Abstract
The transformation of the upper aerodigestive tract – oral cavity, pharynx and larynx – serves the functions of eating, speaking and breathing during sleeping and waking hours. These life-sustaining functions may be produced by a central neural sensorimotor system that shares certain neuroanatomic networks while maintaining separate neural functional systems and network structures. Current understanding of development, maturation, underlying neural correlates and integrative factors are discussed in light of currently available imaging modalities and recently emerging interventions. Exercise and an array of additional treatments together appear to provide promising translational pathways for evidence-based innovation, novel habilitation and rehabilitation strategies and delay, or even prevent neuromuscular decline cross-cutting functions and supporting quality of life throughout increasingly enduring lifespans.
Keywords: dysphagia, dysarthria, obstructive sleep apnea, sarcopenia, exercise, neural maturation, neuromuscular plasticity
Introduction
The physical acts of eating, speaking, and breathing during sleep and waking, can be viewed as a series of transformations of the upper aerodigestive tract. The oral cavity, pharynx and larynx collectively serve the functions of respiration, swallowing and speech production. A set of neural effector commands, activating more than 100 muscle contractions, control various peripheral structures (Fig. 1a and 1b) that perform the bulbar innervated functions of swallowing, speaking and/or breathing. A primary interest of clinicians and researchers concerned with the head and neck is to comprehend muscle tone, forces/pressures and structural movements in terms that permit inferences about the nature of underlying control mechanisms. Such understanding holds promise for translation to enhanced treatment development with the potential for transference across specific head and neck functions. The movements of the semi-independent parts of the peripheral mechanism(s) act to generate air pressures and flows with aerodynamic measurement, and, for instance, in the case of speech production, speech acoustics and phonetics provide levels of the process from which simultaneous measures may be obtained. While biological and biophysical levels of measurement also provide insights into the processes of swallowing, the peripheral mechanism(s) in fact, ceases air pressure generation and flow and alternative outcomes measures specific to bolus flow (direction, duration and clearance) prove of great value in understanding the system’s properties and function from acquisition through maturation to senescence in humans.
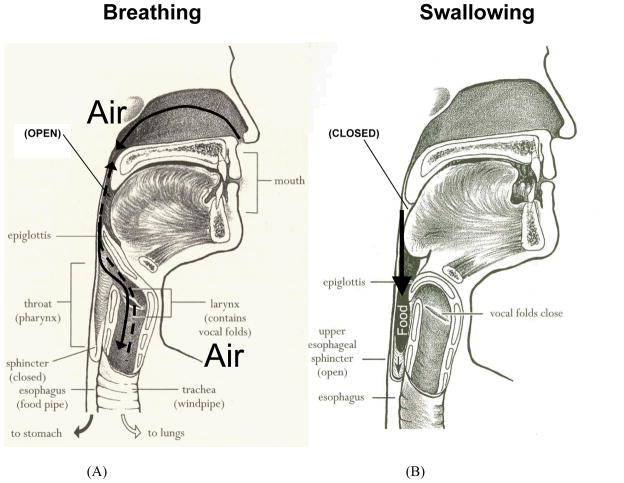
Figure 1.
The upper aerodigestive tract has two primary functions: breathing and swallowing. (A) Upper aerodigestive tract valves positioned (open) for directing airflow through systems. (B) Upper aerodigestive tract valves positioned (closed) for safely directing bolus flow. (Adapted from Easy to Swallow, Easy to Chew Cookbook: Over 150 Tasty and Nutritious Recipes for People Who Have Difficulty Swallowing by Weihofen D, Robbins J, and Sullivan PA. 2002, with permission of John Wiley & Sons, Inc.)
Studies of anatomic and physiologic development have demonstrated age-related changes from infancy to adulthood that serve as a critical background for clinical assessment and treatment of disorders of the aerodigestive tract. Improved understanding of acquisition of upper aerodigestive tract functions may shed light on common underpinnings that explain shared features of the speech, swallowing and respiratory sensorimotor systems, such as the subconscious manner with which movements are made. In the speech domain, traditionally considered to be a sequence of discrete units or segments, attempts to impose segment boundaries on articulatory events quickly are confounded by the fact that movements overlap one another in complex fashion and gave rise to the concept of co-articulation,1 emerging with maturity, now fairly well documented and accepted. In swallowing, analogous phenomena are recently emerging2 and shedding light on temporal courses and attainment of adult neuromuscular control of bulbar innervated functions. seeming to a great extent dependent on the individual’s peripheral, structural and nervous system maturation. Oral and pharyngeal phases of swallowing are more accurate references to bolus location than the oral, pharyngeal and laryngeal co-varying, anticipatory and sometimes co-occurring physiologic events responsible for bolus flow direction, timing and clearance. The study of neural maturation is challenging.
Yakolev’s distinction (1962) between development, growth and maturation form a working definition of neural maturation.3 Yakolev explained growth and maturation as subordinate and additive to his concept of development:
The development of the nervous system follows a sequence of morphological events which reflect and correlate with the changes in the internal state, outward form and dynamic relations of the organism to the environment. All these changes are subsumed in the conceptions of growth and maturation of the biological action systems. The conception of maturation, however, has a broader connotation of an exponential process of the progressive organization of functions and of their morphological substrata which go on through the life span of the individual… (p. 3, italics added)3
Yakolev’s definition on neural maturation contains both morphologic and functional components (i.e., a process of “progressive organization of functions and their morphological substrata”). This definition fits well with the concept of “systemogenesis”.4 Anokhin hypothesized that motor behavior was governed by a number of functional systems within the nervous system.4, 5 A functional system was made up of a group of nervous system structures that developed an “action-system specificity” not unlike Davenport et al’s behavioral central assembly system.5 For example, the neuroanatomy and neurophysiology subserving swallowing would constitute a functional system and that subserving speech production would be another. Given this conception, swallowing and speech and even sleep-related breathing may be produced by a central neural motor system that share certain neuroanatomic networks while maintaining separate neural functional systems and network structures. These systems are said to develop on different schedules, according to the needs of the organism. The functional system for speech motor control, and emerging evidence in support of such findings for human swallowing, indicate that aspects performed by the mature adult are not present at birth. Further, aspects of sleep regulation show profound changes during development, “significant maturational changes in sleep patterns occur after birth in mammals6…” Indeed, work over recent decades indicates morphologic features and anatomical relationships (i.e., protected airway at birth which repositions) evolve over years of life with acquisition, and moreover, lifelong performance, a continuous and nonlinear process (Figure 2). Sensitive periods of nonlinearity occur when neural, musculoskeletal, environmental and cognitive changes merge in the individual. With regard to early development, the point in time at which a particular number of these factors combine can result in changes in performance that occur as quantum leaps and have been referred to as critical periods.7
Figure 2.
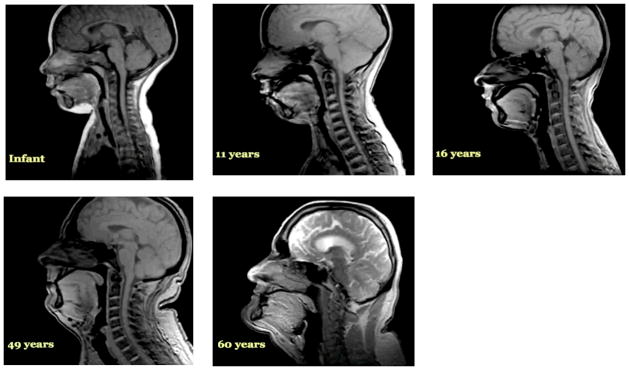
The continuous and non-linear lifelong evolution of morphologic features represented by MRIs of distinct individuals of different ages.
While critical or sensitive periods for acquisition of oropharyngeal sensorimotor control have received attention over recent decades, the concept has been emphasized for childhood development when neuroplasticity and synaptogenesis were believed most available and much more limited in its application to the middle and late years of the lifespan. Acknowledgement of the status of the adult’s neurosensorimotor maturation, musculoskeletal system, and related functional capabilities reflecting “sensitive periods” for upper aerodigestive tract precision in performance of bulbar innervated functions is becoming more prominent in the literature as the world’s population lives longer. Changes in performance are becoming apparent and taking their toll on health status and quality of life in the senior years, placing extraordinary demands on healthcare globally. The prominence of aspiration pneumonia as the third leading cause of death from infection in the US in people over the age of 80 years seems to signal senescent neuroplasticity losing its potency in the face of diminished control and decoupling of the otherwise tightly integrated neuromuscular underpinnings that facilitate precise coordination of the expression of the bulbar innervated functions of the oropharynx and airway maintenance mechanisms.8
Underlying “Integrative” Factors
In the US alone, nearly 8000 Americans are turning 60 every day.9 While the capacity to swallow, communicate effectively, and sleep soundly are basic human needs and pleasures, nearly 40% of Americans over 60 experience dysphagia,10 18 million suffer from sleep apnea11 and 51% of adults with acquired communication disorders demonstrate motor speech impairments.12 Awareness of these circumstances is coincident with a growing health concern regarding sarcopenia: the age-related reduction in skeletal muscle mass and cross-sectional area reduction in the number or size of muscle fibers and a transformation or selective loss of specific muscle fiber types.13 Muscle size appears to be related to changes in neuromuscular function across the lifespan, with age-related atrophic changes contributing to strength reductions.14, 15
Sarcopenia in cranial muscles contributes to age-related decline in the critical functions of the head and neck.16–19 The tongue is a major contributor to speech, swallowing and respiratory activity and therefore has become a focus of research on aging effects. Specific lingual findings in persons over age 60, compared to younger cohorts, include significantly increased fatty and connective tissue and increased amyloid deposits in blood vessels located in tongue muscle and sub-epithelial layers. Further, compared with young adults, aged individuals show decreased tongue mobility and lingual suction pressure, decreased thickness,20 decline in perceived intensity of local pressure on the tongue,21 and decrease in maximum voluntary isometric tongue pressures.22 It is likely that these age-associated lingual changes reflect the underlying condition of sarcopenia in head and neck muscles. That is, diminished muscle mass may be causally related to diminished lingual strength with aging, affecting temporal patterns of muscular action (weak muscle cannot move as quickly as strong23) and leading to age-related changes in bolus flow outcomes. Variability in oropharyngeal airway size and shape, blood flow, and tissue characteristics likely play a role in susceptibility to motor speech disorders and obstructive sleep apnea (OSA). However, sarcopenia has been largely ignored as a contributing factor and warrants attention, particularly with regard to OSA and the otherwise augmented activities of particularly key contributors such as genioglossus during wakefulness and interactions with pharyngeal dilators.24
Neural Correlates
Upper aerodigestive tract maturation appears to be centrally as well as peripherally driven. Fine and gross motor functions slow with age as does the reaction time to sensory stimuli.25, 26 The healthy older swallow is slow.18, 27–29 Lengthened duration of swallow is observed largely before the more automatic pharyngeal phase of the swallow is initiated, which indicates that the more volitional oral phase or a brainstem-mediated transitional period from oral to the more “hardwired” pharyngeal events, or both, are particularly affected. In those over age 65, the initiation of laryngeal and pharyngeal events, including laryngeal vestibule (and hence, airway) closure, are significantly delayed relative to bolus flow compared to adults younger than 45 years of age.27 Although the specific neural underpinnings are not yet confirmed, it can be hypothesized that the more voluntary oral events become “uncoupled” from the more “neurally hardwired” brainstem pharyngeal response which includes airway protection. Thus, in older healthy adults it is not uncommon for the bolus to be adjacent to an open airway by pooling or pocketing in the pharyngeal recesses, for more time than in younger adults, increasing the risk of aspiration and associated adverse consequences due to ineffective deglutition [Figure 3a–c (young) and 3d–f (old)].
Figure 3.
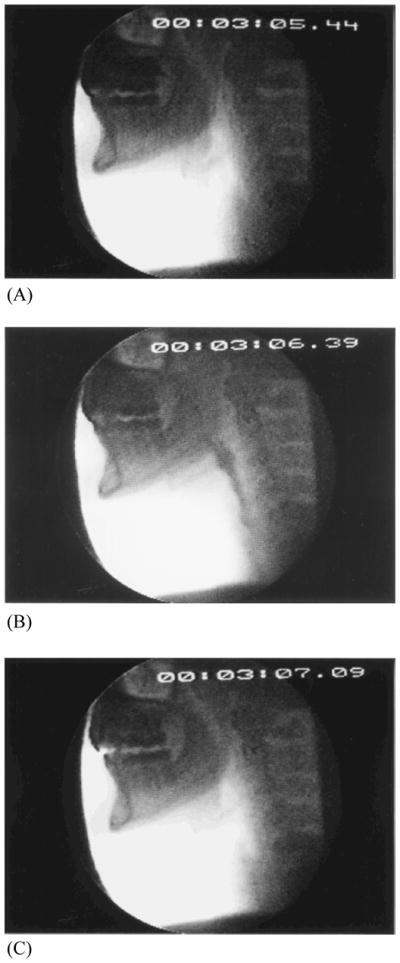
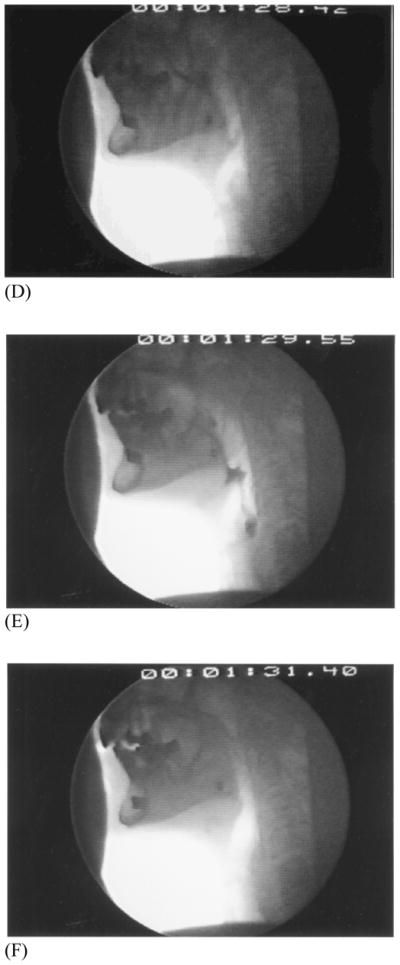
Healthy young swallowing documented with videofluoroscopy. (A) Bolus in oral cavity, ready to be swallowed. (B) Bolus appears as a “column” of material swiftly moving through the pharynx. (C) Oropharynx cleared of material when the swallow is completed. Healthy old swallowing documented with videofluoroscopy. (D) Bolus in mouth ready for swallowing. (E) Bolus pooled in vallecula and pyriform sinus during delayed onset of pharyngeal response. (F) Bolus cleared of material when the swallow is completed. (Adapted with permission from Robbins JA. Normal swallowing and aging. Semin Neurol. 1996; 16(4):309.)
Neuroimaging studies using cranial MRIs in healthy adults show a relationship between slower swallowing and the increased number and severity of periventricular white-matter hyperintensities (PVHs) in the brain, supporting the concept that voluntary control of swallowing is mediated by corticobulbar pathways within the periventricular white matter.30 The appearance of and the degree of these PVHs increase with age and may explain, at least in part, the relatively asymptomatic decline in oropharyngeal motor performance observed in older people. Cerebral atrophy, blood flow changes, and other age-related conditions must also be factored into the process of presbyphagia progression. Thus, central as well as peripheral contributions may modify bulbar innervated functions across the life span.
While muscle growth occurs with youthful development, muscle atrophy occurs with aging and is likely a key determinant in the reductions in strength and endurance observed in senescent muscles. In the upper airway, aging is often associated with functional declines in swallowing status,17, 31, 32 phonatory ability33, 34 and upper airway maintenance during sleep.35 The underlying mechanism for these observed changes with aging may be a loss of skeletal muscle mass and strength that affects both magnitude and timing of muscle force generation and timing.18, 36 Despite findings of decreased isometric maximum lingual strength with aging, no significant differences are found between young and older groups in specific maximum pressure generation during swallowing22, 37 indicating that swallowing pressure reserve, the relationship between isometric (maximal) and swallowing (submaximal) pressures, is reduced with age.
While implications of these reserve reductions remain unclear, decreased functional swallowing reserve may leave older individuals more vulnerable and without the capacity to compensate like younger individuals, when insults occur along the length of the neuraxis to the neural networks for the oropharynx (e.g., stroke). It has been suggested that the observed changes in lingual anatomy and function from anterior to posterior tongue may reflect property changes such as increased number of muscle fibers per motor neuron (as in other muscle tissue)37 or smaller maximal protrusive tetanic forces.38 Such changes may lead to enlarged larger regions of muscle fibers acting as a unit, which may decrease the lingual “degrees of freedom”.37 These changes may lead to decreased adaptability of the oropharyngeal system to other cumulative effects of healthy aging increasing risk for diminished homeostenosis across bulbar innervated functions including swallowing, sleep-related breathing and perhaps, in some cases of increased neurophysiologic aging, include speech production.
Although the mechanisms responsible for oropharyngeal dysphagia, dysarthria and OSA are apparently complex, those for the latter conditions do appear to compensate for etiologies during wakefulness, including increased activity in a number of upper airway muscles such as the genioglossus and tensor palatini. The available evidence suggests that during wakefulness there is neuromuscular compensation in dilator muscles. Unfortunately the basic neural mechanisms during compensation are not well understood and are even less clear during sleep.
Exercise
Work elucidating age-related changes within the oropharyngeal mechanism has provided the foundation for treatment strategies that may reduce, retard or quite possibly reverse the decline that was once believed to be inevitable. Data are accumulating indicating a capacity for increased tongue strength with specific, systematic isometric lingual exercises in young,39 healthy old40 and stroke patients.41 There appears to be a threshold of intensity (overload)42 required to elicit strength and neural changes.43 Evidence is building indicating that the intensity of strengthening therapy and additional fundamental exercise parameters including repetition,44 which have proven successful for striated limb musculature42 are now contributing to important outcomes for the bulbar integrated striated system. The evidence suggests that low intensity treatment is unlikely to support maximum behavioral or neural plasticity. The solutions are to discover optimal treatments that have proven successful for striated limb musculature based in sports medicine literature and then to translate them to delivery models that allow achievement of intensity and repetition most consistent with progressive resistance tongue exercise programs that have been successfully applied to individuals with oropharyngeal swallowing impairment.40, 41
In patients post-stroke, an 8-week isometric lingual exercise regimen resulted in decreased airway invasion, decreased post-swallow residue and more rapid bolus transit.41 Improvements in swallowing outcomes have been reported in studies as limited in duration as four weeks,39 however studies of longer duration (8–9 weeks), report continued improvements in pressure gains through protocol completion.40, 41, 45 It has been hypothesized that the initial rise in pressure generation and reduction in aspiration observed after the first 4 weeks of exercise reflect changes in the neural underpinnings of swallowing, whereas their later improvements in lingual strength and associated outcomes at 8 weeks implicate the positive effects of the intervention on muscle hypertrophy.41, 46 These results, along with recent functional magnetic imaging findings47 (see Malandraki et al, this issue) indicate that exercise protocols, when of sufficient duration, intensity and repetition capitalize on central as well as peripheral changes. Additionally, the transference of progressive isometric resistance lingual exercises to swallowing and speech intelligibility48 suggest modification of neuromuscular underpinnings that transcend specificity of function. The potential for exercise induced airway remodeling, as evidenced by at a minimum, change in muscle fiber cross sectional area15 and lingual volume41, 49 holds promise for modifying airway patency during sleep.50 Oropharyngeal exercises recently have been demonstrated to reduce OSA severity and symptoms and are put forth as “a promising treatment for moderate OSA”.50
Future Directions
We are living in times that are characterized by a surge in new imaging techniques, novel physiologic recording methods and creative systematic behavioral interventions directed at modifying bulbar innervated functions, to date, orthogonally applied and interpreted. The generalization of systematically translated oropharyngeal exercises along with an array of other interventions including electrical and transmagnetic stimulation, must be viewed with caution and conducted with all of the scientific rigor that new pharmaceutical and technologically-based treatment trials warrant. Defining groups of individuals who may benefit from relatively non-invasive, yet systematically designed, carefully controlled, rigorous exercise programs that positively affect quality of life, which certainly breathing, sleeping, eating and speaking comprise, provides a potential and promising pathway for innovation and evidence-based investigation.
Experiments designed to answer hypotheses based questions will play an important role in elucidating the integrative neural systems underlying vital aerodigestive tract functions. While facilitating developmental coupling/habilitation, and injury-related rehabilitation, such an approach will perhaps in the not-too-distant future even prevent the eventual neuromuscular decoupling that appears to be emerging along an increasingly extensive continuum, translating into improved function and increased endurance38, 51 throughout the currently evolving lifespan.
Acknowledgments
This material is the result of work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service, VA Merit Grant C4796R. This is GRECC Manuscript #2011-08.
We express appreciation to the many members of our constantly evolving clinical research program including Jacqueline Hind, Mark Nicosia, Angela Hewitt, Georgia Malandraki, Ianessa Humbert, Stephanie Kays, John Byce, Jay Rosenbek and Abby Duane for their contributions; also to Ray Kent for his excellent editing expertise and steadfast support.
Footnotes
Disclaimer: The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
COI: Dr. Robbins discloses a relationship with Swallow Solutions, LLC, which is focused on clinical research in dysphagia.
References
- 1.Kent RD, Minifie FD. Coarticulation in recent speech production models. J Phonetics. 1977;5:115–133. [Google Scholar]
- 2.Zamir Z, Ren J, Hogan WJ, Shaker R. Coordination of deglutitive vocal cord closure and oral-pharyngeal swallowing events in the elderly. Eur J Gastroenterol Hepatol. 1996;8:425–429. [PubMed] [Google Scholar]
- 3.Yakovlev PI. Morphological criteria of growth and maturation of the nervous system in man. Res Publ Assoc Res Nerv Ment Dis. 1962;39:3–46. [PubMed] [Google Scholar]
- 4.Anokhin PK. studies on physiological properties of synaptic systems of the cerebral cortex. Tr Inst Norm Patol Fiziol. 1964;117:98–99. [PubMed] [Google Scholar]
- 5.Davenport P, Bolser D, Morris K. Swallowing remodeling of respiratory neural networks. Head and Neck. 2011 doi: 10.1002/hed.21845. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benca R, Duncan MJ, Frank E, McClung C, Nelson RJ, Vicentic A. Biological rhythms, higher brain function, and behavior: Gaps, opportunities, and challenges. Brain Res Rev. 2009;62:57–70. doi: 10.1016/j.brainresrev.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netsell R. A neurobiologic view of speech production and the dysarthrias. San Diego, CA: College Hill Press; 1986. [Google Scholar]
- 8.LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of u.S. Older people: The role of chronic conditions, health behaviors, and nutritional status. Public Health Reports. 1989;104:350–360. [PMC free article] [PubMed] [Google Scholar]
- 9.US Census Bureau. [Accessed May 4, 2011];Facts for features (cb06-ffse.01–2) 2006 http://www.census.gov/newsroom/releases/pdf/cb06-ffse01-2.pdf.
- 10.Robbins J, Kays S, McCallum S. Team management of dysphagia in the institutional setting. J Nutr Elder. 2007;26:59–104. doi: 10.1300/J052v26n01_04. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health (NHLBI) Sleep apnea linked to increased risk of death. [Accessed May 5, 2011];NIH News. 2008 Aug 1; http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2580.
- 12.Justice M. Communication sciences and disorders: An introduction. New Jersey: Allyn & Bacon; 2006. [Google Scholar]
- 13.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 14.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 15.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52:732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum BJ, Bodner L. Aging and oral motor function: Evidence for altered performance among older persons. J Dent Res. 1983;62:2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- 17.Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: Radiologic findings in 56 cases. AJR Am J Roentgenol. 1991;156:1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- 18.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 19.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of speech, language, and hearing research: JSLHR. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 20.Sonies B, Stone M, Shawler T. Speech and swallowing in the elderly. Gerontol. 1984;3:115–123. doi: 10.1111/j.1741-2358.1984.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 21.Weiffenbach JM, Tylenda CA, Baum BJ. Oral sensory changes in aging. J Gerontol. 1990;45:M121–125. doi: 10.1093/geronj/45.4.m121. [DOI] [PubMed] [Google Scholar]
- 22.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. The journals of gerontology. Series A, Biological sciences and medical sciences. 1995;50:M257–262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 23.Luschei E. Development of objective standards of non-speech oral strength and performance: An advocates’s view. In: Moore C, Yorkston K, Beukelman D, editors. Dysarthria and apraxia of speech. Baltimore: Brooks Publishing; 1991. [Google Scholar]
- 24.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welford AT. Reaction time, speed of performance, and age. Ann N Y Acad Sci. 1988;515:1–17. doi: 10.1111/j.1749-6632.1988.tb32958.x. [DOI] [PubMed] [Google Scholar]
- 26.Birren IE, Woods AM, Williams MV. Brain function in old age. Berlin: Springer-Verlag; 1979. Speed of behavior as an indicator of age changes and the integrity of the nervous system; pp. 10–44. [Google Scholar]
- 27.Tracy F, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugla C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4:90–94. doi: 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 28.Shaw D, Cook IJ, Dent J, Simula M, Panagopoulos V, Gabb M, Shearman D. Age influences oropharyngeal and upper esophageal sphincter function during swallowing. Gastroenterology. 1990;98:A390. [Google Scholar]
- 29.Shaw D, Cook IJ, Gabb M, Holloway R, Simula M, Panagopoulos V, Dent J. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol Gastrointest Liver Physiol. 1995;268:G389–396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 30.Levine R, Robbins JA, Maser A. Periventricular white matter changes and oropharyngeal swallowing in normal individuals. Dysphagia. 1992;7:142–147. doi: 10.1007/BF02493446. [DOI] [PubMed] [Google Scholar]
- 31.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 32.Schindler JS, Kelly JH. Swallowing disorders in the elderly. Laryngoscope. 2002;112:589–602. doi: 10.1097/00005537-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Ramig LA, Ringel RL. Effects of physiological aging on selected acoustic characteristics of voice. J Speech Hear Res. 1983;26:22–30. doi: 10.1044/jshr.2601.22. [DOI] [PubMed] [Google Scholar]
- 34.Honjo I, Isshiki N. Laryngoscopic and voice characteristics of aged persons. Arch Otolaryngol. 1980;106:149–150. doi: 10.1001/archotol.1980.00790270013003. [DOI] [PubMed] [Google Scholar]
- 35.Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: Relationship to apnoea/hypopnoea index and age. Journal of sleep research. 2000;9:389–393. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 36.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 37.Nicosia MA, Hind JA, Roecker EB, Carnes M, Robbins JA. Age effects on the temporal evolution of isometric and swallowing pressure. The journals of gerontology. Series A, Biological sciences and medical sciences. 2000;55A:M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 38.Nagai H, Russell JA, Jackson MA, Connor NP. Effect of aging on tongue protrusion forces in rats. Dysphagia. 2008;23:116–121. doi: 10.1007/s00455-007-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarus C, Logemann J, Huang C, Rademaker A. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatrica et Logopedica. 2003;55:199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 40.Robbins J, Gangnon R, Theis S, Kays SA, Hind J. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 41.Robbins J, Kays SA, Gangnon R, Hewitt A, Hind J. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88:150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Powers S, Howley E. Exercise physiology: Theory and application to fitness and performance. McGraw-Hill Humanities/Social Sciences/Languages; 2008. [Google Scholar]
- 43.Lisman J, Spruston N. Postsynaptic depolarization requirements for ltp and ltd: A critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8:839–841. doi: 10.1038/nn0705-839. [DOI] [PubMed] [Google Scholar]
- 44.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 45.Clark HM, O’Brien K, Calleja A, Corrie SN. Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52:1034–1047. doi: 10.1044/1092-4388(2009/08-0062). [DOI] [PubMed] [Google Scholar]
- 46.Jones DA, Rutherford OM, Parker DF. Physiological changes in skeletal muscle as a result of strength training. Q J Exp Physiol. 1989;74:233–256. doi: 10.1113/expphysiol.1989.sp003268. [DOI] [PubMed] [Google Scholar]
- 47.Malandraki GA, Johnson S, Robbins J. Functional magnetic resonance imaging of swallowing: From neurophysiology to neuroplasticity. Head and Neck. 2011 doi: 10.1002/hed.21903. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan P, Hind J, Roecker E, Carnes M, Doyle J, Dengel GA, Robbins J. Lingual exercise protocol for head and neck cancer: A case study, poster presentation. 9th Annual Meeting of the Dysphagia Research Society; Savannah, GA. 2000. [Google Scholar]
- 49.Humbert IA, Reeder SB, Porcaro EJ, Kays SA, Brittain JH, Robbins J. Simultaneous estimation of tongue volume and fat fraction using ideal-fse. J Magn Reson Imaging. 2008;28:504–508. doi: 10.1002/jmri.21431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guimaraes KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179:962–966. doi: 10.1164/rccm.200806-981OC. [DOI] [PubMed] [Google Scholar]
- 51.Kays S, Hind J, Gangnon R, Robbins J. Effects of dining on tongue endurance and swallowing-related outcomes. JSLHR. 2010;53:898–907. doi: 10.1044/1092-4388(2009/09-0048). [DOI] [PMC free article] [PubMed] [Google Scholar]