between the TxA and WtA (Supplementary material online, Table S2), but only 2 genes differed between the TxV and WtV (Supplementary material online, Figure S2). In the TxV vs. TxA comparison, 801 genes were significantly (adjusted P < 0.05) differentially expressed (Supplementary material online, Figure S2C), while 665 genes were significantly differentially expressed in the WtV vs. WtA comparison (Supplementary material online, Figure S2D). In Figure 4A, a plot of the log ratios of gene expression from the TxA vs. WtA comparison (X-axis) against those from the TxV vs. WtV comparison (Y-axis) demonstrate a much wider spread along the X-axis than the Y-axis, indicating a more profound TGF-β1 effect in the atria. Of the 82 genes that had a significant interaction effect (adjusted P-values < 0.05) between the two experimental factors—chambers (atria and ventricle) and TGF-β1 genotypes (Tx and Wt)—80 genes displayed TGF-β1-induced expression change in the atria compared with only 2 genes in the ventricle. These genes were divided into categories based on pathways and functional groups using Gene Ontology and GenMapp 2.0 (http://www.genmapp.org) software and are shown in Supplementary material online, Table S2.
Figure 4.
Continued.
Figure 4.
Results of the mRNA micro-array experiments in the TGF-β1 transgenic mice. (A) Scatter plot of estimated log ratios of gene expression from the TxA vs. WtA comparison (X-axis) are plotted against those from the TxV vs. WtV comparison (Y-axis). Differentially expressed genes having adjusted P-values (Bonferroni correction) < 0.05 are highlighted in red (atria) or blue (ventricle). Between the two experimental factors, chambers (atria and ventricle) and TGF-β1 genotypes (Tx and Wt), an overwhelming majority display a larger TGF-β1 induced expression fold change in the atria (red points) compared with the ventricle (blue points). (b–d) Gene expression patterns in atria and ventricles of Wt and Tx mice. Each column represents the results from duplicate hybridizations, as follows: WtA vs. WtV; TxV vs. WtV; TxA vs. TxV; TxA vs. WtA. Normalized data values depicted in shades of red and green represent elevated and repressed expression, respectively. (B) Grouping of genes showing the significant difference in the TxA vs. WtA comparison. (C) Grouping of genes showing significant differences in the TxA vs. TxV comparison. (D) Representative genes important in the TGF-β1 signalling cascade and fibrosis. Differentially expressed genes having adjusted P-values (Bonferroni correction) < 0.05 are highlighted in red or green.
Comparisons of gene expression across Wt and Tx mice were performed using MeV 4.0 (http://mev.tm4.org; Figure 4B–D). Compared with WtA, most of the genes that were up-regulated in the TxA involve cell growth, growth factor binding, oxidoreductase activity, the TGF-β receptor signalling pathway, and the ECM (Figure 4B). None of these genes was altered in the TxV compared with WtV. Most of the genes showing a difference between TxA vs. TxV were also different in the WtA vs. WtV comparison (third column compared with first column in Figure 4C). A large number of genes were uniquely altered in the TxA compared with the TxV (i.e. not different in the WtA vs. WtV; columns 2 and 4 in Figure 4B). Figure 4D shows the differential expression of genes related to fibrosis and TGF-β1 signalling. Compared with WtA, AP-1 regulated genes and ECM genes were significantly up-regulated in TxA, but no different between TxV and WtV.
Following identification of 80 genes that were differentially expressed in the atria of Tx and Wt mice, we utilized the software program Gene Map Annotator and Pathway Profiler (GenMAPP) to group these genes into pathways on the basis of their known biological functions. Given the underlying pathophysiology of our Tx mouse model, we elected to focus on genes that belonged to biological pathways involved in TGF-β signalling, fibrosis, and extracellular matrix remodelling (the list of gene groups that we felt met these criteria are listed in Supplementary material online, Table S2). This approach led to the selection of 10 of the original 80 genes for further analysis.
In order to help ensure that this approach did not omit any genes of significance, we also selected 50 additional genes for further analysis based on evidence from the literature supporting their critical involvement in these biological pathways.
These 60 genes believed to have pathophysiologic significance and representative genes with differential expression in the TxA were selected for validation by quantitative real-time RT-PCR (Supplementary material online, Table S3). The direction of expression and relative levels were similar in the qRT-PCR and the array experiments for all genes, with good correlation between the two methods (r2 = 0.81), confirming the marked increase in AP-1-regulated genes in TxA compared with WtA and TxV (Supplementary material online, Figure S1A–D).
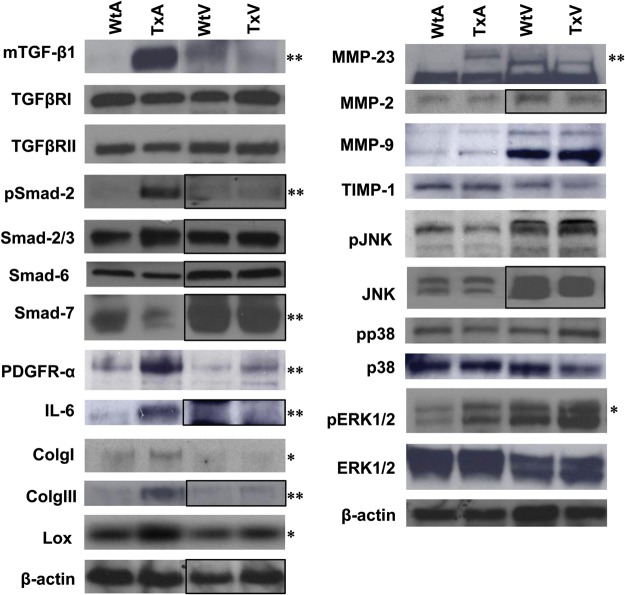
Figure 5 shows typical blots and Supplementary material online, Figure S3 shows the composite normalized (to β-actin) densitometry values for immunoblotting of 22 proteins (4 measuring phosphorylated state) important in TGF-β1 signalling. None of the ECM proteins (TIMP, MMP, collagen), Smad2 or 3 levels, or TGF-β receptor 1 or 2 levels were different in the TxV. Phospho-Smad2 was increased significantly only in the TxA group, demonstrating that the atrial vs. ventricular differences in TGF-β1 signalling are occurring at the receptor level (either effectors of TGF-β1-binding or receptor kinase activity). It should also be noted that levels of Smad7, an inhibitory Smad, are markedly reduced in transgenic atria relative to wild-type atria. Consistent with the array and qRT-PCR data, many of the AP-1 regulated proteins (IL-6, Lox, Col-I, Col-III) were increased in the TxA, but not in the TxV.
Figure 5.
Western blots for 22 proteins in Wt and Tx mice atria and ventricles. A black box indicates that separate immunoblots have been transposed. Pooled data are shown in Supplementary material online, Figure S3. *P < 0.05; **P < 0.001 vs. control.
4. Discussion
Building on previous animal data,6,9 human atrial tissue studies suggest that TGF-β1 is important in human AF. Consistent with animal models showing that heart failure induces atrial fibrosis via TGF-β18,9,26 failing human hearts exhibited elevation of TGF-β1 in atria compared with ventricles, with no atrial vs. ventricular differences observed in non-failing hearts. This finding is consistent with our transgenic mouse model in which an MHC-driven constitutively active TGF-β1 transgene is overexpressed equally in the atria and ventricle but produces isolated atrial fibrosis and a vulnerable substrate for AF. The molecular fingerprint of this model demonstrated that, although levels of the signalling proteins (TGF-β1 receptors, Smads, and AP-1) were similar in atria and ventricles, there was no increased ventricular TGF-β1 signalling. In contrast, increased atrial TGF-β1 signalling was present, with AP-1 activation occurring via the classical Smad pathway from TGF-β receptor 1–2 activation. Notably, levels of Smad7, which has been previously characterized as an inhibitory Smad,27,28 were also markedly reduced in TxA. This is a known self-amplifying effect of TGF-β1 signalling and is another sign that TGF-β signalling is occurring in the atria but not in the ventricle. The isolated atrial fibrosis therefore occurs due to differences in TGF-β receptor binding and/or receptor kinase activity, likely from a protein augmenting this process in the atria and/or inhibiting in the ventricle.
Fibrosis is an important substrate for AF. Atrial fibrosis and vulnerability to AF has been shown in patients with lone atrial fibrillation,7,9 heart failure,29 and valve disease.7 In a canine congestive heart failure model, atrial fibrosis results in abnormal atrial conduction and increased vulnerability to AF.5,30 In an ovine hypertension model, atrial fibrosis provides a ready substrate for AF,31 and drugs that limit fibrosis in animal models prevent AF.5,32 Animal studies have demonstrated the importance of TGF-β1 in atrial remodelling,5,10 and inhibition of TGF-β1 prevents atrial fibrosis, atrial conduction abnormalities, and vulnerability to AF in the dogs with ventricular tachypacing-induced congestive heart failure model.5 While TGF-β1 is a key mediator of atrial fibrosis in animal models,5,16 the role of TGF-β1 in human AF has not been previously established. We found that atrial TGF-β1 levels were increased in AF patients. Examination of TGF-β1 levels restricted to patients that later developed AF demonstrated that elevated TGF-β1 preceded the onset of AF, providing evidence for causality.
Atria appear to be particularly susceptible to fibrosis. In the canine heart failure model, profound, progressive, and persistent atrial fibrosis occurs, with little fibrosis in the ventricle.8,33 AF patients can develop atrial fibrosis or markers of fibrosis prior to the development of any ventricular dysfunction or fibrosis.7,34–36 AF can precede the development of congestive heart failure in up to 40% of cases37 and often precedes ventricular hypertrophy or dysfunction in patients with hypertension or ageing.38,39 We have demonstrated that heart failure patients exhibit elevated active TGF-β1 levels and TGF-β1-regulated gene expression in the atria but not ventricles, similar to the findings in the canine heart failure model.
To identify molecular differences that may be responsible for this atrial susceptibility to fibrosis, we performed an expression microarray study in our transgenic mouse model. Eighty genes were altered by the TGF-β1 transgene in the atria, but only 2 genes were altered in the ventricle. Consistent with the histologic findings, many of the genes differentially expressed in the atria were mediators of fibrosis (such as collagen and other ECM proteins). Other AP-1-regulated genes (e.g. Col-I, Fn-1, PAI-1, and Lox) were up-regulated in the atria but not the ventricle, demonstrating that the transcription factor AP-1 was being selectively modulated by TGF-β1 in the atria. While there were no significant differences in TGF-βR1 or 2 or Smad protein levels between atria and ventricles, Smad2 phosphorylation was significantly increased in the TxA but not in the TxV. Therefore, the fibrosis occurring in the atria is mediated by the classical Smad pathway. Additional evidence for this is that a TGF-βR1-kinase inhibitor prevented the increase in collagen expression in the TxA. The selective TxA increase in phospho-Smad2 suggests that atrial susceptibility to TGF-β1-induced fibrosis is due to modulators of TGF-β1 receptor binding or modifiers of receptor kinase activity. These effects are likely mediated by a paracrine phenomenon through TGF-β1 production from myocytes and subsequent stimulation of fibroblasts. In our mouse model, both atrial and ventricular myocytes over-produce active TGFβ1 (driven by MHC promoter), yet only the atrial fibroblasts seem to be susceptible to TGFβ1 signalling in the normal state (without other pathophysiologic stimuli). Pathophysiologic stimulation of the ventricle with ischaemia, infarction, or pressure overload might increase TGF-β1 signalling in the ventricle, but in the absence of such pathophysiology, it appears that the atria are uniquely vulnerable to TGF-β1 signalling via the Smad pathway. In fact, the microarray data indicate that many of the transcripts resulting from TGF-β1 stimulation are changed in a similar manner (but to a lesser extent) in the WtA compared with WtV as they are in the TxA compared TxV (Figure 5), suggesting a baseline atrial response to TGF-β1.
The microarray experiments revealed several candidate proteins that may contribute to the differential atrial and ventricular response to TGF-β1. For example, endoglin, a protein that modulates TGF-β receptor activity and important in cardiac fibrosis40 was expressed at much higher levels in the atria than in the ventricle. Connective tissue growth factor, which potentiates TGF-β1 receptor binding and activity and appears to be up-regulated in regions of scar formation in MI41–44 was also expressed at much higher levels in the atria than in the ventricle. Finally, natriuretic peptide signalling may also be important: B-type natriuretic peptide inhibits fibroblast transformation and collagen production in isolated fibroblast cultures stimulated with TGF-β45 and an atrial natriuretic peptide frameshift mutation has been found to be important in familial AF.46 Our microarray data demonstrate significant atrial vs. ventricular differences in several natriuretic peptides and their receptors.
Our tissue studies suggest that TGF-β1 is important in human AF and that heart failure patients have a selective increase in atrial TGF-β1. In a mouse model overexpressing constitutively active TGF-β1 equally in the atria and ventricle, the resultant atrial fibrosis is an attractive target for therapeutics to treat or possibly prevent AF. We have demonstrated that the atrial fibrosis is mediated via TGF-β receptor 1/2 complex, the classical Smad pathway, and AP-1. The atrial susceptibility to TGF-β1-mediated fibrosis in the absence of pathophysiologic stimuli (such as MI) appears to be due to augmented TGF-β receptor binding or kinase activity. Pharmacologic interventions aimed at interrupting the specific pathways responsible may provide a novel and particularly selective approach to treating AF.
4.1. Limitations
Our study has several limitations that should be considered. First, it is now apparent that AF likely results due to a combination of triggers as well as an abnormal atrial substrate. This study focuses on the vulnerable substrate of atrial fibrillation upon which triggers act to create self-sustaining AF. While fibrosis may also be important for creating triggers, the mouse model studied did not have spontaneous AF. Other animal models of AF do not exhibit spontaneous AF. In addition, there may be other pathways in addition to canonical TGF-β pathway that may be important in producing atrial fibrillation under different physiologic circumstances or other forms of AF (e.g. lone AF or vagal AF) in which fibrosis may not be a central cause of abnormal substrate. The study of atrial fibrosis, in general, provides a focus on the substrate aspect of this complex disease. Indeed, none of the previously reported animal models exhibiting atrial fibrosis, including the mice in this study, exhibits spontaneous AF.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by NIH (grant RO1-HL072854 to J.E.O., RC1-HL099789 to J.E.O.), and the Stephen and Nancy Grand Fund. G.M.M. was supported by the NIH (NCRR, KL2 RR024130), a component of the NIH, and the Joseph Drown Foundation. Microarray experiments were performed at the UCSF SABRE Center with the support of the NIH/NCRR UCSF-CTSI (grant number UL1 RR024131).
Supplementary Material
Acknowledgements
The TGF-β receptor I inhibitor Ki26894 was a gift from Dr Toshiyuki Shimizu (KIRIN Pharma Company, Japan). MLECs were gifted from Dr Dan Rifkin, New York University and mouse anti-TGF-β1 antibody 1D11 was gifted Dr Dean Sheppard Lab, University of California at San Francisco. Thank you to Dr George Hulley who helped with data analysis.
Conflict of interest: none declared.
References
- 1.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi: 10.1016/s0735-1097(00)01107-4. doi:10.1016/S0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. doi:10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. doi:10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 4.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. doi:10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 5.Lee KW, Everett TH, 4th, Rahmutula D, Guerra JM, Wilson E, Ding C, et al. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320. doi:10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verheule S, Sato T, Everett T, Engle SK, Otten D, Rubart-von der Lohe M, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. doi:10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. doi:10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. doi:10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Marcus GM, Yang Y, Varosy PD, Ordovas K, Tseng ZH, Badhwar N, et al. Regional left atrial voltage in patients with atrial fibrillation. Heart Rhythm. 2007;4:138–144. doi: 10.1016/j.hrthm.2006.10.017. doi:10.1016/j.hrthm.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardin S, Libby E, Pelletier P, Le Bouter S, Shiroshita-Takeshita A, Le Meur N, et al. Contrasting gene expression profiles in two canine models of atrial fibrillation. Circ Res. 2007;100:425–433. doi: 10.1161/01.RES.0000258428.09589.1a. [DOI] [PubMed] [Google Scholar]
- 11.On YK, Jeon ES, Lee SY, Shin DH, Choi JO, Sung J, et al. Plasma transforming growth factor beta1 as a biochemical marker to predict the persistence of atrial fibrillation after the surgical maze procedure. J Thorac Cardiovasc Surg. 2009;137:1515–1520. doi: 10.1016/j.jtcvs.2008.10.022. doi:10.1016/j.jtcvs.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Kim SK, Pak HN, Park JH, Ko KJ, Lee JS, Choi JI, et al. Clinical and serological predictors for the recurrence of atrial fibrillation after electrical cardioversion. Europace. 2009;11:1632–1638. doi: 10.1093/europace/eup321. doi:10.1093/europace/eup321. [DOI] [PubMed] [Google Scholar]
- 13.Behnes M, Hoffmann U, Lang S, Weiss C, Ahmad-Nejad P, Neumaier M, et al. Transforming growth factor beta 1 (TGF-beta 1) in atrial fibrillation and acute congestive heart failure. Clin Res Cardiol. 2011;100:335–342. doi: 10.1007/s00392-010-0248-1. doi:10.1007/s00392-010-0248-1. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Park JH, Kim JY, Choi JI, Joung B, Lee MH, et al. High plasma concentrations of transforming growth factor-beta and tissue inhibitor of metalloproteinase-1: potential non-invasive predictors for electroanatomical remodeling of atrium in patients with non-valvular atrial fibrillation. Circ J. 2011;75:557–564. doi: 10.1253/circj.cj-10-0758. doi:10.1253/circj.CJ-10-0758. [DOI] [PubMed] [Google Scholar]
- 15.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. doi:10.1161/01.CIR.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 16.Verheule S, Wilson E, Banthia S, Everett TH, 4th, Shanbhag S, Sih HJ, et al. Direction-dependent conduction abnormalities in a canine model of atrial fibrillation due to chronic atrial dilatation. Am J Physiol Heart Circ Physiol. 2004;287:H634–H644. doi: 10.1152/ajpheart.00014.2004. doi:10.1152/ajpheart.00014.2004. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. doi:10.1161/01.RES.86.5.571. [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. doi:10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 19.Cao L, Wu J, Gardner DG. Atrial natriuretic peptide suppresses the transcription of its guanylyl cyclase-linked receptor. J Biol Chem. 1995;270:24891–24897. doi: 10.1074/jbc.270.42.24891. doi:10.1074/jbc.270.42.24891. [DOI] [PubMed] [Google Scholar]
- 20.Ehata S, Hanyu A, Fujime M, Katsuno Y, Fukunaga E, Goto K, et al. Ki26894, a novel transforming growth factor-beta type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Sci. 2007;98:127–133. doi: 10.1111/j.1349-7006.2006.00357.x. doi:10.1111/j.1349-7006.2006.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. doi:10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen DT, Ding C, Wilson E, Marcus GM, Olgin JE. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm. 2010;7:1438–1445. doi: 10.1016/j.hrthm.2010.04.030. doi:10.1016/j.hrthm.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Salama G, Kanai A, Efimov IR. Subthreshold stimulation of Purkinje fibers interrupts ventricular tachycardia in intact hearts. Experimental study with voltage-sensitive dyes and imaging techniques. Circ Res. 1994;74:604–619. doi: 10.1161/01.res.74.4.604. doi:10.1161/01.RES.74.4.604. [DOI] [PubMed] [Google Scholar]
- 24.Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans Biomed Eng. 1998;45:563–571. doi: 10.1109/10.668746. doi:10.1109/10.668746. [DOI] [PubMed] [Google Scholar]
- 25.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 26.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–2678. doi: 10.1161/01.cir.0000016826.62813.f5. doi:10.1161/01.CIR.0000016826.62813.F5. [DOI] [PubMed] [Google Scholar]
- 27.He X, Gao X, Peng L, Wang S, Zhu Y, Ma H, et al. Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7. Circ Res. 2011;108:164–175. doi: 10.1161/CIRCRESAHA.110.234369. doi:10.1161/CIRCRESAHA.110.234369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Hao J, Jones SC, Yee MS, Roth JC, Dixon IM. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H1685–H1696. doi: 10.1152/ajpheart.00266.2001. [DOI] [PubMed] [Google Scholar]
- 29.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. doi:10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. doi:10.1161/01.CIR.100.1.87. [DOI] [PubMed] [Google Scholar]
- 31.Kistler PM, Sanders P, Dodic M, Spence SJ, Samuel CS, Zhao C, et al. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J. 2006;27:3045–3056. doi: 10.1093/eurheartj/ehl360. doi:10.1093/eurheartj/ehl360. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. doi:10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 33.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, et al. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. doi:10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 34.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. doi: 10.1016/j.ahj.2007.09.006. doi:10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JY, Lai YJ, Yeh HI, Chen CL, Sun S, Wu SJ, et al. Atrial gap junctions, NF-kappaB and fibrosis in patients undergoing coronary artery bypass surgery: the relationship with postoperative atrial fibrillation. Cardiology. 2009;112:81–88. doi: 10.1159/000141012. doi:10.1159/000141012. [DOI] [PubMed] [Google Scholar]
- 36.Everett TH, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4:S24–S27. doi: 10.1016/j.hrthm.2006.12.040. doi:10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. doi:10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 38.Spach MS. Mounting evidence that fibrosis generates a major mechanism for atrial fibrillation. Circ Res. 2007;101:743–745. doi: 10.1161/CIRCRESAHA.107.163956. doi:10.1161/CIRCRESAHA.107.163956. [DOI] [PubMed] [Google Scholar]
- 39.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. doi:10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen K, Mehta JL, Li D, Joseph L, Joseph J. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ Res. 2004;95:1167–1173. doi: 10.1161/01.RES.0000150369.68826.2f. doi:10.1161/01.RES.0000150369.68826.2f. [DOI] [PubMed] [Google Scholar]
- 41.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, et al. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53:1245–1256. doi: 10.1369/jhc.4A6560.2005. doi:10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 43.Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14:681–685. doi: 10.1097/00002281-200211000-00009. doi:10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. doi:10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 45.Kapoun AM, Liang F, O'Young G, Damm DL, Quon D, White RD, et al. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. doi:10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 46.Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. doi: 10.1056/NEJMoa0706300. doi:10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.