Abstract
Female-directed communication in male songbirds has been reasonably well studied; yet, relatively little is known about communication in other social contexts. Songbirds also produce song that is not clearly directed towards another individual (undirected song) when alone or in flocks. Although the precise functions of undirected song may differ across species, this type of song is considered important for flock maintenance, song learning or practice. Past studies show that undirected song is tightly coupled to analgesia and positive affective state, which are both mediated by opioid activity. Furthermore, labeling for the opioid met-enkephalin in the medial preoptic nucleus (POM) correlates positively with undirected song production. We propose that undirected song is facilitated and maintained by opioid receptor activity in the POM and other brain regions involved in affective state, analgesia, and social behavior. To provide insight into this hypothesis, we used immunohistochemistry to examine relationships between undirected song and mu-opioid receptors in male starlings. Polynomial regression analyses revealed significant inverted-U shaped relationships between measures of undirected song and mu-opioid receptor labeling in the POM, medial bed nucleus of the stria terminalis (BSTm), and periaqueductal gray (PAG). These results suggest that low rates of undirected song may stimulate and/or be maintained by mu-opioid receptor activity; however, it may be that sustained levels of mu-opioid receptor activity associated with high rates of undirected song cause mu-opioid receptor down-regulation. The results indicate that mu-opioid receptor activity in POM, BSTm, and PAG may underlie previous links identified between undirected song, analgesia, and affective state.
Keywords: opioids, vocal communication, reward, birdsong, motivation, mu-opioid receptor
1. Introduction
Across the animal kingdom, vocal communication is critical for successful social interactions. Although female-directed courtship vocalizations produced by males have been relatively well-studied, less is known about communication in other social contexts. In some songbirds, song is observed at high rates in large affiliative flocks (Eens, 1997; Riters et al., 2000; Zann, 1996). Although facilitated by social contact (Jesse and Riebel, 2012), this type of song does not (at least to a human observer) appear to be directed towards a specific individual (Dunn and Zann, 1996a; Hessler and Doupe, 1999; Jarvis et al., 1998; Zann, 1996). In studies of zebra finches, song that is not clearly directed towards conspecifics is referred to as “undirected”, and we will use the term here. Although the precise function of undirected song may differ across species, this type of song is proposed to play a role in flock maintenance (e.g., starlings (Hausberger et al., 1995)), song learning or practice (e.g., zebra finches and starlings (Eens, 1997; Kao et al., 2005)), and may be used by females for future mating decisions (e.g., zebra finches (Dunn and Zann, 1996b; Holveck and Riebel, 2007; Holveck and Riebel, 2010)).
Multiple lines of evidence link opioids to undirected birdsong. First, positive correlations were identified between labeling for the opioid protein met-enkephalin (mENK) in the medial preoptic nucleus (POM) and undirected song produced by male European starlings (with a similar trend identified for the ventral tegmental area (VTA); p=0.06) (Riters et al., 2005). Opioids in the medial preoptic area induce both analgesia and reward / positive affect, at least in rats (Ãgmo and Gomez, 1991; Tseng et al., 1980; Tseng and Wang, 1992), and recent data link undirected song to both opioid-mediated analgesia and positive affect. Specifically, analgesia measures correlated positively with male starling undirected song rates (Kelm-Nelson et al., 2012), and both male starlings and zebra finches singing high rates of undirected song developed a conditioned place preference for a chamber previously paired with undirected singing behavior (Riters and Stevenson, 2012). These results link undirected song to a positive (or at least a less negative) affective state, which we hypothesize may, in part, be mediated by opioids (Riters, 2012).
The present study was designed to further examine links between opioids and undirected song in male starlings. During fall and winter months (i.e., the non-breeding season) when testosterone concentrations are low, male starlings do not sing to attract mates or defend nesting territories (Riters et al., 2000). However, they sing at high rates as part of affiliative overwintering flocks (Eens, 1997; Riters et al., 2000). Song in these flocks is proposed to function primarily to maintain flock cohesion (Hausberger et al., 1995) and may facilitate song learning in these open ended learners (Chaiken et al., 1994). Although the functions and mechanisms underlying this form of song may differ from that described in zebra finches (Heimovics and Riters, 2005), song in these flocks is not (at least to a human observer) clearly directed towards another individual. We thus consider non-breeding season starling song to be a form of undirected, affiliative communication.
In the present study, we used immunohistochemistry to examine links between undirected song in male starlings with low testosterone singing in flocks and mu-opioid receptor labeling. If previously reported links between undirected song, analgesia and reward are mediated by opioids, then we predict that undirected song production will be linked to mu-opioid receptor labeling in regions in which opioids have been found to induce analgesia and / or reward, which include the POM, the ventral tegmental area (VTA), periaqueductal gray (PAG), bed nucleus of the stria terminalis (BSTm), lateral septum (SL) and periventricular nucleus (PVN) (analgesia: (Altier and Stewart, 1997; Hashimoto et al., 1991; Tseng et al., 1980; Tseng and Wang, 1992; Yaksh et al., 1976) and reward: (Ãgmo and Gomez, 1991; Bozarth and Wise, 1981; Jackson, 2009; Le Merrer et al., 2009; Olmstead and Franklin, 1997; Phillips and LePiane, 1982).
2. Results
2.1 Behavior and testosterone concentrations
Eighteen males were used for the behavioral analysis. Male testes were regressed and testosterone concentrations were in the range typical of the non-breeding season. T concentrations were below detectable levels for all but four males (for males with detectable levels n=4, mean=144.85 pg/mL, SD=207.65). For reference, the average T measure for breeding males is approximately 2930.00 pg/mL (Dawson, 1983). Furthermore, individuals did not show behaviors indicative of birds during the breeding season (i.e., they did not collect nest material, wing wave, or displace other males from nesting sites). Taken together, these results indicate that the hormone and photoperiod states were similar to wild birds in the non-breeding season when male starlings sing high rates of undirected but not female-directed song.
2.2 Area Covered by Mu-opioid Receptor Labeling and Total Song
We ran linear regression analyses to examine relationships between the area for each nucleus covered by mu-opioid receptor immunolabeling (mean total pixel area) and Total Song; (untransformed data range for Total Song, lower limit=0, upper limit=67; median=18.5). In all cases linear regression results were not significant and the data did not fit a linear model (p>0.50; Table 1). Therefore, we ran nonlinear, higher-order curve polynomial regression analyses. Specifically, in separate polynomial regression analyses the mean total pixel area for a brain region was entered as the dependent variable and the Total Song was entered as the predictor variable. Statistical outliers identified in residual analysis plots were removed if they fell outside two times the standard deviation of the mean. This resulted in the removal of one animal for POM (total song=25, mean total pixel area POM=1177.83); one for BSTm and SL (total song=44, mean total pixel area BSTm=34662.8, mean total pixel area SL=12701.8, respectively), and an additional animal for BSTm (total song= 3, mean total pixel area=23484.0). Additionally, due to tissue damage during processing, measures were lacking for some individuals which explains differing sample sizes.
Table 1.
Non-Significant Linear Regression Results of Mu-Opioid Receptor Label (Pixel Area) and Total Song
| Brain Region | F(df) | R2 | Adj. R2 | Standard Error of the Estimate | p-value |
|---|---|---|---|---|---|
| POM | 0.0000139(1, 15) | 0.0000009 | −0.07 | 0.770 | 0.99 |
| PAG | 0.05(1, 14) | 0.001 | −0.08 | 23381.100 | 0.90 |
| BSTm | 0.16 (1, 13) | 0.01 | −0.06 | 0.625 | 0.69 |
| VTA | 0.44 (1, 13) | 0.03 | −0.04 | 9515.550 | 0.52 |
| PVN | 0.30 (1, 16) | 0.02 | −0.04 | 1.349 | 0.59 |
| SL | 0.31 (1, 14) | 0.02 | −0.05 | 2735.632 | 0.59 |
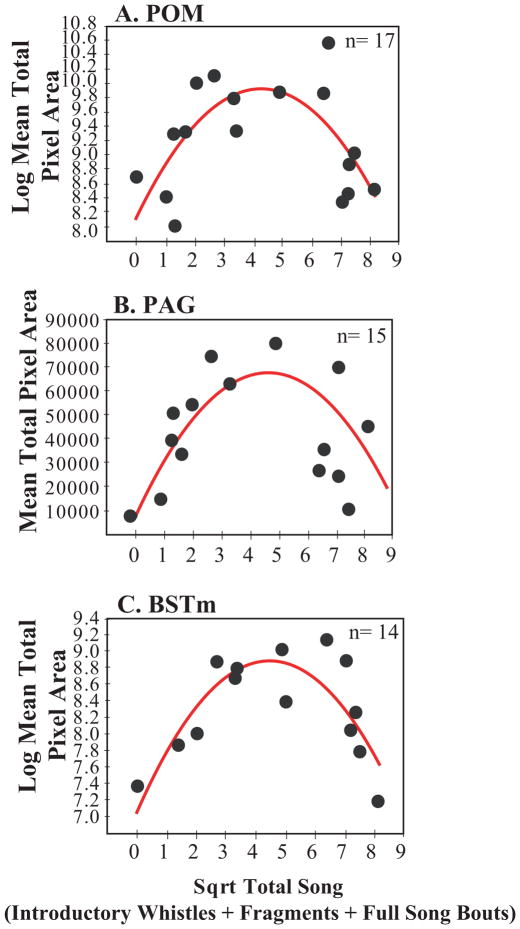
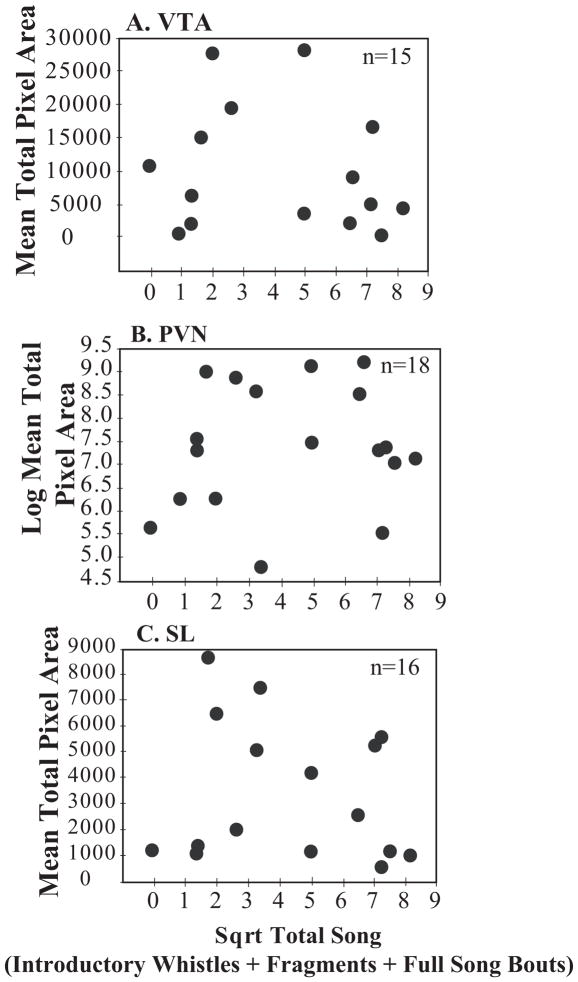
Descriptively, labeling of mu-opioid receptors and Total Song exhibited an inverted U-shaped pattern where mu-opioid receptor labeling in POM, PAG and BSTm were low in both the lowest and highest singers. Results of the polynomial regression analyses revealed significant curvilinear relationships between mu-opioid receptor labeling in POM, PAG, and BSTm and Total Song (Table 2; Figure 1, A–C, Figure 2). There were no significant polynomial relationships between measures of mu-opioid receptor labeling in VTA, PVN, or SL and Total Song (Table 2; Figure 3, A–C).
Table 2.
Polynomial Regression Results of Mu-Opioid Receptor Label (Pixel Area) and Total Song
| Brain Region | N | F(df) | R2 | Adj. R2 | p-value |
|---|---|---|---|---|---|
| POM | 17 | 4.98(1,14) | 0.42 | 0.33 | 0.02 |
| PAG | 15 | 4.92(1, 12) | 0.45 | 0.36 | 0.09 |
| BSTm | 14 | 12.26(1,12) | 0.67 | 0.61 | 0.001 |
| VTA | 15 | 1.55(1,12) | 0.21 | 0.07 | 0.25 |
| PVN | 16 | 1.49(1,15) | 0.17 | 0.05 | 0.25 |
| SL | 16 | 1.31(1,13) | 0.17 | 0.04 | 0.30 |
Figure 1.
Evidence for curvilinear relationships between mu-opioid receptor labeling and undirected song rates. An inverted-U shaped curve showing the measure of undirected song on the x-axis and mean mu-opioid receptor total pixel area on the y-axis in A) POM, B) PAG, and C) BSTm. Individual birds are represented by a single black dot. Sample size indicated in the upper right corner of the figures. Presence of the red regression line indicates a significant relationship p<0.05.
Figure 2.

Photomicrographs illustrating mu-opioid receptor labeling. Representative images (20X) of mu-opioid receptor punctate labeling within POM in individuals that were classified as a A) low singer, B) intermediate singer, or C) high singer. Abbreviation: V= ventricle. Scale bar in bottom left corner.
Figure 3.
Relationships between the area covered by mu-opioid receptor label (mean pixel area) and undirected song. The measure of undirected song is on the x-axis and the mean mu-opioid receptor total pixel area is on the y-axis in A) VTA, B) PVN, C) SL. Individual birds are represented by a single black dot. Sample size indicated in the upper right corner of the figures.
2.3 Area Covered by Mu-opioid Receptor Labeling and General Behaviors
General behaviors (feeding, drinking, and beak wiping) and mu-opioid receptor labeling in each brain region did not display curvilinear tendencies. Therefore, to determine whether labeling could be explained statistically by behavioral variables in addition to Total Song, we investigated the relationship of immunolabeling and non-song behaviors (bouts of feeding, drinking, preening and beak wiping) using linear multiple regression analysis. Specifically, multiple regression analyses were run using the brain region as the dependent variable and the behaviors as the independent variables. Both backward and forward analyses were performed, and in most cases were identical. For VTA both forward and backward analyses resulted in the same (though not identical) significant effects. Results of the forward analysis are provided because this model best explained the data based on the highest adjusted R2, lowest standard error, and the best residual plots.
For measures of the total pixel area covered by mu-opioid receptor labeling, results of the multiple regression analyses revealed bouts of feeding to contribute significantly (negatively) to variance in total pixel area in both PAG (n=16, adj. R2=0.33, feeding beta=−0.74, SE of beta=18491.93, t13=5.14, p=0.03; Figure 4A) and VTA (n=15, adj. R2=0.301, feeding beta=−0.59, SE of beta=7794.5, t13=4.59, p=0.02; Figure 4B). No variables contributed to variance in POM, BSTm, VTA, PVN or SL.
Figure 4.
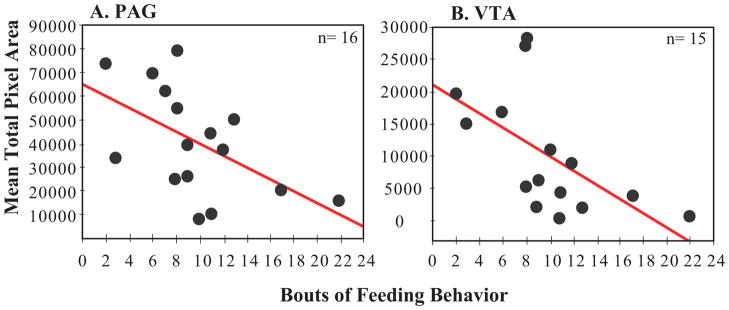
Relationships between the area covered by mu-opioid receptor label (mean pixel area) and bouts of feeding behavior. The total number of bouts of feeding is on the x-axis and mean mu-opioid receptor total pixel area is on the y-axis for A) PAG and B) VTA. Individual birds are represented by a single black dot. Sample size is indicated in the upper right hand corner of the figure. Presence of the red regression line indicates a significant relationship p<0.05.
2.4 Optical Density Measures and Behavior
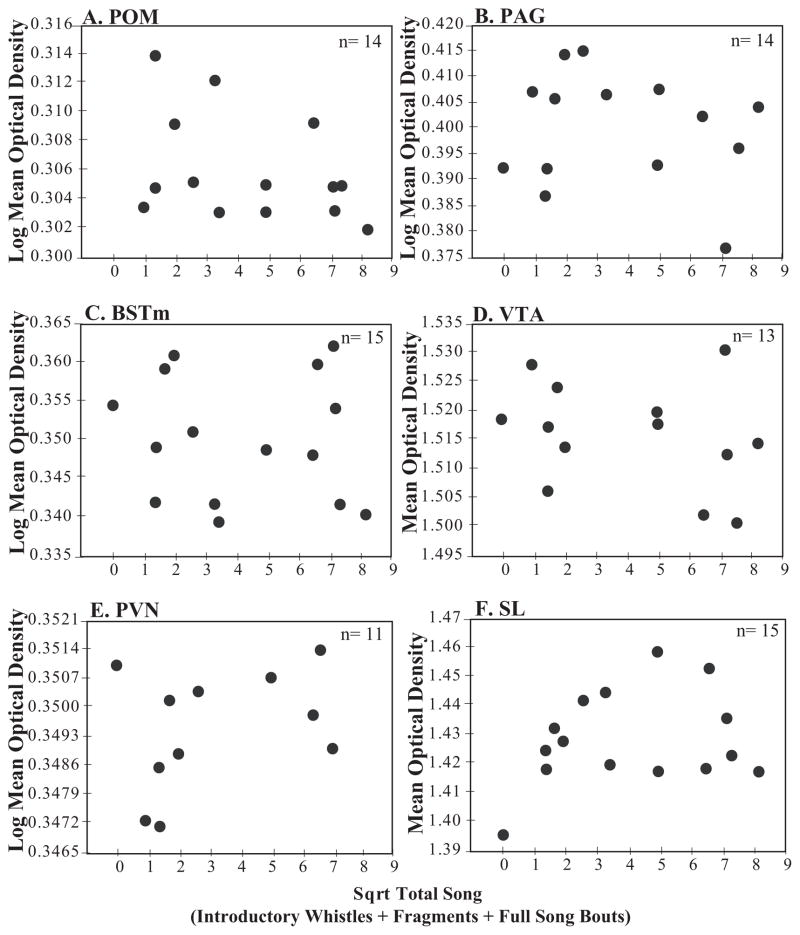
In addition to the pixel area covered by labeled fibers, we analyzed measures of optical density, an index of labeling density determined by measuring the amount of light transmitted through the tissue. No significant relationships were identified between optical density measures and total song (Table 3 and 4, Figure 5) or other behaviors (p > 0.05 for all behaviors) for any of the brain regions of interest.
Table 3.
Non-Significant Linear Regression Results of Mu-Opioid Receptor Label (Optical Density) and Total Song
| Brain Region | F(df) | R2 | Adj. R2 | Standard Error of the Estimate | p-value |
|---|---|---|---|---|---|
| POM | 2.06 (1, 12) | 0.15 | 0.08 | 0.004 | 0.18 |
| PAG | 0.39 (1, 12) | 0.03 | −0.05 | 0.011 | 0.54 |
| BSTm | 0.10 (1, 13) | 0.01 | −0.07 | 0.008 | 0.76 |
| VTA | 1.01 (1, 11) | 0.08 | 0.0004 | 0.009 | 0.34 |
| PVN | 1.30 (1,9) | 0.13 | 0.03 | 0.001 | 0.28 |
| SL | 0.96 (1,13) | 0.13 | −0.003 | 0.016 | 0.35 |
Table 4.
Non-Significant Polynomial Regression Results of Mu-Opioid Receptor Label (Optical Density) and Total Song
| Brain Region | N | F(df) | R2 | Adj. R2 | p-value |
|---|---|---|---|---|---|
| POM | 14 | 1.06(1,11) | 0.16 | 0.009 | 0.38 |
| PAG | 14 | 0.911(1, 11) | 0.14 | 0.01 | 0.43 |
| BSTm | 15 | 0.09(1,12) | 0.02 | 0.14 | 0.91 |
| VTA | 13 | 0.46(1,10) | 0.08 | 0.10 | 0.64 |
| PVN | 11 | 0.58(1,8) | 0.13 | 0.09 | 0.58 |
| SL | 15 | 0.90(1,12) | 0.11 | 0.01 | 0.43 |
Figure 5.
Non-significant relationships between mu-opioid receptor optical densities and undirected song rates. The measure of undirected song on the x-axis and mean mu-opioid receptor optical density on the y-axis in A) POM, B) PAG, C) BSTm, D) VTA, E) PVN, F) SL. Individual birds are represented by a single black dot. Sample size indicated in the upper right corner of the figures.
3. Discussion
In the present study we found links between affiliative, undirected singing behavior and mu-opioid receptor labeling in three regions in which opioids are implicated in analgesia and / or reward (as reviewed in the introduction), POM, PAG and BSTm. Specifically, we report a positive association between mu-opioid receptor labeling in each of these regions in birds singing low to intermediate rates of undirected song. However, there appeared to be a point, after which the highest rates of song were associated with low densities of receptor labeling, resulting in an inverted-U shaped relationship.
3.1 Interpretation of the inverted-U function
We suggest that relatively low rates of undirected song facilitate (and / or are facilitated by) relatively low levels of mu-opioid receptor activity in POM, PAG, and BSTm. Given that mu-opioid receptors down-regulate in response to sustained occupation by enkephalin (Chang et al., 1982; Harrison et al., 1998), it is possible that high levels of opioid release in these regions are associated with high levels of song production which cause mu-opioid receptor down-regulation. Consistent with this possibility, and in contrast to the curvilinear relationships identified for mu-opioid receptors, in a past study immunolabeling for mENK (an endogenous ligand for mu- and delta-opioid receptors) in POM related positively to undirected singing behavior even in the birds singing at the highest rates (Riters et al., 2005). This past study, along with the present results, suggests that when mENK protein is highest (at least in POM) mu-opioid receptor labeling is lowest, consistent with the possibility that sustained song-associated opioid release results in receptor down-regulation.
The possibility that song-associated opioid release results in receptor down-regulation must be examined in future work; however, it is consistent with past studies showing a high level of opioid receptor plasticity. Specifically, in-vitro studies in cultured mouse neuroblastoma cells show that receptor down-regulation can occur in four hours (Chang et al., 1982). Additionally, in mouse cell lines, receptor down-regulation occurred after 72 hours of exposure to high concentrations of morphine (Puttfarcken et al., 1988). In vivo studies in rats also show that when bound by an agonist mu-opioid receptors rapidly internalize (Coolen et al., 2004; Sinchak and Micevych, 2003). For example, mu-opioid receptors identified using immunolabeling were found to internalize as early as 30 minutes after copulation in male rats (Coolen et al., 2004). Finally, past work in dark-eyed juncos has shown that opioid receptor densities change seasonally (Woods et al., 2010). Therefore, it is possible that in male starlings mu-opioid receptor densities change both seasonally (in association with the production of female-directed and undirected singing behavior) as well as immediately in association with singing behavior.
3.2 Mu-opioid receptors in POM, BSTm, and PAG may modify affect and vocal behavior
The POM, BSTm and PAG are components of a reciprocally connected (Absil et al., 2001; Riters and Alger, 2004; Wood and Swann, 2005) opioid-rich (Kelm et al., 2011; Mansour et al., 1994; Pilapil et al., 1987; Riters et al., 2005; Sar et al., 1978; Woods et al., 2010) neural circuit that has been implicated in affiliative social behavior (e.g., (Lonstein and De Vries, 2000; Numan and Numan, 1997)) in birds and mammals (Goodson, 2005; Newman, 1999). In male canaries, cell groups send projections from the PAG to regions controlling song production (Appeltants et al., 2000), and labeling for the immediate early gene egr-1 (often referred to as ZENK in songbird studies) is increased in the PAG in male zebra finches that sing undirected song compared to silent males (Lynch et al., 2008). In birds and mammals, the PAG is proposed to gather and integrate affective information from other brain regions, which it then relays to vocal production (motor) areas so that an animal emits a vocal signal reflective of its emotional state (Dubbeldam and den Boer-Visser, 2002; Gruber-Dujardin, 2010; Jurgens and Pratt, 1979; Lynch et al., 2008). In cats, enkephalin opioids in the PAG suppressed negative, non-affiliative vocalizations (Shaikh et al., 1991), and in monkeys opioid antagonists increased vocal behavior linked to aversive states (Jurgens and Lu, 1993), suggesting a role for opioids in PAG in the inhibition of negative forms of vocal production. Studies in mammals also show that projections from the preoptic area and BSTm to PAG promote positive vocal behaviors (Dujardin and Jurgens, 2006; Kyuhou and Gemba, 1998). Together these data suggest that projections from the POM and BSTm to the PAG may promote positive forms of vocal behavior, including undirected singing.
The link reported here between the POM and undirected singing in starlings is consistent with the results of an increasing number of studies (Alger and Riters, 2006; Heimovics et al., 2009; Riters et al., 2005). Bilateral electrolytic lesions to the POM that suppressed sexually-motivated song increased undirected singing behavior (Alger and Riters, 2006), and met-enkephalin in this region was linked tightly to affiliative song (Riters et al., 2005). Injections of enkephalin opioids directly into the POM of male quail suppressed neuronal activity (Kotegawa et al., 1997). This suggests that increased mu-opioid receptor activity in POM may act to suppress neuronal activity to facilitate undirected singing behavior. This remains to be tested using future site-specific pharmacological manipulations.
The present data are also consistent with past work implicating the BSTm in undirected singing behavior. In male starlings immunolabeling for ZENK in BSTm correlated positively with undirected singing behavior (Heimovics and Riters, 2007). Although the role of opioids in BSTm in vocal production has not been well studied, met-enkephalin in BSTm in cats suppressed negative forms of vocal production (Brutus et al., 1988; Shaikh et al., 1988). Recently in songbirds a population of BSTm neurons (vasotocin-producing) was found to increase activity in response to positively-valenced social stimuli and to reduce activity in response to negatively-valenced social stimuli (Goodson and Wang, 2006; Goodson et al., 2009). These effects were not observed in response to a positive nonsocial stimulus, suggesting that the BSTm responds selectively to positively-valenced social stimuli.
The links between singing behavior and mu-opioid receptor labeling were observed for measures of the area covered by immunolabeling (pixel area) but not measures of optical density. The functional relevance of this difference is not clear. It is possible that an increase in the area covered by mu-opioid receptors relates to an increase in the recruitment of postsynaptic targets across parts of a particular brain region. The lack of relationship between singing and the measure of mu-opioid receptor optical density in contrast may indicate that singing in these areas is not linked to an increase in opioid activity within a single, focal part of a brain region.
3.3 No significant relationships were found between undirected song and mu-opioid receptors in VTA, PVN and SL
Past data in starlings singing undirected song showed a trend for a linear relationship between undirected singing and mENK protein immunolabeling in VTA (p=0.06) (Riters et al., 2005). Here, we report no significant relationship between mu-opioid receptor immunolabeling in VTA and song. We also report no significant relationships between mu-opioid receptor labeling and undirected song in PVN or SL. Although opioids in each of these regions have been found to induce reward and / or analgesia as reviewed in the introduction, the present results do not implicate mu-opioid receptors in these regions in undirected singing behavior in starlings; however additional study is warranted.
3.4 Mu-opioid receptors in PAG and VTA negatively correlated with feeding
Although non-specific behaviors generally did not correlate with measures of mu-opioid receptors in any of the brain regions examined, linear negative correlations were found between mu-opioid receptor labeling (pixel area) in PAG and VTA and feeding behavior. Studies in birds (e.g., pigeons, domestic chicks, and juncos) show that opioids play a role in feeding (Deviche and Schepers, 1983; Deviche et al., 1984; Deviche, 1992; McCormack and Denbow, 1987). Additionally, past studies in rats implicate opioids in both the VTA and PAG in feeding behavior (Jenck et al., 1987; Mucha and Iversen, 1986). In PAG mu-opioid receptors were found to inhibit feeding behavior in rats (Jenck et al., 1987), a finding consistent with the negative correlation between feeding and mu-opioid receptor labeling in PAG that we report here. In contrast, multiple studies show that mu-opioid receptors in VTA stimulate feeding behavior in rats (e.g., (Echo et al., 2002; Jenck et al., 1987; Noel and Wise, 1995), a finding that appears inconsistent with the negative relationship between mu-opioid receptor label in VTA and feeding behavior we report here. Based on past literature, we were not surprised that mu-opioid receptor labeling in PAG and VTA related to feeding behavior; however, the functional meaning of the direction of the correlations is unclear. As reviewed above opioid receptors display extreme plasticity and can become inactive when opioid activity is elevated. Whether this may in part explain our findings for VTA is not clear. Thus future studies involving site-directed opioid manipulations are needed to understand the functional meaning of the correlations we identified between feeding and mu-opioid receptor labeling.
3.5 Conclusions
Based on the present findings and past data, we suggest that in response to positive social and environmental conditions (e.g., in the presence of non-threatening conspecifics, an unlimited food supply, and shelter) opioid release in BSTm and POM induces a positive affective state which is relayed to the PAG. The PAG may integrate this information and transmit it to vocal control regions so that a bird produces undirected song when it is appropriate to do so. Opioids in POM, BSTm, and PAG have been found to induce a positive affective state (at least in rodents), and projections from the preoptic area and BSTm to the PAG in mammals have been found to facilitate positive forms of vocal behavior. Based on these findings, we propose that the link between positive affective state and affiliative singing behavior identified in past work in starlings and zebra finches (Kelm-Nelson et al., 2012; Riters and Stevenson, 2012) may be regulated in part by song-associated opioid release in POM, BSTm, and PAG.
4. Experimental Procedure
4. 1 Animals and Protocols
Eighteen male starlings were captured on a single farm in Madison, WI using baited fly-in traps. After capture, males were housed indoors in stainless steel, single sex cages (91cm × 47cm × 47cm) within the University of Wisconsin’s Department of Zoology indoor animal facilities. Food (Purina Mills Start and Grow Sunfresh Recipe, 61S3-IGH-G) and water were provided ad libitum. For identification, each animal was assigned a numbered as well as a colored leg band.
Protocols used for bird acquisition, housing, and behavioral testing were in adherence to guidelines approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (DHEW Publication 80–23, Revised 8th Edition, 2011, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205) and approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee.
4.2 Light Cycle and Housing
Animals were randomly assigned to one of five social outdoor aviaries (3 or 4 birds per aviary; 2.13m × 2.4m × 1.98m) and allowed to habituate prior to the beginning of behavioral testing. The natural light cycle during the experiment, occurring in fall (early October), was approximately 11hr: 40 min light, decreasing daylight by 3 minutes each day.
Over five consecutive days, each aviary was observed for 20 min by a single observer concealed by dark green and brown camouflage netting and blinds. Each aviary contained four nest boxes and branches for perching. Food and water were provided ad libitum. Each aviary was visually, but not acoustically isolated from the others by the use of camouflage blinds. For seven days prior to the first day of behavioral observations an observer sat in front of each aviary (behind a blind) to allow males to habituate to her presence.
4.3 Behavioral Observations
The testing order of the aviaries was randomized, with observations performed between 9:00 and 13:00 each day. Measures of starling song included bouts of full song (four distinct components including introductory whistles, complex phrases, click-series, and high frequency phrases (Eens, 1997)), bouts of fragments (at least 2 components of song), and bouts of introductory whistles. These measures were summed to create a measure of Total Song. Additionally, bouts of feeding, drinking, preening and beak wiping were recorded. A distinct bout was defined as an event separated by at least 2 seconds.
4.4 T Assay, Tissue Collection, and Immunohistochemistry
Immediately after the last observation, all males in a group were rapidly decapitated. The testes were measured to ensure that they were in a regressed stage, as is typical of the non-breeding season (Falk and Gwinner, 1983). Additionally, a terminal trunk blood sample was taken immediately after sacrifice to confirm fall typical low or undetectable levels of the hormone T. Plasma T was measured with a commercial grade competitive assay (EIA; Cayman Chemical, Ann Arbor, MI, USA, Catalog No. 582701) as described previously in Kelm et al. 2011, and by manufacturer’s instructions.
Brains were removed by dissection, fixed in 5% acrolein overnight, rinsed, cryoprotected in 30% sucrose for 3 days and frozen at −80°C. Using a cryostat, brains were cut in the coronal plane in three, 40μm series and stored in anti-freeze until processing. Series one was used for mu-opioid receptor labeling discussed here.
Brain tissue was processed using immunohistochemistry to identify mu-opioid receptor protein. The primary antibody was an anti- mu-opioid receptor antibody made in rabbit (Abcam, ab10275, 1:5000). The secondary antibody was biotinylated goat anti-rabbit (Vector Laboratories, 1:1000). Briefly, sections were rinsed in phosphate buffered saline (PBS) for 30 min, incubated in 0.5% sodium borohydride solution for 15 min, rinsed in PBS for 20 min, incubated in 0.5% hydrogen peroxide solution for 10 min, rinsed in PBS for 20 min, incubated in 20% normal goat serum (NGS) solution for 1 h, and then incubated in 2% NGS primary solution overnight at room temperature. Sections were then rinsed in PBS-T for 30 min and incubated in 2% NGS biotinylated secondary solution for 90 min at room temperature. Sections were then rinsed in PBS-T for 30 min, incubated in AB solution (Vectastain Elite ABC, Vector Laboratories) for 1 h, rinsed in PBS-T for 30 min, and the avidin–biotin complex was visualized using 3,3′-Diaminobenzidine (DAB) tablets (Sigma Aldrich, St. Louis, MO, USA). Sections were float mounted onto gel-coated slides, dehydrated in a series of alcohols, and cover slipped. Antibody specificity was verified using preadsorption, omitting the primary and via Western blot analysis as reported in Kelm et al. (2011). All brains were processed in a single batch to minimize differences in background labeling.
4.5 Quantification of Brain Regions
A Spot Camera (Diagnostic Instruments, Inc.) connecting a microscope to a computer was used to acquire images of immunolabeled brain tissue. Using METAVUE (Fryer Company, Inc., Huntley, IL, USA) software, the mean total pixel area covered by mu-opioid receptor labeling and mean optical density of labeled tissue were quantified bilaterally from three serial sections for each bird in POM, PAG, BSTm, VTA, PVN, and SL. The locations of these nuclei were based on Heimovics and Riters (2007) (Figure 6). The portion of PAG measured is the area recently suggested as similar to the ventral PAG column in mammals (Kingsbury et al., 2011), which is the portion that regulates analgesia (Bandler and Shipley, 1994). Specifically, using the METAVUE autoscale function, which corrected the exposure of each image as a percentage of the total range of light, the total pixel area provided a measure of highlighted fibers using a specific computer-generated threshold for each individual brain region (within boxes or ovals centered in each region (Table 5)). Additionally, optical density measures (index of labeling density, the amount of light transmitted through the tissue) were also collected. Each threshold selected material that a blind observer agreed to represent mu-opioid receptor labeling. In cases of tissue damage, labeling was quantified either on a fourth section or the individual was dropped from analysis for affected brain areas.
Figure 6.
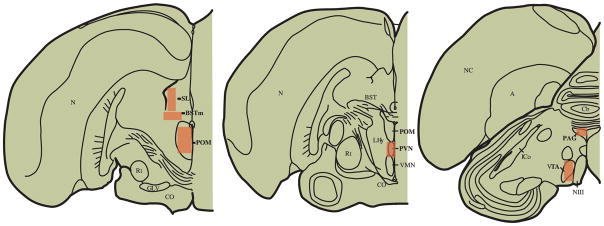
Brain schematic indicating approximate areas in measures of mu-opioid receptor labeling was quantified. Abbreviations: A, arcopallium; BSTm, medial bed nucleus of the stria terminalis Cb, cerebellum; CO, optic chiasm; PAG, periaqueductal gray; GLV, nucleus geniculatus lateralis, pars ventralis; ICo, nucleus intercollicularis; SL, lateral septum; NIII, third cranial nerve; N, nidopallium; NC, caudal nidopallium; POM, medial preoptic nucleus; PVN, paraventricular nucleus of the hypothalamus; Rt, nucleus rotundus; VMN, ventromedial nucleus; VTA, ventral tegmental area.
Table 5.
Box Measurements
| Nucleus | Shape | Size (mm) |
|---|---|---|
| BSTm | Rectangle | Area= 0.67 × 0.37 |
| CG | Circle | Diameter= 0.60 |
| POM | Rectangle | Area= 0.43 × 0.44 |
| PVN | Rectangle | Area= 0.22 × 0.42 |
| SL | Rectangle | Area= 0.36 × 0.54 |
| VTA | Rectangle | Area= 0.38 × 0.53 |
4.6 Statistical Analysis
Data were analyzed using Statistica 6.0 software (StatSoft®, Inc., Tulsa, OK, USA). Lilliefors test for normality and Levene’s test for homogeneity of variance were used to test the assumptions required for the use of parametric statistics. When variables did not meet assumptions they were transformed. Specifically, to improve homogeneity of variance the variable was square root transformed and to improve normality the variable was log transformed. Specific statistical tests are described in the results section (2.0).
Highlights.
Starlings sing variable rates of undirected song in the non-breeding season
POM, BSTm, and PAG mu-opioid receptor label showed inverted-U shaped links to song
Mu-opioid receptor activity may explain links between song and affective state
Acknowledgments
Support/Grant Numbers: R01 MH080225 to LVR and the Zoology Department Bunde Fund (2012) to CKN. Additionally, thank you to Kate Skogen and Chris Elliott for animal care, Bill Feeny for assistance with the illustrations, and Dr. Ben Pawlisch and Sharon Stevenson for help with tissue processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cynthia A. Kelm-Nelson, Email: cakelm@wisc.edu.
Lauren V. Riters, Email: LVRiters@wisc.edu.
References
- Absil P, Riters LV, Balthazart J. Preoptic Aromatase Cells Project to the Mesencephalic Central Gray in the Male Japanese Quail (Coturnix japonica) Horm Behav. 2001;40:369–383. doi: 10.1006/hbeh.2001.1702. [DOI] [PubMed] [Google Scholar]
- Ãgmo A, Gomez M. Conditioned place preference produced by infusion of Met-enkephalin into the medial preoptic area. Brain Res. 1991;550:343–346. doi: 10.1016/0006-8993(91)91339-3. [DOI] [PubMed] [Google Scholar]
- Alger SJ, Riters LV. Lesions to the Medial Preoptic Nucleus Differentially Affect Singing and Nest Box-Directed Behaviors Within and Outside of the Breeding Season in European Starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier Ng, Stewart J. Neuropeptide FF in the VTA blocks the analgesic effects of both intra-VTA morphine and exposure to stress. Brain Res. 1997;758:250–254. doi: 10.1016/s0006-8993(97)00333-8. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVC in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Brutus M, Zuabi S, Siegel A. Effects of D-Ala2-Met5-enkephalinamide microinjections placed into the bed nucleus of the stria terminalis upon affective defense behavior in the cat. Brain Res. 1988;473:147–52. doi: 10.1016/0006-8993(88)90326-5. [DOI] [PubMed] [Google Scholar]
- Chaiken M, Böhner, Marler P. Repertoire Turnover and the Timing of Song Acquisition in European Starlings. Behaviour. 1994;128:25–39. [Google Scholar]
- Chang K-J, Eckel RW, Blanchard SG. Opioid peptides induce reduction of enkephalin receptors in cultured neuroblastoma cells. Nature. 1982;296:446–448. doi: 10.1038/296446a0. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of [mu] opioid receptors in the medial preoptic area following copulation in male rats. Neurosci. 2004;124:11–21. doi: 10.1016/j.neuroscience.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen Comp Endocr. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- Deviche P, Schepers G. Naloxone treatment attenuates food but not water intake in domestic pigeons. Psychopharmacology. 1983;82:122–126. doi: 10.1007/BF00426394. [DOI] [PubMed] [Google Scholar]
- Deviche P, Melmer G, Schepers G. Evidence that naloxone attenuates the consumption of food by domestic pigeons through a central influence. Neuropharmacology. 1984;23:1173–1178. doi: 10.1016/0028-3908(84)90235-1. [DOI] [PubMed] [Google Scholar]
- Deviche P. Regulation of Food Intake in a Migratory Songbird (Junco hyemalis): Participation of Endorphinergic Mechanisms. Ornis Scand. 1992;23:260–263. [Google Scholar]
- Dubbeldam JL, den Boer-Visser AM. The central mesencephalic grey in birds: nucleus intercollicularis and substantia grisea centralis. Brain Res Bull. 2002;57:349–352. doi: 10.1016/s0361-9230(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Dujardin E, Jurgens U. Call type-specific differences in vocalization-related afferents to the periaqueductal gray of squirrel monkeys (Saimiri sciureus) Behav Brain Res. 2006;168:26–36. doi: 10.1016/j.bbr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Dunn AM, Zann RA. Undirected Song in Wild Zebra Finch Flocks: Contexts and Effects of Mate Removal. Ethology. 1996a;102:529–539. [Google Scholar]
- Dunn AM, Zann RA. Undirected Song Encourages the Breeding Female Zebra Finch to Remain in the Nest. Ethology. 1996b;102:540–548. [Google Scholar]
- Echo JA, Lamonte N, Ackerman TF, Bodnar RJ. Alterations in food intake elicited by GABA and opioid agonists and antagonists administered into the ventral tegmental area region of rats. Physiol Behav. 2002;76:107–116. doi: 10.1016/s0031-9384(02)00690-x. [DOI] [PubMed] [Google Scholar]
- Eens M. Advances in the Study of Behavior. Vol. 26. Academic Press; 1997. Understanding the complex song of the European starling: An integrated approach; pp. 355–434. [Google Scholar]
- Falk H, Gwinner E. Photoperiodic control of testicular regression in the European starling. Naturwissenschaften. 1983;70:257–258. [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. P Natl Acad Sci. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Dujardin E. Role of the periaqueductal gray in expressing vocalization. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization: An Integrative Neuroscience Approach. Academic Press; 2010. pp. 313–327. [Google Scholar]
- Harrison LM, Kastin AJ, Zadina JE. Opiate tolerance and dependence: receptors, G-proteins, and antiopiates. Peptides. 1998;19:1603–1630. doi: 10.1016/s0196-9781(98)00126-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ohgami S, Yonemasu Y. The Role of the Paraventricular Nucleus and Pituitary Gland in Morphine Analgesia. Neurol Med-Chir. 1991;31:629–634. doi: 10.2176/nmc.31.629. [DOI] [PubMed] [Google Scholar]
- Hausberger M, Richard-Yris MA, Henry L, Lepage L, Schmidt I. Song Sharing Reflects the Social Organization in a Captive Group of European Starlings (Sturnus vulgaris) J Comp Psychol. 1995;109:222–241. [Google Scholar]
- Heimovics SA, Riters LV. ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris) Behav Brain Res. 2007;176:333–343. doi: 10.1016/j.bbr.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neurosci. 2009;159:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Holveck M-J, Riebel K. Preferred songs predict preferred males: consistency and repeatability of zebra finch females across three test contexts. Anim Behav. 2007;74:297–309. [Google Scholar]
- Holveck M-J, Riebel K. Low-quality females prefer low-quality males when choosing a mate. Proc R Soc B. 2010;277:153–160. doi: 10.1098/rspb.2009.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ED. Psychology. University of Michigan; Ann Arbor: 2009. The extended amygdala in appetitive motivation for reward: Role of the bed nucleus of the stria terminalis. [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For Whom The Bird Sings: Context-Dependent Gene Expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Jenck F, Quirion R, Wise RA. Opioid receptor subtypes associated with ventral tegmental facilitation and periaqueductal gray inhibition of feeding. Brain Res. 1987;423:39–44. doi: 10.1016/0006-8993(87)90822-5. [DOI] [PubMed] [Google Scholar]
- Jesse F, Riebel K. Social facilitation of male song by male and female conspecifics in the zebra finch, Taeniopygia guttata. Behav Process. 2012;91:262–266. doi: 10.1016/j.beproc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Pratt R. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 1979;167:367–378. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Lu CL. The effects of periaqueductally injected transmitter antagonists on forebrain-elicited vocalization in the squirrel monkey. Eur J Neurosci. 1993;5:735–41. doi: 10.1111/j.1460-9568.1993.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:636–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Stevenson SA, Riters LV. Context-Dependent Links between Song Production and Opioid-Mediated Analgesia in Male European Starlings (Sturnus vulgaris) PLoS one. 2012;7:e46721. doi: 10.1371/journal.pone.0046721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm CA, Forbes-Lorman RM, Auger CJ, Riters LV. Mu-opioid receptor densities are depleted in regions implicated in agonistic and sexual behavior in male European starlings (Sturnus vulgaris) defending nest sites and courting females. Behav Brain Res. 2011;219:15–22. doi: 10.1016/j.bbr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-Like Organization of the Avian Midbrain Central Gray and a Reappraisal of the Intercollicular Nucleus. PLoS One. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotegawa T, Abe T, Tsutsui K. Inhibitory role of opioid peptides in the regulation of aggressive and sexual behaviors in male Japanese quails. J Exp Zool. 1997;277:146–154. doi: 10.1002/(sici)1097-010x(19970201)277:2<146::aid-jez6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kyuhou S, Gemba H. Two vocalization-related subregions in the midbrain periaqueductal gray of the guinea pig. Neuroreport. 1998;9:1607–10. doi: 10.1097/00001756-199805110-00064. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker J, Befort K, Kieffer BL. Reward Processing by the Opioid System in the Brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Maternal behaviour in lactating rats stimulates c-fos in glutamate decarboxylase-synthesizing neurons of the medial preoptic area, ventral bed nucleus of the stria terminalis, and ventrocaudal periaqueductal gray. Neurosci. 2000;100:557–568. doi: 10.1016/s0306-4522(00)00287-6. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Diekamp B, Ball GF. Catecholaminergic cell groups and vocal communication in male songbirds. Physiol Behav. 2008;93:870–876. doi: 10.1016/j.physbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- McCormack JF, Denbow DM. The effects of opioid antagonists on ingestive behavior in the domestic fowl. Pharmacol Biochem Behav. 1987;27:25–33. doi: 10.1016/0091-3057(87)90472-2. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–224. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Noel MB, Wise RA. Ventral tegmental injections of a selective μ or δ opioid enhance feeding in food-deprived rats. Brain Res. 1995;673:304–312. doi: 10.1016/0006-8993(94)01442-k. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Projection Sites of Medial Preoptic Area and Ventral Bed Nucleus of the Stria Terminalis Neurons that Express Fos during Maternal Behavior in Female Rats. J Neuroendocrinol. 1997;9:369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KBJ. The development of a conditioned place preference to morphine: Effects of lesions of various CNS sites. Behav Neurosci. 1997;111:1313–1323. doi: 10.1037//0735-7044.111.6.1313. [DOI] [PubMed] [Google Scholar]
- Phillips AG, LePiane FG. Reward produced by microinjection of (d-Ala2), Met5-enkephalinamide into the ventral tegmental area. Behav Brain Res. 1982;5:225–229. doi: 10.1016/0166-4328(82)90057-2. [DOI] [PubMed] [Google Scholar]
- Pilapil C, Welner S, Magnan J, Gauthier S, Quirion R. Autoradiographic distribution of multiple classes of opioid receptor binding sites in human forebrain. Brain Res Bull. 1987;19:611–615. doi: 10.1016/0361-9230(87)90080-3. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Werling LL, Cox BM. Effects of chronic morphine exposure on opioid inhibition of adenylyl cyclase in 7315c cell membranes: a useful model for the study of tolerance at mu opioid receptors. Mol Pharmacol. 1988;33:520–527. [PubMed] [Google Scholar]
- Riters L, Alger S. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrin. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Stevenson SA. Reward and vocal production: Song-associated place preference in songbirds. Physiol Behav. 2012;33:194–209. doi: 10.1016/j.physbeh.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar M, Stumpf WE, Miller RJ, Chang K-J, Cuatrecasas P. Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol. 1978;182:17–37. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- Shaikh MB, Shaikh AB, Siegel A. Opioid peptides within the midbrain periaqueductal gray suppress affective defense behavior in the cat. Peptides. 1988;9:999–1004. doi: 10.1016/0196-9781(88)90080-0. [DOI] [PubMed] [Google Scholar]
- Shaikh MB, Lu CL, Siegel A. An enkephalinergic mechanism involved in amygdaloid suppression of affective defence behavior elicited from the midbrain periaqueductal gray in the cat. Brain Res. 1991;559:109–17. doi: 10.1016/0006-8993(91)90293-5. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Wei ET, Loh HH, Li CH. Beta-Endorphin: central sites of analgesia, catalepsy and body temperature changes in rats. J Pharmacol Exp Ther. 1980;214:328–332. [PubMed] [Google Scholar]
- Tseng LF, Wang Q. Forebrain sites differentially sensitive to beta-endorphin and morphine for analgesia and release of Met-enkephalin in the pentobarbital-anesthesized rat. J Pharmacol Exp Ther. 1992;261:1028–1036. [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: Subnuclei and connections of the posterior division. Neurosci. 2005;135:155–179. doi: 10.1016/j.neuroscience.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Woods JK, Deviche P, Corbitt C. Opioid receptor densities analyzed across seasons in the POM and VTA of the dark-eyed junco, Junco hyemalis. J Chem Neuroanat. 2010;40:123–129. doi: 10.1016/j.jchemneu.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: Observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: A synthesis of field and laboratory studies. Oxford University Press; 1996. [Google Scholar]