Abstract
Aims
Old age and diabetes are risk factors that often coexist increasing the vulnerability of the heart to the lethal effects of ischaemia–reperfusion injury (IRI). However, to our knowledge, no investigations have examined IRI and cardioprotective signalling in animal models bearing these co-morbidities concomitantly. The ability of the heart to recover following IRI is greatly dependent on its innate cardioprotective potential, in which a central role is played by Akt. We aimed to investigate in an aging diabetic rat model, the susceptibility of the heart to IRI, the achievability of ischaemic preconditioning (IPC) against this lethal event, and the changes in Akt signalling, as the main prosurvival intracellular pathway.
Methods and results
Our data showed that the isolated hearts of aged, diabetic Goto-Kakizaki rats were more susceptible to sub-lethal injury and not amenable to cardioprotection via IPC, compared with younger diabetic rat hearts. Western blot analysis of the heart tissue suggested a chronic up-regulation of Akt phosphorylation, and reduced expression of the mitochondrial regulator PGC-1α and of the anti-oxidant enzyme catalase, potentially due to the Akt up-regulation. Moreover, no further activation of Akt could be achieved following IPC.
Conclusion
An increased susceptibility to IRI in the aged, diabetic heart could be a consequence of impaired Akt signalling due to chronic Akt phosphorylation. Additional Akt phosphorylation required for IPC protection may therefore not be possible in the aged, diabetic rat heart and may explain why this cardioprotective manoeuvre cannot be achieved in these hearts.
Keywords: Myocardial infarct, Age, Diabetes, Akt, Ischaemic preconditioning
1. Introduction
Both aging and type 2 diabetes mellitus (T2DM) are recognized risk factors for heart failure, associated with an increased prevalence of ischaemic events and poor clinical recovery following acute myocardial infarction (AMI).1,2 Given the worse clinical outcomes in old, diabetic patients with ischaemic heart disease, novel therapeutic strategies for protecting the heart against the detrimental effects of acute IRI are required to improve clinical outcomes in this patient group. Surprisingly, there are limited investigations using animal models exhibiting both these co-morbidities, the reasons for this are unclear and may be a consequence of the costs associated with the maintenance of an aging, diabetic colony. Studies investigating cardioprotection in aging, diabetic models could provide a vital link in the translational process of basic research into clinical practice.
There has been great discrepancy between clinical and animal data, regarding either the susceptibility of the diabetic heart to ischaemia–reperfusion injury (IRI) or the ability to condition the heart against this lethal event.3 In normoglycaemic young animal models, the role of pro-survival PI3K/Akt (protein kinase B) signalling in endogenous cardioprotection against IRI has been well documented, first, as an anti-apoptotic kinase4 and secondly, as a key element of the reperfusion injury salvage kinase (RISK) pathway that is initiated following ischaemic or pharmacological conditioning.5 This signalling pathway has been reported to be defective in the diabetic myocardium, with an impaired level of basal Akt phosphorylation seen in both diabetic animal heart tissue6 and human tissue,7 compared with non-diabetic. This impairment could lead to a plethora of detrimental effects on the balance of anti- and pro-apoptotic intracellular signalling, potentially rendering the diabetic heart more susceptible to AMI. Surprisingly, Fazel et al. showed that diabetic patients presenting in the clinic with AMI had similar or lower release of cardiac markers of injury, such as troponin or creatinine kinase myocardial band, which suggested a smaller infarction. However, these diabetic patients exhibiting lower levels of infarct markers experienced worse clinical outcomes compared with the non-diabetic patients.8
Based on these apparent contradictory data, we hypothesized that the diabetic heart may develop smaller infarcts, but could be more susceptible to ischaemic damage, i.e. the threshold at which infarct size translates into worse clinical outcomes is lower for diabetic patients. Furthermore, this increased susceptibility could be related to modified levels of endogenous phosphorylated-Akt levels in the aged, diabetic heart. Using an inbred lean model of T2DM, Goto-Kakizaki (GK) rat and its original source, the normoglycaemic Wistar rat, at different ages (between 3 and 18 months), we aimed to determine whether the aging, diabetic heart was (i) more susceptible to ischaemic damage and (ii) amenable to cardioprotection by IPC. Based on the data we obtained from these initial experiments, we further investigated the potential signalling systems which could affect the endogenous cardioprotective abilities of the heart with age and diabetes. In this regard, we initially assessed the activation of Akt by phosphorylation, and subsequently, we evaluated the levels of PGC-1α (a key transcriptional regulator) and catalase (an indicator of anti-oxidant defence), which can both be directly or indirectly controlled by Akt.
2. Methods
2.1. Animals
Male GK rats (a mild, non-obese, insulin-resistant diabetic model9,10) were obtained from Taconic (Denmark), and Male Wistar rats (normoglycaemic) were obtained from Charles River UK Ltd (Margate, UK). They were kept in house until they reached 3, 8, 12, or 18 months of age; all animals received humane care in accordance with the United Kingdom Animal Scientific Procedures Act of 1986 (project license no. 70/7140). Fasting blood glucose levels (Accu-chek system, Roche) and HbA1c (A1C now+ test kit. Bayer, UK) were measured at least 48 h prior to excision of the heart to avoid the up-regulation of cardioprotective stress proteins.11 Body weight and heart weight were recorded on the experimental day.
2.2. Langendorff isolated heart perfusions
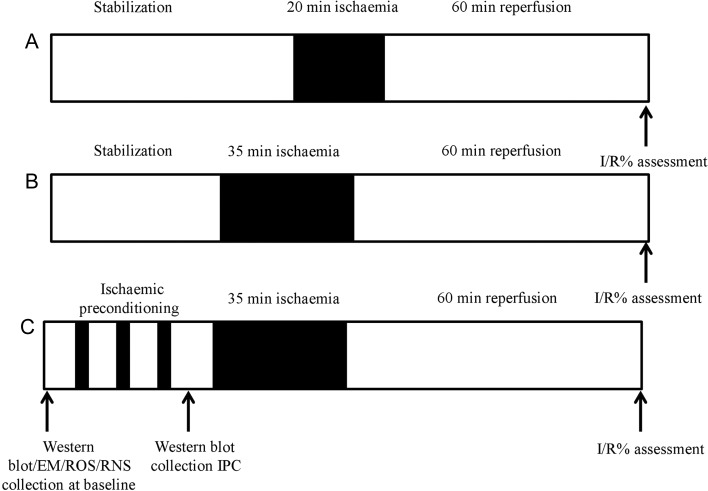
For determination of infarct size, GK and Wistar rats of 3, 8, 12, and 18 months of age were anesthetized with sodium pentobarbital (55 mg/kg intraperitoneally) and heparin (300 IU). Hearts were rapidly excised; the aorta located and cannulated, allowing for retrograde perfusion with Krebs-Henseleit buffer on the Langendorff system, in constant pressure; method described in detail.12 A latex, water filled balloon was inserted into the left ventricle to measure functional parameters, heart rate (HR), and left ventricular developed pressure (LVDP), used to calculate workload of the heart, also known as rate pressure product (RPP). For infarct analysis (n = 6 per group), hearts were randomly assigned to receive sub-lethal (20 min regional ischaemia), lethal (35 min regional ischaemia), or IPC (three cycles of 5 min ischaemia/10 min reperfusion prior to lethal ischaemia), all followed by 60 min reperfusion (summarized in Figure 1). Regional ischaemia was induced by tightening of a snare around the left anterior descending coronary artery and reperfusion by releasing the snare. Infarct size was measured after re-tightening of the snare and infusing Evans blue dye (0.25%) to delineate the myocardium at risk. Hearts were then sectioned transversely and stained by immersion in triphenyltetrazolium chloride solution (1% in phosphate buffer), which reacts with intracellular dehydrogenases in viable tissue forming a red precipitate, leaving the necrotic tissue off-white. The slices were then fixed in 10% formalin overnight and scanned into the computer for analysis. Image J was used to calculate the volume of infarction within the risk zone (I/R%).
Figure 1.
Scheme of Langendorff experiments performed. For infarct analysis (n = 6 per group), hearts were randomly assigned to receive (A) sub-lethal (20 min regional ischaemia), (B) lethal (35 min regional ischaemia), or (C) IPC (three cycles of 5 min ischaemia/10 min reperfusion prior to lethal ischaemia), all followed by 60 min reperfusion. Hearts were collected for WB analysis either at baseline or following IPC.
2.3. Western blot analysis
Western blot (WB) analysis samples were collected to assess protein expression and activation in aging, non-diabetic and aging, diabetic hearts. Hearts from 3- to 18-month-old GK and Wistar rats were collected at baseline (following 2 min on the perfusion apparatus to clear blood) or following three cycles of IPC ((n = 3 per group, protocols summarized in Figure 1C). At the end of the procedure, the hearts were immediately freeze-clamped in liquid nitrogen and frozen at −80°C. For analysis, the heart samples were homogenized in phosphate-buffered saline-based buffer (pH 7.4) containing Calbiochem protease inhibitor cocktail III ethylenediaminetetraacetic acid (EDTA) free (1:1000), Sigma phosphatase inhibitor cocktail III (1:100), and EDTA 1 mM. Protein content was then determined using bicinchoninic acid protein assay reagent (Sigma, UK) and protein levels corrected accordingly to ensure equal protein loading. Equal volumes of heart homogenate were added to Laemmli lysis buffer (Sigma) containing 5% β-mercaptoethanol and were denatured by heating to 80–90°C for 10 min. Baseline samples were assessed for phosphorylated-Akt (Ser-473) (Akt-P), total-Akt (Akt-T), PGC-1α, and catalase expression. IPC samples were assessed for phosphorylated-Akt (Ser-473) and total-Akt using WB analysis as described in detail previously.12 Akt-P, Akt-T, and catalase antibodies were purchased from Cell Signaling, UK, PGC-1α was purchased from Santa Cruz Biotechnology and α-tubulin (used as a loading control) purchased from Abcam, UK.
2.4. Measurement of free radicals in heart tissue
Intracellular reactive oxygen species (ROS) and reactive nitrogen species (RNS) are well-known molecules that play a vital role in disrupting cellular signalling during oxidative stress.13 Experiments were performed using the OxiSelect in vitro ROS/RNS Assay Kit (Cell Bio Labs) to measure total free radical presence in samples from 3- and 18-month-old GK and Wistar rat hearts. The assay used a specific fluorogenic, ROS/RNS probe dichlorodihydrofluorescin DiOxyQ (DCFH-DiOxyQ), which reacted with free radicals within the samples including hydrogen peroxide (H2O2), peroxyl radical (ROO·), nitric oxide (NO), and peroxynitrite anion (ONOO·). Fluorescence was proportional to the concentration of ROS/RNS within the samples. Relative fluorescence was measured using a 96-well plate reader FLUOstar Omega (BMG LabTech) at 480 nm excitation/530 nm emission. Unknown samples were compared against a H2O2 or DCF standard (for RNS).
2.5. Electron microscopy
For electron microscopy (EM) visualization of mitochondria in the aging and/or diabetes, hearts from the 3- and 18-month-old Wistar and GK rats were collected at baseline (following 2 min on the perfusion apparatus to clear blood) and immersed in EM fixative (n = 4 per group). To analyse mitochondrial appearance, the heart samples were coded and two ultrathin sections from each heart were prepared by a blinded operator. The sections were viewed with a Joel 1010 transition electron microscope (Joel Ltd, Warwickshire, UK). Between 6 and 8 electron micrograph photos of interfibrillar mitochondria (IFM) were taken from each section. Subsequently, the codes were revealed and the change in patterns analysed.
2.6. Statistical analysis
Values are presented as mean ± standard error mean (SEM). For statistical comparison between two groups, Student's t-test was used; for comparison of more than two groups, data were analysed by one-way analysis of variance (ANOVA), followed by a Tukey post hoc comparison using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences within the data were considered statistically significant when P < 0.05.
3. Results
3.1. Blood glucose and HbA1c
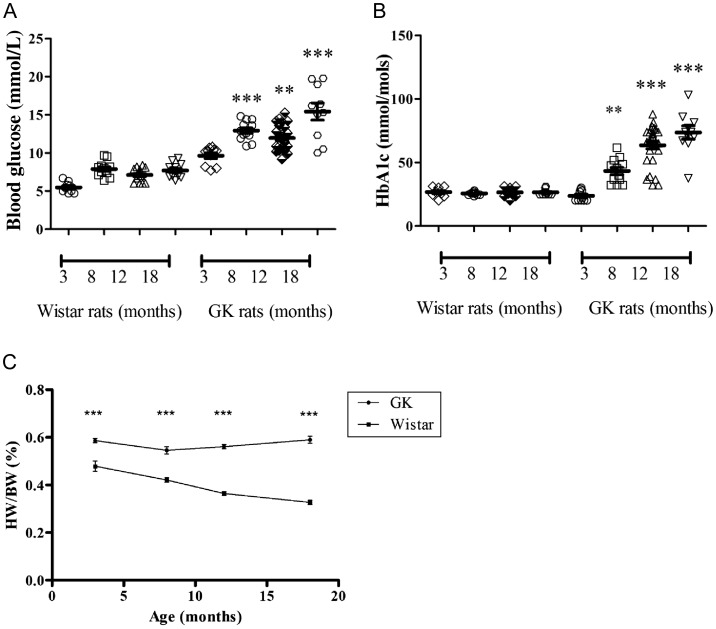
Clinically, patients are considered diabetic with HbA1c reading of >48 mmol/mol. However, due to rapidly expanding blood volumes in young growing animals, the HbA1C fraction may be kept ‘artificially’ low by the constant expansion of red cell numbers. Therefore, in our study, the diabetic status was considered at HbA1c values of >40 mmol/mol, a status achieved at approximately 8 months of age in the GK rat. Blood glucose and HbA1c levels increased with age in the GK rat (P < 0.05). In the 3-month age group, blood glucose levels were within diabetic range (9.6 ± 0.4 mmol/L); however, HbA1c was within the non-diabetic threshold (23.7 mmol/mol). GK rats had higher blood glucose and HbA1c levels than their counterpart Wistars, with the exception of the 3-month GK group having similar HbA1c levels to the 3-month Wistar group. Data are summarized in Figure 2A and B.
Figure 2.
Biological and phenotypic changes in the aging and diabetic heart (A). Fasting blood glucose levels (Accu-chek system, Roche) and (B) HbA1c (A1C now+ test kit. Bayer, UK) were taken at least 48 h prior to excision of the heart. Data are shown as mean ± SEM, n ≥ 10. **P < 0.005, ***P < 0.001 vs. 3-month GK measurements. One-way ANOVA and Tukey's post hoc analysis were used for statistical analysis between all groups. (C) Heart/body weight ratio (HW/BW%) was calculated following the experimental investigations. Data were analysed using Pearson's regression analysis.
3.2. Phenotypic changes associated with age and diabetes
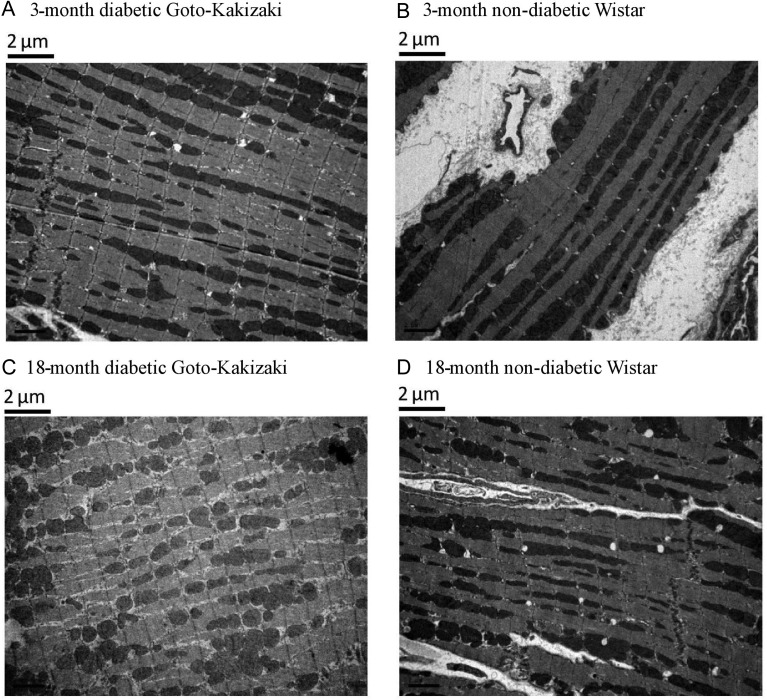
The diabetic GK rats were lighter than the non-diabetic Wistar rats in all age groups; however, their heart/body weight ratio (HW/BW%) was significantly greater (P < 0.001). HW/BW% decreased significantly in the Wistar rats over time (Pearson R2 0.97; P = 0.013), driven by a large increase in body mass; this trend was absent in the GK rats (Pearson R2 0.03; P = 0.82), whose body mass stayed relatively constant after the age of 8 months (Figure 2C). These parameters are also summarized in Table 1. More in-depth investigations using EM showed that the appearance and the organization of mitochondria in the aged, diabetic heart are different when compared with the young pre-diabetic rat heart. An IFM in the 3-month-old, pre-diabetic heart appeared organized and more elongated, whereas in the aged, diabetic heart (18 month old) the mitochondria were unorganized and more spherical in shape. The IFM in the 18-month-old Wistar heart appeared less organized than the younger age groups; however, more organized than the old diabetic heart. Representative pictures are shown in Figure 3.
Table 1.
Summary of physiological parameters.
| 3 months |
8 months |
12 months |
18 months |
|||||
|---|---|---|---|---|---|---|---|---|
| Wistar | GK | Wistar | GK | Wistar | GK | Wistar | GK | |
| Heart weight (g) | 2.3 ± 0.5 | 1.6 ± 0.1 | 1.9 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.3 | 2.4 ± 0.2 | 2.1 ± 0.3 | 2.6 ± 0.3 |
| Body mass (g) | 478.9 ± 83 | 276.8 ± 16.1 | 457.6 ± 33.2 | 409.5 ± 32.8 | 592.8 ± 82 | 435.8 ± 37.4 | 642.2 ± 63.6 | 450.6 ± 37.4 |
| Heart/body % | 0.521 | 0.586 | 0.420 | 0.542* | 0.362 | 0.559* | 0.327 | 0.587* |
Body mass was recorded prior to excision of the heart. Heart mass was measured following isolated Langendorff heart preparation. Data are represented as mean ± SEM, n ≥ 18.
*P < 0.05 vs. counterpart Wistar at each age group. One-way ANOVA was used to determine statistical significance.
Figure 3.
Mitochondrial organization is compromised in the aged, diabetic heart. Basal samples demonstrating mitochondrial appearance and organization within the aging and aging-diabetic hearts using EM. In the 3-month GK heart and 3-month Wistar heart, mitochondria appear elongated and organized along the interfibrillar bands of the cell (A and B). In comparison, in the 18-month GK hearts, a loss of mitochondrial organization and more spherical-shaped mitochondria is seen (C). In the 18-month Wistar heart, mitochondrial is still organized and elongated but to a lesser extent than the younger groups (D).
3.3. Age and diabetes contribute to susceptibility to infarction
We compared infarction that developed after 20 and 35 min of ischaemia followed by reperfusion. In our model, 20 min regional ischaemia is not sufficient to develop a significant infarct in normoglycaemic hearts. In fact, this model is often used to investigate stunning14 and not cell death.
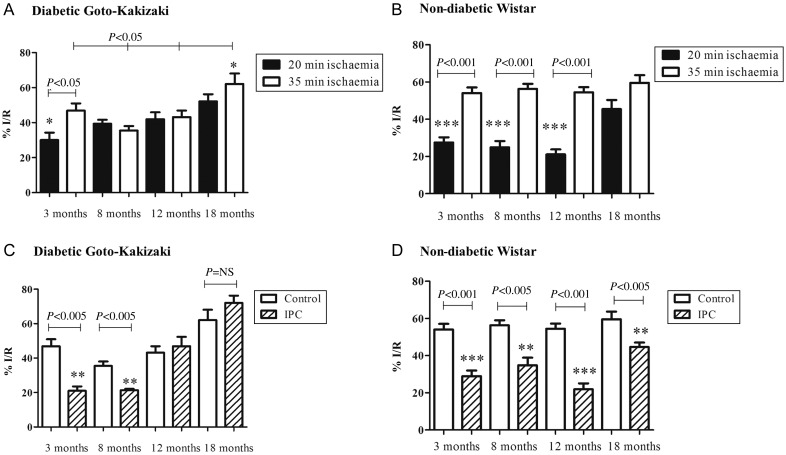
The ‘pre-diabetic’ 3-month GK rat hearts were less vulnerable to a 20 min ischaemic insult (I/R%: 30 ± 4.3) than to a longer 35 min ischaemic insult (I/R%: 46.6 ± 4.1). However, the 8-month-old GK rat heart following both 20 and 35 min ischaemia developed a similar infarct (I/R%; 35.5 ± 2.5 vs. 39.4 ± 2.3, P = NS). Similar data were obtained in the 12- and 18-month age groups, which developed comparable infarct sizes following both 20 and 35 min ischaemia: I/R% was 43.2 ± 3.7 (20 min) vs. 42.0 ± 3.9 (35 min) (P = NS); I/R% 55.5 ± 4.7 (20 min) vs. 62.1 ± 6.0 (35 min), (P = NS). Interestingly, at the oldest age, the infarct sizes were significantly greater compared with the younger age groups (39.4 ± 2.3%, 42.0 ± 3.9, vs. 62.1 ± 6.0, P < 0.005). Data are summarized in Figure 4A. In contrast, the non-diabetic Wistar rat hearts subjected to 20 min of ischaemia in 3-, 8-, and 12-month age groups developed significantly less damage, compared with 35 min ischaemia. However, in the 18-month group, there is no significant difference in the infarction following 20 or 35 min ischaemic insult. Data are summarized in Figure 4B. These data seem to indicate that the age-induced susceptibility to infarction is manifested earlier in the diabetic rats.
Figure 4.
The susceptibility to sub-lethal ischaemia and the effectiveness of IPC on infarct size in the setting of aging and diabetes. Infarct size is expressed as the ischaemic volume within the area at risk of the left ventricle (I/R%). Hearts were either stabilized for 40 min and subjected to 20 min (black bars) or 35 min (white bars) of coronary artery occlusion followed by 60 min reperfusion or subjected to three cycles of 5 min ischaemia/reperfusion, then subjected or 35 min of coronary artery occlusion followed by 60 min reperfusion (striped bars). The diabetic GK rat heart (A) exhibits an increased susceptibility to 20 min ischaemia between 8 and 18 months. Whereas the youngest GK group along with the non-diabetic Wistar rat hearts (B) are not as susceptible to shorter ischaemia. The young, diabetic GK rat hearts are still amenable to protection by IPC, however as the diabetic heart ages, IPC is no longer effective and is in fact detrimental at 18 months (C). Whereas the non-diabetic Wistar rat heart is amenable to protection by three cycles of IPC, though the effectiveness decreases with age (D) Data are shown as mean ± SEM, n ≥ 6. One-way ANOVA and Tukey's post hoc analysis were used for statistical analysis between all groups.
3.4. Impairment of cardiac function in the aging, diabetic heart
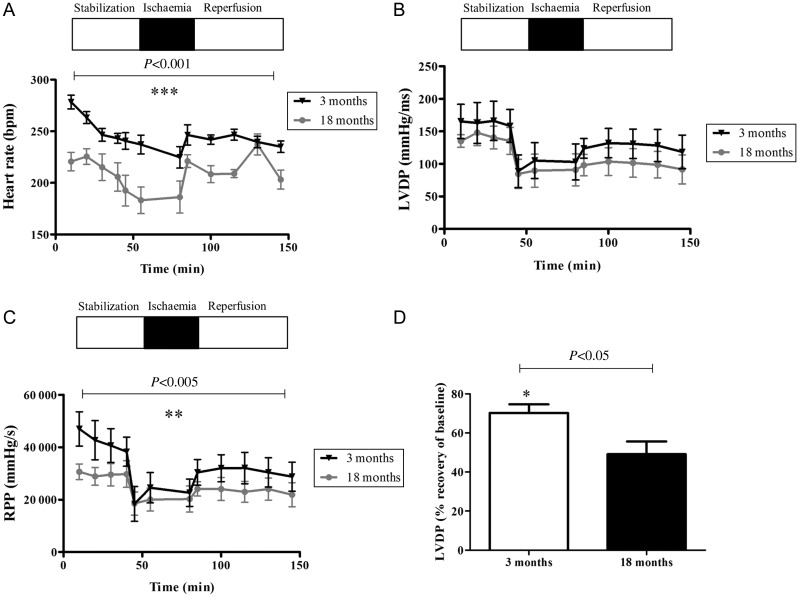
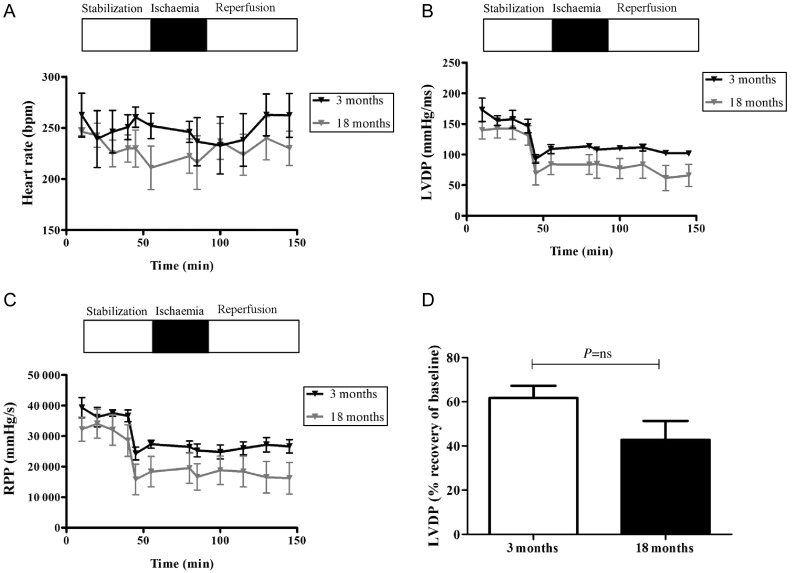
Functional parameters of the isolated hearts were monitored throughout the experimental protocol, but for clarity purposes, only the 3- and 18-month GK and Wistar groups are shown. The 18-month diabetic group had a significantly lower HR and LVDP, leading to a reduced RPP output during stabilization compared with the 3-month diabetic group. At the end of reperfusion, LVDP in the 3-month group recovered to 70% of the baseline compared with only 49% recovery in the 18-month group. Data are summarized in Figure 5. Interestingly, in the non-diabetic group, no significant differences in cardiac function were noted between 3 and 18 months. Data are summarized in Figure 6.
Figure 5.
Cardiac function assessment in the aging, diabetic heart. The old, diabetic GK rat hearts have decreased resting cardiac function and diminished ability to recover in response to ischaemia/reperfusion. (A) HR, (B) LVDP, and (C) RPP were calculated as HR × LVDP as a measure of cardiac workload. (D) Recovery of LVDP (% resting LVDP/end of reperfusion LVDP). Data are shown as mean ± SEM, n ≥ 6. Student's t-test was used for statistical analysis between 3- and 18-month groups.
Figure 6.
Cardiac function assessment in the aging, non-diabetic heart. The old, non-diabetic Wistar rat hearts exhibit no significant differences in cardiac function compared with young, non-diabetic hearts in response to ischaemia/reperfusion. (A) HR, (B) LVDP, and (C) RPP were calculated as HR × LVDP as a measure of cardiac workload. (D) Recovery of LVDP (% resting LVDP/end of reperfusion LVDP). Data are shown as mean ± SEM, n ≥ 6. Student's t-test was used for statistical analysis between 3- and 18-month groups.
3.5. Cardioprotective effect of ischaemic preconditioning is lost in the aged, diabetic heart
In both rat strains at 3 and 8 months of age, the ischaemic preconditioning (IPC) protocol we used was successful in limiting the damage caused by 35 min ischaemia. IPC caused a 46 and 38% reduction in infarct size in 3- and 8-month Wistar (non-diabetic) hearts and a 55 and 40% reduction in 3- and 8-month GK diabetic hearts when compared with control groups (P < 0.005 for all groups vs. their respective controls). At 12 and 18 months of age, the non-diabetic heart was protected by IPC, albeit to a less extent in the 18-month group (38.2 vs. 25% reduction in infarct size). However, the diabetic heart was not amenable to preconditioning protection in either the 12- or 18-month groups (I/R% for the 12-month group, 42.0 ± 3.9 vs. 46.9 ± 5.5 and I/R% for the 18-month group, 62.1 ± 5.9 vs. 69.1 ± 4.3, P = NS; Figure 4C and D).
3.6. Age alters the expression and activation of key pro-survival signalling proteins in the diabetic heart
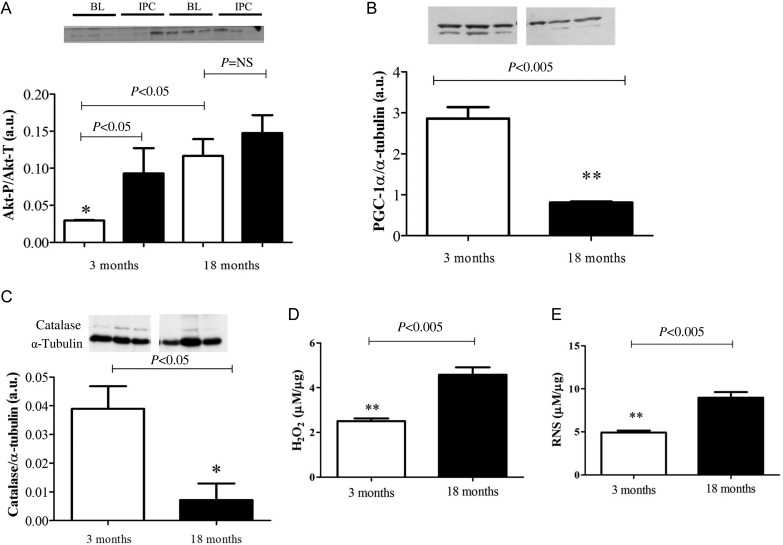
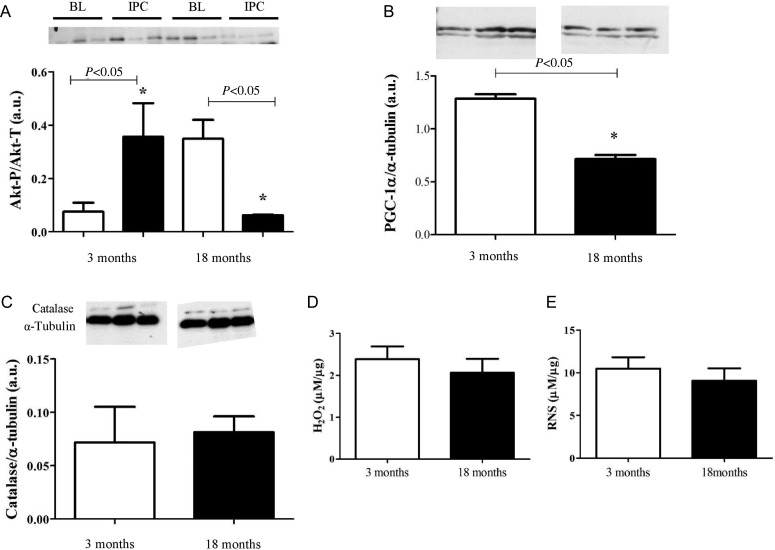
Akt is essential for cardioprotection via IPC. IPC induces Akt phosphorylation,5 therefore activation, and in this state the enzyme is cardioprotective via its multiple anti-apoptotic actions15 and anti-necrotic actions.16 Surprisingly, a progressive rise in baseline Akt phosphorylation was seen in the aging GK rat heart, with a significant increase observed at 18 months compared with 3 months (Arbitrary units (A.U.): 1.3 ± 0.2 vs. 0.4 ± 0.3, P < 0.05). Furthermore, in the 18-month group, IPC failed to increase Akt-P above baseline levels (Figure 7A). Downstream to Akt-P, an age-related decrease was seen in the expression of PGC-1α (A.U.: 2.9 ± 0.3 at 3 months vs. 0.8 ± 0.01 at 18 months). Moreover, the expression of catalase, an anti-oxidant enzyme, which can be transcriptionally regulated by PGC-1α,17 and catalyses the breakdown of H2O2 was extremely low in the 18-month age group (Figure 7B and C). Interestingly, in the 18-month non-diabetic heart, Akt phosphorylation was also increased compared with the 3-month group. However, following IPC, phosphorylation was significantly lower than baseline. The expression of PGC-1α was reduced in these hearts, but not to the same extent as the diabetic heart. Moreover, no difference was seen in catalase levels (Figure 8).
Figure 7.
Age alters the expression and activation of key signalling proteins in the diabetic heart (A). Phosphorylation of Akt increases with age in the diabetic heart, with no further activation following IPC. White bars represent samples taken at baseline (BL), black bars represent samples taken following three cycles of IPC. Values determined by densitometry of Akt phosphorylation (Akt-P) corrected to respective α-tubulin over the relative expression of Akt-total (Akt-T) corrected to respective α-tubulin. Data are shown as mean ± SEM, n = 3 per group. One-way ANOVA and Tukey's post hoc analysis were used for statistical analysis. (B) PGC-1α expression and (C) catalase expression decreases with age in the diabetic heart. (D) Hydrogen peroxide (H2O2) and (E) RNS increase with age in the diabetic heart. Data are shown as mean ± SEM, n = 4 per group. Student's t-test was used for statistical analysis.
Figure 8.
Key signalling proteins in the non-diabetic heart are differentially expressed and activated in the aging, non-diabetic heart. (A) Phosphorylation of Akt increases with age in the non-diabetic heart, with a decrease in activation following IPC. White bars represent samples taken at baseline (BL), black bars represent samples taken following three cycles of IPC. Values determined by densitometry of Akt phosphorylation (Akt-P) corrected to respective α-tubulin over the relative expression of Akt-total (Akt-T) corrected to respective α-tubulin. Data are shown as mean ± SEM, n = 3 per group. One-way ANOVA and Tukey's post hoc analysis were used for statistical analysis. (B) PGC-1α expression decreased with age. However, no changes in catalase expression or hydrogen peroxide (H2O2) or RNS were seen with age in the non-diabetic heart (C–E). Data are shown as mean ± SEM, n = 4 per group. Student's t-test was used for statistical analysis.
3.7. Oxidative stress is increased in the aged, diabetic heart
Oxidative stress caused by an excessive accumulation of ROS or RNS are implemented in myocardial injury.13 We found that basal levels of H2O2 and RNS were significantly increased in the 18-month diabetic heart compared with the 3-month diabetic heart (Figure 7D and E), whereas no significant changes were noted in the aging, non-diabetic heart (Figure 8D and E).
4. Discussion
Despite the clinical data suggesting that the diabetic heart is more susceptible to acute ischaemic damage,18 the animal data have been conflicting with experimental studies showing more,19–22 equal,23–25 or less6,26–29 sensitivity to this injury. These controversies were the subject of discussions and reviews; however, there is no general consensus in this matter as yet.3,30 In our opinion, one of the main contributors to this confusion could be due to the choice of the experimental models. As far as we know, this is the first study which investigated the myocardial IRI and cardioprotection in a model exhibiting concomitantly two aggravating factors: diabetes and age. In the GK rat model, as in humans, diabetes develops in time, becoming further established with age;31 however, there has been limited investigations of myocardial IRI in this setting. In parallel with the clinical setting of diabetes, our data demonstrate that the older diabetic hearts were more susceptible to ischaemia: infarct size was increased in response to a shorter ischaemic insult in diabetic adult and aged hearts, and the older hearts developed larger infarcts compared with the non-diabetic hearts. Furthermore, this is the first study that has shown that an increased stimulus of IPC previously shown to protect the young diabetic and aged non-diabetic heart is unsuccessful in a producing a cardioprotective effect in a model exhibiting both old age and diabetes. We found that basal phosphorylation of the pro-survival kinase, Akt, was up-regulated in these old, diabetic hearts, and could not be further activated following IPC. These changes seem to be attributed to aging in combination with diabetes following the observations that the aging, non-diabetic heart exhibited a different response to IRI and IPC protocols, alongside dissimilar protein expression and activation.
It is largely accepted that Akt activation by phosphorylation is strongly implicated in the cardioprotective response to IRI,32 and that this signalling cascade leads to inhibition of mitochondrial permeability transition pore opening,6 reducing the necrotic effects of reperfusion. However, it is clear that the increase in baseline Akt-P we saw within the aging, diabetic heart did not serve a cardioprotective role in this setting. Interestingly, the aged, non-diabetic heart also demonstrated an increased phosphorylation of Akt at baseline compared with its 3-month counterparts. It is worth pointing out that, in spite of the fact that acute Akt phosphorylation could play a crucial role in the survival of a stressed cell,15 it has been shown that a chronic increase in basal phosphorylation of Akt may have detrimental effects. One of these effects was described by Hunter et al.33 who suggested that a chronic increase in Akt phosphorylation in aged, non-diabetic rats could induce hypertrophy. In this investigation, the HW/BW % did not increase with age in the GK rat (Table 1, Figure 2C). Hypertrophy has been demonstrated to increase the susceptibility to IRI;34 however, the young and aged diabetic hearts exhibit diverse responses to IPC and shortened ischaemia with similar HW/BW%. This suggests that hypertrophy alone is not responsible for these differences in this setting.
Another detrimental effect of chronic Akt phosphorylation may be the indirect down-regulation of the transcriptional co-activator PGC-1α.35 PGC-1α is a transcriptional co-activator and a key regulator of energy metabolism and mitochondrial biogenesis36 and also regulates ROS detoxification systems.37 Efficient control of these systems plays a vital role in increasing the tolerance of the heart to IRI; regulatory proteins such as PGC-1α allow the mitochondria to maintain its energetics and to reduce oxidative stress when subjected to IRI.38 Interestingly, fundamental studies by Duncan39 suggest that alterations in PGC-1α activity in the setting of diabetes could have detrimental consequences, leading to an increased susceptibility to cardiovascular disease. Our results showed an age-related decrease in PGC-1α expression in diabetic hearts, with 18-month old GK rats expressing significantly less PGC-1α compared with their 3-month counterparts. Moreover, we saw a decreased functional capacity of the heart in the 18-month-old GK rat, a possible consequence of decreased mitochondrial energetics in the heart driven by decreased PGC-1α expression. In comparison, the increased phosphorylation of Akt in the aged, non-diabetic heart was not associated with changes in these downstream pathways, suggesting that this may be a diabetes-associated phenomenon.
It has been demonstrated by other groups that Akt phosphorylation can reduce the activation of PGC-1α, directly by phosphorylating PGC-1α at Ser57035 and indirectly by inactivation of FoxO1, a stimulator of the PGC-1α promoter.40 Interestingly, Tonks et al.41 demonstrated that Akt phosphorylation was impaired in insulin-resistant human muscle, and that hyperinsulinaemia inactivated FoxO1 preventing its transcriptional activity. In addition, Akt has close regulatory links with other members of the FoxO family such as FoxO3. Olmos et al.17 showed that the transcriptional regulator Foxo3b and the co-activator PGC-1α interact directly to drive the expression of anti-oxidant genes such as catalase supporting the down-regulation of catalase we observed in the aged, diabetic heart. It is possible that the less organized, almost spherical intrafibrillar mitochondria we saw in these hearts, is a consequence of decreased PGC-1α and catalase expression. This study focused on chronic modifications related to baseline changes in Akt phosphorylation; therefore, the expression levels of PGC-1α were not assessed following an acute IPC stimulus. One could speculate that the altered expression of PGC-1α could also result in the lack of protection seen by IPC. Interestingly, PGC-1α expression has been implicated in the protective effect of late or delayed preconditioning.42
A main process that contributes to the damage caused by IRI is the rapid production of ROS upon reperfusion of the myocardium.43 The availability of endogenous anti-oxidants, such as catalase, to counterbalance this release of ROS, is crucial to reduce the myocardial damage.44 Diabetes is accompanied by an increased production of intracellular ROS,45 and a compensatory increase in catalase.46 However, it is suggested that the increase in the level of the anti-oxidant enzymes is likely to be insufficient to completely counteract ROS overproduction.46 Interestingly, our results suggest that in young diabetic hearts, where diabetes is not fully established the level of H2O2 and RNS are low and catalase level may be sufficient to counteract oxidative stress. However, it appears that this anti-oxidant defence is lost with age, possibly due to the down-regulation of catalase via high Akt-P and low PGC-1α levels.
Having confirmed that differences exist between GKs' and Wistars' susceptibility to IRI across all ages, we moved on to investigate if the endogenous cardioprotective potential is also affected by age in the diabetic heart. Previously, it was demonstrated that the young, diabetic heart is still amenable to IPC protection, but the threshold is raised, therefore an increased stimulus is required.47 Yellon and colleagues6 found that this increased IPC stimulus was required to achieve an essential level of Akt phosphorylation necessary to mediate cardioprotection. Similar to the studies examining cardioprotection in diabetes, conditioning the aged heart has also remained a controversial issue. Yellon and colleagues48 showed that the Sprague-Dawley aging heart required an increased IPC stimulus to elicit cardioprotection. When combining the two co-morbidities, age and diabetes, IPC protection cannot be achieved using a similar protocol. One could argue that an even higher IPC stimulus is required for protection to be elicited in these aged, diabetic hearts. However, our data showed that the 18-month diabetic hearts undergoing three cycles of IPC exhibited more damage following ischaemia than their respective control; therefore, it is unlikely that an increased stimulus would be effective. It seems that the initial increased threshold for cardioprotection observed in young diabetic hearts evolves with age towards the complete loss of this ability. We found that the basal level of phosphorylated—therefore activated, Akt was increased with age and no further increment was observed following a preconditioning protocol in aged, diabetic hearts. Interestingly, a similar increase in phosphorylated-Akt had been linked to the aged heart.49 We also demonstrate an age-related increase in phosphorylated-Akt in the aged, non-diabetic heart. Following an IPC stimulus, however, activation of Akt was surprisingly decreased. Hearts from the 18-month group were still protected to some degree against IRI, suggesting that, in the absence of diabetes, an alternative pathway, independent of Akt, could be activated in response to an IPC stimulus that can elicit some but not maximal protection. The upstream cause of chronic Akt phosphorylation was not investigated in this study; however, it appears that this impairment caused by insulin resistance41 leads to detrimental outcomes, augmented in combination with old age. It is noticeable, in our opinion, that old age and diabetes associate with impact on signalling pathways that mediate IPC.
We are aware that there are many possible mechanisms responsible for the loss of cardioprotection in the aging,50 as well as in the diabetic myocardium.51 From the network of intracellular signalling pathways that could affect the myocardial susceptibility to infarction, we focused our study on Akt which is a key regulator of survival in general and of myocardial preservation following IRI , in particular. The GK rat model is representative of a lean, normotensive model of diabetes, and therefore does not provide a platform to assess the potential implications of other co-morbidities such as hypertension34 or hypercholesterolaemia/hyperlipidaemia,52 previously shown to impact on IRI. The non-diabetic, Wistar rat accumulates body weight with age, but does not appear to develop severe hyperglycaemia. The subtle differences between the aged Wistar and GK rats may suggest that diabetes-independent factors also contribute to damaged caused by IRI.
In conclusion, we report an increased susceptibility of the aged, diabetic rat heart to IRI and the inability of these hearts to be preconditioned against this insult. We suggest that one of the major causes of these negative outcomes is the chronically augmented phosphorylation of Akt in the aged, diabetic myocardium, which may lead to detrimental downstream effects such as the down-regulation of PGC-1α and catalase, leading to compromised defence against oxidative stress. Moreover, there is no biochemical reserve for sufficient additional Akt phosphorylation to be activated by preconditioning, and hence this form of cardioprotection is lost. Further experimental exploration of signalling deficits in the aged, diabetic heart could support the development of more clinically applicable conditioning strategies, such as pharmacological, remote, or post-conditioning against IRI.
Funding
This work was supported by the British Heart Foundation (program grant RG/08/015/26411 and FS/09/058/27987). This work was undertaken at University College London Hospital/University College London (UCLH/UCL) who received a proportion of funding from the Department of Health's National Institute of Health Research (NIHR) Biomedical Research Centres funding scheme to which D.M.Y. is a senior inverstigator.
Acknowledgements
The authors thank Louise Casson and her colleagues at the central unit of Biological Services Unit, UCL, London, for the help in maintaining the animal colonies and blood testing. We also thank Mark Turmaine, UCL, for the preparation of samples for electron microscopy imaging.
Conflict of interest: none declared.
References
- 1.Marso SP, Miller T, Rutherford BD, Gibbons RJ, Qureshi M, Kalynych A, et al. Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD Trial) Am J Cardiol. 2007;100:206–210. doi: 10.1016/j.amjcard.2007.02.080. doi:10.1016/j.amjcard.2007.02.080. [DOI] [PubMed] [Google Scholar]
- 2.Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol. 2011;57:9–17. doi: 10.1016/j.jacc.2010.08.623. doi:10.1016/j.jacc.2010.08.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittington HJ, Babu GG, Mocanu MM, Yellon DM, Hausenloy DJ. The diabetic heart: too sweet for its own good? Cardiol Res Pract. 2012;2012:845698. doi: 10.1155/2012/845698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. doi:10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. doi:10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 6.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. doi:10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 7.Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM. Preconditioning the diabetic human myocardium. J Cell Mol Med. 2010;14:1740–1746. doi: 10.1111/j.1582-4934.2009.00796.x. doi:10.1111/j.1582-4934.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazel R, Fang J, Kline-Rogers E, Smith DE, Eagle KA, Mukherjee D. Prognostic value of elevated biomarkers in diabetic and non-diabetic patients admitted for acute coronary syndromes. Heart. 2005;91:388–390. doi: 10.1136/hrt.2003.032797. doi:10.1136/hrt.2003.032797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22:359–370. doi: 10.1111/j.1464-5491.2005.01499.x. doi:10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451–472. [PubMed] [Google Scholar]
- 11.Shamaei-Tousi A, Halcox JP, Henderson B. Stressing the obvious? Cell stress and cell stress proteins in cardiovascular disease. Cardiovasc Res. 2007;74:19–28. doi: 10.1016/j.cardiores.2006.10.025. doi:10.1016/j.cardiores.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther. 2012;27:5–16. doi: 10.1007/s10557-012-6425-x. [DOI] [PubMed] [Google Scholar]
- 13.Ansley DM, Wang B. Oxidative stress and myocardial injury in the diabetic heart. J Pathol. 2013;229:232–241. doi: 10.1002/path.4113. doi:10.1002/path.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreyra AE, Conway RS, Wilson AC, Chen WH, Schmidling MJ, Kostis JB. Attenuation of myocardial stunning in isolated rat hearts by a 21-aminosteroid lazaroid (U74389G) J Cardiovasc Pharmacol. 1996;28:659–664. doi: 10.1097/00005344-199611000-00008. doi:10.1097/00005344-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. doi:10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Miura T, Tanno M. The mPTP and its regulatory proteins: final common targets of signalling pathways for protection against necrosis. Cardiovasc Res. 2012;94:181–189. doi: 10.1093/cvr/cvr302. doi:10.1093/cvr/cvr302. [DOI] [PubMed] [Google Scholar]
- 17.Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, et al. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. doi:10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J. 2012;14(Suppl. B):B4–B13. [Google Scholar]
- 19.Marfella R, D'Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, et al. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45:1172–1181. doi: 10.1007/s00125-002-0882-x. doi:10.1007/s00125-002-0882-x. [DOI] [PubMed] [Google Scholar]
- 20.Kersten JR, Toller WG, Gross ER, Pagel PS, Warltier DC. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol. 2000;278:H1218–H1224. doi: 10.1152/ajpheart.2000.278.4.H1218. [DOI] [PubMed] [Google Scholar]
- 21.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, et al. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. doi:10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 22.Ebel D, Mullenheim J, Frassdorf J, Heinen A, Huhn R, Bohlen T, et al. Effect of acute hyperglycaemia and diabetes mellitus with and without short-term insulin treatment on myocardial ischaemic late preconditioning in the rabbit heart in vivo. Pflugers Arch. 2003;446:175–182. doi: 10.1007/s00424-003-1051-x. [DOI] [PubMed] [Google Scholar]
- 23.Bulhak AA, Jung C, Ostenson CG, Lundberg JO, Sjoquist PO, Pernow J. PPAR-alpha activation protects the type 2 diabetic myocardium against ischemia-reperfusion injury: involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol Heart Circ Physiol. 2009;296:H719–H727. doi: 10.1152/ajpheart.00394.2008. doi:10.1152/ajpheart.00394.2008. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto S, Cho S, Tosaka S, Ureshino H, Maekawa T, Hara T, et al. Pharmacological preconditioning in type 2 diabetic rat hearts: the roles of mitochondrial ATP-sensitive potassium channels and the phosphatidylinositol 3-kinase-Akt pathway. Cardiovasc Drugs Ther. 2009;23:263–270. doi: 10.1007/s10557-009-6184-5. doi:10.1007/s10557-009-6184-5. [DOI] [PubMed] [Google Scholar]
- 25.Ravingerova T, Neckar J, Kolar F. Ischemic tolerance of rat hearts in acute and chronic phases of experimental diabetes. Mol Cell Biochem. 2003;249:167–174. doi: 10.1023/a:1024751109196. doi:10.1023/A:1024751109196. [DOI] [PubMed] [Google Scholar]
- 26.Ooie T, Takahashi N, Nawata T, Arikawa M, Yamanaka K, Kajimoto M, et al. Ischemia-induced translocation of protein kinase C-epsilon mediates cardioprotection in the streptozotocin-induced diabetic rat. Circ J. 2003;67:955–961. doi: 10.1253/circj.67.955. doi:10.1253/circj.67.955. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira PJ, Rolo AP, Seica R, Palmeira CM, Santos MS, Moreno AJ. Decreased susceptibility of heart mitochondria from diabetic GK rats to mitochondrial permeability transition induced by calcium phosphate. Biosci Rep. 2001;21:45–53. doi: 10.1023/a:1010482017540. doi:10.1023/A:1010482017540. [DOI] [PubMed] [Google Scholar]
- 28.Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, et al. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia. 2004;47:1716–1721. doi: 10.1007/s00125-004-1514-4. doi:10.1007/s00125-004-1514-4. [DOI] [PubMed] [Google Scholar]
- 29.Hadour G, Ferrera R, Sebbag L, Forrat R, Delaye J, de Lorgeril M. Improved myocardial tolerance to ischaemia in the diabetic rabbit. J Mol Cell Cardiol. 1998;30:1869–1875. doi: 10.1006/jmcc.1998.0751. doi:10.1006/jmcc.1998.0751. [DOI] [PubMed] [Google Scholar]
- 30.Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res. 1997;34:104–112. doi: 10.1016/s0008-6363(97)00018-7. doi:10.1016/S0008-6363(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 31.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl. 2):S151–S156. doi: 10.2337/dc09-S301. doi:10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. doi:10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol. 2007;292:R800–R809. doi: 10.1152/ajpregu.00374.2006. doi:10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- 34.Yano T, Miki T, Tanno M, Kuno A, Itoh T, Takada A, et al. Hypertensive hypertrophied myocardium is vulnerable to infarction and refractory to erythropoietin-induced protection. Hypertension. 2011;57:110–115. doi: 10.1161/HYPERTENSIONAHA.110.158469. doi:10.1161/HYPERTENSIONAHA.110.158469. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. doi:10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884S–8890. doi: 10.3945/ajcn.110.001917. doi:10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. doi:10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 38.McLeod CJ, Pagel I, Sack MN. The mitochondrial biogenesis regulatory program in cardiac adaptation to ischemia–a putative target for therapeutic intervention. Trends Cardiovasc Med. 2005;15:118–123. doi: 10.1016/j.tcm.2005.05.001. doi:10.1016/j.tcm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Duncan JG. Peroxisome proliferator activated receptor-alpha (PPARalpha) and PPAR gamma coactivator-1alpha (PGC-1alpha) regulation of cardiac metabolism in diabetes. Pediatr Cardiol. 2011;32:323–328. doi: 10.1007/s00246-011-9889-8. doi:10.1007/s00246-011-9889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Southgate RJ, Bruce CR, Carey AL, Steinberg GR, Walder K, Monks R, et al. PGC-1alpha gene expression is down-regulated by Akt- mediated phosphorylation and nuclear exclusion of FoxO1 in insulin-stimulated skeletal muscle. FASEB J. 2005;19:2072–2074. doi: 10.1096/fj.05-3993fje. [DOI] [PubMed] [Google Scholar]
- 41.Tonks KT, Ng Y, Miller S, Coster AC, Samocha-Bonet D, Iseli TJ, et al. Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia. 2013;56:875–885. doi: 10.1007/s00125-012-2811-y. [DOI] [PubMed] [Google Scholar]
- 42.McLeod CJ, Jeyabalan AP, Minners JO, Clevenger R, Hoyt RF, Jr, Sack MN. Delayed ischemic preconditioning activates nuclear-encoded electron-transfer-chain gene expression in parallel with enhanced postanoxic mitochondrial respiratory recovery. Circulation. 2004;110:534–539. doi: 10.1161/01.CIR.0000136997.53612.6C. doi:10.1161/01.CIR.0000136997.53612.6C. [DOI] [PubMed] [Google Scholar]
- 43.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. doi:10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 44.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000;47:446–456. doi: 10.1016/s0008-6363(00)00078-x. doi:10.1016/S0008-6363(00)00078-X. [DOI] [PubMed] [Google Scholar]
- 45.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. doi:10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qujeq D, Rezvani T. Catalase (antioxidant enzyme) activity in streptozotocin-induced diabetic rats. Int J Diabetes Metab. 2007;15:22–24. [Google Scholar]
- 47.Bouchard JF, Lamontagne D. Protection afforded by preconditioning to the diabetic heart against ischaemic injury. Cardiovasc Res. 1998;37:82–90. doi: 10.1016/s0008-6363(97)00234-4. doi:10.1016/S0008-6363(97)00234-4. [DOI] [PubMed] [Google Scholar]
- 48.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281:H1630–H1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 49.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. doi:10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 50.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. doi:10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 51.Yin X, Zheng Y, Zhai X, Zhao X, Cai L. Diabetic inhibition of preconditioning- and postconditioning-mediated myocardial protection against ischemia/reperfusion injury. Exp Diabetes Res. 2012;2012:198048. doi: 10.1155/2012/198048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferdinandy P. Myocardial ischaemia/reperfusion injury and preconditioning: effects of hypercholesterolaemia/hyperlipidaemia. Br J Pharmacol. 2003;138:283–285. doi: 10.1038/sj.bjp.0705097. doi:10.1038/sj.bjp.0705097. [DOI] [PMC free article] [PubMed] [Google Scholar]