Abstract
Objective
To determine the sex and age distribution of aquaporin-4 (AQP4) autoimmunity using data derived from clinical service laboratory testing of 56 464 patient samples.
Design
Observational analysis.
Setting
Mayo Clinic Neuroimmunology Laboratory.
Patients
Between October 1, 2005, and January 4, 2011, 56 464 patients were tested for AQP4-IgG; 2960 (5.2%) patients were seropositive.
Main Outcome Measure
Seropositivity for AQP4-IgG.
Results
Patients seropositive for AQP4-IgG were older than seronegative patients (mean [SD] age, 46 [16] vs 42 [15] years, respectively; P<.001). More females than males were tested (37 662 vs 16 810, respectively; P<.001). Among 2743 seropositive patients, 146 (5.3%) were pediatric (aged ≤18 years) and 333 (12.1%) were elderly (aged ≥65 years). The sex distribution of seropositive patients was 2465 females and 306 males (absolute female:male ratio, 8.1:1; P<.001). After adjusting for the number of females tested, an excess of females persisted (adjusted female:male ratio, 3.6:1). Female predominance for AQP4-IgG was more striking in adults (absolute female:male ratio, 8.4:1; adjusted female:male ratio, 3.5:1) than in pediatric patients (absolute female: male ratio, 4.3:1; adjusted female:male ratio, 2.9:1) (P<.001). Elderly women were more likely to be seropositive than individuals in other age categories (13.1% vs 6.0%, respectively; P<.001). The proportion of AQP4-IgG–seropositive individuals (detection rate), defined by decade of age, increased exponentially in women after age 50 years.
Conclusions
Seropositivity for AQP4-IgG occurs predominantly in females, particularly in individuals older than 18 years. Among seropositive patients, 1 in 6 is in the extremes of age. The detection rate of AQP4-IgG increased in women after age 50 years.
Neuromyelitis optica (NMO) is an inflammatory autoimmune demyelinating disease of the central nervous system characterized by relapsing optic neuritis and transverse myelitis and may cause blindness and paraplegia.1 It is frequently misdiagnosed as multiple sclerosis. It is the first inflammatory demyelinating central nervous system disorder for which a specific target antigen, the astrocytic water channel aquaporin-4 (AQP4), has been identified.2 The AQP4-specific autoantibody (NMO-IgG) is a clinically validated serum biomarker that distinguishes relapsing NMO and its partial or inaugural forms (NMO spectrum disorders) from multiple sclerosis.2,3 Epidemiological data unanimously indicate that women are more prone to develop NMO, but the magnitude of the female prevalence varies considerably between studies.4 Studies using clinical diagnostic criteria to identify patients with NMO1,5 tend to report lower female:male ratios (range, 2.4–4.1:1),6–10 while studies identifying individuals on the basis of AQP4-IgG seropositivity report significantly higher female:male ratios (range 7–12.1:1)11–13 (Table). Although children13,14 and elderly individuals15 are recognized to be vulnerable to developing NMO, study of sex differences among patients of different ages has been limited by inadequately powered sample sizes.
Table 1.
Table Comparison of Selected Studies Reporting Sex and Age Distribution for Patients With Neuromyelitis Optica or Aquaporin-4 Autoimmunity
| Source | Inclusion Criteria | Study Population (Patients, No.) | Female:Male Ratio | Onset Age, y | Positive AQP4-IgG Status, % |
|---|---|---|---|---|---|
| Collongues et al,7 2010 | Clinical diagnosis5 | Adult (125) | 3:1 | Mean, 34.5 (range, 4–66)a | 54b |
| Rivera et al,9 2008 | Clinical diagnosis1 | Adult (34) | 2.4:1 | Mean, 34 | Not tested |
| Bichuetti et al,10 2009 | Mostly clinical | Adult (41) | 2.4:1 | Mean, 32.6 (range, 20–60) | 41c |
| Ghezzi et al,8 2004 | Clinical diagnosis | Adult, pediatric (46) | 4.1:1 | Mean ± SD, 40 ± 16.3 (range, 12–77) | Not tested |
| Wingerchuk and Weinshenker,6 2003 | Clinical diagnosis1 | Adult, pediatric (80) | 2.5:1 | Median, 29 (monophasic) or 41 (relapsing) | Not tested |
| Jarius et al,12 2011 | AQP4-IgG seropositivity | Adult, pediatric (89) | 12.1:1 | Median, 39.5 (range, 14–79)d | 100 |
| Nagaishi et al,11 2011 | AQP4-IgG seropositivity | Adult, pediatric (583) | 10.7:1 | Median, 44 (range, 3–86) | 100 |
| McKeon et al,13 2008 | AQP4-IgG seropositivity | Pediatric (88) | 7:1 | Median, 12 (range, 4–18) | 100 |
| Current study | AQP4-IgG seropositivity | Adult, pediatric (56 464) | Unadjusted, 8.1:1; adjusted, 3.6:1 | Mean, 46 (16)d | 100 |
Abbreviation: AQP4, aquaporin-4.
Among the patients, 9.6% had onset before age 18 years and 16% had onset after age 50 years.
A total of 111 patients were tested.
A total of 17 patients were tested.
Refers to patient age at the time of AQP4-IgG testing.
From 2004 until 2011, the Mayo Clinic Neuroimmunology Laboratory served as the only clinical reference center for AQP4-IgG evaluation in the United States. The aim of this study is to determine the sex and age distribution of AQP4 autoimmunity using data derived from AQP4-IgG testing in this laboratory.
METHODS
Between October 1, 2005 (formal date of service testing implementation), and January 4, 2011, 56 464 serum or cerebrospinal fluid specimens were tested for AQP4-IgG. Only 1 specimen was counted for patients with more than 1 specimen tested (whichever was first positive) and the age recorded was when AQP4-IgG was first detected. Seropositivity for AQP4-IgG was detected in 2960 patients (5.2%). Information on sex and age was available from the test request form for 2771 and 2743 patients, respectively. For the 53 504 patients whose results were negative, sex and age information was available for 51 701 and 50 853 patients, respectively. To ascertain the clinical manifestations of AQP4 autoimmunity, clinical records of Mayo Clinic patients (95 of the 2960 seropositive study patients [3.2%]) were reviewed. Fifty-five percent of the Mayo Clinic patients fulfilled 2006 clinical diagnostic criteria for NMO5 (excluding AQP4-IgG status), and 45% had an NMO spectrum disorder3 (including monophasic or recurrent optic neuritis or transverse myelitis, or circumventricular organ or cerebral syndromes).
Testing for AQP4-IgG was performed by a clinically validated indirect immunofluorescence assay on a substrate comprising normal adult mouse brain, kidney, and gut tissues.2,16 The study protocol was reviewed and approved by the Mayo Clinic Institutional Review Board.
Descriptive summaries, for age as a continuous variable, were reported as mean (standard deviation) and range. Categorical variables were summarized as frequencies and percentages. Comparison of age by seropositive status was performed using 2-sample t test, while comparisons between 2 categorical variables, such as seropositivity status vs sex or distribution of sex across age groups, were performed using χ2 tests. Statistical analysis was performed using SAS version 9.2 statistical software (SAS Institute, Inc). All tests were 2-sided, and P < .05 was considered statistically significant.
RESULTS
The mean (SD) age of individuals for whom AQP4-IgG testing was requested was 42.3 (14.9) years (range, 1–99 years); testing was requested more frequently for females than males (37 662 females vs 16 810 males; sex ratio, 2.2:1; P < .001). Seropositive patients were older than seronegative patients (mean [SD] age, 46 [16] vs 42 [15] years, respectively; P < .001).
FEMALE PREDOMINANCE IN AQP4 AUTOIMMUNITY
Of 2771 seropositive patients, 2465 were female and 306 were male (absolute female:male ratio, 8.1:1; P < .001). Subgroup analysis of Mayo Clinic seropositive patients revealed a comparable female-predominant absolute female:male ratio (6.9:1). Of the 51 701 seronegative patients, 35 197 were female and 16 504 were male (absolute female:male ratio, 2.1:1; P < .001). Corrections were performed to take into account the disproportionate number of females tested. After these adjustments, the female predominance persisted (female:male ratio, 3.6:1; 2465 seropositive females of 37 662 total females [6.5%] vs 306 seropositive males of 16 810 total males [1.8%]).
FEMALE PREDOMINANCE HIGHEST IN ELDERLY PATIENTS AND LOWEST IN CHILDREN
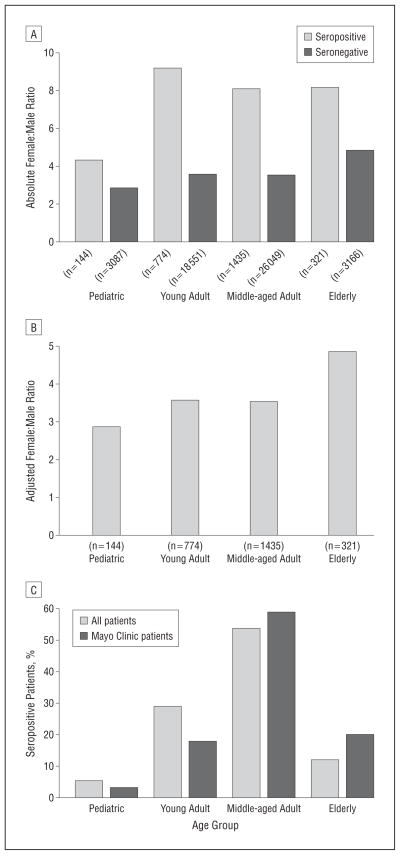
To investigate the sex differences for seropositive patients according to age, we stratified individuals to 4 age groups (Figure 1A and B): pediatric (≤18 years), young adult (19–39 years), middle-aged adult (40–64 years), and elderly (≥65 years). Female predominance was more striking in adult patients (aged ≥19 years; absolute female: male ratio, 8.4:1; female:male ratio adjusted for bias in test request frequency, 3.5:1) compared with pediatric patients (absolute female:male ratio, 4.3:1; adjusted female:male ratio, 2.9:1) (P < .001). Subgroup analysis of Mayo Clinic seropositive patients revealed similar sex differences stratified by age when compared with the primary cohort.
Figure 1.
Female preponderance and extremes of age of aquaporin-4 autoimmunity. A, The female:male ratios of 2771 patients testing positive for aquaporin-4–IgG are compared in 4 age groups: pediatric (≤18 years), young adult (19–39 years), middle-aged adult (40–64 years), and elderly (≥65 years). The absolute female:male sex ratios of seropositive patients are 4.3:1 in the pediatric group, 9.2:1 in young adults, 8.1:1 in middle-aged adults, and 8.2:1 in the elderly group. B, The corrected sex ratios of seropositive patients are 2.9:1 in the pediatric group, 3.6:1 in young adults, 3.5:1 in middle-aged adults, and 4.9:1 in the elderly group. C, The age distribution of 2743 patients testing positive for aquaporin-4–IgG is shown. Subgroup analysis findings of the Mayo Clinic cohort are comparable.
EXTREMES OF AGE ACCOUNT FOR 1 IN 6 PATIENTS WITH AQP4 AUTOIMMUNITY
Pediatric and elderly patients accounted for 12.6% of those tested. Of the 2743 seropositive patients, 146 (5.3%) were pediatric and 333 (12.1%) were elderly. Thus, approximately 1 in 6 AQP4-IgG–seropositive patients were at the extremes of age. Findings were comparable for the Mayo Clinic cohort (Figure 1C).
INCREASED DETECTION RATE OF AQP4-IgG IN WOMEN OLDER THAN 50 YEARS
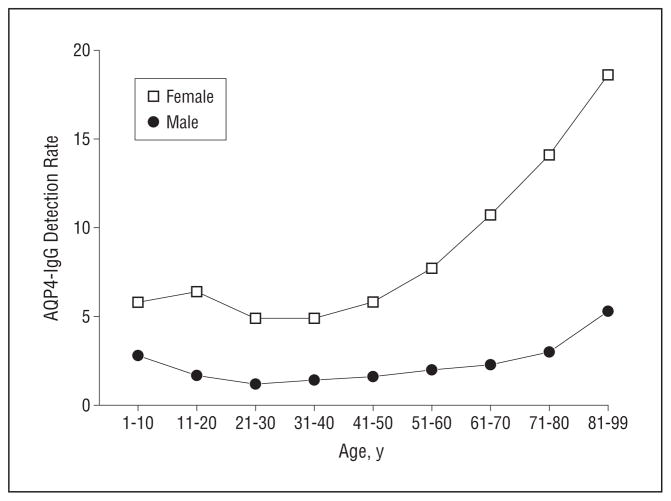
Women in the elderly group were more likely to test positive than those in other age categories (13.1% vs 6.0%, respectively; P < .001). A more detailed analysis of detection rates (proportion of seropositive individuals defined by decade of age) revealed an exponential increase in the AQP4-IgG detection rate after age 50 years, especially for women (Figure 2).
Figure 2.
Analysis of detection rates (proportion of seropositive individuals defined by decade of age) of aquaporin-4 (AQP4)–IgG in females and males revealed a higher detection rate for females compared with males. An increased likelihood of detecting AQP4 autoimmunity was found in females after age 50 years, with the female detection rate increasing exponentially from ages 51 to 60 years.
COMMENT
This seroepidemiologic study of AQP4-IgG status among 56 464 patients tested on a service basis reports several important new observations: (1) females are more likely to be seropositive than males, even after correcting for excess testing in females, and this female predominance is more striking in adults than in children; (2) one-sixth of AQP4-IgG seropositivity was detected in children and elderly patients; and (3) the detection rate of AQP4-IgG increases exponentially in women older than 50 years.
Previous studies of female:male ratios in patients with NMO have reported values ranging from 2.4:1 to 12.1:1. These inconsistencies are likely explained by differences in 1 or more of the following: patient acquisition (clinically acquired vs referral for serological evaluation), regional or international patient referral patterns (to either general neurology or demyelinating disease clinics and testing laboratories), and disease biology (among AQP4-IgG–seropositive and AQP4-IgG–seronegative patients). Some clinic-based studies have reported the proportion of AQP4-IgG–seropositive patients but do not mention sex ratios among seropositive and seronegative patients.7,10 We recently reported a clinic-based study of 99 consecutive Mayo Clinic patients with NMO treated with azathioprine; the female:male ratios in that cohort were 4:1 in all 93 AQP4-IgG–tested patients, 6:1 in AQP4-IgG–seropositive patients, and 2:1 in AQP4-IgG–seronegative patients.17 Thus, while a female:male ratio of 8.1:1 in the current study may be an overestimate, current evidence points to the female preponderance being stronger among cohorts of seropositive patients than among clinically acquired cohorts and seronegative patients. Future population-based serological studies of demyelinating disorders will add further to our understanding.
Although the evidence for humoral immune mechanisms underlying the pathogenesis of NMO is compelling at this stage, the etiology remains unknown. Genetic susceptibility,18 infections,19 and cancer associations20 have been implicated. Our observation of an exponentially increasing AQP4-IgG detection rate in elderly women (aged >50 years) compared with elderly men suggests that aging and/or sex hormones may influence the development of AQP4 autoimmunity and its associated spectrum of inflammatory central nervous system disorders. Furthermore, the female predominance was greatest in elderly patients and least in children. These findings are in contrast with those by Nagaishi et al,11 which suggested a decreasing female predominance with age. It is unclear whether the small sample size of the latter study, which comprised 9 patients younger than 15 years and 60 patients older than 60 years, could have led to erroneous representations of sex differences in different age groups.
In a disease typically regarded as disproportionately affecting middle-aged patients, we found that approximately one-sixth were at the extremes of age (pediatric and elderly). These findings agree with observations reported in a French study7: 25% of patients with NMO had disease onset before age 19 years or later than age 50 years. The heterogeneous cerebral presentations of AQP4 autoimmunity in the pediatric group13 underscore the importance of serological testing to aid clinical distinction from acute disseminated encephalomyelitis. The high rate of AQP4-IgG detection in elderly patients, particularly women, is also noteworthy. Although aging and menopause could contribute to immune dysfunction leading to AQP4 autoimmunity, it remains possible that this observation may relate to different clinical thresholds for AQP4-IgG testing applied for different age groups, ie, lower threshold for testing in younger individuals compared with elderly individuals. These findings emphasize the importance of considering NMO spectrum disorders in the differential diagnosis of a new-onset central nervous system disorder occurring at the extremes of age.
Our study has several limitations. The study population comprises patients with heterogeneous clinical manifestations for which AQP4-IgG testing was requested. Details of these manifestations, however, were not available for this analysis except for the Mayo Clinic patients. Furthermore, some seropositive cases may have been missed owing to limited assay sensitivities or immunosuppressant therapy reducing antibody levels below the assay detection threshold. Notwithstanding, this study, which reports observations from the only clinical laboratory in the United States performing this antibody test on a service basis until 2011, raises pertinent questions relating to the influence of sex hormones and aging on AQP4 autoimmunity. The cross-sectional study design does not provide information on disease onset and its evolution. Future prospective studies are warranted to investigate their contributions to production of AQP4-IgG and clinical manifestations of NMO spectrum disorders.
Acknowledgments
Funding/Support: This work was supported by the Mayo Clinic Foundation, a grant from the Guthy-Jackson Charitable Foundation, and grant NS065829 from the National Institutes of Health.
Footnotes
Additional Contributions: Debby Cheung, BS, provided technical assistance, Karen Brekke provided study coordination, and Evelyn Posthumus and Connie Brekke provided manuscript assistance.
Authors Contributions: Study concept and design: Quek and Pittock. Acquisition of data: Quek, McKeon, Jiao, Weinshenker, Lucchinetti, Shuster, and Pittock. Analysis and interpretation of data: Quek, McKeon, Lennon, Mandrekar, Iorio, Costanzi, Wingerchuk, and Pittock. Drafting of the manuscript: Quek and Pittock. Critical revision of the manuscript for important intellectual content: Quek, McKeon, Lennon, Mandrekar, Iorio, Jiao, Costanzi, Weinshenker, Wingerchuk, Lucchinetti, Shuster, and Pittock. Statistical analysis: Quek and Mandrekar. Obtained funding: Pittock. Administrative, technical, and material support: Jiao. Study supervision: Pittock.
Financial Disclosure: Dr Lennon is a named inventor on a patent (7101679 issued 2006) relating to AQP4 antibodies for diagnosis of NMO and receives royalties for this technology; is a named inventor on patents (12/ 678 350 filed 2010 and 12/573 942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; and receives research support from the Guthy-Jackson Charitable Foundation and National Institutes of Health. Dr Weinshenker serves on data safety monitoring boards for Novartis and Biogen Idec; serves on the editorial boards of the Canadian Journal of Neurological Sciences, Multiple Sclerosis Journal, and Turkish Journal of Neurology; has received research support from Genzyme Corp and the Guthy-Jackson Charitable Foundation; and receives license royalties for a patent relating to AQP4 antibodies for diagnosis of NMO. Dr Wingerchuk has received research support from Alexion Pharmaceutics, Inc, Caridian BCT, Genzyme, Genentech, and the Guthy-Jackson Charitable Foundation. Dr Lucchinetti receives royalties from the publication of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); receives research support from the National Institutes of Health, the National Multiple Sclerosis Society, and the Guthy-Jackson Charitable Foundation; and receives license royalties for a patent relating to AQP4 antibodies for diagnosis of NMO. Dr Pittock is a named inventor on patents (12/678 350 filed 2010 and 12/ 573 942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker and receives research support from Alexion Pharmaceuticals, Inc, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health.
References
- 1.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53(5):1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 2.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364 (9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM. Neuromyelitis optica: effect of gender. J Neurol Sci. 2009;286 (1–2):18–23. doi: 10.1016/j.jns.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology. 2003;60(5):848–853. doi: 10.1212/01.wnl.0000049912.02954.2c. [DOI] [PubMed] [Google Scholar]
- 7.Collongues N, Marignier R, Zéphir H, et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology. 2010;74(9):736–742. doi: 10.1212/WNL.0b013e3181d31e35. [DOI] [PubMed] [Google Scholar]
- 8.Ghezzi A, Bergamaschi R, Martinelli V, et al. Italian Devic’s Study Group (IDESG) Clinical characteristics, course and prognosis of relapsing Devic’s neuromyelitis optica. J Neurol. 2004;251(1):47–52. doi: 10.1007/s00415-004-0271-0. [DOI] [PubMed] [Google Scholar]
- 9.Rivera JF, Kurtzke JF, Booth VJA, Corona T. Characteristics of Devic’s disease (neuromyelitis optica) in Mexico. J Neurol. 2008;255(5):710–715. doi: 10.1007/s00415-008-0781-2. [DOI] [PubMed] [Google Scholar]
- 10.Bichuetti DB, Oliveira EM, Souza NA, Rivero RL, Gabbai AA. Neuromyelitis optica in Brazil: a study on clinical and prognostic factors. Mult Scler. 2009;15 (5):613–619. doi: 10.1177/1352458508101935. [DOI] [PubMed] [Google Scholar]
- 11.Nagaishi A, Takagi M, Umemura A, et al. Clinical features of neuromyelitis optica in a large Japanese cohort: comparison between phenotypes. J Neurol Neurosurg Psychiatry. 2011;82(12):1360–1364. doi: 10.1136/jnnp-2011-300403. [DOI] [PubMed] [Google Scholar]
- 12.Jarius S, Paul F, Franciotta D, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. 2011;306(1–2):82–90. doi: 10.1016/j.jns.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 13.McKeon A, Lennon VA, Lotze T, et al. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71(2):93–100. doi: 10.1212/01.wnl.0000314832.24682.c6. [DOI] [PubMed] [Google Scholar]
- 14.Banwell B, Tenembaum S, Lennon VA, et al. Neuromyelitis optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology. 2008;70(5):344–352. doi: 10.1212/01.wnl.0000284600.80782.d5. [DOI] [PubMed] [Google Scholar]
- 15.Filley CM, Sternberg PE, Norenberg MD. Neuromyelitis optica in the elderly. Arch Neurol. 1984;41(6):670–672. doi: 10.1001/archneur.1984.04210080082019. [DOI] [PubMed] [Google Scholar]
- 16.McKeon A, Fryer JP, Apiwattanakul M, et al. Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol. 2009;66(9):1134–1138. doi: 10.1001/archneurol.2009.178. [DOI] [PubMed] [Google Scholar]
- 17.Costanzi C, Matiello M, Lucchinetti CF, et al. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77(7):659–666. doi: 10.1212/WNL.0b013e31822a2780. [DOI] [PubMed] [Google Scholar]
- 18.Matiello M, Kim HJ, Kim W, et al. Familial neuromyelitis optica. Neurology. 2010;75(4):310–315. doi: 10.1212/WNL.0b013e3181ea9f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga M, Takahashi T, Kawai M, Fujihara K, Kanda T. A serological analysis of viral and bacterial infections associated with neuromyelitis optica. J Neurol Sci. 2011;300(1–2):19–22. doi: 10.1016/j.jns.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Pittock SJ, Lennon VA. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch Neurol. 2008;65(5):629–632. doi: 10.1001/archneur.65.5.629. [DOI] [PubMed] [Google Scholar]