Abstract
Recent evidence suggests that non-selective cannabinoid receptor agonists may regulate serotonin 2A (5-HT2A) receptor neurotransmission in brain. The molecular mechanisms of this regulation are unknown but could involve cannabinoid-induced enhanced interaction between 5-HT2A and dopamine D2 (D2) receptors. Here, we present experimental evidence that Sprague-Dawley rats treated with a non-selective cannabinoid receptor agonist (CP55,940, 50μg/kg, 7days, i.p.) showed enhanced co-immunoprecipitation of 5-HT2A and D2 receptors and enhanced membrane-associated expression of D2 and 5-HT2A receptors in prefrontal cortex (PFCx). Furthermore, 5-HT2A receptor mRNA levels were increased in PFCx suggesting a cannabinoid-induced upregulation of 5-HT2A receptors. To date, two cannabinoids receptors have been found in brain, CB1 and CB2 receptors. We used selective cannabinoid agonists in a neuronal cell line to study mechanisms that could mediate this 5-HT2A receptor upregulation. We found that selective CB2 receptor agonists upregulate 5-HT2A receptors by a mechanism that seems to involve activation of Gαi G-proteins, ERK1/2, and AP-1 transcription factor. We hypothesize that the enhanced cannabinoid-induced interaction between 5-HT2A and D2 receptors and in 5-HT2A and D2 receptors protein levels in the PFCx might provide a molecular mechanism by which activation of cannabinoid receptors might be contribute to the pathophysiology of some cognitive and mood disorders.
Keywords: Serotonin, prefrontal cortex, 5-HT2A receptor, D2 receptor, G proteins, cannabis sativa
Introduction
Serotonin 2A (5-HT2A) and dopamine D2 (D2) receptors are molecular targets in the treatment of various neuropsychiatric disorders such as depression, anxiety, and schizophrenia (Carrasco and Van de Kar 2003; Celada et al. 2004; De Almeida et al. 2008; Lawford et al. 2006; Schiller et al. 2006; Weisstaub et al. 2004). For instance, the therapeutic benefits of atypical antipsychotics (which are more potent 5-HT2A receptor antagonists than D2 receptor antagonists) and antidepressants are proposed to be mediated by antagonism and subsequent desensitization of 5-HT2A and D2 receptors signaling in several brain areas, including prefrontal cortex (PFCx) (De Almeida et al. 2008; Singh et al. 2007). Noteworthy, recent evidence indicates that post-synaptically located 5-HT2A and D2 receptors can assemble into functionally interacting heteromers in PFCx (Albizu et al. 2011; Borroto-Escuela et al. 2010). Although the molecular mechanisms that regulate this 5-HT2A and D2 receptor interaction have not been clearly established, this 5-HT2A-D2 receptor complex might have a key significance in understanding the pathophysiology of several neuropsychiatric disorders and the mechanism of action of drugs used to treat them. Indeed, atypical antipsychotics target this heteromer decreasing its formation (Lukasiewicz et al. 2011)
The clinical implications of the formation of a 5-HT2A-D2 receptor complex in PFCx have not been identified. However, dimerization of 5-HT2A and D2 receptors may provide a mechanism by which these receptors might regulate each other’s activity. Indeed, activation of D2 receptors would enhance the affinity of 5-HT2A receptors to specific agonists and could modify the signaling of 5-HT2A receptors, as it has been recently suggested (Albizu et al. 2011). Specifically, recent studies reported that the activity of 5-HT2A receptors in PFCx would be synergistically enhanced by the formation of this 5-HT2A-D2 receptor complex (Borroto-Escuela et al. 2010; Fuxe et al. 2010). Therefore, it is possible that drug-treatments that modify the expression of either 5-HT2A or D2 receptors could modify the formation of this 5-HT2A-D2 receptor complex.
Recent behavioral studies suggest that chronic exposure to a non-selective cannabinoid agonist is associated with enhanced activity of 5-HT2A receptors in brain (Hill et al. 2006). It was reported that rats treated with HU-210, a non-selective cannabinoid receptor agonist, exhibited enhanced the 5-HT2A receptor mediated-head twitches (Hill et al. 2006). This behavioral test has been widely used as a model of activity of 5-HT2A receptors in PFCx (Darmani and Reeves 1996; Willins and Meltzer 1997). If exposure to cannabinoids modifies the expression of cortical 5-HT2A receptors in PFCx, they could also modify the 5-HT2A-D2 heteromer formation in this brain area. Here, we focus on determining the effect of exposure to cannabinoid agonists on the interaction between 5-HT2A and D2 receptors and the expression of 5-HT2A and D2 receptors in rat PFCx.
The biological effects of cannabinoids in brain are produced mainly through G-protein coupled cannabinoid receptors, CB1 and CB2 receptors (Howlett 2005). While CB1 receptors were initially identified in brain, early reports identified CB2 receptors only in immune cells (Barrio et al. 2011; Fribourg et al. 2011; Galiegue et al. 1995; Munro et al. 1993). However, recent studies have established the expression of CB2 receptors in normal neurons in cortex, amygdala, hypothalamus, hippocampus, etc. (Barrio et al. 2011; Fribourg et al. 2011; Garcia-Gutierrez et al. 2010; Gong et al. 2006). CB1 and CB2 receptors couple to Gαi/o G-proteins (Felder et al. 2006; Gong et al. 2006; Herkenham 1991), and would activate ERK in a protein kinase C (PKC)-dependent manner (Bouaboula et al. 1996; Onaivi et al. 2008). Here, we also used cultured cells to explore some molecular mechanisms that could contribute to the cannabinoid-induced upregulation of 5-HT2A receptors.
Our results suggest that chronic cannabinoid exposure could enhance the formation and activity of 5-HT2A-D2 receptor heteromers in rat PFCx. This could provide a molecular mechanism by which chronic use of cannabinoids might contribute to the pathophysiology of some neuropsychiatric disorders associated with dysfunction of 5-HT2A and D2 neurotransmission in brain limbic areas such as PFCx.
Materials and Methods
Drugs
(−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP 55,940), a CB1 and CB2 receptor agonist, N-(Piperidin-1-yl)-1-(2,4-dichlorophenyl)-1,4-dihydro-6-methylindeno[1,2-c]pyrazole-3-carboxamide (GP 1a), a highly selective CB2 receptor agonist; 3-(1,1-Dimethylbutyl)-1-deoxy-Δ8-tetrahydrocannabinol (JWH 133), a selective CB2 receptor agonist;[6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)-methanone (AM 630), a selective CB2 receptor antagonist; Pertussis Toxin (PTX); N,N-Dimethyl-(3R,4aR,5S,6aS,10S,10aR,10bS)-5-(acetyloxy)-3-ethenyldodecahydro-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-1H-naphtho[2,1-b]pyran-6-yl ester β-alanine hydrochloride (NKH 477), a potent activator of adenyl cyclase; 2-[1-(3-Dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF 109203X), a very potent and selective inhibitor of protein kinase c; 5,6,7,13-Tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile (Go 6967), a potent protein kinase c inhibitor; (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-1a,1b,4,4a,5,7a,7b,8,9,9a-Decahydro-4a,7b-dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-1H-cyclopropa[3,4]benz[1,2-e]azulen-9,9a-diyl butanoic acid ester (Phorbol 12,13-dibutylrate, PDBu), a protein kinase c activator; (E,E,Z,E)-3-Methyl-7-(4-methylphenyl)-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid (SR 11302), an inhibitor of activating protein-1 transcription factor activity and N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA), a highly selective CB1 receptor agonist, were purchased from Tocris (Ellisville, MO). Naphthol AS-E phosphate, a CREB inhibitor, was purchased from Sigma-Aldrich Inc. (St. Louis, MO).
Animal Experimental Protocol
Male Sprague-Dawley rats (225–275 g; Harlan Laboratories, Indianapolis, IN) were housed two per cage in a temperature-, humidity-, and light-controlled room (12 hr light/dark cycle, lights on 7:00 AM–19:00 PM). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the University of Kansas Institutional Animal Care and Use Committee (IACUC).
After arrival, the rats were allowed to acclimate to their environment for at least 4 days prior to the start of the treatment period. Eight rats were randomly assigned to each group, cage mates were assigned to the same treatment group. The body weight of each rat was recorded every other day. All solutions were made fresh before administration and rats were injected with either vehicle (Tween-80/ethanol/saline (1:1:18); 1ml/kg, i.p.) or CP 55,940 (0.05 mg/kg, i.p.) once a day for 7 days. Rats were sacrificed by decapitation 48 h after the last CP 55,940 injection. The brains were immediately removed and the PFCx was dissected and frozen in dry ice.
Co-Immunoprecipitation
Co-immunoprecipitation (co-IP) was done using the Thermo Scientific Pierce co-IP kit following manufacturer’s protocol. 5-HT2A receptor antibody was a generous gift from Dr. Nancy A. Muma and the D2 receptor antibody was purchased from Santa Cruz, CA. Briefly, 5-HT2A receptor antibody or D2 receptor antibody was first immobilized for 2 hours using AminoLink Plus coupling resin. The resin was washed and incubated with pre-cleared prefrontal cortex lysate (300 μg) from vehicle and CP 55,940 treated rats overnight. A negative control in this assay included a non-reactive resin that was also incubated with either 5-HT2A or D2 receptor antibodies. In this control, the coupling resin is not amine-reactive preventing covalent immobilization of the primary antibody onto the resin. This inactive resin was provided with the IP kit to assess non-specific binding in samples that received the same treatment as the co-IP samples, including 5-HT2A receptor antibody or D2 receptor antibody. After the overnight incubation of all the prefrontal cortex lysates from vehicle- and CP55,940-treated samples with either active or inactive resins, the resins were washed (3x) and the protein eluted using elution buffer. Samples were analyzed by Western blot using 5-HT2A receptor antibody or D2 receptor antibody. The specificity of the 5-HT2A and D2 receptor antibody has been verified in the literature (Montezinho et al. 2006; Nam and Kim 2008; Singh et al. 2007).
Western Blot
Membrane-associated proteins were isolated using the ProteoExtract™ Native Membrane Protein Extraction kit (Calbiochem, La Jolla, CA). Nuclear-associated proteins were isolated using NE-PER ® Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, IL). Samples containing 5 μg of protein were separated by sodium dodecyl-polyacrylamide gel electrophoresis containing 0.1% SDS, 12.5% acrylamide/bisacrylamide (30:0.2), 4.6 M urea, and 275 mM Tris, pH 8.7. Gels were transferred electrophoretically by semi-dry blot to nitrocellulose membranes. After incubation with a blocking buffer (phosphate buffered saline containing 0.2% casein and 0.1% Tween 20), immunodetection was performed at 4°C overnight using primary antibody. c-Fos antibody was purchased from Santa Cruz, CA. The anti-dopamine D2 receptor, cytoplasmic domain, long form antibody was purchased from Millipore (Billerica, MA) and the Dopamine D2 Receptor (Short Isoform 239–246) antibody was purchased from Acris Antibodies GmbH (Germany). The specificity of the antibodies has been verified in the literature (Baltzis et al. 2004; Boundy et al. 1993; Montezinho et al. 2006; Nam and Kim 2008; Singh et al. 2007). Antibodies were used at the following dilutions: c-Fos (1:1,000), D2L (1:1,000), D2S (1:1,000), 5-HT2A (1:5,000) and D2 (1:1,000). The overnight incubation was followed by incubation with peroxidase-labeled secondary antibody for 1 hour at room temperature. The membranes were incubated with enhanced chemiluminescence substrate solution (Amersham Biosciences Inc., Piscataway, NJ). Protein loading for each lane was verified using an anti-actin antibody (Santa Cruz Biotechnology, Inc.). Negative controls included either the omission of primary antibody or addition of preimmune rabbit immunoglobulins.
Film Analysis
Films were analyzed densitometrically with values calculated from the integrated optical density (IOD) of each band using Scion Image software (Scion Corporation, Frederick, MD, USA). The gray scale density readings were calibrated using a transmission step-wedge standard. The integrated optical density (IOD) of each band was calculated as the sum of the optical densities of all the pixels within the area of the band outlined. An adjacent area was used to calculate the background optical density of the film. The IOD for the film background was subtracted from the IOD for each band. The resulting IOD for each protein was then divided by the amount of protein loaded on the corresponding lane, and each sample was expressed as IOD per microgram of protein. Each sample was measured on three independent gels. All samples were standardized to controls and normalized to their respective actin levels.
Quantitative Real-Time PCR
Total RNA was isolated from either cell culture or prefrontal cortex tissue using the RNeasy Mini Kit (Qiagen, Valencia, CA) protocol as described by the manufacturer. Total mRNA was reverse transcribed to generate cDNA. Quantitative real time PCR reactions were prepared using QuantiFast SYBR Green PCR Kit (Qiagen, Valencia, CA), a 4% (v/v) concentration of cDNA product, and forward and reverse primers at a final concentration of 0.35 mM. All reactions were performed in triplicate using the ABI 7500 fast real time PCR system (Applied Biosystems, Foster City, CA). A negative control lacking cDNA or any known DNA template was included for each primer pair. The primers used in this manuscript were: 5-HT2A (F:5′-AACGGTCCATCCACAGAG-3′ and R:5′-AACAGGAAGAACACGATGC-3′), D2 (F:5′-CACCACGGCCTACATAGCAA-3′ and R:5′-GGCGTGCCCATTCTTCTCT-3′), and GAPDH (F: 5′-TGGAGTCTACTGGCGTCTTCAC-3′ and R:5′-GGCATGGACTGTGGTCATGA-3′). These primers have been previously validated in the literature (Kindlundh-Hogberg et al. 2006; Roessner et al. 2010; Singh et al. 2009; Zhang et al. 2008).
In all real-time PCR experiments, measurements were made from the number of cycles required to reach the threshold fluorescence intensity [cycle threshold (Ct)]. Ct values for each reaction were subtracted from Ct values for GADPH and then subtracted from Ct values for vehicle-treated animals that served as a baseline, and the result was referred to as ΔΔCt. Fold changes in gene expression were calculated as 2-ΔΔCt to reflect the fact that, under optimal conditions, the amount of PCR product doubles with each amplification cycle. Results were normalized to those obtained for amplifications of the same cDNA samples using primers designed against GADPH, which acts as an internal standard, and averaged for each treatment group.
Cell Culture Protocol
We purchased CLU213 cells from Cedarlane Laboratories (Burlington, NC). We selected this neuronal cell line because: (1) it coexpresses 5-HT2A, D2, CB1 and CB2 receptors; and (2) the preliminary results in our lab showed that it reproduces the effect of sustained cannabinoid exposure in vivo experiments. This was confirmed in experiments reported in this paper (Fig.2E and 2F). Although many in vitro cannabinoids studies use transformed cells that overexpress neurotransmitter receptors, we chose this neuronal cell line because it endogenously expresses 5-HT2A, D2, CB1 and CB2 receptors. Therefore, we anticipate that the results depicted in this manuscript could be a good model of the mechanisms underlying 5-HT2A upregulation in vivo.
CLU213 cells were grown on 100-mm2 plates treated with polystyrene (Corning Incorporated, Corning, NY) and maintained in 5% CO2 at 37°C, in Dulbecco’s modified eagle medium (DMEM; Mediatech Inc, Manassas, VA) containing 10% fetal bovine serum (FBS; Thermo Scientific, Logan, UT).
Effect of Non-Selective and Selective CB1 and CB2 Receptor Agonists on 5-HT2A and D2 Receptor mRNA
CLU213 cells were incubated with either vehicle (ethanol 0.01% final concentration), CP55,940 (CB1 and CB2 agonist, 1 nM) (Thomas et al. 1998; Wiley et al. 1995); ACEA (CB1 agonist, 15 nM) (Hillard et al. 1999; Rutkowska and Jachimczuk 2004); or GP 1a (CB2 agonist, 1nM) (Gorantla et al. 2010; Murineddu et al. 2006) for 24 hours. mRNA was isolated and qRT-PCR for 5-HT2A and D2 mRNA were performed as described above.
Effect of Highly Selective CB2 Receptor Agonists on 5-HT2A Receptor mRNA in Cultured Cells
CLU213 cells were pretreated with either vehicle (ethanol 0.01% final concentration) or 1 μM AM 630 (Barrio et al. 2011), a highly selective CB2 receptor antagonist. Twenty minutes later cells were treated with either vehicle or one of the following highly selective CB2 agonists, 30 nM JWH 133 (Barrio et al. 2011; Zarruk et al. 2011) or 1nM GP 1a (Gorantla et al. 2010; Murineddu et al. 2006). 24 hours later mRNA was isolated and qRT-PCR for 5-HT2A mRNA was performed as previously described.
Effect of Pertussis Toxin (PTX) on GP 1a-Induced Increases in 5-HT2A receptor mRNA and Protein Levels
CLU213 cells were treated with either vehicle (PBS) or PTX (100 ng/ml)(Bokoch et al. 1983; Casey et al. 1989). Twenty minutes later cells were treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1 nM) for 24 hours. mRNA was isolated and qRT-PCR for 5-HT2A was performed as described above.
In a different experiment, CLU213 cells were treated with either vehicle (PBS) or PTX (100 ng/ml) for 20 minutes. Cells were then incubated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1 nM) for 72 hours. Cells were washed (3x) with PBS every 24 hours and fresh vehicle or GP 1a were added. Expression of membrane-associated 5-HT2A receptors was determined by Western blot as previously described.
Effect of a selective ERK1/2 inhibitor (PD 198306) or Adenylyl Cyclase Activator (NKH 477) on GP 1a-Induced Increases in 5-HT2A Receptor mRNA
CLU213 cells were treated with either vehicle (ethanol 0.01% final concentration), NKH 477 (20 μM) (Sobolewski et al. 2004; Toya et al. 1998) or PD 198306 (200 nM) (Ciruela et al. 2003; Pelletier et al. 2003). Twenty minutes later cells were incubated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1 nM) for 24 hours. mRNA was isolated and qRT-PCR for 5-HT2A was performed as described.
Effect of PKC Inhibitors on GP 1a-Induced Increases in 5-HT2A Receptor mRNA
CLU213 cells were treated with either vehicle (ethanol 0.01% final concentration), GF 109203X (5 μM)(Jacobson et al. 1995; Toullec et al. 1991), or Go 6967 (10 nM)(Martiny-Baron et al. 1993) for 20 min. Cells were then treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1 nM) for 24 hours. mRNA was isolated and qRT-PCR for 5-HT2A was performed as described above.
Effect of PKC Activator on GP 1a-Induced Increases in 5-HT2A Receptor mRNA
CLU213 cells were treated with either vehicle (DMSO 0.01% final concentration), PDBu (1 μM), or PDBu (30 nM)(Burns et al. 1990; Kim et al. 2005) for 20 minutes. Cells were then treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1 nM) for 24 hours. mRNA was isolated and qRT-PCR for 5-HT2A was performed as described above.
Effect of Transcription Factor Inhibitors on GP 1a-Induced Upregulation of 5-HT2A Receptors
CLU213 cells were treated with either vehicle (ethanol 0.01%), Naphthol AS-E phosphate (10 μM)(Best et al. 2004) or SR 11302 (1 μM)(Fanjul et al. 1994; Huang et al. 1997) for 20 minutes. Cells were then treated with either vehicle (ethanol 0.01%) or GP 1a (1 nM) for 24 hours. mRNA was isolated and qRT-PCR for 5-HT2A was performed as previously described.
Effect of a Selective ERK1/2 inhibitor on GP 1a-Induced Increases in Nuclear levels of c-Fos Protein
CLU213 cells were treated with either vehicle (ethanol 0.01% final concentration) or PD 198306 (200 nM)(Ciruela et al. 2003; Pelletier et al. 2003)for 20 minutes. Cells were then incubated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1 nM) for 15 minutes. After 15 minutes of incubation, cells were collected and nuclear-associated proteins were isolated. Expression of nuclear-associated c-Fos was determined by Western blot as previously described.
Statistics
All data are expressed as the mean ± S.E.M., where n indicates the number of rats per group. Data was analyzed by an unpaired Student’s t-test or ANOVA (Newman-Keuls post-hoc test). GB-STAT software (Dynamic Microsystems, Inc., Silver Spring, MD, USA) was used for all statistical analyses.
Results
Effect of CP 55,940 Treatment on the Co-Immunoprecipitation of 5-HT2A and D2 Receptors in Rat PFCx
We used co-immunoprecipitation protocols to study the effect of CP55,940 on the physical interaction between 5-HT2A and D2 receptors in rat PFCx (Fig.1). PFCx lysate of rats treated with either vehicle or CP 55,940 (a non-selective CB1/CB2 receptor agonist) for 7 days was used in this experiment as described in Methods. We used either D2 or 5-HT2A receptor antibodies as baits in two different co-immunoprecipitation experiments. In the first experiment, we used active columns to precipitate 5-HT2A receptors using D2 receptors as bait (Fig.1A, lanes 1 and 2). We also used inactive columns, unable to bind D2 receptor antibody as control (Fig.1A, lanes 3 and 4), as described in methods. We found that 5-HT2A receptors co-precipitate with D2 receptors when we used D2 receptors as bait. Indeed, We found an enhanced co-immunoprecipitation of 5-HT2A and D2 receptors in PCx of CP55,940-treated rats compared with vehicle controls (approx. 200% increase, Fig.1A lanes 1 and 2 for vehicle or CP55,940 samples, respectively). No co-precipitation of 5-HT2A and D2 receptors was detected when using inactive columns (Fig.1A, lanes 3 and 4). Similarly, we found an approx. two-fold increased co-precipitation of D2 receptors with 5-HT2A receptors in PFCx lysate of CP55,940-treated rats compared to controls when we used 5-HT2A receptor as a bait (Fig.1B, lanes 5 and 6 for vehicle of CP55,940 samples, respectively). No co-precipitation of 5-HT2A and D2 receptors was detected when using inactive columns (Fig.1B, lanes 7 and 8). This evidence suggests that CP55,940 treatment enhances formation of a 5-HT2A-D2 receptor heteromer in rat PFCx.
Figure 1. CP 55,940-induced enhanced co-immunoprecipitation of 5-HT2A and D2 receptors in rat PFCx.
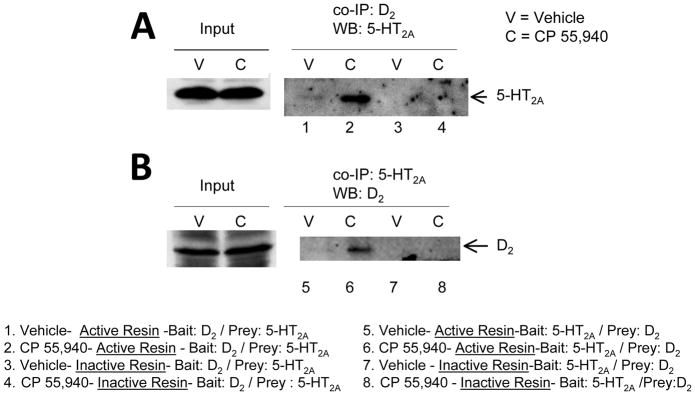
(A) Enhanced immunoprecipitation of the 5-HT2A receptor (Lane 2) compared to vehicle-treated controls (Lane 1). (B) Enhanced immunoprecipitation of the D2 (Lane 6) receptor compared to vehicle-treated controls (Lane 5). Negative controls (Lanes 3, 4, 7, and 8) received the same concentration of D2 or 5-HT2A receptor antibody except that the coupling resin was replaced with control agarose resin that is not amine reactive. All columns were incubated with prefrontal cortex lysate (300 μg) from vehicle (Lanes 1,3,5, and 7) or CP 55,940 (2, 4, 6, and 8) treated rats. Prefrontal cortex lysate (45 μg of protein) was used as an input control for both immunoprecipitations.
Effect of Chronic CP 55,940 Treatment on the Protein Expression of D2 and 5-HT2A Receptors in Rat PFCx
CP55,940 enhanced expression of post-synaptically located D2 and 5-HT2A receptors could underlie the enhanced co-immunoprecipitation of these receptors detected in Fig.1. In our next experiments, we studied the effect of CP55,940 exposure on the membrane-associated protein levels of 5-HT2A and D2 receptors. There are two alternatively spliced isoforms of the D2 receptor that are codified for the same gene (Doly et al. 2004; Khan et al. 1998; Usiello et al. 2000). These are the dopamine D2 receptor Long (D2L) and short (D2S) isoforms that differ by a 29 amino acid insert in the third cytoplasmic loop (Dal Toso et al. 1989). The D2S receptor (M.Wt 48 kDa) is mainly presynaptically localized while the D2L receptor (M.Wt 50 kDa) and the 5-HT2A receptor (M.Wt 42 kDa) are mainly located postsynaptically (Doly et al. 2004; Khan et al. 1998; Usiello et al. 2000).
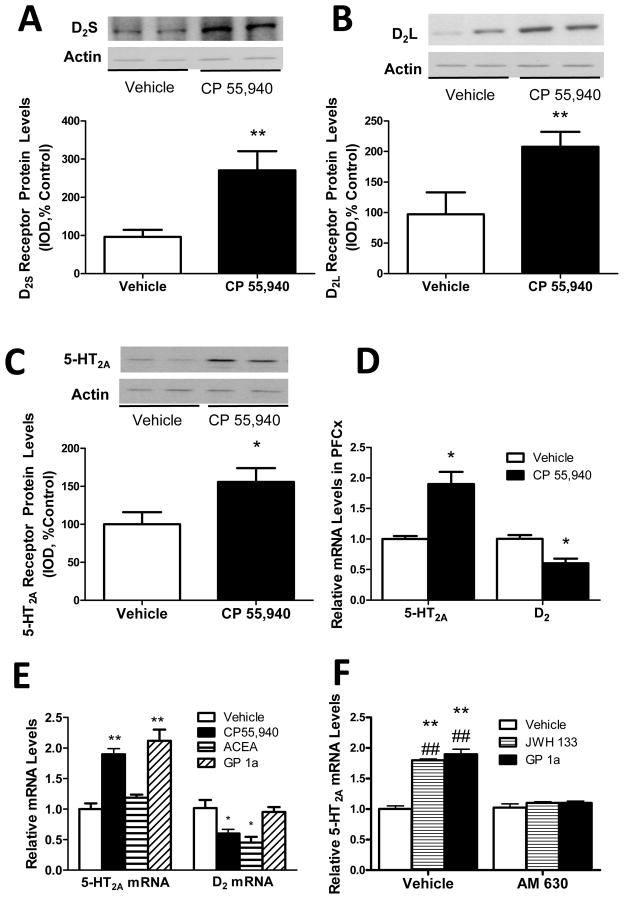
Chronic administration of CP55,940 produced significant increases in membrane-associated levels of D2S receptors (Fig.2A), D2L receptors (Fig. 2B), and 5-HT2A receptors (Fig. 2C) in rat PFCx. Membrane-associated levels of D2L and 5-HT2A receptors increased between 60% and 100% compared to vehicle-treated animals (p<0.01, t 3.264, df 10 and p<0.05, t 2.55, df 10, respectively) while D2S receptor levels increased almost three-fold compared to vehicle treated controls (p<0.05, t 2.299, df 10). Actin was used as a control for protein loading in all these Western blots. We also determined the effect of chronic CP 55,940 treatment on 5-HT2A and D2 mRNA levels in rat PFCx. 5-HT2A receptor mRNA was significantly (p<0.05) increased (approx.90% increase) in PFCx of CP55,940-treated rats compared to vehicle-treated controls (Fig. 2D). Interestingly, D2 receptor mRNA was significantly (p<0.05) reduced (approx. 45% reduction) in PFCx of CP 55,940 treated rats compared to vehicle-treated controls.
Figure 2. CP 55,940-induced increased membrane-associated expression of 5-HT2A and D2 receptors in rat PFCx.
(A) Increased membrane-associated D2S receptor protein levels in PFCx of CP 55,940 treated rats. (**p<0.01 significant effect of CP 55,940 treatment compared to vehicle-treated controls). (B) Increased membrane-associated D2L receptor protein levels in PFCx of CP 55,940 treated rats. (**p<0.01 significant effect of CP 55,940 treatment compared to vehicle-treated controls). (C) Increased membrane-associated 5-HT2A receptor protein levels in PFCx of CP 55,940 treated rats. (*p<0.05 significant effect of CP 55,940 treatment compared to vehicle-treated controls). (D) Increased 5-HT2A receptor mRNA levels and reduced D2 receptor mRNA levels in PFCx of CP 55,940 treat rats. (*p<0.05 significant effect of CP 55,940 treatment compared to vehicle-treated controls). (E) Increased 5-HT2A receptor mRNA levels in CP 55,940 or GP 1a treated cells (**p<0.01 significant effect of CP 55,490 or GP 1a treatment compared to vehicle-treated controls) and reduced D2 receptor mRNA levels in CP 55,940 or ACEA treated cells (*p<0.05 significant effect of CP 55,940 or ACEA treatment compared to vehicle-treated controls). (F) AM 630 pretreatment prevents GP 1a and JWH 133-induced increases in 5-HT2A receptor mRNA. **p<0.01, significant effect of GP 1a or JWH 133 treatment on 5-HT2A receptor mRNA levels compared to vehicle-treated controls. ##p<0.01, significant effect of AM 630 pretreatment on the GP 1a or JWH 133-induced upregulation of 5-HT2A receptors. Representative Western blots are shown in this figure and IOD was calculated as described in Experimental Procedures. The data represent mean ± SEM (n=6–8).
Effect of non-selective and selective cannabinoid agonists on the 5-HT2A and D2 mRNA levels in a neuronal cell line
We used a neuronal cell line, CLU213 cells, in our next experiments to better examine the mechanisms involved in the cannabinoid-induced upregulation of 5-HT2A receptors. CLU213 cells express 5-HT2A, D2, CB1 and CB2 receptors. In these experiments we tested the effect of either a non-selective cannabinoid agonist (CP55,940) (Thomas et al. 1998; Wiley et al. 1995); a selective CB1 receptor agonist (ACEA) (Hillard et al. 1999; Rutkowska and Jachimczuk 2004); or a selective CB2 receptor agonist (GP 1a) (Gorantla et al. 2010; Murineddu et al. 2006).
We found that either CP55,940 or GP 1a produced a significant (p<0.01) upregulation of 5-HT2A receptor mRNA levels in CLU213 cells (Fig. 2E). Cells treated with either CP55,940 or GP 1a exhibited an approx. two-fold increase in 5-HT2A receptor mRNA levels compared to controls. No significant differences (p>0.05) in the 5-HT2A receptor mRNA levels were detected between cells treated with either CP55,940 or GP 1a. The CB1 agonist ACEA did not have significant effects on 5-HT2A receptor mRNA levels (Fig. 2E). On the other hand, cells treated with either CP55,940 or ACEA exhibited a significant (p<0.05) downregulation of D2 mRNA levels in CLU213 cells. Cells treated with CP55,940 exhibited an approx. 60% reduction (P<0.05) in D2 mRNA levels while cells treated with ACEA exhibited an approx. 52% reduction (P<0.05) in D2 mRNA levels. No significant differences (p>0.05) in D2 mRNA levels were detected between cells treated with either CP55,940 or ACEA.
Since we detected a very strong regulation of 5-HT2A receptor mRNA induced by GP 1a, a highly selective CB2 receptor agonist, we also studied the effect of other selective CB2 agonist and antagonist on 5-HT2A upregulation. In this experiment cells were pretreated with either vehicle or AM 630, a selective CB2 antagonist. Twenty minutes later the cells were incubated with either vehicle, JWH 133 or GP 1a as described in Methods. We found that both JWH 133 and GP 1a produced a significant (p<0.01) upregulation of 5-HT2A receptor mRNA in CLU213 cells (Fig.2F). There were no significant (p>0.05) differences between the 5-HT2A upregulation induced by JWH 133 or GP 1a. This strong 5-HT2A mRNA upregulation induced by these CB2 receptor agonists was significantly (p<0.01) inhibited in cells pretreated with a selective CB2 antagonist, AM 630 (Fig.2F). No significant (p>0.05) differences in 5-HT2A mRNA were found between vehicle treated cells and cells pretreated with AM 630 and later treated with either vehicle, JWH 133, or GP 1a (Fig.2F). The two-way ANOVA for 5-HT2A mRNA showed a significant main effect of AM 630 pretreatment (F1,134.75, p<0.0001) and CB2 agonists treatment (F1,65.98, p<0.0001). There was also a significant interaction between AM 630 pretreatment and CB2 agonists treatment (F1,40.03 p<0.0001).
Effect of G-Protein and ERK1/2 Signaling Inhibitors on the GP 1a-Induced Upregulation of 5-HT2A Receptors in CLU213 cells
Our next experiments were designed to identify some signaling components that would mediate the upregulation of 5-HT2A receptors by the CB2 receptor agonist GP 1a. Previous reports suggested that CB1 and CB2 cannabinoid receptors couple to Gαi/o G-proteins receptors to inhibit adenylyl cyclase activation and to induce the activation of the ERK1/2 signaling cascade (Bouaboula et al. 1996). Here we used PTX to prevent the GP 1a-induced activation of Gαi/o G-proteins (Bouaboula et al. 1996). PTX-induced ADP-ribosylation of Gαi/o subunits mediates the inactivation of their signaling by interfering with Gα/receptor coupling (Bokoch et al. 1983; Casey et al. 1989).
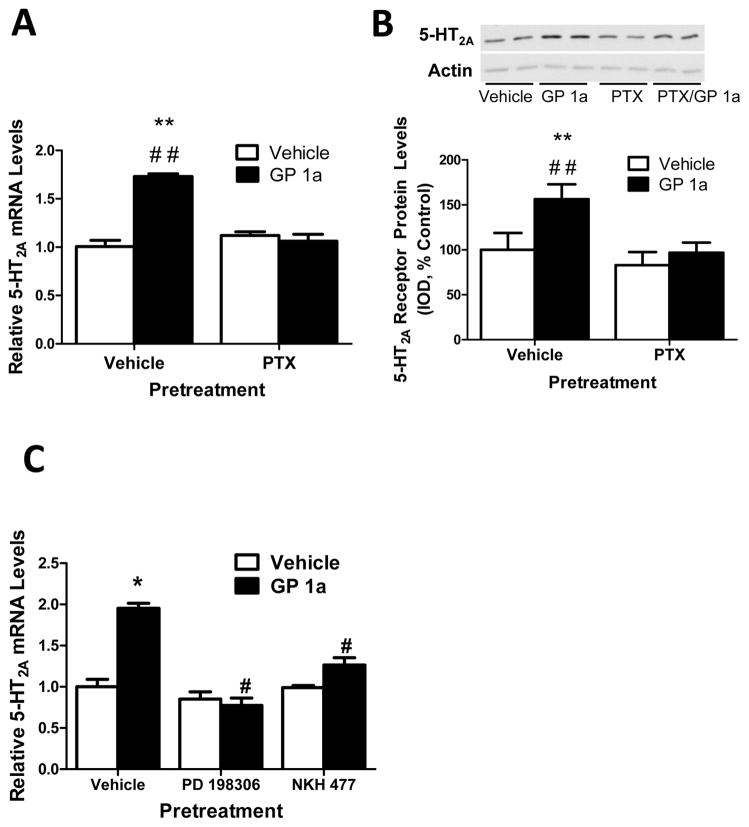
Figure 3 illustrates the effect of PTX pretreatment on GP 1a-induced upregulation of 5-HT2A receptors in CLU213 cells. CLU213 cells were pretreated with either vehicle or PTX (100 ng/ml) for 20 minutes then vehicle or GP 1a (1nM) was added to the media. We found that in vehicle pretreated cells, GP 1a significantly (p<0.01) increased 5-HT2A mRNA levels (two-fold increase) over controls (Fig.3A). This effect of GP 1a was prevented (p<0.01) in cells pretreated with PTX. No significant (p>0.05) effect of PTX was found in basal 5-HT2A receptor mRNA levels. The two-way ANOVA for 5-HT2A mRNA showed significant main effects of PTX pretreatment (F1,23.52, p<0.0004) and GP 1a treatment (F1,34.11 p<0.0001). There was a significant interaction between PTX pretreatment and GP 1a treatment (F1,47.74 p<0.0001).
Figure 3. GP 1a, a selective CB2 receptor agonist, upregulated 5-HT2A receptors via Gαi G-protein in CLU213 cells.
(A) Pertussis toxin (100 ng/ml) prevents GP 1a-induced increases in 5-HT2A receptor mRNA. **p<0.01, significant effect of GP 1a treatment on 5-HT2A receptor mRNA levels compared to vehicle-treated controls. ##p<0.01, significant effect of pertussis toxin pretreatment on the GP 1a-induced upregulation of 5-HT2A receptors. (B) Pertussis toxin (100 ng/ml) prevents GP 1a-induced increases in membrane-associated 5-HT2A receptor protein expression. **p<0.01, significant effect of GP 1a treatment on 5-HT2A receptor protein levels compared to vehicle-treated controls. ##p<0.01, significant effect of pertussis toxin pretreatment on the GP 1a-induced increases in membrane-associated 5-HT2A receptor protein levels. (C) An inhibitor of ERK1/2 (PD 198306) prevents GP 1a-induced increases in 5-HT2A receptor mRNA and an activator of adenylyl cyclase (NHK 477) prevents GP 1a-induced increases in 5-HT2A receptor mRNA. *p<0.05, significant effect of GP 1a treatment on 5-HT2A receptor mRNA levels compared to vehicle-treated controls. #p<0.05, significant effect of PD 198306 or NKH 477 pretreatment on the GP 1a-induced increases in 5-HT2A receptor mRNA. The data represent mean ± SEM (n=3).
In Figure 3B, CLU213 cells were treated with either vehicle or PTX (100 ng/ml) then vehicle or GP 1a (1 nM) was added to the media 20 min later. Membrane-associated 5-HT2A receptor protein expression was measured in these cells after 3 days of incubation with GP 1a, as described in Methods. We found that in vehicle pretreated cells, GP 1a significantly (p<0.01) increased 5-HT2A receptor protein levels (approx. 60% increase) over controls (Fig. 3B). The effect of GP 1a on 5-HT2A receptor protein levels was prevented (p<0.01) in cells pretreated with PTX. No significant (p>0.05) effect of PTX was found on basal 5-HT2A receptor protein levels. The two-way ANOVA for 5-HT2A receptor protein levels showed significant main effects of PTX pretreatment (F1,23.18, p<0.0001) and GP 1a treatment (F1,19.34, p<0.0003). There was a significant interaction between PTX pretreatment and GP 1a treatment (F1,7.14, p<0.0151). These data suggest that the GP 1a-induced upregulation of 5-HT2A receptors is mediated by a Gαi/o G-protein mechanism.
Coupling of CB2 cannabinoid receptors to Gαi/o G-proteins mediates the increases in ERK signaling and also the inhibition adenylate cyclase that results in reduced cAMP levels (Felder et al. 2006). In our next experiment, we studied the effect of a ERK1/2 inhibitor (PD 198306) (Pelletier et al. 2003) and an adenylyl cyclase activator (NHK 477)(Sobolewski et al. 2004) on the GP 1a-induced upregulation of 5-HT2A receptor mRNA. CLU213 cells were treated with either vehicle, PD 198306 (200nM) or NKH 477 (20 μM). Twenty min later cells were treated with either vehicle or GP 1a (1nM) for 24 hours. Consistent with our previous findings, GP 1a significantly (p<0.05) increased 5-HT2A mRNA levels (approx. two-fold increase) over controls (Fig.3C). The effect of GP 1a was prevented (p<0.05) in cells pretreated with either PD 198306 or NKH 477. No significant (p>0.05) effect of PD 198306 or NKH 477 was found on basal 5-HT2A receptor mRNA levels (Fig.3C). These results suggest that the GP 1a-induced upregulation of 5-HT2A receptors is dependent on ERK1/2 activation and prevented by activation of adenylyl cyclase.
Effect of PKC on GP 1a-Induced Upregulation of 5-HT2A Receptor mRNA
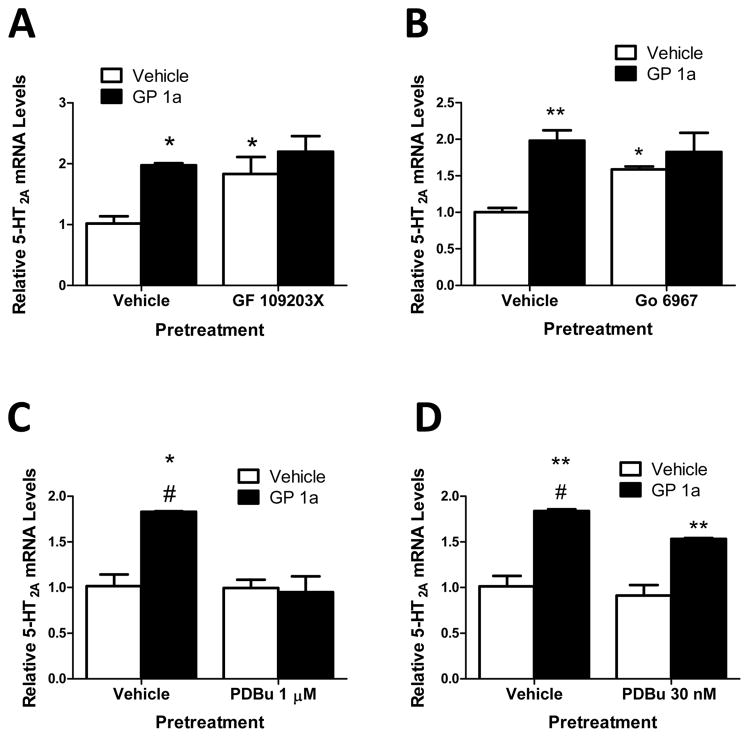
Figure 4A and 4B illustrate the effect of PKC inhibition on GP 1a-induced 5-HT2A receptor upregulation. Bouaboula et al. (1996) proposed that cannabinoid receptors activate the ERK1/2 signaling cascade through PKC activation (Bouaboula et al. 1996). Additionally, they reported evidenced to suggest that Ca2+− dependent PKC isoforms could be involved in CB2 signal transduction which are not involved in CB1 signal transduction (Bouaboula et al. 1996). Here we studied the effect of two different PKC inhibitors (GF 109203X and Go 6967) on GP 1a-induced increases in 5-HT2A receptor mRNA levels. GF 109203X does not discriminate between Ca2+ -dependent and -independent isoforms of PKC (IC50 values are 0.0084, 0.0180, 0.210, 0.132, and 5.8 μM for α, β1, δ, ε and ζ isoforms, respectively) (Toullec et al. 1991) while Go 6967 selectively inhibits Ca2+ -dependent isoforms PKCα and PKCβ1 (IC50 values are 2.3 and 6.2 nM, respectively) (Martiny-Baron et al. 1993).
Figure 4. Ca2+ -independent and -dependent isoforms of PKC regulate 5-HT2A receptor mRNA levels in CLU213 cells.
(A) Inhibition of Ca2+ -independent and –dependent isoforms of PKC (GF 109203X) enhanced basal levels of 5-HT2A receptor mRNA. *p<0.05, significant effect of GP 1a treatment, GF 109203X pretreatment, and GP 1a/GF 109203X treatment compared to vehicle-treated controls. (B) Inhibition of Ca2+ -dependent isoforms of PKC (Go 6967) enhanced basal levels of 5-HT2A receptor mRNA. **p<0.01, significant effect of GP 1a treatment, Go 6967 pretreatment, and GP 1a/Go 6967 treatment compared to vehicle-treated controls. (C) Activation of Ca2+ -independent and -dependent isoforms of PKC (PDBu) prevented GP 1a-induced increases in 5-HT2A receptor mRNA. *p<0.05, significant effect of GP 1a treatment compared to vehicle-treated controls. #p<0.05, significant effect of PDBu pretreatment on GP 1a-induced increases in 5-HT2A receptor mRNA. (D) Activation of Ca2+ -dependent isoforms of PKC did not prevent GP 1a-induced increases in 5-HT2A receptor mRNA. **p<0.01, significant effect of GP 1a treatment compared to vehicle-treated controls. The data represent mean ± SEM (n=3).
In Figure 4A, CLU213 cells were pretreated with either vehicle or GF 109203X (5 μM) for 20 minutes and then treated with vehicle or GP 1a (1 nm). In this experiment, 5 μM GF 109203X should produce a substantial inhibition of most PKC isoforms. We found that GP 1a significantly (p<0.05) increased 5-HT2A receptor mRNA levels (two-fold increase) in vehicle pretreated cells compared to vehicle treated controls (Fig 4A). Furthermore, GF 109203X pretreatment significantly (p < 0.05) increased basal 5-HT2A receptor mRNA levels (two-fold increase) over vehicle treated controls and had no significant effect (p>0.05) on GP 1a-induced increases in 5-HT2A receptor mRNA levels. The two-way ANOVA for 5-HT2A mRNA showed significant main effects of GF 109203X pretreatment (F1,6.68, p<0.0324) and GP 1a treatment (F1,10.82 p<0.011). There was no significant interaction between GF 109203X pretreatment and GP 1a treatment (F1,2.17 p>0.05).
In order to address the role of PKC Ca2+-dependent isoforms on the regulation of 5-HT2A receptor mRNA, CLU213 cells were pretreated with either vehicle or Go 6967 (10 nm) for 20 minutes then treated with either vehicle or GP 1a (1 nm) for 24 h. GP 1a significantly (p<0.01) increased 5-HT2A receptor mRNA levels (two-fold increase) compared to vehicle pretreated controls (Fig 4B). Pretreatment with Go 6967 significantly (p <0.01) increased basal 5-HT2A receptor mRNA levels (58% increase) over vehicle pretreated controls while Go 6967 pretreatment did not have a significant effect (p>0.05) on GP 1a-induced upregulation of 5-HT2A receptors mRNA. The two-way ANOVA for 5-HT2A receptor mRNA showed a main effect of Go 6967 pretreatment (F1, 5.85, p<0.0418) and a main effect of GP 1a treatment (F1,16.15, p<0.0038). There was no significant interaction between Go 6967 pretreatment and GP 1a treatment (F1,2.03, p>0.05).
Next we examined the effect a PKC activator, PDBu (Kd values are 1 μM, 0.98 μM, 26 nM, 11 nM, and 9 nM for ε, δ, β1, α, and ζ isoforms, respectively) (Burns et al. 1990), has on GP 1a-induced increases in 5-HT2A receptor mRNA. We used two doses of PDBu in our experiments, 1 μM and 30 nM. We expect to activate all the different isoforms with the 1 μM dose and selectively activate the β1, α, and ζ isoforms (Ca2+-dependent isoforms) with the lowest dose (30 nM) (Burns et al. 1990).
CLU213 cells were pretreated with either vehicle or PDBu (1μM) for 20 minutes.Cells were then treated with either vehicle or GP 1a (1 nm). GP 1a significantly (p<0.05) increased 5-HT2A receptor mRNA levels (two-fold increase, Fig 4C). This effect of GP 1a was prevented (p<0.05) in cells pretreated with PDBu. No significant (p>0.05) effect of PDBu was found on basal 5-HT2A receptor mRNA levels. The two-way ANOVA for 5-HT2A receptor mRNA showed significant main effects of PDBu pretreatment (F1,6.12, p<0.0385) and GP 1a treatment (F1,6.10 p<0.0375). There was a significant interaction between PDBu pretreatment and GP 1a treatment (F1,5.38 p<0.0489).
In order to examine the effect Ca2+-dependent PKC isoforms on GP 1a-induced increases in 5-HT2A receptor mRNA, we used a concentration of PDBu (30 nm) that activated the Ca2+-dependent isoforms (Burns et al. 1990). GP 1a significantly (p<0.01) increased 5-HT2A receptor mRNA levels (two-fold increase, Fig 4D). There was no significant (p>0.05) effect of PDBu 30 nm found on basal 5-HT2A receptor mRNA levels and PDBu pretreatment significantly reduced (approx. 20% decrease, p<0.05) the GP 1a-induced upregulation of 5-HT2A receptors. The two-way ANOVA for 5-HT2A receptor mRNA showed significant main effects of PDBu pretreatment (F1,6.26 p<0.0368) and GP 1a treatment (F1,79.39 p<0.0001). There was no significant interaction between PDBu pretreatment and GP 1a treatment (F1,1.62 p<0.2385). The use of PKC activators seems to suggest that both Ca2+-dependent and Ca2+-independent PKC isoforms play a role preventing the GP 1a-induced upregulation of 5-HT2A receptor mRNA.
Effect of CREB and AP-1 Transcription Factors Inhibitors on the GP 1a- Induced Upregulation of 5-HT2A Receptor mRNA
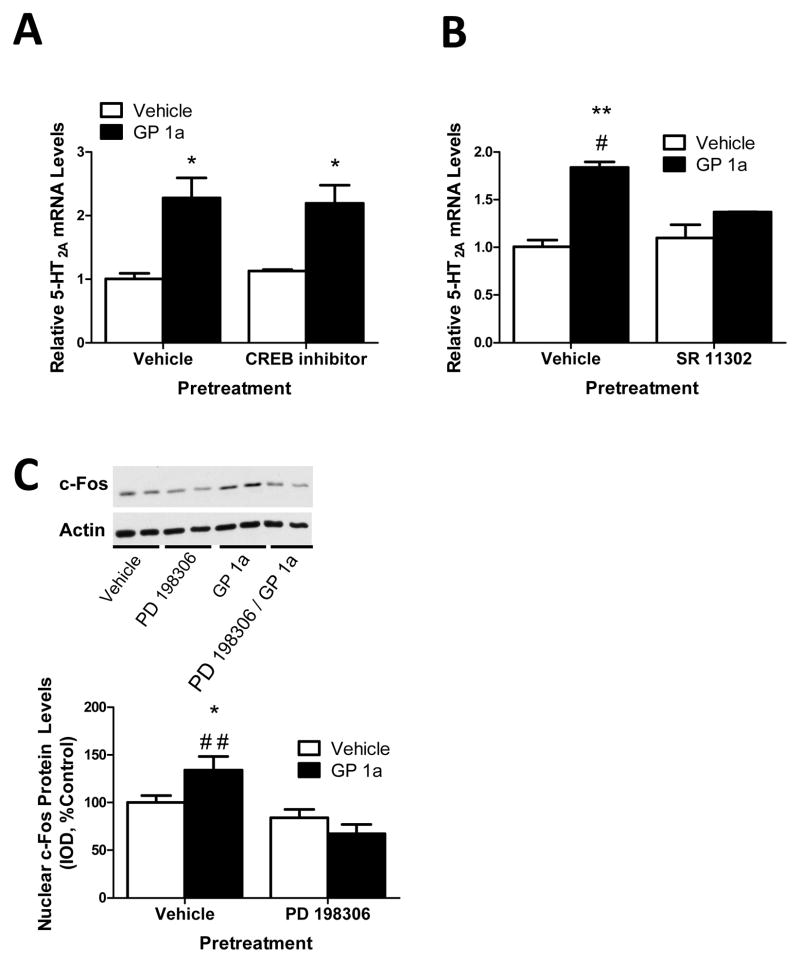
Figure 5 illustrates the effect of CREB or AP-1 transcription factor inhibitor pretreatment on GP 1a-induced upregulation of 5-HT2A receptor mRNA in CLU213 cells. Here we wanted to identify possible transcription factor(s) that would contribute to GP 1a-induced increases of 5-HT2A receptor mRNA. In our previous experiments we showed that the GP 1a-induced upregulation of 5-HT2A receptors is prevented by PD 198306, an inhibitor of ERK1/2 activation. Activation of ERK involved the phosphorylation of this protein in the cytoplasm and its translocation to the nucleus (Campbell et al. 1995; Chang et al. 2003; Seger and Krebs 1995). In the nucleus, phosphorylated ERK (pERK) can activate several transcription factors such as CREB, c-Fos, ELK-1, SP-1, and EGR-1 (Campbell et al. 1995; Chang et al. 2003; Seger and Krebs 1995). The transcription factors CREB and AP-1 have consensus sequences within the promoter region of the rat 5-HT2A receptor gene (Chalecka-Franaszek et al. 1999; Du et al. 1994; Ferry and Molinoff 1996). Therefore, we decided to test the effects of inhibitors of these transcription factors on the GP 1a-induced upregulation of 5-HT2A receptor mRNA.
Figure 5. CB2 receptor-induced upregulation of 5-HT2A receptor involves AP-1 and c-fos, but not CREB, activation.
(A) Inhibition of CREB activation did not prevent or significantly reduce GP 1a-induced increases in 5-HT2A receptor mRNA. *p<0.05, significant effect of GP 1a and CREB/GP 1a treatment on 5-HT2A receptor mRNA levels compared to vehicle-treated controls. (B) CB2 receptor-mediated upregulation of 5-HT2A receptor involves AP-1 transcription factor activity. **p<0.01, significant effect of GP 1a treatment compared to vehicle-treated controls. #p<0.05, significant effect of AP-1 transcription factor inhibitor pretreatment on GP 1a-induced increases in 5-HT2A receptor mRNA. (C) Inhibition of GP 1a mediated increases in nuclear c-fos protein levels via a selective ERK1/2 inhibitor (PD 198306). *p<0.05, significant effect of GP 1a treatment on nuclear c-fos levels compared to vehicle treated controls. ##p<0.01, significant effect of PD 198306 pretreatment on GP 1a-induced increases in nuclear c-fos levels. The data represent mean ± SEM (n=3).
CREB is a transcription factor that binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing transcription of downstream genes (Chrivia et al. 1993; Kwok et al. 1994). c-Fos belongs to the immediate early gene family of transcription factors. Members of the Fos family dimerize with c-jun to form the AP-1 transcription factors, which can upregulate transcription of various genes (Karin 1995; Karin et al. 1997). In our first experiment, we study the effect of CREB inhibitor pretreatment on the GP 1a-induced upregulation of 5-HT2A receptors.
CLU213 cells were treated with either vehicle or Naphthol AS-E phosphate (10 μM) for 20 minutes and then treated with vehicle or GP 1a (1 nm). Naphthol AS-E phosphate blocks cAMP-induction of CREB-dependent gene transcription (Ki 10 μM) (Best et al. 2004). We found that Naphthol AS-E phosphate did not inhibit or decrease GP 1a-induced increases in 5-HT2A receptor mRNA (Fig. 5A). No significant (p>0.05) effect of Naphthol AS-E phosphate was found on basal 5-HT2A mRNA levels. The two-way ANOVA for 5-HT2A receptor mRNA showed no significant main effect of Naphthol AS-E phosphate pretreatment (F1,0.006, p>0.9384) and a significant main effect of GP 1a treatment (F1,28.91 p<0.0007). There was no significant interaction between Naphthol AS-E pretreatment and GP 1a treatment (F1,0.23 p>0.6453). These data indicate that CREB is not involved in GP 1a-induced 5-HT2A receptor upregulation.
We then studied the effect of AP-1 inhibition on GP 1a-induced increases in 5-HT2A receptor mRNA. CLU213 cells were treated with either vehicle or SR 11302 (1 μM) for 20 min then vehicle or GP 1a (1nM) was added to the incubation media. SR 11302 is retinoid that transrepresses AP-1 without transactivating the retinoic acid response element (Emax 1 μM) (Fanjul et al. 1994). As expected, GP 1a induced a significant (p<0.05) increase in 5-HT2A mRNA levels (approx. two fold increase in 5-HT2A mRNA) (Fig.5B). SR 11302 pretreatment significantly reduced (approximately 55% decrease, p<0.05) the GP 1a-induced upregulation of the 5-HT2A receptor mRNA (Fig. 5B). No significant (p>0.05) effect of SR 11302 was found in basal 5-HT2A mRNA levels. The two-way ANOVA for 5-HT2A mRNA did not show a significant main effects of SR 11302 pretreatment (F1,2.89, p>0.1271) and did show a significant effect of GP 1a treatment (F1,32.80 p<0.0004). There was a significant interaction between SR 11302 pretreatment and GP 1a treatment (F1,7.48 p<0.0256).
Our data seems to indicate that GP 1a-induced upregulation of 5-HT2A receptors would be mediated, at least in part, by ERK1/2 and AP-1 activation. Here we examined whether inhibition of ERK1/2 can prevent the GP 1a-induced increases in the nuclear-associated protein levels of c-fos. CLU213 cells were treated with either vehicle or PD 198306 (200 nm) for 20 minutes and then treated with either vehicle or GP 1a (1 nm) for 15 minutes. As mentioned above, PD 198306 is a potent inhibitor of ERK1/2 (IC50 100 nM) (Pelletier et al. 2003). We found that in vehicle pretreated cells GP 1a significantly (p<0.05) increased c-fos levels over controls (Fig. 5C). Indeed, GP 1A induced an approx. 40% increase in the nuclear-associated protein levels of c-fos. This effect of GP 1a was prevented (p<0.01) in cells pretreated with PD 198306 (Fig.5C). No significant (p>0.05) effect of PD 198306 was found on basal 5-HT2A mRNA levels. The two-way ANOVA for 5-HT2A mRNA showed significant main effects of PD 198306 pretreatment (F1,15.74, p<0.0008) and GP 1a treatment (F1,6.09 p<0.0147). There was a significant interaction between PD 198306 pretreatment and GP 1a treatment (F1,5.95 p<0.0241).
Discussion
G protein-coupled receptors (GPCRs) can exist as dimers or part of larger oligomeric complexes (Lohse 2006; Milligan 2004). Interestingly, recent reports from several independent groups suggest that 5-HT2A and D2 receptors co-expressed in the same cells could form 5-HT2A-D2 receptor heterodimers (Albizu et al. 2011; Borroto-Escuela et al. 2010; Lukasiewicz et al. 2011; Lukasiewicz et al. 2010). This 5-HT2A-D2 receptor complex would be found in cultured cells that co-express these monoamine receptors such as CLU213 cells and in several brain areas such as PFCx, substantia nigra, etc (Lukasiewicz et al. 2010). Our results suggest that exposure to CP55,940, a non-selective cannabinoid CB1/CB2 receptor agonist (Thomas et al. 1998; Wiley et al. 1995), increases the interaction between 5-HT2A and D2 receptors in rat PFCx (Fig.1). Indeed, we found increased co-immunoprecipitation of 5-HT2A and D2 receptors in PFCx samples of CP55,940 treated rats compared to vehicle controls. Co-immunoprecipitation has been successfully used by some groups to demonstrate the interaction between these two monoamine receptors in cultured cells and in vivo (Albizu et al. 2011; Lukasiewicz et al. 2010). The nature of this interaction between 5-HT2A and D2 receptors in PFCx is still not well defined but it could be favored by the high degree of co-localization of 5-HT2A and D2 receptors in this brain area and by specific domains in the third intracellular loop and the C-tail of the D2 and 5-HT2A receptors, respectively (Lukasiewicz et al. 2010).
The CP55,940-enhanced co-immunoprecipitation between 5-HT2A and D2 receptors in PFCx seems to be mediated by increased protein levels of membrane-associated levels of 5-HT2A and D2 receptors in this area of the limbic brain. This was shown by increased membrane-associated protein levels of D2L, D2S, and 5-HT2A receptors in PFCx of CP55,940 treated rats compared to control (Fig.2A, 2B and 2C). Moreover, we found increased 5-HT2A receptor mRNA in PFCx of CP55,940 treated rats compared to controls (Fig.2D) suggesting that increases in 5-HT2A receptor expression most likely occurs through cannabinoid-mediated enhanced transcription of the 5-HT2A receptor gene. Our evidence also indicates that exposure to CP55,940 induced decreases in D2 mRNA levels in PFCx (Fig.2D). This latter study suggests that the CP55,940-mediated increases in D2 receptor protein in rat PFCx may be occurring through mechanisms such as increased trafficking of D2 receptors from the cytosol to the membrane and/or through decreased degradation of D2 receptors. Noteworthy, typical antipsychotics such as haloperidol increase D2 receptor protein levels independently of D2 mRNA levels even after several days of treatment (Albizua et al. 2011; Cottet et al. 2011). These latter studies suggest that trafficking of D2 receptors might play an important role in the regulation of membrane-associated levels of this monoamine receptor. More importantly, recent studies in human tissue using nonselective cannabinoid agonists also support the hypothesis that activation of cannabinoid receptors downregulate D2 mRNA expression (Wang et al. 2004). Δ9-THC, the main psychoactive component of cannabis sativa (marijuana) is a nonselective CB1 and CB2 receptor agonist (Dresen et al. 2010). Wang et al (2004) reported that expression of D2 receptor mRNA is decreased in several brain areas of human fetal specimens from mothers with documented evidence of cannabis use during pregnancy (Wang et al. 2004), suggesting that stimulation of cannabinoid receptors mediates a (Wang et al. 2004) reduction in D2 mRNA levels in mesocorticolimbic neural systems (Wang et al. 2004).
In this manuscript, we used a neuronal cell line and selective CB1 and CB2 receptor agonists to determine the contribution of these receptors to the regulation of D2 and 5-HT2A receptor mRNA levels in cultured cells (Fig.2E). D2 receptor mRNA levels were decreased in neuronal cells treated with either CP55,940 a nonselective CB1/CB2 agonist or ACEA a selective CB1 agonist (Hillard et al. 1999; Rutkowska and Jachimczuk 2004) (Fig.2E). GP 1a a selective CB2 agonist (Gorantla et al. 2010; Murineddu et al. 2006) did not modify D2 mRNA levels in cultured cells (Fig.2E). These studies suggests that the effect of CP55,940 on D2 mRNA would be mediated by activation of CB1 receptors. On the other hand, activation of CB2 receptors seems to mediate the CP55,940-mediated upregulation of 5-HT2A receptor mRNA (Fig.2E and 2F). Cells treated with either of the following highly selective CB2 agonists, JWH 133 (Barrio et al. 2011; Zarruk et al. 2011) or GP 1a (Gorantla et al. 2010; Murineddu et al. 2006), upregulated 5-HT2A receptor mRNA levels in cultured cells compared to vehicle controls. ACEA did not modify 5-HT2A mRNA levels in this cell line. Supporting these results, AM 630 a highly selective CB2 antagonist (Barrio et al. 2011) prevented the JWH 133- or the GP 1a-induced upregulation of 5-HT2A mRNA in CLU213 cells. AM 630 shows an approximate 165-fold selectivity over CB2 receptors compared to CB1 receptors (Barrio et al. 2011).
The results presented here suggest that CB2, but not CB1, receptor agonists mediate the upregulation of 5-HT2A receptors. Interestingly, there has been some controversy regarding the expression of CB2 receptors in brain. Indeed, CB2 receptors were initially identified in the periphery but not in the brain (Abood and Martin 1996; Demuth and Molleman 2006). Brain expression of CB2 receptors has been much less well established and characterized in comparison to the expression of brain CB1 receptors. Later studies have identified CB2 receptors in several brain areas including: cortex, hippocampus, amygdala, substantia nigra, cerebellum etc (Garcia-Gutierrez et al. 2010; Gong et al. 2006). Furthermore, recent studies reported that there are functional CB2 receptors in the medial prefrontal cortex and that CB2 receptors are mainly localized in post-synaptic neurons (Brusco et al. 2008; den Boon et al. 2012; Onaivi et al. 2008). These findings have led to a re-evaluation of the possible roles that CB2 receptors may play in the brain. Interestingly, deletion of the CB2 receptor induces schizophrenia-related behaviors in mice and chronic treatment with a selective CB2 agonist (JWH 133) increases anxiety in mice (Garcia-Gutierrez et al. 2011; Onaivi et al. 2008; Ortega-Alvaro et al. 2011). Here we found that a selective CB2 receptor agonist induced increases in 5-HT2A receptor mRNA and protein expression in a neuronal cell model. It is possible that CB2 receptors that are co-localized with 5-HT2A receptors in the PFCx could be driving the upregulation of 5-HT2A receptors in the PFCx of animals chronically treated with CP 55,940. However, it is currently unknown whether CB2 receptors co-localize with 5-HT2A receptors in PFCx.
GP 1a and JWH 133, two CB2 receptor agonists, induce an approximate two-fold increase in 5-HT2A receptor mRNA and protein (Fig.2F). Similar increases in expression of 5-HT2A receptor protein levels have been associated with exposure to drugs of abuse and estrogen (Akash et al. 2008; Cyr et al. 2000; Horner et al. 2011). In our next experiments we used GP 1a to study some of the molecular mechanisms involved in the upregulation of 5-HT2A receptors by CB2 receptor agonists. For these experiments, we selected GP 1a because it shows higher CB2/CB1 receptor selectivity compared to JWH 133 (>5,000- and 165-fold CB2/CB1 selectivity, respectively) (Gorantla et al. 2010; Murineddu et al. 2006). First, we examined the role of Gαi G-protein and PKC in the upregulation of 5-HT2A receptors in a neuronal cell line. CB2 receptors couple to PTX-sensitive Gαi G-proteins to mediate: (1) the inhibition of adenylyl cyclase; and (2) the activation of ERK1/2 signaling pathway (Bouaboula et al. 1996). PTX catalyses the ADP-ribosylation of specific Gαi subunits preventing the receptor–G-protein interaction (Bouaboula et al. 1996). Our results indicate that PTX prevented the GP 1a-induced increases in 5-HT2A mRNA and protein levels (Fig.3A and 3B). Additionally, we found that the GP 1a-induced increases of 5-HT2A receptor mRNA levels are prevented by: (1) inhibition of ERK1/2 activation by PD198306; and by (2) activation of adenylyl cyclase by NKH 477 (Fig.3C) in cultured cells. Our results seem to indicate that the GP 1a-induced 5-HT2A upregulation would involve the ERK1/2 activation by PTX-sensitive Gαi G-proteins.
While our results point to the role of Gαi G-proteins and ERK1/2 in the GP 1a-induced upregulation of 5-HT2A receptors, the role of PKC is not clear. Based on previous reports we expected a main role of PKC in mediating the GP 1a-induced activation of ERK signaling (Bouaboula et al. 1996). We found that non-selective PKC inhibitors and selective calcium-dependent PKC inhibitors did not prevent or significantly reduce the GP 1a-induced 5-HT2A upregulation (Fig.3A and 3B). Moreover, 5-HT2A mRNA basal levels were increased by exposure to either of these PKC inhibitors (calcium dependent and independent inhibitors). Furthermore, activation of calcium dependent and independent isoforms with 1μM PDBu or the selective activation of calcium dependent PKC isoforms with 30 nM PDBu (Burns et al. 1990) (Fig.3C and 3D) significantly reduced GP 1a-induced increases in 5-HT2A mRNA levels. Specifically, activation of both calcium dependent and independent isoforms completely inhibited the GP 1a-induced upregulation of 5-HT2A mRNA, while inhibition of selective calcium dependent isoforms partially prevented it. Hence, these findings do not support a role for different isoforms of PKC as a signaling component in the GP 1a-induced upregulation of 5-HT2A receptor signaling but rather they point to a regulatory role of PKC in this signaling pathway. This could be because PKC isoforms are expressed in a tissue-specific manner and individual isoforms play cell-type specific roles in cellular responses as reported (Mischak et al. 1991). Moreover, activation of certain PKC isoforms inhibits gene transcription (Esteve et al. 2002; Newton 1995; Page et al. 2002) and that could prevent the GP 1a-induced increases in 5-HT2A receptor mRNA levels. In summary, it appears that the GP 1a-mediated activation of ERK1/2 would not be mediated by PKC isoforms but it could involve the direct activation of the ERK signaling pathway by scaffold proteins such as β-arrestins (Lefkowitz and Shenoy 2005).
Activation of ERK signaling stimulates several transcription factors such as CREB, c-Fos, ELK-1, SP-1, and EGR-1 (Campbell et al. 1995; Chang et al. 2003; Seger and Krebs 1995). AP-1 is a heterodimeric protein composed of proteins belonging to the c-Fos and c-Jun family. Interestingly, CREB and AP-1 have consensus sequences within the promoter region of the rat 5-HT2A receptor gene (Chalecka-Franaszek et al. 1999; Du et al. 1994; Ferry and Molinoff 1996). Therefore, we tested the effects of inhibitors of these transcription factors on the GP 1a-induced upregulation of 5-HT2A receptor mRNA. Our results suggest that inhibition of AP-1, but not the CREB, activation significantly decreased the GP 1a-induced upregulation of 5-HT2A receptors (Fig.5A and 5B). The partial inhibition of the GP 1a-induced increases in 5-HT2A mRNA levels by SR 11302 suggest that other transcription factors yet to be identified could also contribute to this upregulation. Supporting this hypothesis, we also found that inhibition of ERK1/2 by PD 198306 prevented the GP 1a-induced activation of c-fos (Fig.5C). Although further research is needed, SP-1 could also mediate the GP 1a-induced upregulation of 5-HT2A mRNA. This transcription factor is also activated by the ERK signaling cascade and has a consensus sequence within the rat 5-HT2A receptor promoter region (Ferry et al. 1993; Seger and Krebs 1995).
Exposure to cannabinoids has been associated in the pathophysiology of several neuropsychiatric disorders such as anxiety, depression and schizophrenia (Crippa et al. 2009; Henquet et al. 2005; Kuepper et al. 2011; Large et al. 2011). As stated above, these diseases have been also associated with dysregulation of 5-HT2A and D2 receptor signaling. A causal link has not been found between chronic cannabis use and the etiology of these neuropsychiatric disorders. Recent evidence suggests that chronic use of cannabis may precipitate these disorders in individuals who are prone to developing them (Crippa et al. 2009; Kuepper et al. 2011; Large et al. 2011). Yet a mechanism by which chronic use of cannabis may precipitate these disorders has not been identified. Furthermore, the long term effects of chronic synthetic cannabinoid agonist use, which are now commonly included in herbal incenses and are many times more potent than Δ9-THC (Dresen et al. 2010), have yet to be addressed. We provide evidence here that exposure to cannabinoids might enhance the formation and activity of 5-HT2A-D2 receptor heterodimers in PFCx. This would involve increases in membrane-associated levels of 5-HT2A and D2 receptors in this brain area. In a neuronal cell line we also found that CB2, but not CB1 agonists, seems to mediate this increase in 5-HT2A mRNA. We hypothesize that this CB2 receptor agonist-induced upregulation of 5-HT2A receptors could provide a molecular mechanism by which chronic use of cannabinoids might precipitate the onset of some cognitive and mood disorders in individuals predisposed to developing them.
Acknowledgments
Funding Acknowledgements:
This work was supported by National Institute of Health/National Institute on Drug Abuse DA024329 and University of Kansas Startup Funds.
Footnotes
Conflict of Interest Statement
The Authors declare that there is no conflict of interest.
References
- Abood ME, Martin BR. Molecular neurobiology of the cannabinoid receptor. IntRevNeurobiol. 1996;39:197–221. doi: 10.1016/s0074-7742(08)60667-4. [DOI] [PubMed] [Google Scholar]
- Akash KG, Balarama KS, Paulose CS. Enhanced 5-HT(2A) receptor status in the hypothalamus and corpus striatum of ethanol-treated rats. Cell Mol Neurobiol. 2008;28:1017–25. doi: 10.1007/s10571-008-9281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizu L, Holloway T, Gonzalez-Maeso J, Sealfon SC. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology. 2011;61:770–7. doi: 10.1016/j.neuropharm.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizua E, Gallardo M, Barrio S, Rapado I, Jimenez A, Ayala R, Rueda D, Sanchez-Espiridion B, Puigdecanet E, Espinet B, Florensa L, Besses C, Martinez-Lopez J. Differential expression of JAK2 and Src kinase genes in response to hydroxyurea treatment in polycythemia vera and essential thrombocythemia. Ann Hematol. 2011;90:939–46. doi: 10.1007/s00277-011-1179-2. [DOI] [PubMed] [Google Scholar]
- Baltzis D, Qu LK, Papadopoulou S, Blais JD, Bell JC, Sonenberg N, Koromilas AE. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2alpha kinases PERK and PKR. J Virol. 2004;78:12747–61. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio S, Gallardo M, Albizua E, Jimenez A, Rapado I, Ayala R, Gilsanz F, Martin-Subero JI, Martinez-Lopez J. Epigenomic profiling in polycythaemia vera and essential thrombocythaemia shows low levels of aberrant DNA methylation. Journal of clinical pathology. 2011;64:1010–3. doi: 10.1136/jclinpath-2011-200175. [DOI] [PubMed] [Google Scholar]
- Best JL, Amezcua CA, Mayr B, Flechner L, Murawsky CM, Emerson B, Zor T, Gardner KH, Montminy M. Identification of small-molecule antagonists that inhibit an activator: coactivator interaction. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17622–7. doi: 10.1073/pnas.0406374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, Katada T, Northup JK, Hewlett EL, Gilman AG. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983;258:2072–5. [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Marcellino D, Ciruela F, Agnati LF, Fuxe K. Dopamine D2 and 5-hydroxytryptamine 5-HT(A) receptors assemble into functionally interacting heteromers. Biochemical and Biophysical Research Communications. 2010;401:605–10. doi: 10.1016/j.bbrc.2010.09.110. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le FG, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. EurJBiochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Luedtke RR, Artymyshyn RP, Filtz TM, Molinoff PB. Development of polyclonal anti-D2 dopamine receptor antibodies using sequence-specific peptides. Mol Pharmacol. 1993;43:666–76. [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008;62:944–9. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- Burns DJ, Bloomenthal J, Lee MH, Bell RM. Expression of the alpha, beta II, and gamma protein kinase C isozymes in the baculovirus-insect cell expression system. Purification and characterization of the individual isoforms. J Biol Chem. 1990;265:12044–51. [PubMed] [Google Scholar]
- Campbell JS, Seger R, Graves JD, Graves LM, Jensen AM, Krebs EG. The MAP kinase cascade. Recent Progress in Hormone Research. 1995;50:131–59. doi: 10.1016/b978-0-12-571150-0.50011-1. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. EurJPharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Casey PJ, Graziano MP, Gilman AG. G protein beta gamma subunits from bovine brain and retina: equivalent catalytic support of ADP-ribosylation of alpha subunits by pertussis toxin but differential interactions with Gs alpha. Biochemistry. 1989;28:611–6. doi: 10.1021/bi00428a029. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. JPsychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chen H, Chuang DM. 5-Hydroxytryptamine2A receptor stimulation induces activator protein-1 and cyclic AMP-responsive element binding with cyclic AMP-responsive element-binding protein and Jun D as common components in cerebellar neurons. Neuroscience. 1999;88:885–898. doi: 10.1016/s0306-4522(98)00269-3. [DOI] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–9. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Ciruela A, Dixon AK, Bramwell S, Gonzalez MI, Pinnock RD, Lee K. Identification of MEK1 as a novel target for the treatment of neuropathic pain. BrJPharmacol. 2003;138:751–756. doi: 10.1038/sj.bjp.0705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottet M, Albizu L, Comps-Agrar L, Trinquet E, Pin JP, Mouillac B, Durroux T. Time resolved FRET strategy with fluorescent ligands to analyze receptor interactions in native tissues: application to GPCR oligomerization. Methods Mol Biol. 2011;746:373–87. doi: 10.1007/978-1-61779-126-0_21. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martin-Santos R, Bhattacharyya S, Atakan Z, McGuire P, Fusar-Poli P. Cannabis and anxiety: a critical review of the evidence. HumPsychopharmacol. 2009;24:515–523. doi: 10.1002/hup.1048. [DOI] [PubMed] [Google Scholar]
- Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology. 2000;23:69–78. doi: 10.1016/S0893-133X(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH. The dopamine D2 receptor: two molecular forms generated by alternative splicing. Embo J. 1989;8:4025–34. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Reeves SL. The mechanism by which the selective 5-HT 1A receptor antagonist S-(−)UH 301 produces head-twitches in mice. Pharmacology, Biochemistry and Behavior. 1996;55:1–10. doi: 10.1016/0091-3057(96)00072-x. [DOI] [PubMed] [Google Scholar]
- De Almeida J, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. ProgBrain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A. 2012;109:3534–9. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly S, Madeira A, Fischer J, Brisorgueil MJ, Daval G, Bernard R, Verge D, Conrath M. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J Comp Neurol. 2004;472:496–511. doi: 10.1002/cne.20082. [DOI] [PubMed] [Google Scholar]
- Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. Journal of mass spectrometry : JMS. 2010;45:1186–94. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- Du YL, Wilcox BD, Teitler M, Jeffrey JJ. Isolation and characterization of the rat 5-hydroxytryptamine type 2 receptor promoter: constitutive and inducible activity in myometrial smooth muscle cells. Molecular Pharmacology. 1994;45:1125–31. [PubMed] [Google Scholar]
- Esteve PO, Chicoine E, Robledo O, Aoudjit F, Descoteaux A, Potworowski EF, St-Pierre Y. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem. 2002;277:35150–5. doi: 10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, Graupner G, Lu XP, Pfahl M. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature. 1994;372:107–11. doi: 10.1038/372107a0. [DOI] [PubMed] [Google Scholar]
- Felder CC, ckason-Chesterfield AK, Moore SA. Cannabinoids biology: the search for new therapeutic targets. MolInterv. 2006;6:149–161. doi: 10.1124/mi.6.3.6. [DOI] [PubMed] [Google Scholar]
- Ferry RC, Molinoff PB. Regulation of 5-HT 2A receptor mRNA in P11 cells. BehavBrain Res. 1996;73:187–191. doi: 10.1016/0166-4328(96)00094-0. [DOI] [PubMed] [Google Scholar]
- Ferry RC, Unsworth CD, Molinoff PB. Effects of agonists, partial agonists, and antagonists on the regulation of 5-hydroxytryptamine 2 receptors in P11 cells. MolPharmacol. 1993;43:726–733. [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, Mackerell AD, Jr, Brezina V, Sealfon SC, Filizola M, Gonzalez-Maeso J, Logothetis DE. Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell. 2011;147:1011–23. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Borroto-Escuela DO, Frankowska M, Ferraro L, Guidolin D, Ciruela F, Agnati LF. The changing world of G protein-coupled receptors: from monomers to dimers and receptor mosaics with allosteric receptor-receptor interactions. Journal of Receptor and Signal Transduction Research. 2010;30:272–83. doi: 10.3109/10799893.2010.506191. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB(2) receptors induces anxiolytic-like actions associated to alterations in GABA(A) receptors. Br J Pharmacol. 2011;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol. 2010;160:1773–84. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Makarov E, Roy D, Finke-Dwyer J, Murrin LC, Gendelman HE, Poluektova L. Immunoregulation of a CB2 receptor agonist in a murine model of neuroAIDS. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2010;5:456–68. doi: 10.1007/s11481-010-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van OJ. The environment and schizophrenia: the role of cannabis use. SchizophrBull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Characterization and localization of cannabinoid receptors in brain: an in vitro technique using slide-mounted tissue sections. NIDA ResMonogr. 1991;112:129–145. [PubMed] [Google Scholar]
- Hill MN, Sun JC, Tse MT, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. IntJNeuropsychopharmacol. 2006;9:277–286. doi: 10.1017/S1461145705005651. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–33. [PubMed] [Google Scholar]
- Horner KA, Gilbert YE, Noble ES. Differential regulation of 5-HT(2A) receptor mRNA expression following withdrawal from a chronic escalating dose regimen of D-amphetamine. Brain Res. 2011;1390:10–20. doi: 10.1016/j.brainres.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. HandbExpPharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A. 1997;94:5826–30. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson PB, Kuchera SL, Metz A, Schachtele C, Imre K, Schrier DJ. Anti-inflammatory properties of Go 6850: a selective inhibitor of protein kinase C. J Pharmacol Exp Ther. 1995;275:995–1002. [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–6. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Current Opinion in Cell Biology. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7731–6. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Ahn KS, Han H, Choung SY, Choi SY, Kim IH. Decursin and PDBu: two PKC activators distinctively acting in the megakaryocytic differentiation of K562 human erythroleukemia cells. Leuk Res. 2005;29:1407–13. doi: 10.1016/j.leukres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kindlundh-Hogberg AM, Svenningsson P, Schioth HB. Quantitative mapping shows that serotonin rather than dopamine receptor mRNA expressions are affected after repeated intermittent administration of MDMA in rat brain. Neuropharmacology. 2006;51:838–847. doi: 10.1016/j.neuropharm.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Kuepper R, van OJ, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok RP, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SG, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–6. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-analysis. Archives of General Psychiatry. 2011;68:555–61. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–5. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lohse MJ. G protein-coupled receptors: too many dimers? Nat Methods. 2006;3:972–3. doi: 10.1038/nmeth1206-972. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz S, Faron-Gorecka A, Kedracka-Krok S, Dziedzicka-Wasylewska M. Effect of clozapine on the dimerization of serotonin 5-HT(2A) receptor and its genetic variant 5-HT(2A)H425Y with dopamine D(2) receptor. European Journal of Pharmacology. 2011;659:114–23. doi: 10.1016/j.ejphar.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz S, Polit A, Kedracka-Krok S, Wedzony K, Mackowiak M, Dziedzicka-Wasylewska M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochimica et Biophysica Acta. 2010;1803:1347–58. doi: 10.1016/j.bbamcr.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–7. [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Molecular Pharmacology. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- Mischak H, Kolch W, Goodnight J, Davidson WF, Rapp U, Rose-John S, Mushinski JF. Expression of protein kinase C genes in hemopoietic cells is cell-type- and B cell-differentiation stage specific. J Immunol. 1991;147:3981–7. [PubMed] [Google Scholar]
- Montezinho LP, Castro MM, Duarte CB, Penschuck S, Geraldes CF, Mork A. The interaction between dopamine D2-like and beta-adrenergic receptors in the prefrontal cortex is altered by mood-stabilizing agents. J Neurochem. 2006;96:1336–48. doi: 10.1111/j.1471-4159.2005.03654.x. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murineddu G, Lazzari P, Ruiu S, Sanna A, Loriga G, Manca I, Falzoi M, Dessi C, Curzu MM, Chelucci G, Pani L, Pinna GA. Tricyclic pyrazoles. 4. Synthesis and biological evaluation of analogues of the robust and selective CB2 cannabinoid ligand 1-(2′,4′-dichlorophenyl)-6-methyl-N-piperidin-1-yl-1,4-dihydroindeno[1,2-c ]pyrazole-3-carboxamide. Journal of Medicinal Chemistry. 2006;49:7502–12. doi: 10.1021/jm060920d. [DOI] [PubMed] [Google Scholar]
- Nam J, Kim K. Abnormal motor function and the expression of striatal dopamine D2 receptors in manganese-treated mice. Biological & pharmaceutical bulletin. 2008;31:1894–7. doi: 10.1248/bpb.31.1894. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–8. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain Neuronal CB2 Cannabinoid Receptors in Drug Abuse and Depression: From Mice to Human Subjects. PLoSONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K, Li J, Corbit KC, Rumilla KM, Soh JW, Weinstein IB, Albanese C, Pestell RG, Rosner MR, Hershenson MB. Regulation of airway smooth muscle cyclin D1 transcription by protein kinase C-delta. American journal of respiratory cell and molecular biology. 2002;27:204–13. doi: 10.1165/ajrcmb.27.2.20010016oc. [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Fernandes JC, Brunet J, Moldovan F, Schrier D, Flory C, Martel-Pelletier J. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum. 2003;48:1582–1593. doi: 10.1002/art.11014. [DOI] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, Becker A, Rothenberger A, Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–91. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Jachimczuk O. Antidepressant--like properties of ACEA (arachidonyl-2-chloroethylamide), the selective agonist of CB1 receptors. Acta poloniae pharmaceutica. 2004;61:165–7. [PubMed] [Google Scholar]
- Schiller L, Donix M, Jahkel M, Oehler J. Serotonin 1A and 2A receptor densities, neurochemical and behavioural characteristics in two closely related mice strains after long-term isolation. ProgNeuropsychopharmacolBiolPsychiatry. 2006;30:492–503. doi: 10.1016/j.pnpbp.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. Faseb J. 1995;9:726–35. [PubMed] [Google Scholar]
- Singh R, Jia C, Garcia F, Carrasco G, Battaglia G, Muma N. Activation of the JAK-STAT pathway by olanzapine is necessary for desensitization of serotonin2A receptor-stimulated phospholipase C signaling in rat frontal cortex but not serotonin2A receptor-stimulated hormone release. J Psychopharmacol. 2009;24:1079–88. doi: 10.1177/0269881109103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Shi J, Zemaitaitis BW, Muma NA. Olanzapine increases RGS7 protein expression via stimulation of the Janus tyrosine kinase-signal transducer and activator of transcription signaling cascade. Journal of Pharmacology and Experimental Therapeutics. 2007;322:133–140. doi: 10.1124/jpet.107.120386. [DOI] [PubMed] [Google Scholar]
- Sobolewski A, Jourdan KB, Upton PD, Long L, Morrell NW. Mechanism of cicaprost-induced desensitization in rat pulmonary artery smooth muscle cells involves a PKA-mediated inhibition of adenylyl cyclase. American journal of physiology Lung cellular and molecular physiology. 2004;287:L352–9. doi: 10.1152/ajplung.00270.2003. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–92. [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–81. [PubMed] [Google Scholar]
- Toya Y, Schwencke C, Ishikawa Y. Forskolin derivatives with increased selectivity for cardiac adenylyl cyclase. J Mol Cell Cardiol. 1998;30:97–108. doi: 10.1006/jmcc.1997.0575. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Anderson V, Minkoff H, Hurd YL. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. 2004;56:909–15. doi: 10.1016/j.biopsych.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Lira A, Zhou M, Bradley-Moore M, Merker R, Gingrich JA. Altered anxiety-related behaviors in 5-HT2A receptor knock out mice. Neuroscience Abst. 2004:468.10. [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995;34:669–76. doi: 10.1016/0028-3908(95)00027-4. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT 2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. Journal of Pharmacology and Experimental Therapeutics. 1997;282:699–706. [PubMed] [Google Scholar]
- Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, Torres M, Burguete MC, Manzanares J, Lizasoain I, Moro MA. Cannabinoid Type 2 Receptor Activation Downregulates Stroke-Induced Classic and Alternative Brain Macrophage/Microglial Activation Concomitant to Neuroprotection. Stroke. 2011;43:211–9. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Zhang XF, Zhang JQ, Tian YM, Xue H, Yang N, Zhu JX. Beta-adrenoceptors, but not dopamine receptors, mediate dopamine-induced ion transport in late distal colon of rats. Cell Tissue Res. 2008;334:25–35. doi: 10.1007/s00441-008-0661-1. [DOI] [PubMed] [Google Scholar]