Abstract
Background
Myocardial infarct size is a strong predictor of cardiovascular events. Intravenous metoprolol before coronary reperfusion has been shown to reduce infarct size; however, it is unknown whether oral metoprolol initiated early after reperfusion, as clinical guidelines recommend, is similarly cardioprotective. We compared the extent of myocardial salvage associated with intravenous pre-reperfusion-metoprolol administration in comparison with oral post-reperfusion-metoprolol or placebo. We also studied the effect on suspected markers of reperfusion injury.
Methods
Thirty Yorkshire-pigs underwent a reperfused myocardial infarction, being randomized to pre-reperfusion-metoprolol, post-reperfusion-metoprolol or placebo. Cardiac magnetic resonance imaging was performed in eighteen pigs at day 3 for the quantification of salvaged myocardium. The amounts of at-risk and infarcted myocardium were quantified using T2-weighted and post-contrast delayed enhancement imaging, respectively. Twelve animals were sacrificed after 24 h for reperfusion injury analysis.
Results
The pre-reperfusion-metoprolol group had significantly larger salvaged myocardium than the post-reperfusion-metoprolol or the placebo groups (31±4%, 13±6%, and 7±3% of myocardium at-risk respectively). Post-mortem analyses suggest lesser myocardial reperfusion injury in the pre-reperfusion-metoprolol in comparison with the other 2 groups (lower neutrophil infiltration, decreased myocardial apoptosis, and higher activation of the salvage-kinase phospho-Akt). Salvaged myocardium and reperfusion injury pair wise comparisons proved there were significant differences between the pre-reperfusion-metoprolol and the other 2 groups, but not among the latter two.
Conclusions
The intravenous administration of metoprolol before coronary reperfusion results in larger myocardial salvage than its oral administration initiated early after reperfusion. If confirmed in the clinical setting, the timing and route of β-blocker initiation could be revisited.
Keywords: Myocardial infarction, Reperfusion injury, Beta-blockers, MRI, Cardioprotection, Myocardial salvage
1. Introduction
The intravenous (i.v.) administration of the β-blocker metoprolol during ongoing ischemia has been shown to increase myocardial salvage [1]. However, the mechanisms responsible for the observed cardioprotection remain elusive. Clinical guidelines recommend the oral administration of β-blockers in patients with acute myocardial infarction (MI) [2]; though, whether the oral administration of metoprolol, initiated early after coronary reperfusion, as current clinical practice guidelines recommend [2], exerts similar effect on myocardial salvage is also unknown.
Indirect evidence suggest that the early i.v. initiation of β-blockers before mechanical reperfusion result in less cardiovascular events and improved myocardial recovery [3], however a study systematically comparing the pre-reperfusion β-blocker administration vs. the oral post-reperfusion route of administration is lacking. The understanding of the impact of the time of β-blocker initiation may have clinical implications, since the early i.v. route of β-blocker initiation is not encouraged [2,4].
Recent literature supports the notion that metoprolol can block the migration of neutrophils both in vitro [5] and in vivo [6], and also display antiapoptotic properties in different conditions like heart failure [7]. Both mechanisms (neutrophil infiltration and apoptosis) have been proposed as major contributors to reperfusion injury [8,9]. Therefore, we hypothesized that the cardioprotective effect of metoprolol could be mediated via reduction in reperfusion injury, and therefore restricted to its administration before the restoration of coronary flow.
The aims of the present work were to compare the extent of myocardial salvage associated with the pre-reperfusion i.v. administration of metoprolol in comparison with its oral post-reperfusion initiation. We additionally studied the effect of metoprolol administration on suspected markers of myocardial reperfusion injury.
2. Materials and methods
2.1. Study design
A reperfused anterior MI was induced in thirty male Yorkshire Albino pigs (weight 28±0.5 kg). Animals were randomized 1:1:1 to 1) i.v. metoprolol before reperfusion followed by oral metoprolol after reperfusion (pre-reperfusion-metoprolol group), 2) i.v. placebo before reperfusion followed by oral metoprolol after reperfusion (post-reperfusion-metoprolol group) or 3) i.v. placebo before reperfusion alone (placebo group). Eighteen animals underwent cardiac magnetic resonance imaging (MRI) 72h post-MI for the quantification of left ventricular (LV) functional parameters, area of myocardium at-risk, and MI size, as previously validated [1]. Twelve pigs (4 in each group) were sacrificed 24h after reperfusion for further evaluation of reperfusion injury. The study protocol was approved by the Mount Sinai School of Medicine animal research committee. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals.”
2.2. Experimental procedures
Acute MIs were experimentally created as previously described [1]. In summary, anaesthesia was induced by intramuscular injection of ketamine (30 mg/kg), xylazine (2.2 mg/kg), and atropine (0.05 mg/kg). Animals underwent endotracheal intubation, and anesthesia was maintained by isofluorane inhalation. A continuous infusion of amiodarone (300 mg, 75 mg/h) was initiated at the beginning of the procedure in all pigs as prophylaxis for malignant ventricular arrhythmias [1]. The left anterior descending (LAD) coronary artery was occluded for 90min with an angioplasty balloon via percutaneous femoral approach. The site of occlusion was immediately distal to the origin of the first diagonal branch. Thirty minutes after coronary occlusion, 7.5 mg of i.v. metoprolol were infused in the pre-reperfusion-metoprolol group: three 2.5 mg boluses of metoprolol tartrate (1 mg/ml ampuls, Hospira Inc., Lake Forest, IL, USA) every 3–5min. Animals in the post-reperfusion-metoprolol and the placebo groups received equal amounts of placebo (saline) infusion following the same timing and intervals. The medication (injectable metoprolol or placebo) was prepared in non-labelled syringes before the MI induction, and administered by operators blinded to the randomization.
Animals allocated to the pre- and the post-reperfusion-metoprolol groups received 50 mg of oral metoprolol (metoprolol tartrate, UDL Laboratories, Inc., Rockford, IL, USA) every 12h for the entire duration of each protocol, starting after reperfusion. Buprenorphine (0.03 mg/kg) and cefazoline (25 mg/kg) were additionally administered every 12h in all animals.
2.3. Non-invasive MRI protocol
For the MRI studies, pigs were anesthetized by intramuscular injection of ketamine, xylazine, and atropine. Anaesthesia was maintained by continuous propofol i.v. infusion, and animals were kept under mechanical ventilation. MRI studies were performed with a 1.5T magnet (Magneton Sonata©, Siemens Medical Solutions, Erlangen, Germany) by operators blinded to the study arm, following a protocol previously reported [1]. In summary, images were acquired with electrocardiographic gating and during suspended respiration. First, contiguous short-axis cine images covering the LV from base to apex (slice thickness 6 mm, no gap) were acquired for the quantification of functional LV parameters. Oedema imaging (for the quantification of area of myocardium at-risk) [10] was performed with a T2-weighted, triple inversion-recovery fast spin-echo sequence. Delayed enhancement (DE) imaging was performed 15min after the administration of 0.2 mmol/kg of gadopentate dimeglumine using an inversion-recovery fast gradient echo sequence. The slice positions for both T2-weighted and DE acquisitions matched those of the cine images. Detailed imaging parameters have been previously reported [1].
2.4. MRI data analysis
All MRI images were analyzed blinded to the study allocation. LV function analysis was performed using dedicated software (Argus©, Siemens Medical Solutions). Epicardial and endocardial borders were traced in each cine image to obtain LV end-diastolic volume (LVEDV), end-systolic volume (LVESV) and LVEF. The area of myocardium at-risk was defined as the extent of the LV demonstrating high signal intensity on T2-weighted images [11]. MI size was quantified from the extent of abnormal DE [12]. Oedema and DE were defined as those myocardial regions demonstrating signal intensity 3 standard deviations above the average signal of normal, remote myocardium, and quantified as previously validated by using prototype analysis software (VPT©, Siemens Corporate Research, New Jersey) [1]. MI size and salvaged myocardium are expressed as percentage of the area of myocardium at-risk and/or the LV. See Fig. 1.
Fig. 1.
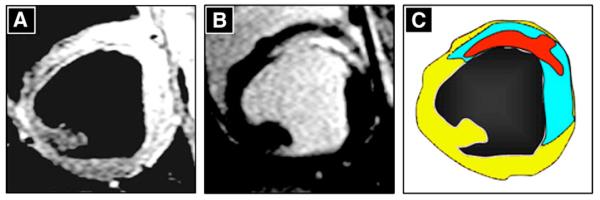
Visualization of cardioprotection by magnetic resonance imaging (MRI). MRI short axis images obtained at the same left ventricular level 3 days after MI induction in a pre-reperfusion-metoprolol-treated animal. Panel A shows a T2-weighted, fast spin-echo image, where the hyperintense area indicates the presence of edema. Panel B shows a delayed enhancement image after contrast administration, depicting the infarcted area (bright). In panel C, the area at risk (edema, blue) and infarcted area (red inside the blue region) shown in panels A and B are merged. Non-ischemic myocardium is shown in yellow. Note the large salvaged myocardium (blue area surrounding the infarcted red zone).
2.5. Reperfusion injury analysis
Reperfusion injury was examined by studying 3 different processes: 1) myocardial inflammation (neutrophil infiltration in the ischemic area) [13], 2) myocardial apoptosis [9], and 3) activation of kinase Akt, a protein representative of the Reperfusion Injury Salvage Kinase (RISK) pathway [14]. Twenty-four hours after reperfusion, twelve animals (4 in each group) were heparinised (100 IU/kg) and euthanized with pentobarbital (Sleepaway®, Fort Dodge. 75 mg/kg). The LAD was re-occluded (immediately distal to the first diagonal branch), and hearts retro-perfused with Evans Blue. Small pieces from the ischemic myocardium (negative for Evans Blue) were cut, washed carefully in sterile PBS and immediately frozen in liquid nitrogen and stored at −80 °C until processed for myeloperoxidase (MPO) activity and protein expression.
Neutrophil infiltration of the ischemic area was determined by semi-quantitative colorimetric determination of MPO activity by using a commercially available kit (Myeloperoxidase Assay Kit, CytoStore, Calgary, Alberta, Canada). Frozen myocardial tissue was pulverized and 50 μg of tissue were weighed and homogenized in the sample buffer enclosed in the kit. After adding the development reagent, the absorbance (at 450 nm) was measured at 1 min intervals. Results are expressed as MPO units per mg of tissue. Apoptosis was measured by assessing the protein expression of cleaved caspase-3. Finally, Phospho-Akt (P-Akt) protein levels were assessed in order to explore the degree of activation of the RISK pathway.
Protein expression was performed by Western blot analyses as previously reported [15]. Antibodies were purchased from StressGen (caspase-3) and cell signaling (P-Akt).
2.6. Statistical analysis
Data are expressed as mean±standard error of the mean. Statistical comparisons of means were made by ANOVA. When differences were found, Turkey’s multiple pair wise comparisons tests were performed. A value of p<0.05 (two-tailed) was considered statistically significant. All statistical analyses were performed with the statistical software package SPSS 15.0 (SPSS Inc., Chicago, IL, USA). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
3. Results
All three groups showed a similar mean heart rate during the procedure (66±2, 69±3 and 66±3bpm for the pre-reperfusion-, post-reperfusion-metoprolol and placebo groups, respectively; P = NS).
3.1. Effect of different regimes of metoprolol administration on LV function and myocardial salvage
The results of MRI-derived parameters are presented in Table 1. In summary, there were no significant differences in LV volumes or LV ejection fraction (LVEF) 72h post-MI amongst the 3 study groups. Similarly, the extent of myocardium at-risk did not significantly differ. Myocardial salvage was significantly larger only in the pre-reperfusion-metoprolol group. The extent of salvaged myocardium was 31±4% of the area at risk in the pre-reperfusion-metoprolol group, compared to 13±6% in the post-reperfusion-metoprolol group (p=0.04 vs. pre-reperfusion-metoprolol) and 7±3% in placebo group (p=0.005 vs. pre-reperfusion-metoprolol). There were no statistically significant differences in myocardial salvage between the post-reperfusion-metoprolol and placebo groups. See Fig. 2.
Table 1.
Magnetic resonance imaging-derived parameters
| Pre-reperfusion- metoprolol |
Post-reperfusion- metoprolol |
Placebo | |
|---|---|---|---|
| LVEDV (ml) | 92±5 | 97±7 | 116±12 |
| LVESV (ml) | 58±2 | 63±6 | 75±13 |
| LVEF (%) | 36.2±2.9 | 35.3±3.6 | 34.3±5.3 |
| Myocardium at-risk (% of LV) | 37.6±2.1 | 40.1±4.3 | 32.1±1.9 |
| Salvaged myocardium (% of myocardium at-risk) |
30.8±4.0*† | 13.1±6.2 | 7.4±3.2 |
LV: left ventricle; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume.
p=0.04 vs. post-reperfusion-metoprolol group.
p=0.005 vs. placebo group.
All other p values were not significant.
Data is expressed as mean±standard error of the mean. N=18 (6 per group).
Fig. 2.
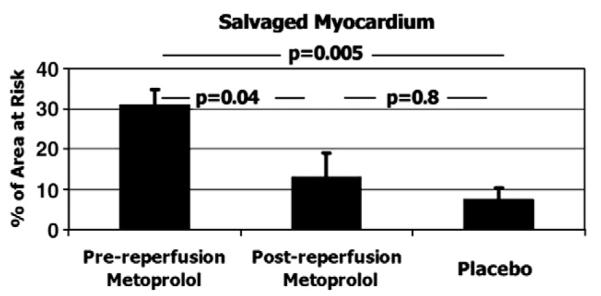
MRI-based quantification of salvaged myocardium. Data is expressed as mean±standard error of the mean. N=18 (6 per group).
3.2. Effect of different regimes of metoprolol administration on reperfusion injury
Neutrophil-related inflammation was significantly lower in the pre-reperfusion-metoprolol group compared with the other two groups: 0.27±0.02 MPO units in pre-reperfusion-metoprolol, 0.56±0.05 in post-reperfusion-metoprolol (p=0.016 vs. pre-reperfusion-metoprolol), and 0.54±0.02 in placebo (p=0.036 vs. pre-reperfusion-metoprolol). There were no significant differences between the post-reperfusion-metoprolol and placebo groups. See Fig. 3.
Fig. 3.
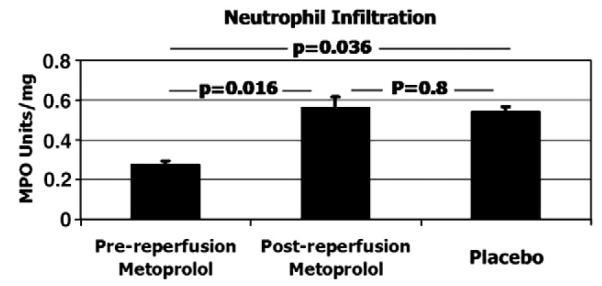
Neutrophil infiltration in the ischemic area. Myeloperoxidase (MPO) activity was determined by semi-quantitative colorimetric determination. Data is expressed as mean±standard error of the mean of MPO activity units. N=12 (4 per group).
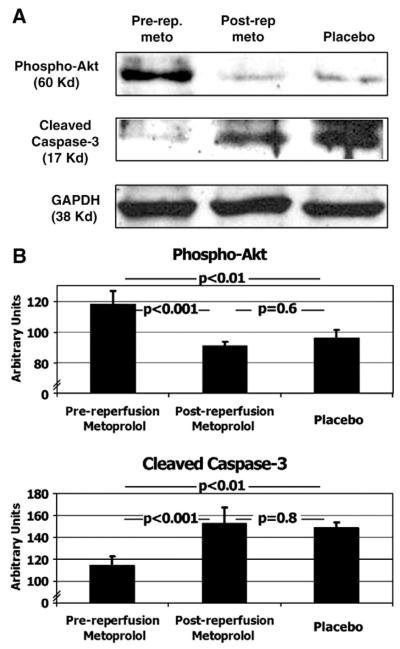
A similar effect was observed for rates of apoptosis in the ischemic myocardium. Animals allocated to the pre-reperfusion-metoprolol group showed significantly lower levels of cleaved caspase-3 in the ischemic area compared to the other two groups: 115±9 arbitrary units in pre-reperfusion-metoprolol, 153±15 in post-reperfusion-metoprolol (p<0.001 vs. pre-reperfusion-metoprolol), and 149±4 in placebo (p<0.01 vs. pre-reperfusion-metoprolol). Again, there were no significant differences between the post-reperfusion-metoprolol and placebo groups, See Fig. 4.
Fig. 4.
Protein expression of markers of reperfusion injury. Western blotting (Panel A), and its quantification by densitometric units (Panel B, expressed as mean±standard error of the mean) of phospho-Akt and cleaved caspase-3 in animals allocated to the pre-reperfusion-metoprolol, post-reperfusion-metoprolol and placebo. See also text. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). N=12 (4 per group).
Conversely, the pre-reperfusion administration of metoprolol resulted in a significantly higher degree of RISK pathway activation, as evaluated by P-Akt protein expression. Levels of P-Akt in the pre-reperfusion-metoprolol group were significantly higher compared to the other 2 groups: 118±9, 91±3, and 96±5 arbitrary units respectively. Differences were significant between the pre-reperfusion-metoprolol group and the other 2 groups (p<0.001 and p<0.01 respectively), but not between the post-reperfusion-metoprolol and placebo groups. See Fig. 4.
4. Discussion
Myocardial infarct size has been shown to be a strong predictor of cardiovascular events [16]. Beyond early reperfusion (the major determinant of cardioprotection [17]), therapies that can reduce the size of MI are strongly needed [18].
β-blockers are of clinical value in the setting of acute MI, with a large body of evidence showing mortality reductions when administered early [19]. The use of oral β-blockade constitutes a class I indication in clinical practice guidelines [20]; however, its i.v. administration during the acute phase of MI has not been universally adopted[21,22]. Furthermore, due to the current lack of substantiation of benefits, the early i.v. route of administration has been recently discouraged [2,4]. The cardioprotective effect of many interventions has been shown to be restricted to its administration either before or at early stages of reperfusion. Following our previous observation the administration of metoprolol increases myocardial salvage [1], we wanted to investigate whether its cardioprotective effect was restricted to its administration before reperfusion. Using state-of-the-art MRI, we have found that the pre-reperfusion administration of i.v. metoprolol results in a significant ≈2.5-fold increase in myocardial salvage in comparison with its oral administration only after reperfusion. To the best of our knowledge, this is the first study systematically comparing the pre-reperfusion β-blocker administration vs. the oral post-reperfusion route of administration.
In the human setting, the impact of prior β-blockade on MI size was analyzed by Sharma et al. [23]. They showed that patients that were already on β-blockers before an AMI had consistently smaller MIs than those who were not on β-blockers before the ischemic event. Even though these results, added to the pre-clinical data of the current study, might represent an argument to prescribe β-blockers in high risk patients, its practical value is unknown. Conversely, the impact of i.v. β-blockade before reperfusion (and after AMI initiation) was studied in a post-hoc retrospective analysis of the CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trial [3]. Despite there was no assessment of MI size, the 30-mortality was significantly lower in the group of patients receiving i.v. β-blockers before reperfusion [3]. In addition, the myocardial recovery at 7 months was significantly improved in the pre-reperfusion i.v.β-blocker patients [3]. Our results are in agreement with these, showing that the timing of β-blocker initiation has a significant impact on the final size of MI, being the latter a strong predictor of cardiovascular events [16]. If our results are replicated in the clinical area, the timing and route of administration of β-blockers might be revisited for patients with no contraindications.
The mechanism by which metoprolol is cardioprotective remains unknown. Potential mechanisms include the reduction of oxygen demand, secondary to heart rate reduction during ongoing ischemia, the increase of collateral flow or the reduction of reperfusion injury. In a previous study we observed that the MI reduction granted by metoprolol was independent to its negative chronotropic effect [1] in an animal model lacking collateral coronary blood flow [24]. Here, we have confirmed that the effect on myocardial salvage is independent to the heart rate achieved during ischemia, which was similar in all 3 groups. The similar heart rate in all 3 groups, regardless metoprolol infusion, was due to the administration of amiodarone as prophylaxis for malignant ventricular arrhtymias. Therefore, we speculated that metoprolol could reduce the extent of necrosis via reduction of reperfusion injury. Early neutrophil infiltration and myocardial apoptosis are among the specific pathological events associated with reperfusion injury in various organs, including heart, brain and kidney [8,9,13,25,26]. Conversely, the activation of a “programmed defense mechanism” called the RISK pathway protects the myocardium from reperfusion injury. The degree of activation of this pathway modulates the extent of myocardial salvage and thus the resulting MI size [14,27].
We observed that myocardial neutrophil infiltration was significantly reduced only in the pre-reperfusion-metoprolol group. These results suggest anti-inflammatory effects of metoprolol in ischemia–reperfusion injury, in agreement with similar observations reported after spinal cord trauma [6]. Furthermore, previous in vitro studies have evidenced the inhibition of neutrophils chemotaxis by metoprolol [5], suggesting a direct effect of the latter on neutrophils. Even though early neutrophil infiltration is considered a specific feature of ischemia/reperfusion injury, it cannot be completely ruled out that the lower inflammation was the result of a reduced ischemic insult.
In addition, we have found a significant reduction in the protein expression of cleaved caspase-3, suggesting a reduction in myocardial apoptotic death when metoprolol is initiated before reperfusion. Apoptotic cell death after an acute MI is a recognized phenomenon linked to reperfusion [26]. In fact, the administration of inhibitors of apoptosis after the onset of ischemia but before reperfusion has been shown to limit MI size [28]. This effect is not restricted to the heart. Daemen and colleagues proved that the pre-reperfusion administration of a caspase-inhibitor diminished the reperfusion injury in a model of renal ischemia. Interestingly, when the caspase-inhibitor was administered few hours after reperfusion, these reno-protective effects were completely abrogated [8]. In our work, when metoprolol was administered after reperfusion, its salutary effects on apoptosis limitation were also abrogated. The antiapoptotic activity of metoprolol has been also shown in experimental models of heart failure [7]. Our results confirm the antiapoptotic properties of metoprolol in different cardiac conditions. In this regard, another β-blocker, carvedilol, has been shown to prevent the myocardial ischemia/reperfusion-induced apoptosis in a rabbit model of MI when administered before coronary reperfusion[29]. Our results confirm the latter by showing the anti reperfusion-induced apoptotic myocardial death by a different β-blocker agent. Altogether, our results suggest that the administration of metoprolol during ongoing ischemia may reduce the ischemia–reperfusion injury, contributing to the infarct size reduction achieved by metoprolol.
5. Conclusions
In an animal model of acute myocardial infarction, we have studied the effect of metoprolol on myocardial infarct size and on suspected markers of reperfusion injury. Here we show that the cardioprotective effect of metoprolol, as evaluated 3 days post-AMI, is restricted to its administration prior to reperfusion (during ongoing ischemia). The cardioprotection granted by the intravenous pre-reperfusion-metoprolol administration was associated with a significant reduction in myocardial apoptosis and neutrophil infiltration, suggesting that metoprolol’s cardioprotective effects could be mediated, at least in part, via reduction in reperfusion injury. If confirmed in human studies, the timing and route of β-blockade in acute myocardial infarction might be revisited.
Acknowledgements
BI was granted by the Working Group on Ischemic Heart Disease of the Spanish Society of Cardiology, SP by a Fellowship of Fundación CajaMadrid, and GV is a Juan de la Cierva investigator of MEC. GV and LB are members of CIBEROBN-Institute Carlos III and are funded by PNS 2006-10091 from MEC, Spain.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [30].
References
- [1].Ibanez B, Prat-Gonzalez S, Speidl WS, et al. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation. 2007;115:2909–16. doi: 10.1161/CIRCULATIONAHA.106.679639. [DOI] [PubMed] [Google Scholar]
- [2].Antman EM, Hand M, Armstrong PW, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- [3].Halkin A, Grines CL, Cox DA, et al. Impact of intravenous beta-blockade before primary angioplasty on survival in patients undergoing mechanical reperfusion therapy for acute myocardial infarction. J Am Coll Cardiol. 2004;43:1780–7. doi: 10.1016/j.jacc.2003.10.068. [DOI] [PubMed] [Google Scholar]
- [4].Bates ER. Role of intravenous beta-blockers in the treatment of ST-elevation myocardial infarction: of mice (dogs, pigs) and men. Circulation. 2007;115:2904–6. doi: 10.1161/CIRCULATIONAHA.107.707968. [DOI] [PubMed] [Google Scholar]
- [5].Dunzendorfer S, Wiedermann CJ. Modulation of neutrophil migration and superoxide anion release by metoprolol. J Mol Cell Cardiol. 2000;32:915–24. doi: 10.1006/jmcc.2000.1148. [DOI] [PubMed] [Google Scholar]
- [6].Beril Gok H, Solaroglu I, Okutan O, Cimen B, Kaptanoglu E, Palaoglu S. Metoprolol treatment decreases tissue myeloperoxidase activity after spinal cord injury in rats. J Clin Neurosci. 2007;14:138–42. doi: 10.1016/j.jocn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- [7].Prabhu SD, Wang G, Luo J, Gu Y, Ping P, Chandrasekar B. Beta-adrenergic receptor blockade modulates Bcl-X(S) expression and reduces apoptosis in failing myocardium. J Mol Cell Cardiol. 2003;35:483–93. doi: 10.1016/s0022-2828(03)00052-x. [DOI] [PubMed] [Google Scholar]
- [8].Daemen MA, van ’t Veer C, Denecker G. Inhibition of apoptosis induced by ischemia–reperfusion prevents inflammation. J Clin Invest. 1999;104:541–9. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhao ZQ, Nakamura M, Wang NP, et al. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000;45:651–60. doi: 10.1016/s0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- [10].Garcia-Dorado D, Oliveras J, Gili J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–9. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- [11].Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- [12].Kim RJ, Judd RM, Chen EL, Fieno DS, Parrish TB, Lima JA. Relationship of elevated 23Na magnetic resonance image intensity to infarct size after acute reperfused myocardial infarction. Circulation. 1999;100:185–92. doi: 10.1161/01.cir.100.2.185. [DOI] [PubMed] [Google Scholar]
- [13].Vedder NB, Winn RK, Rice CL, Chi EY, Arfors KE, Harlan JM. Inhibition of leukocyte adherence by anti-CD18 monoclonal antibody attenuates reperfusion injury in the rabbit ear. Proc Natl Acad Sci U S A. 1990;87:2643–6. doi: 10.1073/pnas.87.7.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–60. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- [15].Ibanez B, Vilahur G, Cimmino G, et al. Rapid change in plaque size, composition and molecular footprint following recombinant ApoA-IMilano (ETC-216) administration. Magnetic resonance imaging study in an experimental model of atherosclerosis. J Am Coll Cardiol. 2008;51:1104–9. doi: 10.1016/j.jacc.2007.09.071. [DOI] [PubMed] [Google Scholar]
- [16].Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730–6. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- [17].Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- [18].Yellon DM, Baxter GF. Protecting the ischaemic and reperfused myocardium in acute myocardial infarction: distant dream or near reality? Heart. 2000;83:381–7. doi: 10.1136/heart.83.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol. 2004;44:276–86. doi: 10.1016/j.jacc.2004.03.068. [DOI] [PubMed] [Google Scholar]
- [20].Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:e82–e292. [PubMed] [Google Scholar]
- [21].Ellerbeck EF, Bhimaraj A, Hall S. Impact of organizational infrastructure on beta-blocker and aspirin therapy for acute myocardial infarction. Am Heart J. 2006;152:579–84. doi: 10.1016/j.ahj.2006.02.011. [DOI] [PubMed] [Google Scholar]
- [22].Daly C, Clemens F, Lopez-Sendon JL, et al. The impact of guideline compliant medical therapy on clinical outcome in patients with stable angina: findings from the Euro Heart Survey of stable angina. Eur Heart J. 2006;27:1298–304. doi: 10.1093/eurheartj/ehl005. [DOI] [PubMed] [Google Scholar]
- [23].Sharma SK, Kini A, Marmur JD, Fuster V. Cardioprotective effect of prior beta-blocker therapy in reducing creatine kinase-MB elevation after coronary intervention: benefit is extended to improvement in intermediate-term survival. Circulation. 2000;102:166–72. doi: 10.1161/01.cir.102.2.166. [DOI] [PubMed] [Google Scholar]
- [24].Chorro FJ, Such-Belenguer L, Lopez-Merino V. Animal models of cardiovascular disease. Rev Esp Cardiol. 2009;62:69–84. [PubMed] [Google Scholar]
- [25].Basso C, Thiene G. The pathophysiology of myocardial reperfusion: a pathologist’s perspective. Heart. 2006;92:1559–62. doi: 10.1136/hrt.2005.086959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao ZQ, Velez DA, Wang NP, et al. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis. 2001;6:279–90. doi: 10.1023/a:1011335525219. [DOI] [PubMed] [Google Scholar]
- [27].Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–9. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- [28].Zhao ZQ, Morris CD, Budde JM, et al. Inhibition of myocardial apoptosis reduces infarct size and improves regional contractile dysfunction during reperfusion. Cardiovasc Res. 2003;59:132–42. doi: 10.1016/s0008-6363(03)00344-4. [DOI] [PubMed] [Google Scholar]
- [29].Yue TL, Ma XL, Wang X, et al. Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res. 1998;82:166–74. doi: 10.1161/01.res.82.2.166. [DOI] [PubMed] [Google Scholar]
- [30].Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–50. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]