Abstract
Aims
Expression of the heat shock protein 22 (Hsp22) in the heart stimulates cardiac cell survival through activation of the Akt pathway and expression of the inducible nitric oxide (NO) synthase (iNOS), the mediator of ischaemic preconditioning and the most powerful prophylaxis against cardiac cell death. The goal of the present study was to elucidate the downstream effector by which Hsp22 and Akt increase iNOS expression. We tested both in vivo and in vitro the hypothesis that such an effector is the valosin-containing protein (VCP), an Akt substrate, which activates the transcription factor NF-κB, using a transgenic mouse with cardiac-specific over-expression of Hsp22, as well as isolated rat cardiac myocytes.
Methods and results
Using two-dimensional gel electrophoresis and mass spectrometry combined with immunoprecipitation, we found that Hsp22 and Akt co-localize and interact together with VCP. Adeno-mediated over-expression of VCP in isolated cardiac myocytes activated NF-κB and dose-dependently increased the expression of iNOS, which was abolished upon NF-κB inhibition. Over-expression of a dominant-negative (DN) mutant of VCP did not increase iNOS expression. VCP, but not its DN mutant, protected against chelerythrine-induced apoptosis, which was suppressed by inhibition of either NF-κB or iNOS. VCP-mediated activation of the NF-κB/iNOS pathway was also prevented upon inhibition of Akt.
Conclusion
We conclude that the Akt substrate, VCP, mediates the increased expression of iNOS downstream from Hsp22 through an NF-κB-dependent mechanism.
Keywords: H11 kinase, Ischaemia, Nitric oxide synthase, Preconditioning, Valosin-containing protein
1. Introduction
The cardiac expression of the small molecular weight heat shock protein (Hsp) H11 kinase/Hsp22 increases in response to myocardial ischaemia.1,2 Cardiac-specific over-expression of Hsp22 in vivo in a transgenic (TG) mouse model3 provides protection against myocardial ischaemia equally powerful to ischaemic preconditioning.4 We showed previously that these protective effects of Hsp22 correlate with the activation of Akt.3–5 Hsp22 physically associates with Akt, and both proteins accumulate at the nuclear membrane of myocytes from the TG mouse.4 We also showed that Hsp22 over-expression leads to the increased expression of the inducible isoform of nitric oxide (NO) synthase (iNOS),4 the effector of the second window of ischaemic preconditioning.6 Furthermore, inhibition of iNOS abolishes the cardioprotection conferred by Hsp22.7 However, the mechanistic link between Hsp22 and iNOS expression remains unclear.
The purpose of the present investigation was to elucidate further the pathway of Hsp22-mediated induction of iNOS, and to determine whether such induction is Akt-dependent. We hypothesized that the valosin-containing protein (VCP) plays a critical role in that process. VCP is a ubiquitously expressed and highly conserved member of the type II AAA (ATPases associated with various cellular activities) family with an apparent molecular weight of 97 kDa, and which contains four main domains: a N-terminal domain binding ubiquitinated substrates,8 two ATPase domains (D1 and D2), and a C-terminal domain involved in nuclear localization.9 VCP participates in various cellular processes requiring ubiquitination and proteasome degradation, including cell cycle control, transcriptional regulation, apoptosis, membrane fusion, and endoplasmic reticulum (ER)-associated degradation.9,10 Increased expression of VCP correlates with cell growth and survival, particularly in cancer cells,11 whereas its depletion, oxidation, or mutation lead to apoptosis triggered by ER stress.12–14 At the molecular level, VCP is activated by Akt,15,16 and it activates the transcription factor NF-κB10 responsible for iNOS induction.17 However, despite the extensive information obtained from cell lines and cancer tissues, the potential role of VCP in the heart remains unknown. In the present study, we tested the hypothesis that VCP represents the link between Hsp22-mediated activation of Akt and NF-κB-induced expression of iNOS in cardiac myocytes, thereby playing a central role in the mechanisms of cardiac cell survival promoted by Hsp22.
2. Methods
2.1. Animal model
Three-month-old male TG mice with cardiac-specific over-expression of the haemagglutinin-tagged human Hsp22 and their wild-type (WT) littermates were used. Characteristics of the TG mouse were described before.3,4 Animals were euthanized with 100 mg/kg pentobarbital. The investigation conforms with the ‘Guide for the Care and Use of Laboratory Animals’ published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and with the local ethics review board.
2.2. Protein extraction and immunoblotting
Proteins were extracted at 4°C in a buffer4 supplemented with protease and phosphatase inhibitors, followed by centrifugation at 12 000 g for 20 min at 4°C. Sub-cellular fractions were prepared by differential centrifugation of tissue homogenized in hypotonic buffer as before.18 Briefly, sub-cellular fractions were prepared by differential centrifugation of tissue homogenized manually in a Dounce homogenizer using hypotonic buffer. After an initial spin at 100 g (5 min) to discard the cellular debris and unbroken cells, the nuclear fraction was pelleted at low-speed centrifugation (500 g, 10 min). The supernatant was further centrifuged (10 000 g, 10 min) to pellet the mitochondrial fraction. The resulting supernatant was ultracentrifuged (100 000 g, 90 min) to obtain a cytosolic fraction (supernatant) and a microsomal fraction (pellet). The nuclear, mitochondrial, and microsomal fractions were washed three times with 1× phosphate-buffered saline and resuspended in buffer (150 mM NaCl, 1% NP40, 0.5% deoxycholate, 0.1% sodium dodecyl sulphate, SDS, 50 mM Tris—pH 8.0).
Proteins were denatured by boiling, resolved on SDS–polyacrylamide gel electrophoresis (PAGE) gels, and transferred onto nitrocellulose membranes. Correct separation of the fractions was verified by western blotting for cytosol, (glucose-6-phosphate dehydrogenase, G6PDH; Sigma-Aldrich, St Louis, MO, USA, 1:10 000), nucleus (histone 1; Cell Signaling, 1:1000), mitochondria (NDUFA9; Invitrogen 1:1000), and microsomes (calnexin; Abcam 1:1000). Immunoblots were performed with the corresponding antibodies (Cell Signaling, 1:1000 for VCP, Akt, p65, STAT3, p-STAT3 Y705; Sigma-Aldrich, 1:1000 for α-actinin; and Invitrogen, 1:200 for iNOS) and incubated overnight at 4°C. The Hsp22 antibody was generated as reported before.4 After washing and incubation with the secondary antibody, detection was performed by chemiluminescence (Dupont/NEN, Boston, MA, USA), and quantified by densitometry.
Immunoprecipitation was performed using 30 μL of phosphate-buffered saline (PBS)-washed protein A-sepharose incubated overnight at 4°C with 1 μg of antibody, as before.4 Proteins were denatured, resolved on SDS–polyacrylamide gels, and transferred onto nitrocellulose membranes. After incubation with the secondary antibody, the signal was detected by enhanced chemiluminescence (Dupont/NEN), and quantified by densitometry.
2.3. Two-dimensional gel electrophoresis and mass spectroscopy
Left ventricular tissue samples were homogenized in a two-dimensional compatible buffer (7 M urea, 2 M thiourea, 4% 3- (3-cholamidopropyl)dimethylammonio-1-propanesulfonate, 0.5% Biolytes, 1% Triton, 1% dithiothreitol, and phosphatase and protease inhibitors). Following the protein assay, 100 μg of proteins were separated in the first dimension according to their isoelectric point by isoelectric focusing (IEF), and then resolved in the second dimension, according to the molecular weight by SDS–PAGE.19 IEF was performed using Immobilized pH Gradient strips with a pH range of 3–10 on a BioRad IEF cell with a programmed voltage gradient. SDS–PAGE was performed on 8 and 12% polyacrylamide gels to detect proteins in different ranges of molecular weight. Matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF MS, Applied Biosystems) was used to identify proteins using the ‘Peptide Mass Mapping’ method.20 Measured peptide masses were imported into protein ‘Peptide Mass Mapping’ identification programmes for identification using search programmes (ProFound, Mascot, and MS-Fit).
2.4. Cell culture
Neonatal rat cardiac myocytes were cultured as before3 from 2-day-old Sprague-Dawley rat pups (Charles River Laboratories, Wilmington, MA, USA) and plated at a density of 106 cells/cm2. Myocytes were dispersed from the ventricles by five digestions of 15 min each, with 0.1% collagenase type IV (Worthington Biochem, Lakewood, NJ, USA), 0.1% trypsin (GIBCO), and 15 µg/mL DNase I (Sigma-Aldrich). Cell suspensions were applied on a discontinuous Percoll gradient (1.050/1.060/1.082 g/mL), and a myocyte layer was collected between densities 1.060 and 1.082. These cells were washed in cell culture medium containing Dulbecco's Modified Eagle Medium (DMEM)/F12 (1:1) (Sigma-Aldrich) with 17 mM NaHCO3, 2 mM glutamine, and 50 μg/mL gentamycin and centrifuged. These cells were pre-plated for 30 min to remove fibroblasts. The cell culture medium was changed to serum-free medium after 24 h. Myocytes were further cultured under serum-free conditions for another 24 h before the experiments were performed.
All inhibitors were added to the medium for 24 h. Inhibition of NF-κB was performed by the addition of 1 μM SN50 (Sigma-Aldrich). Inhibition of NO synthase was performed by the addition of 10 μM l-N-nitro-arginine (L-NNA), 100 μM aminoguanidine (AG), or 100 μM 1400W inhibitor (all from Sigma-Aldrich). Akt inhibition was performed upon incubation with 10 μM of Akt inhibitor IX (Calbiochem, San Diego, CA, USA).
Apoptotic cell death was stimulated upon incubation with 1 μM chelerythrine (Sigma-Aldrich) for 4 h. Apoptosis was measured both by enzyme-linked colorimetric assay (caspase-3 activation for early-stage apoptosis) and by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (DNA degradation for late-stage apoptosis).21 For TUNEL staining, incorporation of biotin-16-dUTP was measured with FITC-ExtrAvidin (Sigma-Aldrich). Slides were read at ×40 objective field. Nuclear counterstaining was performed with Vecto 4′-6-diamino-2-phenylindole (DAPI). TUNEL-positive and DAPI-positive cells were counted on at least 10 different fields. The number of apoptotic cells was calculated as a ratio of TUNEL/DAPI.21
2.5. Adeno-mediated over-expression of VCP
The VCP coding sequence was amplified by PCR from a human heart library. A VCP dominant-negative (DN) mutant was generated, in which the cloned sequence lacks the first 600 nucleotides encoding the N-terminus domain, as characterized before.8 The sequences were ligated downstream from the cytomegalovirus promoter of the AdEasy XL adenoviral vector system (Stratagene), followed by homologous recombination with the adenoviral backbone plasmid.3 The recombinant adenovirus was propagated in HEK 293 cells and titered. The adenovirus harbouring the Hsp22 sequence was described before.3 An adenovirus harbouring LacZ was prepared similarly and used as a negative control as before.21 Myocytes were collected 48 h after adenovirus infection.
2.6. DNA binding activity
Cardiac myocytes were transfected with 20 moi Cignal lentivirus particles designed for NF-κB reporter assays (SABiosciences, Frederick, MD), using the SureENTRY Transduction Reagent (SABiosciences). After 48 h of incubation, cells were infected with the adenovirus harbouring the VCP sequence or with the β-Gal control for 36 h, after which the cells were collected for the measurement of firefly luciferase activity using the Luciferase Reporter Gene Assay (Roche, Indianapolis, IN, USA) in an automated 96-well plate luminometre. The results were normalized with respect to the cell number according to the manufacturer's instructions, and reported as a percentage of β-Gal control.
2.7. Immunofluorescence
Cardiac myocytes were cultured confluently on a four-chamber slide. The cells were washed with PBS and fixed in methanol at −20°C for 10 min. After fixation, the slides were washed in PBS and blocked with 5% bovine serum albumin for 1 h, and incubated with primary antibody. After washing three times, the cells were incubated with a fluorescein-labeled secondary antibody, washed again, and mounted in a Vecto DAPI medium for fluorescent microscopic observation at a ×40 objective field.
2.8. Statistical analysis
Results are the mean ± SEM for the number of samples indicated in the figure legends. Comparison was performed using Student's t-test. Two-way analysis of variance with Fisher correction was performed for multigroup comparisons. A value of P < 0.05 was considered significant.
3. Results
3.1. Characterization of VCP expression in the heart in vivo
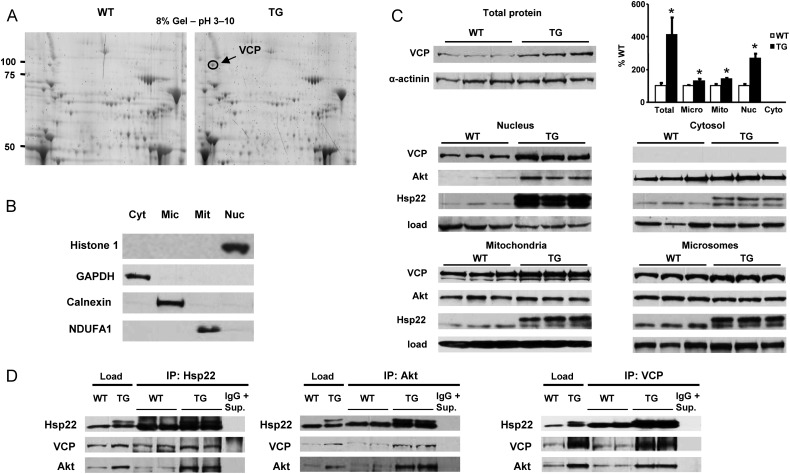
We showed previously that cardiac-specific over-expression of Hsp22 is accompanied by an accumulation and co-localization of Hsp22 and Akt in the peri-nuclear area, where both proteins interact.4 We investigated whether other proteins might also accumulate and interact with Hsp22 and Akt in the peri-nuclear space of myocytes from hearts of TG mice with Hsp22 over-expression. Hearts from 3-month-old WT and Hsp22 TG mice were submitted to sub-cellular fractionation and separated into nuclear, mitochondrial, microsomal, and cytosolic fractions. Proteins from the nuclear fraction of both WT and TG mice were separated by two-dimensional (2D) gel electrophoresis (Figure 1A). Sixteen different spots were submitted to in-gel tryptic digestion and identified by mass spectrometry. The identities of proteins that showed increased abundance in the nuclear fractions from TG mice are reported in Table 1. Among these proteins are Hsp22, other small molecular weight Hsps, and VCP (circled in Figure 1A).
Figure 1.
(A) Two-dimensional gel electrophoresis with 8% polyacrylamide gels in a pH range from 3 (left) to 10 (right), using nuclear fractions from hearts of WT and TG mice. The panel represents a representative example in each group for n = 3 per group. The VCP spot is circled. Other identified spots are reported in Table 1. (B) Sub-cellular fractionation of hearts from WT and TG mice between cytosol (Cyt), microsomes (Mic), mitochondria (Mit), and nuclei (Nuc). Specific markers show the purity of the fractions. (C) Quantitation of VCP expression in total protein extracts, and of VCP, Akt, and Hsp22 in sub-cellular fractions. The loading controls correspond to the markers shown in B. The bar graph represents the mean ± SEM for n = 3 per group. *P < 0.05 vs. corresponding WT. (D) Immunoprecipitation of VCP, Akt, and Hsp22 with specific antibodies, followed by western blotting for the three proteins in nuclear fractions from hearts of WT and TG mice. Experiments are in duplicate to show the reproducibility. Protein loading is indicated in each case. After immunoprecipitation, the resulting supernatant was submitted to a second immunoprecipitation (IgG + Sup) as a negative control for specificity.
Table 1.
Identification of proteins found by 2D gel electrophoresis
| Protein name | Gi ID | Total ion C.I% |
|---|---|---|
| Proteins enriched in nuclear fraction of TG mice | ||
| GRP 78 | 2506545 | 100 |
| Valosin-containing protein | 6005942 | 100 |
| Hsp73 | 42542422 | 100 |
| Hsp70.2 | 31560686 | 100 |
| Hsp74 | 6754256 | 100 |
| H-2 class II histocompatibility antigen | 758148 | 99 |
| Alpha-fetoprotein | 191765 | 100 |
| Immt protein | 51874032 | 85 |
| Regulator of G-protein signalling 18 | 12738845 | 99 |
| Lymphoid-restricted membrane protein | 34328142 | 100 |
| Oestrogen-related receptor alpha | 1916861 | 99 |
| Toll-like receptor 5 precursor | 20140822 | 97 |
| Hsp22/H11 kinase | 13507646 | 100 |
| HSP27 | 424145 | 100 |
| Alpha B crystallin | 6753530 | 100 |
| Peroxiredoxin 5 protein | 15928397 | 100 |
Gi ID: GenInfo Identifier Identification.
Total Ion C.I%: Total ion confidence interval: a score generated by GPS Explorer to describe the confidence of the identification.
This qualitative experiment was further validated by immunoblotting, both in total cardiac protein extracts and in specific sub-cellular fractions (Figures 1B and C). In total extracts, VCP expression was increased by about four-fold in the hearts of TG mice when compared with WT littermates (Figure 1C). When measured in different sub-cellular fractions, a three-fold increase in abundance of VCP was observed in the nuclear fraction of TG mice compared with WT (Figure 1C), and its distribution was also significantly increased in the mitochondrial and microsomal fractions of hearts from the TG mice (Figure 1B). However, VCP expression could not be detected in the cytosolic fractions, indicating that it is a mostly membrane bound protein (Figure 1C).
To determine whether VCP potentially interacts with the Hsp22/Akt complex, immunoprecipitation experiments were performed using nuclear fractions from the hearts of WT and TG mice. Antibodies against VCP, Hsp22, or Akt were used for immunoprecipitation, followed by western blotting with the other antibodies. Loading controls were included, along with a sample submitted to repeated immunoprecipitation (labelled ‘IgG + Sup.’ in Figure 1D, and used as a negative control for specificity). A co-precipitation between VCP, Akt, and Hsp22 was confirmed after pull down. The co-precipitation between these three proteins was more abundant in hearts from the Hsp22 TG mice than in the WT mice (Figure 1D).
3.2. VCP activates NF-κB
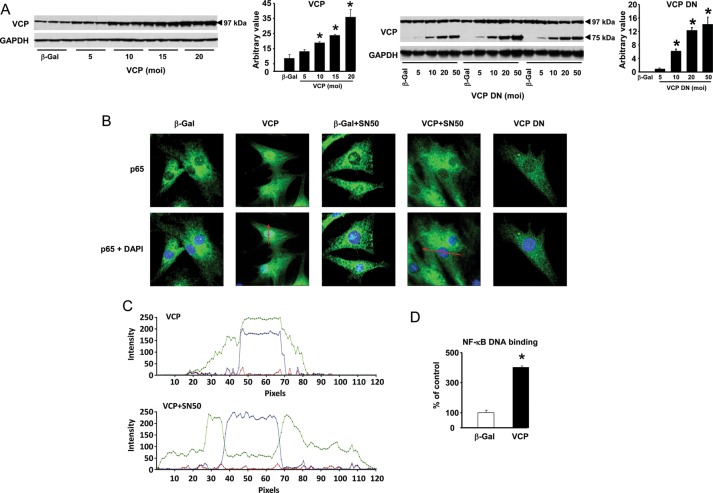
To reproduce the increased abundance of VCP found in the Hsp22 TG mouse, we generated an adenovirus harbouring the coding sequence of VCP (Figure 2A). A second adenovirus was also generated (Figure 2A), harbouring a DN mutant of VCP (VCP DN), which lacks the first 600 nucleotides of the coding sequence responsible for the binding of VCP to ubiquitin and its client proteins normally targeted to the ubiquitin-proteasome system (UPS).8,10
Figure 2.
Over-expression of VCP activates NF-κB. (A) Rat neonatal cardiomyocytes were incubated with various moi of VCP or VCP DN adenovirus. GAPDH was used as a loading control. (B) Immunofluorescence showing the nuclear translocation of the NF-κB subunit p65 upon over-expression of VCP, which is blocked in the presence of the NF-κB inhibitor, SN50. Over-expression of the VCP DN mutant or of the β-Gal control does not affect p65 translocation. The red lines represent the planes of spectral analysis shown in C. (C) Spectral analysis of the immunofluorescence, confirming the difference in distribution of NF-κB (green line) in the presence or in the absence of SN50 upon VCP over-expression. The blue line represents the DAPI staining to localize the nucleus. (D) NF-κB DNA binding activity in cardiac myocytes infected with the adenovirus harbouring the VCP sequence vs. a β-Gal control (n = 3 per group). *P < 0.05 vs. the β-Gal control.
VCP modulates NF-κB activity in cancer cells by targeting the inhibitor of NF-κB (IκBα) to the UPS through its N-terminal domain.8,10,11 We showed before that the expression of Hsp22 modulates the expression and activity of NF-κB.22 Therefore, we investigated whether VCP over-expression activate NF-κB in cardiac myocytes. Compared with a β-Gal control, over-expression of VCP was accompanied by a strong nuclear translocation of the NF-κB subunit p65, which was inhibited by the addition of the NF-κB inhibitor SN50 (Figure 2B). Over-expression of the VCP DN mutant did not modify the sub-cellular distribution of p65 (Figure 2B). Spectral analysis of the immunostainings confirmed the difference in p65 distribution in the presence and in the absence of SN50, upon over-expression of VCP (Figure 2C). Increased transcriptional activity of NF-κB by VCP was confirmed by DNA binding assay, showing a four-fold increase upon VCP over-expression when compared with the β-Gal control (Figure 2D). Therefore, over-expression of VCP, but not VCP DN, activates NF-κB.
3.3. VCP increases iNOS expression via NF-κB
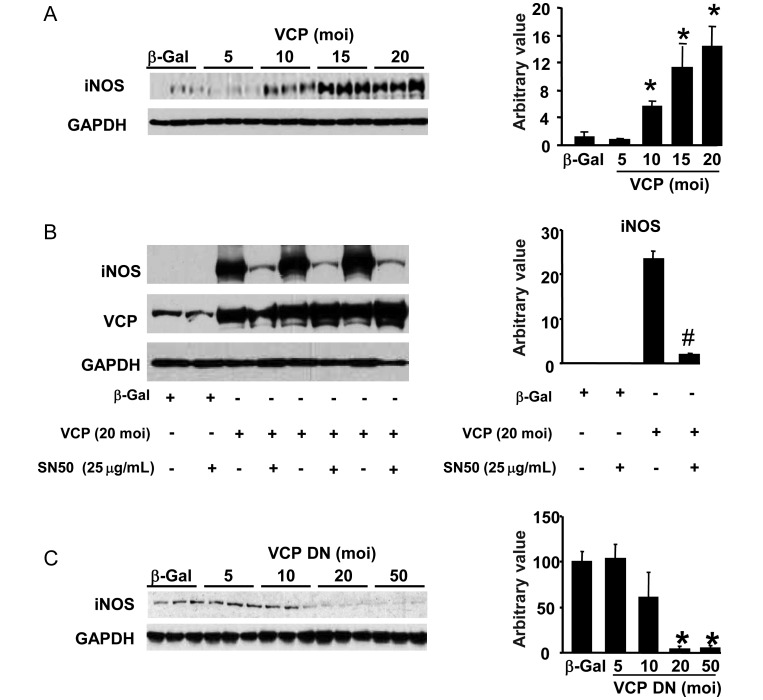
Since Hsp22 expression controls the expression of the inducible isoform of NO synthase (iNOS), a well-known target gene of NF-κB,22 we determined whether VCP participates in iNOS induction. Upon adeno-mediated infection of VCP, expression of iNOS showed a dose-dependent increase (Figure 3A). To determine whether such increase is mediated by NF-κB, cardiac myocytes were infected with 20 moi of VCP adenovirus, and the nuclear translocation of NF-κB was blocked by administration of SN50. In these conditions, the increased expression of iNOS mediated by VCP was abolished (Figure 3B). Reciprocally, over-expression of the VCP DN was unable to increase iNOS expression, and instead it dose-dependently decreased the endogenous levels of iNOS in cardiac myocytes (Figure 3C).
Figure 3.
VCP increases iNOS expression via NF-κB. (A) Rat neonatal cardiomyocytes were incubated with various moi of VCP adenovirus. Western blot analysis shows an increased expression of iNOS in response to over-expression of VCP (n = 3 per group). GAPDH was used as a loading control. (B) Increased iNOS expression in the presence of VCP is abolished by the NF-κB inhibitor, SN50. (C) Over-expression of the VCP DN mutant does not increase, but it significantly decreases iNOS expression (n = 3 per group). *P < 0.05 vs. respective controls; #P < 0.01 vs. corresponding group without SN50.
3.4. Akt participates in VCP-mediated iNOS expression
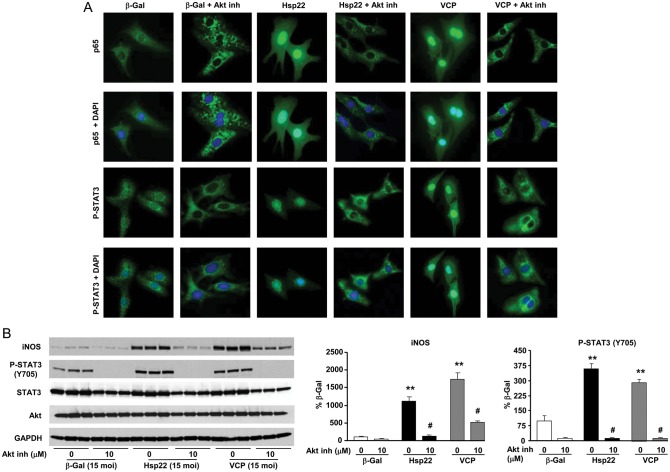
We showed before that Hsp22 over-expression activates Akt.21 We also showed previously that Hsp22 over-expression promotes the nuclear translocation and activation of two transcription factors that increase the expression of iNOS, namely NF-κB and STAT3.4,22 The activation of NF-κB is shown here to be reproduced by VCP. Therefore, we tested whether Akt represents a plausible candidate mediating VCP-mediated induction of iNOS via NF-κB together with STAT3.
This possibility was investigated in cardiac myocytes using the Akt inhibitor IX, a specific inhibitor of the Akt serine/threonine kinase activity. As shown by immunofluorescence in Figure 4A, VCP over-expression increased the nuclear translocation and the tyrosine phosphorylation of STAT3 on Y705, a marker of its transcriptional activation, together with the nuclear translocation of the p65 subunit of NF-κB. VCP-mediated translocation of both transcription factors, as well as Y705 phosphorylation of STAT3, was prevented using Akt inhibition (Figure 4A). In a more quantitative approach, immunoblotting confirmed that VCP increases iNOS expression and tyrosine phosphorylation of STAT3, and that these effects were blocked by the addition of the Akt inhibitor (Figure 4B).
Figure 4.
VCP acts downstream of Akt to promote cardiac cell survival. (A) Immunofluorescence of the NF-κB subunit p65 and Y705 tyrosine phosphorylation of STAT3 in myocytes infected with the adenovirus harbouring β-Gal, Hsp22, or VCP, in the presence or in the absence of Akt inhibitor IX. Nuclear counterstaining was performed by DAPI. (B) Immunoblotting of iNOS, Y705 P-STAT3, total STAT3, and Akt in the same experimental groups as A. GAPDH was used as a loading control. **P < 0.01 vs. β-Gal; #P < 0.05 vs. same group without Akt inhibitor.
3.5. VCP provides iNOS-dependent protection against apoptosis
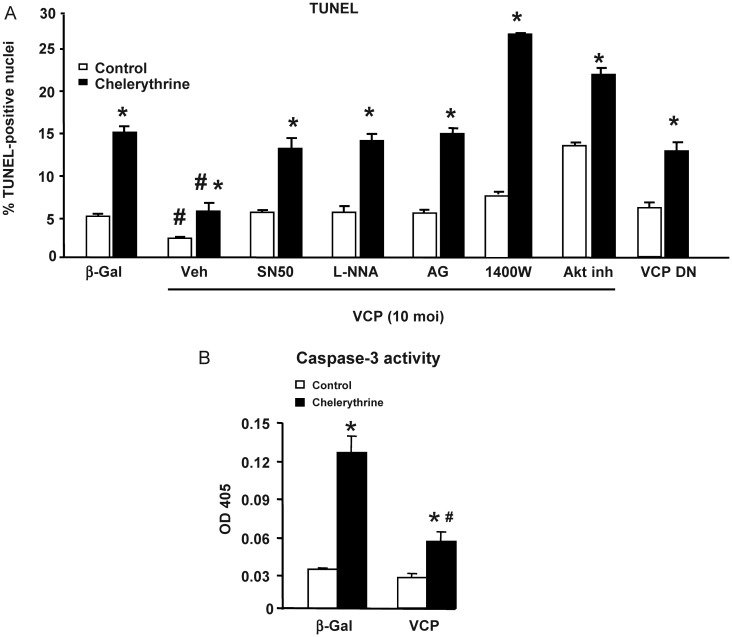
Because increased expression of iNOS in the heart is central to the cardioprotective mechanisms of the delayed window of ischaemic preconditioning,6 we tested whether activation of the NF-κB/iNOS axis by VCP would provide cardioprotection. Cardiac myocytes were exposed to apoptosis by the addition of chelerythrine, and compared with vehicle-treated myocytes. Upon over-expression of VCP, apoptosis was reduced by half even in the absence of chelerythrine when compared with the β-Gal control (Figure 5A). Upon addition of chelerythrine, apoptosis increased by three-fold in the β-Gal group, and this was significantly less in myocytes over-expressing VCP (Figure 5A). In contrast, over-expression of the VCP DN mutant did not decrease apoptosis in control conditions, and did not protect against chelerythrine-induced apoptosis, when compared with the β-Gal control (Figure 5A). The anti-apoptotic effects of VCP over-expression was abolished by the addition of the NF-κB inhibitor, SN50, as well as upon the addition of the pan-NOS inhibitor, L-NNA, or upon the addition of AG or 1400W, which are more specific iNOS inhibitors (Figure 5A). Therefore, VCP provides cardioprotection through the NF-κB/iNOS pathway.
Figure 5.
VCP provides cardioprotection through iNOS. (A) Apoptosis measured by TUNEL in vehicle-treated (open bars) vs. chelerythrine-treated (closed bars) cardiac myocytes, upon over-expression of VCP (in the presence of SN50, L-NNA, AG, 1400 W or Akt inhibitor, compared with vehicle), or of the VCP DN mutant. (B) Apoptosis, as measured by caspase-3 activity in vehicle-treated (open bars) vs. chelerythrine-treated (closed bars) cardiac myocytes, upon over-expression of VCP. *P < 0.05 vs. same group without chelerythrine; #P < 0.05 vs. corresponding β-Gal control.
Because no single method is recognized as a gold standard to measure apoptosis, the TUNEL results presented above were confirmed by a caspase-3 colorimetric assay (Figure 5B). Chelerythrine treatment in the β-Gal control group induced a four-fold increase in caspase activation, while addition of the VCP adenovirus markedly limited such an increase (Figure 5B).
4. Discussion
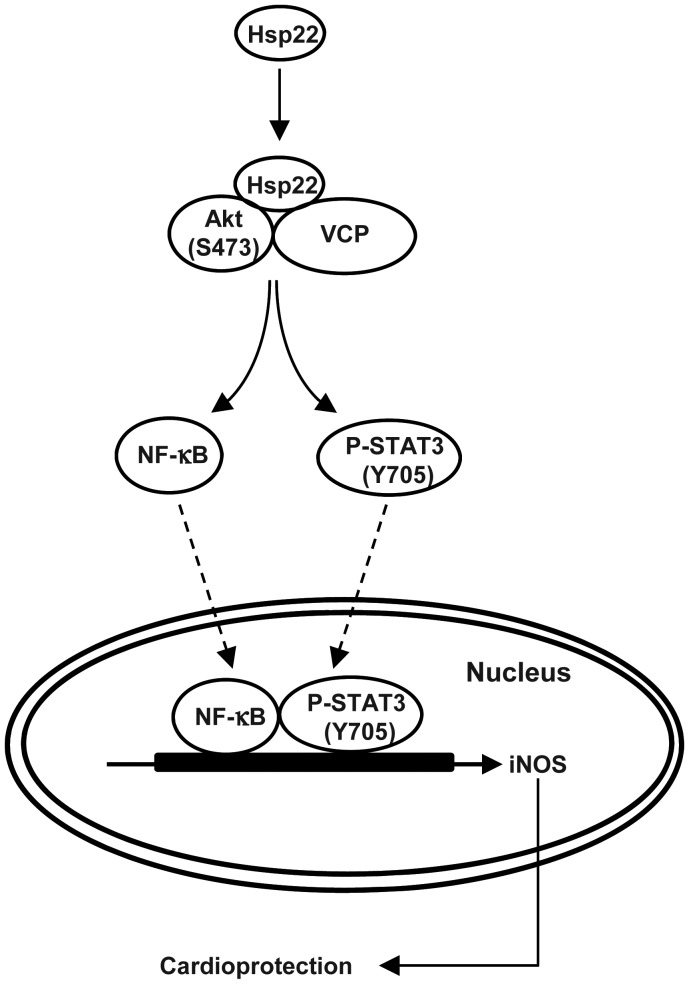
In this study, we characterized the expression and regulation of VCP in the heart, and in particular iNOS-mediated mechanisms of inhibition of apoptosis. We have shown that Hsp22 through Akt stimulates VCP-mediated activation of NF-κB, resulting in iNOS expression and inhibition of apoptosis. These findings are summarized in Figure 6.
Figure 6.
Summary of the findings. VCP interacts with Hsp22 and Akt to activate both transcription factors NF-κB and STAT3, leading to the expression of iNOS.
Using 2D gel electrophoresis followed by mass spectrometry, we identified VCP in Hsp22 TG mice. We found that both Hsp22 and Akt co-localize and co-precipitate with VCP. This interaction between Hsp22, Akt, and VCP predominated in the nuclear fraction of adult mouse cardiac myocytes, and it was markedly increased in the Hsp22 TG mouse. However, immunofluorescence experiments presented here and in our previous publication4 show that the Hsp22/Akt/VCP complex does not penetrate the nuclear compartment, but rather adheres at the nuclear membrane. It is therefore possible that, in addition to the role of Hsp22 in specific signalling pathways, Hsp22 redistributes signalling molecules between different compartments, thereby promoting the formation of a multiprotein complex in a specific sub-cellular locale. Therefore, the identification of molecules participating in this complex by Hsp22 can provide vital information towards understanding the mechanisms of Hsp22-mediated survival.
The identification of VCP and its potential role as a novel player in cardioprotection conferred by Hsp22 is particularly interesting based on the existing literature related to the role of VCP as a survival agent in cancer cells.11 In such cell types, VCP is directly involved in the mechanism of NF-κB activation,8,10 a transcription factor central to cardioprotection by Hsp22. Therefore, we also tested the hypothesis that VCP can trigger the transcriptional activation of NF-κB in the heart upon stimulation by Hsp22. It is known that NF-κB is activated upon cardiac stress, such as overload or ischaemia, by the proteasomal degradation of its specific inhibitor, IκBα, thereby freeing NF-κB to move into the nucleus, where it becomes transcriptionally active.23 However, phosphorylation of IκBα is not sufficient for its degradation, and it has to be physically carried to the ubiquitin-proteasome pathway by a shuttle protein.23 VCP has been shown to act as such a carrier.8,10,24
In addition, we demonstrate in this study that VCP is both sufficient (by over-expression of VCP) and necessary (by over-expression of VCP DN) for NF-κB activation and STAT3, which are both necessary for the transcriptional activation of iNOS expression.22 Interestingly, the VCP-mediated activation of NF-κB and STAT3 is abolished by the inhibition of Akt. Not only have we shown before that Akt mediates the cardioprotection by Hsp22,4,21 but also it has been published previously that three specific serine residues of VCP (Ser 352, Ser746, and Ser748) are phosphorylated by Akt.15,16 Whether such mechanism applies to our conditions will require further investigation. Our experiments involving the Akt inhibitor IX further support the conclusion that Akt activates VCP in the heart. Taken together, these results provide further support to the description of a linear signalling pathway involving Akt, VCP, NF-κB/STAT3, and iNOS, and which results in protection against cell death.
In conclusion, these experiments shed more light on novel signalling mechanisms of cardioprotection related to preconditioning. Identified first as a downstream target of Hsp22, this study highlights the cardioprotective role of VCP, which was previously not known to be cytoprotective in the heart. The relevance of VCP in these mechanisms of cardioprotection is supported by the following lines of evidence. First, we show that VCP, but not its DN mutant, is sufficient to protect against chelerythrine-induced apoptosis. Secondly, this protection was abolished by NF-κB inhibition, the pan-NOS inhibitor, L-NNA, and by more specific iNOS inhibitors. In addition, we also show that VCP is necessary for cardiac cell survival, since over-expression of VCP DN does not confer cardioprotection and actually decreases iNOS expression. We conclude that the Akt substrate, VCP, mediates the increased expression of iNOS downstream from Hsp22 through an NF-κB-dependent mechanism. These data provide more insights as to how VCP expression promotes cell survival, and thereby may improve our therapeutic opportunities to treat ischaemic heart disease.
Conflict of interest: none declared.
Funding
This work was supported by National Institute of Health grants 1R01HL093415-01 and 1R01HL115195-01, and by American Heart Association grants 0230017N and 0835182N.
References
- 1.Depre C, Kim SJ, John AS, Huang Y, Rimoldi OE, Pepper JR, et al. Program of cell survival underlying human and experimental hibernating myocardium. Circ Res. 2004;95:433–440. doi: 10.1161/01.RES.0000138301.42713.18. [DOI] [PubMed] [Google Scholar]
- 2.Depre C, Tomlinson JE, Kudej RK, Gaussin V, Thompson E, Kim SJ, et al. Gene program for cardiac cell survival induced by transient ischemia in conscious pig. Proc Natl Acad Sci USA. 2001;98:9336–9341. doi: 10.1073/pnas.171297498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, et al. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res. 2002;91:1007–1014. doi: 10.1161/01.res.0000044380.54893.4b. [DOI] [PubMed] [Google Scholar]
- 4.Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, et al. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res. 2006;98:280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- 5.Danan IJ, Rashed ER, Depre C. Therapeutic potential of h11 kinase for the ischemic heart. Cardiovasc Drug Revs. 2007;25:14–29. doi: 10.1111/j.1527-3466.2007.00002.x. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Lizano P, Zhao X, Sui X, Dhar SK, Shen YT, et al. Pre-emptive conditioning of the swine heart by h11 kinase/hsp22 provides cardiac protection through inducible nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2011;300:H1303–H1310. doi: 10.1152/ajpheart.00979.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol. 2001;3:740–744. doi: 10.1038/35087056. [DOI] [PubMed] [Google Scholar]
- 9.Braun R, Zischka H. Mechanisms of cdc48/vcp-mediated cell death: From yeast apoptosis to human disease. Biochim Biophys Acta. 2008;1783:1418–1435. doi: 10.1016/j.bbamcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Dai RM, Chen E, Longo DL, Gorbea CM, Li CC. Involvement of valosin-containing protein, an ATPase co-purified with ikappa balpha and 26s proteasome, in ubiquitin-proteasome-mediated degradation of ikappa balpha. J Biol Chem. 1998;273:3562–3573. doi: 10.1074/jbc.273.6.3562. [DOI] [PubMed] [Google Scholar]
- 11.Asai T, Tomita Y, Nakatsuka S, Hoshida Y, Myoui A, Yoshikawa H, et al. Vcp (p97) regulates nfkappab signaling pathway, which is important for metastasis of osteosarcoma cell line. Jpn J Cancer Res. 2002;93:296–304. doi: 10.1111/j.1349-7006.2002.tb02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojcik C, Yano M, DeMartino GN. RNA interference of valosin-containing protein (vcp/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J Cell Sci. 2004;117:281–292. doi: 10.1242/jcs.00841. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Tanaka K, Inoue K, Kakizuka A. Functional ATPase activity of p97/valosin-containing protein (vcp) is required for the quality control of endoplasmic reticulum in neuronally differentiated mammalian pc12 cells. J Biol Chem. 2002;277:47358–47365. doi: 10.1074/jbc.M207783200. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi M, Takata T, Kimura Y, Manno A, Murakami K, Koike M, et al. ATPase activity of p97/valosin-containing protein is regulated by oxidative modification of the evolutionally conserved cysteine 522 residue in walker a motif. J Biol Chem. 2005;280:41332–41341. doi: 10.1074/jbc.M509700200. [DOI] [PubMed] [Google Scholar]
- 15.Vandermoere F, El Yazidi-Belkoura I, Slomianny C, Demont Y, Bidaux G, Adriaenssens E, et al. The valosin-containing protein (vcp) is a target of akt signaling required for cell survival. J Biol Chem. 2006;281:14307–14313. doi: 10.1074/jbc.M510003200. [DOI] [PubMed] [Google Scholar]
- 16.Klein JB, Barati MT, Wu R, Gozal D, Sachleben LR, Jr, Kausar H, et al. Akt-mediated valosin-containing protein 97 phosphorylation regulates its association with ubiquitinated proteins. J Biol Chem. 2005;280:31870–31881. doi: 10.1074/jbc.M501802200. [DOI] [PubMed] [Google Scholar]
- 17.Kleinert H, Pautz A, Linker K, Schwarz P. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Hedhli N, Wang L, Wang Q, Rashed E, Tian Y, Sui X, et al. Proteasome activation during cardiac hypertrophy by the chaperone h11 kinase/hsp22. Cardiovasc Res. 2008;77:497–505. doi: 10.1093/cvr/cvm054. [DOI] [PubMed] [Google Scholar]
- 19.Yan L, Ge H, Li H, Lieber SC, Natividad F, Resuello RRG, et al. Gender-specific proteomic alterations in glycolytic and mitochondrial pathways in aging monkey hearts. J Mol Cell Cardiol. 2004;37:921–929. doi: 10.1016/j.yjmcc.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Yan L, Vatner DE, Kim S-J, Ge H, Masurekar M, Massover WH, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sui X, Li D, Qiu H, Gaussin V, Depre C. Activation of the bone morphogenetic protein receptor by h11kinase/hsp22 promotes cardiac cell growth and survival. Circ Res. 2009;104:887–895. doi: 10.1161/CIRCRESAHA.108.192328. [DOI] [PubMed] [Google Scholar]
- 22.Qiu H, Lizano P, Laure L, Sui X, Rashed E, Park J, et al. H11 kinase/hsp22 deletion impairs both nuclear and mitochondrial functions of stat3 and accelerates the transition into heart failure upon cardiac overload. Circulation. 2011;124:406–415. doi: 10.1161/CIRCULATIONAHA.110.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayden MS, Ghosh S. Signaling to nf-kappab. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 24.Vandermoere F, El Yazidi-Belkoura I, Adriaenssens E, Lemoine J, Hondermarck H. The antiapoptotic effect of fibroblast growth factor-2 is mediated through nuclear factor-kappab activation induced via interaction between Akt and ikappab kinase-beta in breast cancer cells. Oncogene. 2005;24:5482–5491. doi: 10.1038/sj.onc.1208713. [DOI] [PubMed] [Google Scholar]