Abstract
Prevention of chronic graft-versus-host disease (cGVHD) remains a major challenge in allogeneic hematopoietic cell transplantation (HCT), due to limited understanding of cGVHD pathogenesis and lack of appropriate animal models. Here, we report that, in classical acute GVHD models with C57BL/6 donors and MHC-mismatched BALB/c recipients and with C3H.SW donors and MHC-matched C57BL/6 recipients, GVHD recipients surviving for more than 60 days after HCT developed cGVHD characterized by cutaneous fibrosis, tissue damage in the salivary gland and the presence of serum autoantibodies. Donor CD8+ T cells were more potent than CD4+ T cells for inducing cGVHD. The recipient thymus and de novo-generated, donor-derived CD4+ T cells were required for induction of cGVHD by donor CD8+ T cells but not by donor CD4+ T cells. Donor CD8+ T cells preferentially damaged recipient medullary thymic epithelial cells and impaired negative selection, resulting in production of autoreactive CD4+ T cells that perpetuated damage to the thymus and augmented the development of cGVHD. Short-term anti-CD4 monoclonal antibody treatment early after HCT enabled recovery from thymic damage and prevented cGVHD. These results demonstrate that donor CD8+ T cells cause cGVHD solely through thymic-dependent mechanisms, while CD4+ T cells can cause cGVHD through either thymic-dependent or independent mechanisms.
Introduction
Donor CD8+ T cells are more potent than CD4+ T cells in facilitating stem cell engraftment and mediating graft versus lymphoma/leukemia (GVL) effects, but both CD4+ and CD8+ T cells mediate severe graft-versus-host disease (GVHD) in mice and humans (1-12). GVHD can be divided into acute (aGVHD) and chronic (cGVHD) based on different clinical manifestations and histopathology. aGVHD usually begins within 100 days after HCT and is characterized by acute tissue inflammation and infiltration of alloreactive lymphocytes in GVHD target organs such as colon, skin, and liver (13). cGVHD usually begins more than 100 days after HCT as an autoimmune scleroderma- and lupus-like syndrome characterized by autoantibody production, chronic inflammation, and collagen deposition in target tissues (14-18). Chronic GVHD and aGVHD can both affect the skin, liver, and gastrointestinal tract, but cGVHD also affects prototypical target organs such as salivary gland (14-16). Although some cGVHD can occur without prior aGVHD, cGVHD often overlap with persistent, recurrent, and late aGVHD, and most cGVHD occurs after aGVHD (14-16, 19). Many murine models have been used to examine the pathophysiology of aGVHD or cGVHD (20-26), but none of these models clearly reflects the transition from aGVHD to cGVHD that typically occurs in humans. In addition, the role of donor CD8+ T cells in chronic GVHD induction remains unclear, as all mouse chronic GVHD models focus on CD4+ T cells.
Thymic medullary epithelial cells (mTEC) and dendritic cells (DCs) play important roles in central deletion of autoreactive T cells (27, 28). Since cGVHD often follows aGVHD, it has been proposed that cGVHD results from impaired negative selection in the thymus caused by alloreactive T cells during aGVHD, allowing for de novo generation of donor-derived T cells that recognize recipient tissues (29-33), but the role of damaging mTEC has not clearly been documented. Bone marrow cells from MHC II-/- mice give rise to autoreactive CD4+ T cells that mediate cGVHD in recipients conditioned with high dose TBI, due to a defect in thymic DC-mediated negative selection (34). But in this model, the role of thymic epithelial cells remains unknown, and the development of autoantibodies was not reported. These issues have not been addressed in other cGVHD models (20). In the current studies, we explore whether aGVHD mediated by donor CD4+ or CD8+ T cells can develop into characteristic cGVHD in murine models, and we explore the roles of thymic mTEC and DCs in the generation of autoreactive T cells early after HCT.
Materials and Methods
Mice
C57BL/6 and BALB/c mice were purchased from the National Cancer Institute (NCI) animal production program (Frederick, Maryland). Thymectomized and Control euthymic BALB/c as well as CD4+ T- or CD8+ T-deficient C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Rag-2-/- BALB/c and Rag-2-/- C57BL/6 mice were purchased from Taconic Farms, Inc. (Germantown, New York). Mice were maintained in a pathogen-free room in the City of Hope Animal Resource Center (Duarte, CA). All animal protocols were approved by the City of Hope Institutional Animal Care and Use Committee.
Statistical analysis
Clinical cutaneous damage scoring and survival in different groups were compared by using the rank sum test or log-rank test (Prism, version 5.0; GraphPad Software, San Diego, CA). Comparison of two means was analyzed using an unpaired two-tail Student t test.
Antibodies, flow cytometry analysis, and cell sorting
FITC-Ly51 (6C3), FITC-CD45.1 (A20), FITC-I-A/I-E (2G9), FITC-Vβ3 (KJ25), FITC-Vβ4 (KT4), FITC-Vβ5.1/5.2 (MR9-4), FITC-Thy1.2 (30-H12), PE-B220 (RA3-6B2), APC-Cy7-CD8a (53-6.7), APC-Cy7-CD4 (GK-1.5), and PE-streptavidin were purchased from BD Pharmingen (San Diego, CA). eFluor450-EpCAM (G8.8), eFluor450-CD4 (RM4-5), PE-Cy7-CD8a (53-6.7), APC-TCRβ (H57-597), APC-B220 (RA3-6B2), PE-H-2Kb(AF6-88.5.5.3), APC-CD11c(N418), FITC-CD11c(N418) were purchased from eBioscience (San Diego, CA). PE-CCR9 (Clone 242503) was purchased from R & D System (Minneapolis, MN). Biotinylated UEA I was purchased from Vector Laboratories, Inc (Burlingame, CA). APC-CD45 (Clone 30F11) was purchased from Miltenyi Biotec (Auburn, CA). Aqua fluorescent reactive dye for viability analysis was purchased from Invitrogen (Carlsbad, CA). Flow cytometric data were analyzed with FlowJo Software (Treestar, Ashland, OR) as described in our previous publications (35-37)
Induction and assessment of GVHD
Mice were exposed to 850 cGy TBI with the use of a 137Cs source 8 hours before HCT. Recipients were injected with T and B cell-depleted donor BM cells (TBCD-BM) and whole splenocytes or sorted CD4+ T or CD8+ T cells. T and B cells in BM were depleted with the use of biotin-conjugated anti-CD3, anti-CD4, anti-CD8, and anti-B220 mAb and streptavidin-conjugated micro-magnetic beads, followed by passage through an AutoMACS cell sorter (Miltenyi Biotec). The purity of depletion was >99%. CD4+ and CD8+ T cells were isolated from donor spleen suspensions with the use of biotin-conjugated anti-CD4 or anti-CD8 mAb and streptavidin-conjugated micro-magnetic beads, and passage through an AutoMACS cell sorter (Miltenyi Biotec). The purity of the positive fraction exceeded 95%. The assessment and scoring of clinical cutaneous GVHD were described in our previous publications (35, 37, 38).
Histopathology
Tissue specimens were fixed in formalin before embedding in paraffin blocks, cut, and stained with H&E. Slides were examined at 200-400x magnification and visualized with an Olympus BX51 and a Pixera (600CL) cooled charge-coupled device camera (Pixera, Los Gatos, CA). Tissue damage was blindly assessed according to a scoring system described previously (35, 37).
Proliferation assays
De novo-generated CD4+ T cells were sorted with flow cytometry with anti-CD45.1 (marker for de novo-generated donor cells), anti-CD4, and anti-Thy1.2. Proliferating CD4+ T cells were measured as previously reported (35, 37). Briefly, sorted CD4+ T cells (2×105) were incubated with irradiated dendritic cells (1×105) in complete RPMI media for 4 days, with tritiated thymidine deoxyribose added to the culture 18h before harvest. Cultures were established in triplicates. The stimulation index was calculated according to the formula: [(cpm of culture of responder cells with stimulator) – (cpm of culture of responder alone)] / (cpm of culture of responder alone).
Thymic epithelial cell staining
Thymic epithelial cell isolation was performed according to a protocol from Dr. Marcel van den Brink’s laboratory (39). In brief, the thymus was cut into small (<.25 cm2) pieces, and placed in complete RPMI media with collagenase D and DNAse I. Thymic fragments were rapidly pipetted through the aperture of a 1000 uL pipette tip, and incubated in a 37°C water bath to digest the thymus and release epithelial cells from the extracellular matrix. Supernatants were harvested every 15 minutes, and the process was repeated 3 additional times. For the fourth digestion, dispase was added to release additional thymic epithelial cells. Fractions were stained with anti-EpCam, CD45, UE Agglutinin, and Ly-51. Cortical epithelial cells were defined as EpCam+CD45-UEA lo/mid Ly51mid/hi, while medullary epithelial cells were defined as EpCam+CD45-UEAmid/high Ly51 lo/mid. Cells from 6 thymi were combined in order to provide sufficient cells for analysis.
Serum staining of Rag-2-/- mouse skin or salivary gland tissues
Serum autoantibodies were measured by staining Rag-2-/- mouse skin and salivary gland tissues as previously described (40). In brief, cryosections from Rag2-/- C57BL/6 and BALB/c mice were prepared by soaking tissues in 10% formalin for 1h followed by dehydration in a solution of 30% sucrose in PBS. Tissue was then frozen in OCT gel and cryosectioned. Cryosections were placed in acetone at -20°C for 15 minutes, then rehydrated in PBS containing Mg2+. Tissues were blocked with 10% FBS in PBS for 2h. Tissues were then incubated overnight with 5 x-diluted serum and washed again. Tissues were then stained with anti-mouse IgG-AlexaFluor488 and DAPI for 2h and washed. Representative photomicrographs were taken of each tissue at 200x with the use of an Olympus BX51 and a Pixera (600CL) cooled charge-coupled device camera (Pixera, Los Gatos, CA).
Results
Low-dose donor spleen cells enabled recipient survival and development of cGVHD
Transplantation of bone marrow (BM) and spleen cells from C57BL/6 donor into lethally irradiated BALB/c recipients has been considered an aGVHD model for more than three decades (21, 41), and autoreactive CD4+ T cells were detected in the recipients early after HCT (42-44). To test whether cGVHD developed in BALB/c recipients transplanted with C57BL/6 donor BM and spleen cells, T and B cell-depleted (TBCD)-BM cells (2.5 ×106) were injected with or without titrated dose of donor spleen cells (5-1.25 × 106) into lethally irradiated recipients. Recipients were monitored for bodyweight change, clinical manifestations of diarrhea and hair-loss, and survival. Additionally, at days 15 and 60 after HCT, the presence of autoantibodies in the recipient was measured by staining the skin and salivary gland tissues of donor-type Rag-2-/- C57BL/6 and recipient-type Rag-2-/- BALB/c mice, as previously described (40). Histopathology of colon, jejunum, skin, and salivary gland, as well as percentage and yield of CD4+CD8+ thymocytes were also compared.
The recipients given TBCD-BM alone showed gradual recovery of bodyweight and no signs of diarrhea or hair-loss, and all survived for more than 60 days. In contrast, recipients given 5 or 2.5 ×106 spleen cells showed severe bodyweight loss and diarrhea and all died by 30 or 45 days after HCT (Fig. 1A-D). Approximately 60% of recipients given low-dose (1.25 ×106) spleen and TBCD-BM cells showed mild diarrhea, and 85% survived for more than 60 days after HCT, but they showed gradual bodyweight-loss and hair-loss beginning at approximately 45 days after HCT (Fig. 1A-D).
Fig. 1. Low-dose of donor spleen cells induced cGVHD.
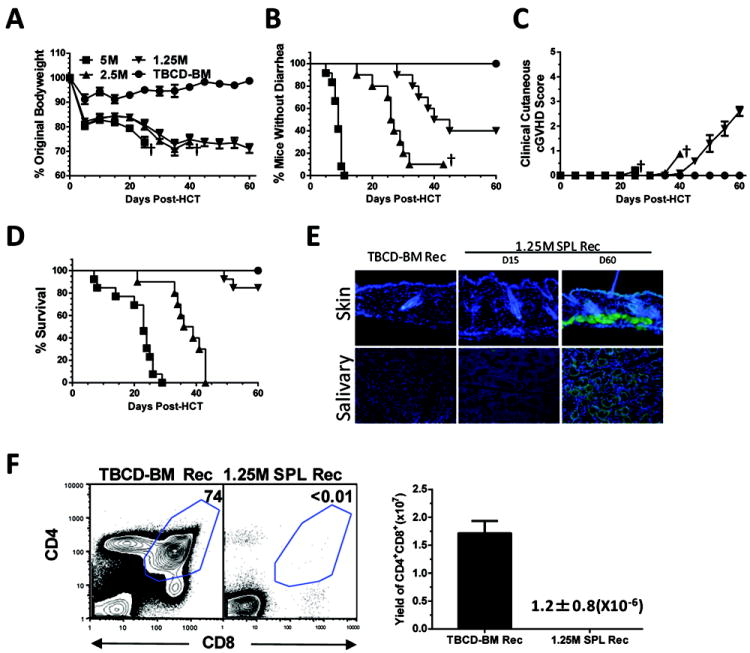
Lethally irradiated BALB/c recipients were transplanted with titrated numbers of spleen cells (5-1.25 ×106) and TBCD-BM cells (2.5×106) from C57BL/6 donors. Control recipients were given TBCD-BM cells alone. Recipients were monitored for clinical GVHD including bodyweight changes, diarrhea, hair-loss, and survival (†, indicating death of all recipients in a group). 15 and 60 days after HCT, recipient serum samples were tested for presence of autoantibodies by staining donor-type Rag-2-/- C57BL/6 skin and salivary gland tissues. 60 days after HCT, recipients were measured for percentage and yield of CD4+CD8+ thymocytes. A-D: Percentage of bodyweight changes, percentage of recipients without diarrhea, clinical cutaneous GVHD score, and percentage of survival. Each group contained 12 recipients combined from three replicate experiments. E: Representative photomicrographs of GVHD recipient serum autoantibody staining of Rag-2-/- skin and salivary gland tissues. DAPI staining is shown in blue, and autoantibody staining is shown in green. One representative result is shown from 8 samples evaluated in each group. F: One representative of CD4+CD8+ thymocytes staining pattern is shown of 8 recipients examined in each group. The mean ± SE of yield of CD4+CD8+ thymocytes (N=8).
Our recent report showed that in a cGVHD model with DBA/2 donors and BALB/c recipients, cGVHD characteristic features became obvious at approximately 45 days after HCT. Other manifestations included serum autoantibodies, expansion of dermis, loss of fat, and increased collagen deposition in the skin, loss or blunting of intestinal crypts in the jejunum, and infiltration and loss of secretory follicles in the salivary gland (35, 37). We tested whether similar manifestations developed in the classical acute GVHD model using C57BL/6 donors and BALB/c recipients. Sera from control GVHD-free BALB/c recipients at 60 days after HCT and sera from GVHD recipients at 15 days after HCT showed little autoantibody staining of donor-type Rag-2-/- C57BL/6 or recipient-type Rag-2-/- BALB/c skin or salivary gland tissues. In contrast, sera from GVHD recipients at 60 days after HCT showed strong staining of the skin and salivary gland tissues of donor-type (Fig. 1E) and recipient-type (Fig S1A). Since the staining of recipient-type tissues could also result from donor-anti-host allo-antibodies, measuring serum autoantibody was focused on using donor-type tissues. By 60 days after HCT, the GVHD recipients had almost undetectable CD4+CD8+ thymocytes, as compared to control recipients (P<0.001, Fig. 1F).
We also compared the histopathology of GVHD recipients and control TBCD-BM recipients at the different time points in prototypical aGVHD-target tissues (i.e., colon), acute and chronic GVHD overlapping target tissues (i.e., jejunum and skin), and prototypical cGVHD target tissues (i.e., salivary gland) (16, 45). Compared to control recipients, colon tissues of GVHD recipients were significantly damaged (P<0.01) by 15 days after HCT, worsened (P<0.01) by 30 days after HCT, but showed evidence of recovery by 60 days after HCT (Fig. 2A).
Fig. 2. Kinetic changes of GVHD target tissue pathology.
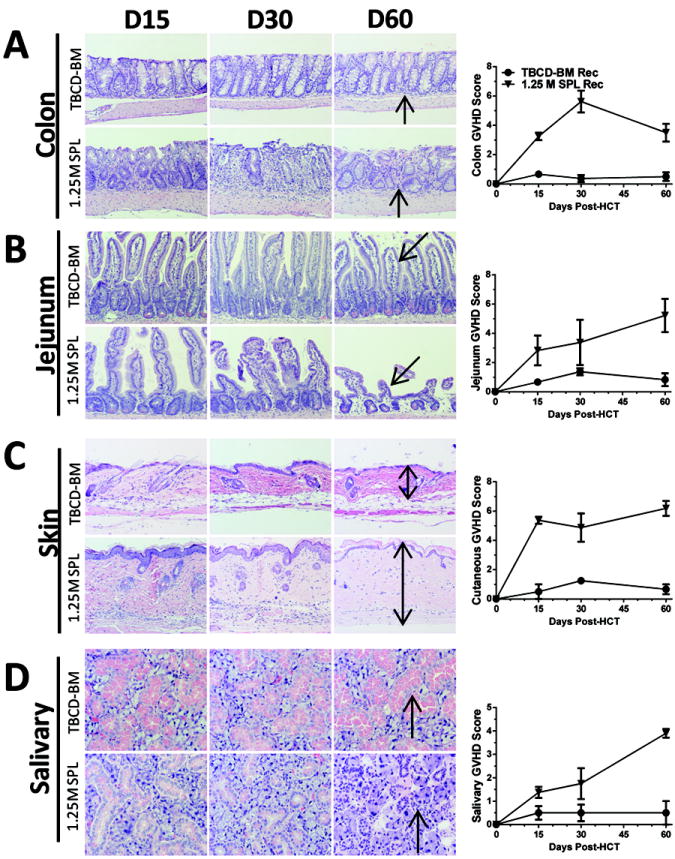
GVHD target tissues of colon (A), jejunum (B), skin (C) and salivary gland (D) were harvested and processed for evaluation of histopathology on days 15, 30, and 60 days after HCT from recipients given donor TBCD-BM alone or additional 1.25 ×106 spleen cells. Arrows indicate the following changes in GVHD recipients as compared to control recipients: infiltration and loss of crypts in the colon; blunting and loss of crypts in the jejunum; expansion of dermis and loss of fat tissues in the skin; infiltration and destruction of secretory follicles in the salivary gland. One representative photomicrograph and mean ± SE of histopathology scores are shown for 6 recipients in each group.
Compared with GVHD-free control TBCD-BM recipients, the jejunum specimens from GVHD recipients were mildly, moderately, and severely damaged at days 15, 30, and 60, respectively, and the difference between days 15 and 60 was statistically significant (P<0.01, Fig. 2B). The skin tissues of GVHD recipients showed epidermal hyperplasia and tissue infiltration at 15 and 30 days after HCT, but these manifestations appeared to be reduced by 60 days after HCT (Fig. 2C). Instead, we observed significant expansion of the dermis with collagen deposition together with loss of hair-follicles and fat tissues (Fig. 2C; Fig.S1B). Compared to GVHD-free control TBCD-BM recipients, salivary gland tissues of GVHD recipients were mildly damaged at day 15, moderately damaged by day 30, and severely damaged by day 60 after HCT (P<0.01, Fig. 2D). Taken together, BALB/c recipients given low-dose donor C57BL/6 spleen cells developed typical clinical and histopathological features of cGVHD by 60 days after HCT.
Acute GVHD in C57BL/6.SJL (H-2Kb, CD45.1) recipients induced by CD8+ T cells from MHC-matched but minor histocompatability antigen-mismatched C3H.SW (H-2Kb, CD45.2) donors has been described as evolving to cGVHD at late time points after HCT (33), but the characteristic features of cGVHD were not reported. In the current study, recipients given CD8+ T-enriched spleen cells showed gradual bodyweight-loss, hair-loss, starting at approximately 30 days after HCT (Fig. S2 A & B), while control recipients given TBCD-BM did not develop these changes. By 60 days after HCT, the GVHD recipients developed characteristic cGVHD features with serum autoantibody production, loss of CD4+CD8+ thymocytes, and chronic tissue damage in the jejunum, skin, and salivary gland (Fig. S2 D-F).
Low-dose C57BL/6 donor CD8+ T cells are more potent than CD4+ T cells in mediating cGVHD, although high-dose CD4+ T cells were more potent at mediating aGVHD
The GVHD model of C57BL/6 donor to BALB/c recipient has been characterized as CD4+ T cell-dependent model (21). We compared the capacity of C57BL/6 donor CD4+ and CD8+ T cells to induce aGVHD and cGVHD in BALB/c recipients. Lethally irradiated recipients were transplanted with donor TBCD-BM with or without titrated numbers (5-0.1 ×106) of donor CD4+ or CD8+ T cells. 5 × 106 donor CD4+ T cells induced severe aGVHD with bodyweight loss and diarrhea, and all recipients died within 20 days after HCT. In contrast, 5 ×106 donor CD8+ T cells induced little diarrhea. All recipients developed severe hair-loss by 45 days after HCT, although most of the recipients survived for more than 60 days (P<0.01, Fig. 3A). Reduction of the donor CD4+ T cell dose decreased early mortality in the recipients. With 0.5 ×106 CD4+ or CD8+ donor T cells, most recipients survived for more that 60 days after HCT. Recipients in both groups showed similar bodyweight-loss and hair-loss (Fig. 3B). Diarrhea was induced with donor CD4+ cells but not with CD8+ T cells (P<0.01, Fig. 3B). With 0.1 ×106 donor CD4+ or CD8+ T cells, bodyweight loss and hair-loss was more severe with CD8+ cells than with CD4+ T cells beginning at 45 days after HCT (P<0.05, Fig. 3C).
Fig. 3. High dose of donor CD4+ T cells induced more severe aGVHD but low-dose of donor CD8+ T cells induced more severe cGVHD.
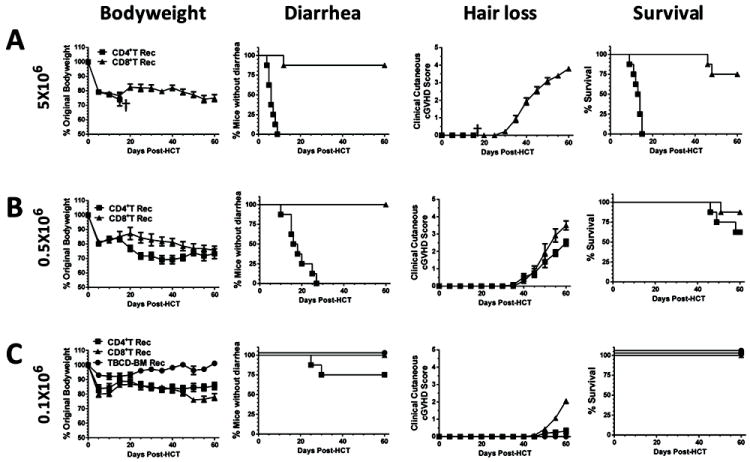
Lethally irradiated BALB/c recipients were injected with titrated numbers (5, 0.5, and 0.1 ×106) of sorted donor CD4+ or CD8+ T cells and TBCD-BM cells from C57BL/6 donors. Recipients given TBCD-BM alone were used as controls. Recipients were monitored for bodyweight change, hair-loss, diarrhea, and survival (†, indicating death of all recipients in a group). There were 8 recipients in each group, combined from two replicate experiments. A: Recipients given 5 ×106 donor CD4+ T cells developed diarrhea, and all died before development of hair-loss; in contrast, recipients given 5 ×106 CD8+ T cells showed little diarrhea, and most survived for more than 60 days, but all developed severe hair-loss. B: Recipients given 0.5 ×106 donor CD4+ T cells developed diarrhea and hair-loss, although most survived for more than 60 days. In contrast, recipients given 0.5 ×106 CD8+ T cells showed severe hair-loss without diarrhea, and most survived for more than 60 days. C: Recipients given 0.1 ×106 donor CD4+ T cells showed mild weight-loss with little hair-loss. In comparison, recipients given 0.1 ×106 CD8+ T cells showed more severe weight-loss and hair-loss 40 days after HCT (P<0.01).
We also measured serum autoantibody, percentage and yield of thymic CD4+CD8+ T cells and histopathology of skin and salivary gland 60 days after HCT in recipients given low numbers (0.1 or 0.5 ×106) of donor CD4+ or CD8+ T cells. Sera from control TBCD-BM recipients showed no autoantibody staining, and sera from recipients given 0.1 ×106 CD4+ T cells showed very weak autoantibody staining (Fig. 4A). In contrast, sera from recipients given 0.1 ×106 CD8+ T cells showed strong autoantibody staining of donor-type Rag-2-/- skin and salivary gland tissues (Fig. 4A). The recipients given 0.1 × 106 donor CD8+ T cells developed clinical chronic GVHD and had tiny thymus with markedly reduced yield of CD4+CD8+ thymocytes, although the percentage of CD4+CD8+ thymocytes was only approximately 2-fod lower as compared to recipients given in 0.1 × 106 CD4+ T cells (P<0.01, Fig. 4B). The skin and salivary gland tissue damage was also markedly more severe in CD8+ T recipients than in CD4+ T recipients (P<0.01, Fig. 4C). On the other hand, the recipients given 0.5 ×106 CD4+ or CD8+ T cells developed similar severity in cGVHD features (data not shown). Taken together, these results indicate that 1) low numbers of donor CD8+ T cells are more potent than CD4+ T cells in causing cGVHD, although high numbers of donor CD4+ T cells are more potent in causing aGVHD; 2) cGVHD mediated by low numbers of donor CD8+ T cells is associated with thymic damage.
Fig. 4. Recipients given 0.1 ×106 CD8+ but not CD4+ T cells developed autoantibody production, thymic damage, and tissue damage.
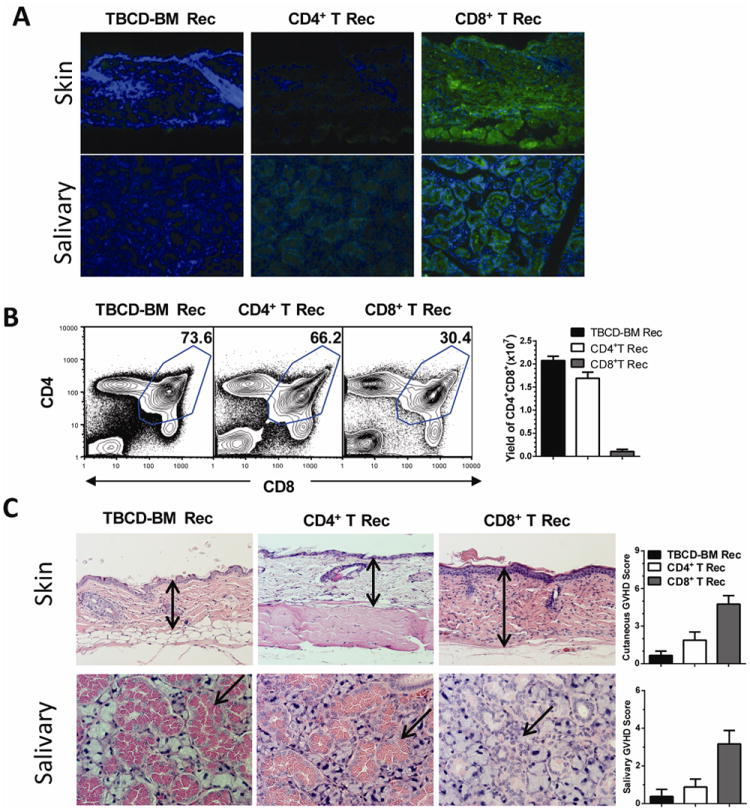
Recipients from Fig. 3C were measured for autoantibodies in serum, percentage and yield of CD4+CD8+ thymocytes, and histopathology in skin and salivary gland at 60 days after HCT. A: Serum autoantibody staining of donor-type Rag-2-/- C57BL/6 skin and salivary gland tissues. DAPI staining is shown in blue. Autoantibody staining is shown in green. One representative photomicrograph is shown of 6 recipients in each group. B: Representative flow cytometry patterns of CD4+CD8+ thymocytes and mean ± SE of yield of CD4+CD8+ thymocytes of 6 recipients in each group. C: Representative photomicrograph and histopathology scores of 6 recipients examined in each group. Arrows indicate the following changes in GVHD recipients as compared to control recipients: expansion of dermis and loss of fat tissues in the skin; infiltration and destruction of secretory follicles in the salivary gland.
Chronic GVHD caused by donor CD8+ but not CD4+ T cells in transplant required the presence of the recipient thymus
We evaluated the role of the recipient thymus in the pathogenesis of cGVHD caused by donor CD4+ and CD8+ T cells. Lethally irradiated, euthymic or thymectomized BALB/c recipients were injected with TBCD-BM with CD4+ or CD8+ T cells (0.5 × 106). To avoid the contamination of mature CD4+ or CD8+ T cells in transplants, CD4+ T cells from CD8+ T-deficient donors or CD8+ T cells from CD4+ T-deficient donors were co-injected with TBCD-BM from wild-type donors. Donor CD8+ T cells induced mild bodyweight-loss without signs of diarrhea in either euthymic or thymectomized recipients early after HCT. Approximately 30 days after HCT, both recipients started to show gradual bodyweight-loss and hair-loss (Fig. 5A-C). At approximately 45 days after HCT, euthymic recipients showed worsening clinical GVHD, but the thymectomized recipients showed gradual recovery, and clinical manifestations of GVHD disappeared by 60 days after HCT (P<0.01, Fig. 5A & C). Sera from euthymic recipients showed strong autoantibody staining, but the sera from thymectomized recipients had no autoantibody staining of donor-type Rag-2-/- skin and salivary gland (Fig 5G). While the former recipients had severe tissue damage in the skin and salivary gland, the latter had little tissue damage by 60 days after HCT (P<0.01, Fig. 5H).
Fig. 5. Donor CD8+ but not CD4+ T cell induction of cGVHD required recipient thymus.
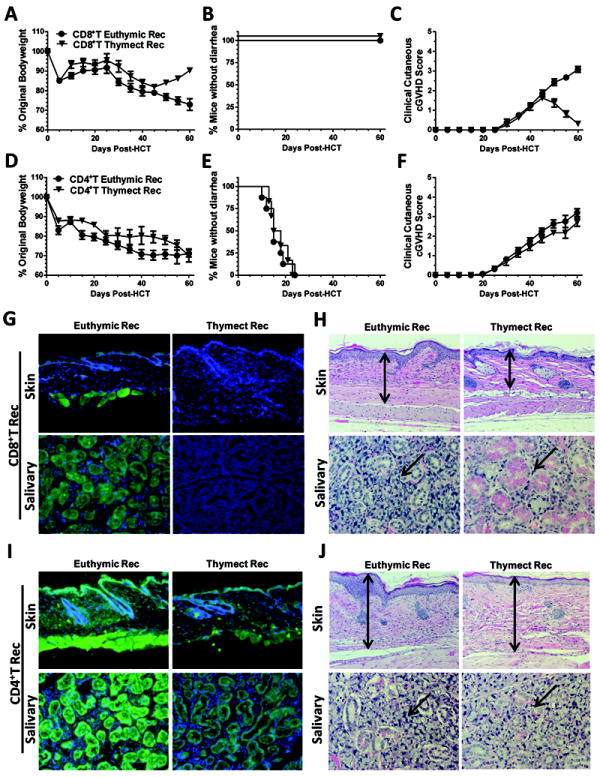
Lethally irradiated, euthymic and thymectomized BALB/c recipients were transplanted with 0.5 ×106 CD4+ T cells from CD8+ T deficient or CD8+ T cells from CD4+ T cell-deficient C57BL/6 and TBCD-BM cells from wild-type C57BL/6 mice. The use of CD4+ or CD8+ T cell-deficient donors avoided contamination of CD4+ or CD8+ T cells in injected CD8+ or CD4+ T cells. Recipients were monitored for bodyweight change, diarrhea, hair-loss, and survival after HCT. Data are combined from two replicate experiments (N=8). At day 60 after HCT, recipient sera were tested for autoantibodies. Recipient skin and salivary gland tissues were used for histopathology. A & D: Percentage bodyweight changes. Compared with euthymic recipients, thymectomized recipients given donor CD8+ T cells showed bodyweight recovery 45 days after HCT (P<0.01), but no difference was observed between thymectomized or euthymic recipients given donor CD4+ T cells. B & E: Percentage of mice without diarrhea. Recipients given donor CD8+ T cells showed no diarrhea, but recipients given donor CD4+ T cells all showed diarrhea. C & F: Hair-loss. Thymectomized recipients that were transplanted with donor CD8+ T cells showed transient weak hair-loss and then re-growth of hair at approximately 45 days after HCT, while euthymic recipients showed worsening hair-loss (P<0.01). Both thymectomized and euthymic recipients given CD4+ T cells showed severe hair-loss. G & I: Serum autoantibody staining of donor-type Rag-2-/- C57BL/6 skin and salivary gland tissues. DAPI staining is shown in blue, and autoantibody staining is shown in green. G shows recipients injected with donor CD8+ T cells. I shows recipients injected with donor CD4+ T cells. Left panels show euthymic recipients, and right-panels show thymectomized recipients. One representative photograph of 6 recipients examined in each group. H & J: Histopathology of skin and salivary gland of euthymic and thymectomized recipients given donor CD8+ or CD4+ T cells. Arrows indicate the following changes in GVHD recipients as compared to GVHD-free recipients: expansion of dermis and loss of fat tissues in the skin; infiltration and destruction of salivary follicles in the salivary gland. A representative photomicrograph from 1 of 6 recipients in each group is shown.
In contrast, donor CD4+ T cells (0.5 × 106) caused early bodyweight-loss and diarrhea in both euthymic and thymectomized recipients (Fig. 5D & E), and both types of recipients started to show increasing hair-loss at approximately 30 days after HCT, with no significant difference between the two groups (Fig. 5F). Sera from both types of recipients showed strong autoantibody staining of the donor-type Rag-2-/- skin and salivary gland tissues (Fig. 5I). Both types of recipients showed similarly severe damage in the skin and salivary gland (Fig. 5J). Taken together, these results indicate that 1) cGVHD caused by donor CD8+ but not CD4+ T cells requires thymic damage in the recipients; 2) donor CD4+ T cells are sufficient to induce cGVHD in the absence of recipient thymus; 3) although CD8+ T cells in transplants are sufficient to induce aGVHD, the subsequent development of cGVHD requires help from de novo-generated donor-derived T cells developing in the GVHD-damaged thymus.
De novo-generated donor-derived CD4+ T cells in recipients given low-dose donor CD8+ T cells play an important role in thymic damage
Low-dose donor CD8+ T cells appeared to be more potent than low-dose donor CD4+ T cells in damaging the recipient thymus (Fig. 4). To explore the mechanisms that account for this difference, we kinetically compared CD4+CD8+ thymocyte regeneration at days 15, 30, 45, and 60 after HCT in recipients given low numbers of donor CD4+ or CD8+ T cells (0.1 ×106).
At day 15 after HCT, the percentage of CD4+CD8+ thymocytes was higher in CD8+ T recipients than in CD4+ T recipients, although lower than in control TBCD-BM recipients (P<0.01, Fig. 6A). The percentage of CD4+CD8+ thymocytes increased in both types of recipients by 30 days after HCT as compared to Day 15 (P<0.01). The percentage of CD4+CD8+ thymocytes in CD4+ T cell recipients continued to increase and reached a plateau by 45 days after HCT, which was similar to control TBCD-BM recipients. In contrast, the percentage of CD4+CD8+ thymocytes in CD8+ T recipients gradually decreased, starting 30 days after HCT, and it was approximately 3-fold lower compared with CD4+ T cell recipients by 60 days after HCT (P<0.01, Fig.6A). This indicates a second-phase thymic damage in the CD8+ T recipients. In addition, anti-donor- and anti-recipient reactivity by de novo-generated donor-derived CD4+ T cells from the spleen was stronger in CD8+ T recipients than in CD4+ T cell recipients (P<0.01, Fig. 6A). These results indicate that 1) thymic regeneration early after HCT is more robust in CD8+ T cell recipients than in CD4+ T cell recipients, and 2) CD8+ T cell recipients showed a second phase of thymic damage, which was associated with de novo generation of donor-derived autoreactive CD4+ T cells.
Fig. 6. Low-dose donor CD8+ T cells was more potent than CD4+ T cells for inducing thymic damage and development of cGVHD.
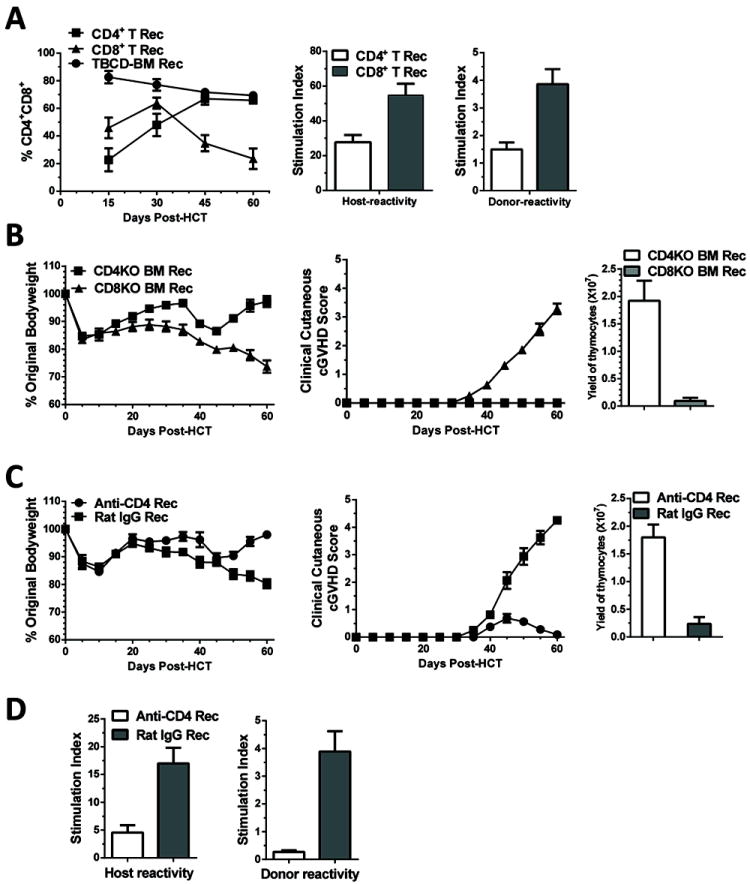
A: Lethally irradiated BALB/c recipients were transplanted with 0.1 ×106 CD4+ or CD8+ T cells in addition to TBCD-BM from donor C57BL/6 mice. Recipients given TBCD-BM alone was used as controls. Percentage of CD4+CD8+ thymocytes was measured on 15, 30, 45, and 60 days after HCT. Mean ± SE of 4 recipients at each time point from each group were combined from two replicate experiments. At 60 days after HCT, de novo-generated donor-derived CD4+ T cells (0.2 ×106) from recipients given donor CD4+ or CD8+ T cells were stimulated with recipient- or donor-derived CD11c+ DCs (0.1 ×106). Proliferation of donor CD4+ T cells was measured with 3H-TdR incorporation. Mean ± SE of the stimulation index was combined from 4 replicate experiments. B: Lethally irradiated BALB/c recipients were transplanted with donor CD8+ T cells (0.5 ×106) with TBCD-BM cells (2.5 ×106) from CD4+ T- or CD8+ T-deficient C57BL/6 donors. Recipients were monitored for bodyweight changes and hair-loss. Thymocyte yield was also compared at 60 days after HCT. As compared to recipients given CD8+ T-deficient BM cells, recipients given CD4+ T-deficient BM cells showed bodyweight increase beginning at approximately 45 days after HCT (P<0.01). There were 8 recipients in each group, combined from two replicate experiments. C: Lethally irradiated recipients were transplanted with donor CD8+ T cells (0.5 ×106) and TBCD-BM cells from wild-type C57BL/6 donors. On days 15 and 30 days after HCT, the recipients were injected with anti-CD4 mAb or control rat-IgG (500μg/mouse). Recipients were monitored for bodyweight changes and hair-loss. At 60 days after HCT, total thymocyte yield was compared. Data were combined from 8 recipients in each group from two replicate experiments. As compared with recipients treated with rat-IgG, recipients treated with anti-CD4 mAb showed bodyweight increase at approximately 45 days after HCT (P<0.01). There were 8 recipients in each group, combined from two replicate experiments. D. De novo-generated CD4+ T cell proliferation in response to recipient- or donor-derived CD11c+ DCs at 60 days after HCT. Mean ± SE of the stimulation index was combined from 4 replicate experiments.
In further experiments, we tested whether de novo-generated CD4+ or CD8+ T cells play a role in causing thymic damage and cGVHD induction. Lethally irradiated BALB/c recipients were transplanted with CD8+ T cells (0.5 ×106) in combination with TBCD-BM (2.5 × 106) from CD4+ or CD8+ T-deficient donors. As expected, recipients given CD4+ T-deficient donor BM cells showed de novo-generated CD8+ T cells with very few de novo-generated CD4+ T cells, while recipients given CD8+ T-deficient donor BM cells showed de novo-generated CD4+ T cells with very few de novo-generated CD8+ T cells (Fig. S3A). The recipients that had only de novo-generated donor-derived CD8+ T cells showed only mild weight loss at 30-45 days after HCT and then recovered. In contrast, the recipients that had only de novo-generated donor-derived CD4+ T cells showed increasing bodyweight-loss and hair-loss, especially beyond 45 days after HCT (P<0.01, Fig 6B & Fig. S3B). These recipients also developed serum autoantibodies and severe damage in the skin and salivary gland (Fig. S3C & D). Consistently, thymocytes yield in the recipients that had only de novo-generated donor-derived CD4+ T was 20 fold lower than in the recipients that had only de novo-generated donor-derived CD8+ T cells (P<0.01, Fig. 6B). These results indicate that de novo produced CD4+ T cells play an important role in causing thymic damage and cGVHD in recipients given low-dose donor CD8+ T cells, whereas de novo-generated CD8+ T cells do not.
We also tested whether injection of anti-CD4 mAb could prevent cGVHD development in recipients given low-dose donor CD8+ T cells. Lethally irradiated recipients were transplanted with donor CD8+ T cells (0.5 × 106) and TBCD-BM cells. 15 and 30 days after HCT, recipients were injected with anti-CD4 mAb or control rat IgG (500 μg/mouse). As compared with rat IgG control, anti-CD4 mAb treatment depleted de novo-generated CD4+ but not CD8+ T cells early after HCT, but the CD4+ T cells recovered by 60 days after HCT (Fig. S4A). Anti-CD4 mAb treatment significantly increased bodyweight and decreased hair-loss, starting at approximately 45 days after HCT (P<0.01, Fig. 6C & Fig. S4B). Anti-CD4 mAb treatment markedly decreased serum autoantibody production (Fig. S4C) and tissue damage in the skin and salivary gland (Fig. S4D). Anti-CD4 treatment also significantly increased the total thymocyte yield (P<0.01, Fig. 6C), and percentage and yield of CD4+CD8+ thymocytes (Fig. S4E). Anti-CD4 mAb treatment markedly reduced the allo- and auto-reactivity of donor-derived CD4+ T cells (P<0.01, Fig. 6D). These results demonstrate that de novo-generated donor-derived CD4+ T cells can mediate thymic damage and cGVHD development, and a short-term depletion of the de novo-generated CD4+ T cells enables thymic recovery from initial damage mediated by donor CD8+ T cells, with consequent prevention of cGVHD.
Donor CD8+ T cells preferentially damaged thymic mTEC and caused defective thymic selection early after HCT
Since recipients given low-dose donor CD8+ T cells appeared to have more de novo-generated autoreactive CD4+ T cells (Fig. 4), we tested whether donor CD8+ T cells preferentially damaged mTECs or reduced thymic DCs early after HCT. Indeed, 15 days after HCT, the percentage and yield of mTECs in the thymus of recipients given donor CD8+ T cells (0.1×106) was 2-3 fold lower than in recipients given donor CD4+ T cells (0.1×106) (P<0.01), although the mTEC yield in both recipients was significantly lower than that in control TBCD-BM recipients (P<0.01, Fig. 7A & B).
Fig. 7. Low numbers of donor CD8+ T cells preferentially damaged mTEC in the recipient thymus and caused defective thymic negative selection.
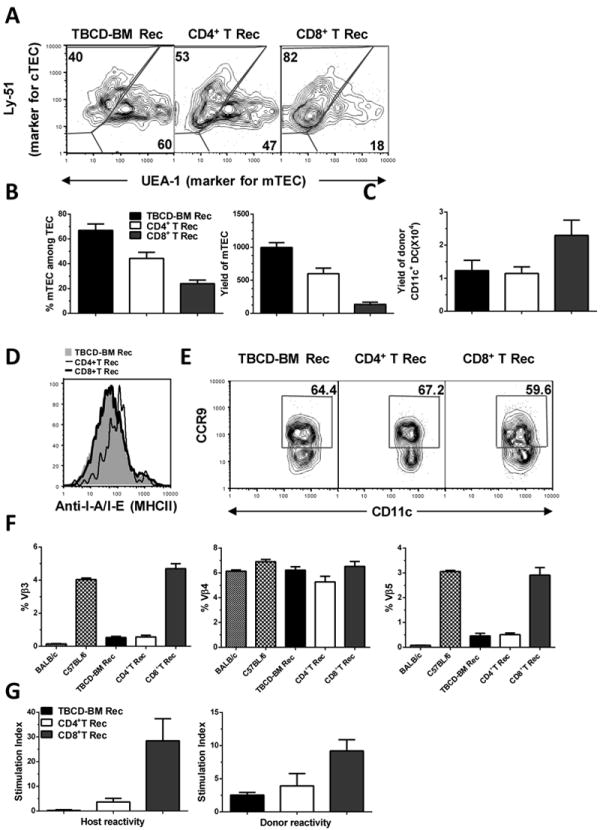
Lethally irradiated BALB/c recipients were transplanted with 0.1 ×106 CD4+ or CD8+ T cells and TBCD-BM cells from C57BL/6 donors. Recipients given TBCD-BM cells alone were used as controls. At 15 days after HCT, recipient thymus was measured for the percentage and yield of mTEC, percentage and yield of thymic CD11c+ DCs, and expression of MHC II and CCR9. At 30 days after HCT, recipient spleen cells were measured for MMTV-mediated clonal deletion of Vβ3, Vβ4, and Vβ5. In addition, flow cytometry-sorted de novo-generated donor-derived CD4+ T cells were measured for donor- and recipient-reactivity. A. Gated thymic epithelial cells are shown in UEA-1 (mTEC marker) versus Ly51 (cTEC marker). A representative flow cytometry pattern and mean ± SE of percentage and yield of mTEC is shown from1 of 4 replicate experiments. In each experiment, 6 thymi were combined from each group in order to obtain a sufficient number of thymic epithelial cells. B. Percentage and yield of mTEC, Mean ± SE, N=4. C. Total yield of donor CD11c+ DCs in the thymus, Mean ± SE, N=6. D. Expression levels of MHC II by CD11c+ DCs. E. Percentage of CCR9+CD11c+ DCs among total CD11c+ DCs. A representative flow cytometry pattern is shown from 1 of 6 samples. F. Percentage changes of Vβ3, Vβ4, and Vβ5 among de novo-generated donor-derived CD4+ T cells. Mean ± SE (N=6) is shown. G. Recipient- and donor-reactivity of de novo-generated donor CD4+ T cells. Flow cytometry-sorted, de novo-generated, donor-derived CD4+ T cells (0.2 ×106) were stimulated with donor- or recipient-type DCs (0.1×106). T cell proliferation was measured with 3H-TDR incorporation. Mean ± SE of the stimulation index from 4 replicate experiments is shown.
On the other hand, the yield of CD11c+ DCs by day 15 after HCT was significantly increased in CD8+ T cell recipients as compared to CD4+ T recipients or control TBCD-BM recipients (P<0.01, Fig. 7C). We found no significant difference by day 30, but we observed an marked reduction by day 45, when the CD8+ T cell recipients showed obvious clinical GVHD (data not shown). DC expression of MHC II and CCR9+ DC subset has been proposed to play an important role in mediating thymic negative selection (46, 47). We found no reduction of MHC II expression by DC or decrease in the percentage of CCR9+ DCs in the thymus of CD8+ T cell recipients by days 15 or 30 after HCT (Fig. 7D & E and data not shown), although we observed a marked reduction by day 45 (data not shown). These results indicate that low numbers of donor CD8+ T cells preferentially damage mTECs in the recipients early after HCT.
In further experiments, we tested whether the reduced numbers of mTECs early after HCT in the recipient thymus was associated with defective negative selection of recipient- and donor-reactive T cells by measuring mouse mammary tumor virus(MMTV)-mediated clonal deletion(48) and T cell proliferation in response to donor- or host-type DCs. At day 15 after HCT, the number of de novo-generated donor-derived CD4+ T cells in the spleen was approximately 0.4-0.8% and then increased to approximately 3-12% by day 30. Vβ3+ and Vβ5+ but not Vβ4+ T cells are deleted in BALB/c mice but not in C57BL/6 mice. At day 30 after HCT, C57BL/6 donor-derived Vβ3+ and Vβ5+ were deleted in GVHD-free BALB/c recipients given TBCD-BM alone or low-dose CD4+ T cells, but they were not deleted in GVHD-recipients given donor CD8+ T cells (P<0.01, Fig. 7F). Additionally, the de novo-generated donor-derived CD4+ T cells from the spleen of CD8+ T cell recipients showed much stronger proliferation in response to stimulation by donor- or recipient-type DCs, as compared to CD4+ T cells from recipients given TBCD-BM or CD4+ T cells (P<0.01, Fig. 7G). Taken together, these results indicate that damage to thymic mTECs caused by donor CD8+ T cells leads to defective negative selection of de novo-generated autoreactive T cells early after HCT.
Discussion
Most cGVHD patients have aGVHD that is controlled with immunosuppressants (49, 50). Separate models of aGVHD and cGVHD have been developed with specific donor-recipient strain combinations (20, 21), but the link between aGVHD and cGVHD has not been well established, and no single model reflects all characteristics of human cGVHD (20). Our current results demonstrate that, similar to in humans, aGVHD can evolve into characteristic cGVHD in mice, as long as the donor T cell dose is appropriate. The key for establishing cGVHD mouse model is not the special donor-recipient combination, instead, it is allowing the recipient to survive aGVHD, giving time for autoreactive T cells to expand and cause cGVHD.
We observed that donor CD8+ T cell-mediated thymic mTEC damage allowed autoreactive CD4+ T cells to escape negative selection in the thymus. Kinetic differences between the presence of autoreactive CD4+ T cells and changes in the numbers of donor DCs or tolerogenic CCR9+ DCs or MHC II expression in DCs suggest that these DC changes do not initiate cGVHD. These results indicate that mTEC damage is sufficient to initiate the generation of autoreactive CD4+ T cells and the development of cGVHD. This observation appears to be inconsistent with previous report that MHC II-deficient TCD-BM cells led to development of autoreactive CD4+ T cells in recipients exposed to high-dose TBI (34). In fact, we observed that MHC II-deficient C57BL/6 or DBA/2 TCD-BM gave rise to cGVHD in BALB/c recipients conditioned with 950 cGy TBI but not in recipients conditioned with 850 cGy TBI (Young and Zeng, unpublished data). Therefore, it is possible that irreversible severe damage to mTEC also contributes to cGVHD development in high-dose TBI-conditioned recipients transplanted with MHC II-deficient donor BM. The mechanisms of the preferential damage of mTEC by donor CD8+ T cells remain unclear. A previous publication showed that donor T cell damage of thymic epithelial cells required cognate cytotoxicity (51); another report showed that thymic mTEC could directly activate the alloreactive T cells (32). In addition, donor CD8+ T cells usually have stronger cytotoxic activity than CD4+ T cells. As T cells enter the thymus in the medullary area adjacent to cortical area (52), it is possible that the preferential damage of mTEC by donor CD8+ T cells is due to the fact that donor T cells that enter the thymus are anatomically closer to mTEC. The interaction between donor DC and host mTEC in GVHD recipients and the role of donor DC abnormality in induction of chronic GVHD remains unclear and is under investigation.
It is of interest that donor CD8+ T cells induced only transient aGVHD that did not develop into cGVHD in thymectomized recipients. This finding is consistent with our observation that de novo-generated donor-derived CD4+ T cells were required for induction of cGVHD in recipients given donor CD8+ T cells alone. The mechanisms explaining why donor CD8+ T cell-mediated GVHD does not evolve into cGVHD remain to be explored. We recently showed that the expansion of donor CD8+ T cells in GVHD target tissues was co-incident with the presence of de novo-generated CD4+ T cells (53). These observations suggest that purified donor CD8+ T cells could be used to facilitate engraftment and mediate GVL effects without cGVHD in elderly patients with little thymic de novo T cell production.
We observed that two injections of anti-CD4 mAb early after HCT depleted de novo-generated donor CD4+ T cells for a short-period of time, and the donor-derived CD4+ T cells recovered at approximately one month after the last antibody injection. Anti-CD4 mAb treatment restored thymic production, prevented production of de novo-generated autoreactive T cells, and prevented cGVHD. This observation is of important potential clinical significance. This short-term anti-CD4 mAb treatment spares donor CD8+ T cells that are needed to facilitate engraftment and mediate GVL effects and prevents cGVHD development.
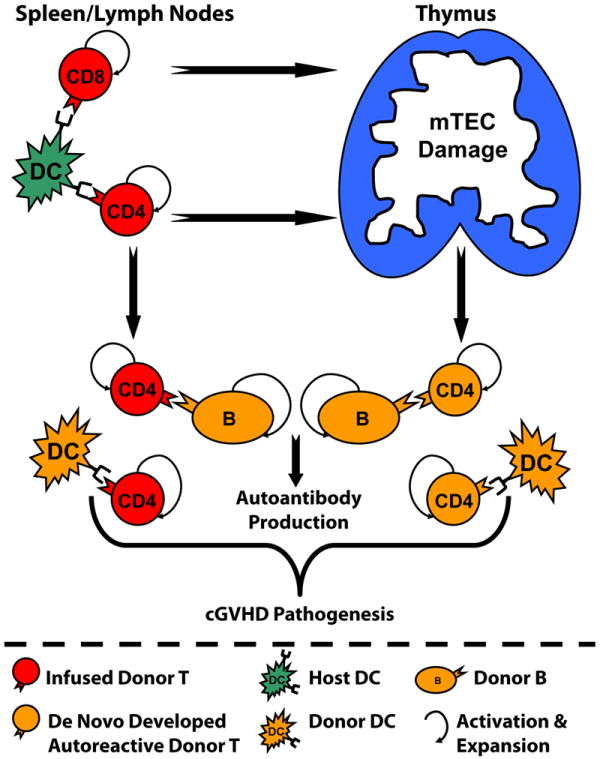
In summary, this report has provided 1) a new approach to establish murine models that reflect the pathogenesis of cGVHD in humans; 2) novel observations that donor CD4+ T cells induce cGVHD in the presence or absence of recipient thymus, while donor CD8+ T cells induce cGVHD only in the presence of the recipient thymus; 3) a demonstration that damage to thymic mTEC early after HCT is sufficient to allow the development of autoreactive T cells and cGVHD; 4) depletion of de novo-generated autoreactive CD4+ T cells can allow recovery of thymic negative selection and prevent the development of cGVHD. Our recent studies also showed that replacing wild-type with Igμ knockout donor TBCD-BM that do not give rise to donor B cells markedly reduced chronic GVHD induced by donor CD8+ T cells (Wu and Zeng et al; unpublished observation). Combining these observations with our previous publications showing that donor B cells play important roles in augmenting chronic GVHD (22, 35, 37), we propose the pathophysiology of chronic GVHD. As described in Fig. 8, donor CD4+ and CD8+ T cells are activated and expanded by host APCs after allogeneic HCT. The alloreactive T cells migrate into the thymus and damage mTEC, resulting in production of autoreactive CD4+ T cells. Autoreactive CD4+ T cells can also derive from CD4+ T cells in transplants, probably through recognizing non-polymorphic antigens (35, 37, 43). Autoreactive T cells interact with donor-derived DCs and B cells, resulting in mutual expansion and autoantibody production as well as cGVHD development. These new insights into cGVHD pathogenesis could guide the development of novel therapies for prevention of cGVHD. For example, strategies that deplete donor CD4+ T cells and/or donor B cells in the graft and de novo-generated CD4+ T cells early after HCT would allow donor CD8+ T cells to facilitate engraftment and mediate GVL effects without causing cGVHD.
Fig. 8. Diagram of chronic GVHD Pathophysiology.
Supplementary Material
Acknowledgments
We thank Dr. Paul Martin at Fred Hutchinson Cancer Center, Seattle for critical review and editing of the manuscript; thank Dr. Marcel van den Brink at Memorial Sloan-Kettering Cancer Center, New York for providing detailed lab protocols for isolation of thymic epithelial cells; thank Lucy Brown and her staff at the COH Flow Cytometry Facility, and Sofia Loera and her staff at the COH Anatomic Pathology Laboratory for their excellent technical assistance.
Grant Support: This work was supported by National Institutes of Health Grant R01AI066008 (to D. Zeng). T. Wu was an international PhD student and his living stipend was provided in part from Chinese National Fundation for Oversea’s PhD Program and from National Natural Science Foundation of China (NSFC 81090413 to J. Wang).
Abbreviations
- HCT
Hematopoietic cell transplantation
- GVHD
Graft-versus-host disease
- GVL
Graft versus leukemia/lymphoma
- DC
Dendritic cells
- mTEC
Medullary thymic epithelial cells
- mAb
Monoclonal antibody
Footnotes
Authorship contributions: T. Wu designed and performed research as well as wrote the manuscript; J. Young assisted in experimental design and performing research. H. Johnston, X. Ni, R. Deng, M. Wang, and A. Wang performed some experiments. J. Racine assisted in experimental design and data analysis. I. Todorov evaluated histopathological slides. J-M Wang: Tao Wu’s PhD advisor co-supervised Tao’s research activity; D. Zeng designed and supervised the research and wrote the manuscript.
All authors declare no conflict of interest
References
- 1.Martin PJ. Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med. 1993;178:703–712. doi: 10.1084/jem.178.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin PJ, Rowley SD, Anasetti C, Chauncey TR, Gooley T, Petersdorf EW, van Burik JA, Flowers ME, Storb R, Appelbaum FR, Hansen JA. A phase I-II clinical trial to evaluate removal of CD4 cells and partial depletion of CD8 cells from donor marrow for HLA-mismatched unrelated recipients. Blood. 1999;94:2192–2199. [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 5.Shlomchik WD, Lee SJ, Couriel D, Pavletic SZ. Transplantation’s greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:2–10. doi: 10.1016/j.bbmt.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Sung AD, Chao NJ. Concise review: acute graft-versus-host disease: immunobiology, prevention, and treatment. Stem Cells Transl Med. 2013;2:25–32. doi: 10.5966/sctm.2012-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature reviews Immunology. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R, Ritz J, Antin JH, Soiffer RJ. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, Strober S. Bone marrow NK1.1(-) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Shizuru JA. Graft-vs.-lymphoma effect in an allogeneic hematopoietic stem cell transplantation model. Biol Blood Marrow Transplant. 1999;5:357–368. doi: 10.1016/s1083-8791(99)70012-1. [DOI] [PubMed] [Google Scholar]
- 12.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler C, A JH. Manifestations and Treatment of Acute Graft-versus-Host Disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. Fourth. Wiley-Blackwell; 2009. pp. 1287–1303. [Google Scholar]
- 14.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Pavletic SZ, Vogelsang GB. Chronic Graft-versus-Host Disease: Clinical Manifestations and Therapy. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. Blackwell Publishing; Hoboken, NJ: 2009. pp. 1304–1324. [Google Scholar]
- 16.Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, Arora M, Weisdorf DJ, Flowers ME, Martin PJ, Palmer J, Jacobsohn D, Pavletic SZ, Vogelsang GB, Lee SJ. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz KR. Pathophysiology of Chronic Graft versus Host Disease. In: Vogelsang GB, Pavletic SZ, editors. Chronic Graft versus Host Disease: Interdisciplinary Management. Cambridge University Press; New York, NY: 2009. pp. 17–30. [Google Scholar]
- 18.Parkman R. Is chronic graft versus host disease an autoimmune disease? Curr Opin Immunol. 1993;5:800–803. doi: 10.1016/0952-7915(93)90140-n. [DOI] [PubMed] [Google Scholar]
- 19.Pavletic SZ, Fowler DH. Are we making progress in GVHD prophylaxis and treatment? In: Burns LJ, Mikhael JR, Schwartz BS, Crowther MA, editors. Hematology 2012. American Society of Hematology Education Program; 2012. pp. 251–264. [DOI] [PubMed] [Google Scholar]
- 20.Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant. 2008;14:365–378. doi: 10.1016/j.bbmt.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korngold R, Friedman TM. Murine Models of Graft versus-Host Disease and Graft-versus-Tumor Effect. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoeitic Cell Tranplantation. 4. John Wiley & Sons; Hoboken, NJ: 2009. pp. 176–187. [Google Scholar]
- 22.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, Strober S, Zeng D. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. J Immunol. 2002;168:3088–3098. doi: 10.4049/jimmunol.168.6.3088. [DOI] [PubMed] [Google Scholar]
- 24.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 25.Via CS, Shearer GM. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol Today. 1988;9:207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- 26.Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A new murine model for bronchiolitis obliterans post-bone marrow transplant. Am J Respir Crit Care Med. 2007;176:713–723. doi: 10.1164/rccm.200702-335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 28.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nature reviews Immunology. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 29.Fukushi N, Arase H, Wang B, Ogasawara K, Gotohda T, Good RA, Onoe K. Thymus: a direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci U S A. 1990;87:6301–6305. doi: 10.1073/pnas.87.16.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi S, Blazar BR, Farrell CL, Danilenko DM, Lacey DL, Weinberg KI, Krenger W, Hollander GA. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 31.van den Brink MR, Moore E, Ferrara JL, Burakoff SJ. Graft-versus-host-disease-associated thymic damage results in the appearance of T cell clones with anti-host reactivity. Transplantation. 2000;69:446–449. doi: 10.1097/00007890-200002150-00026. [DOI] [PubMed] [Google Scholar]
- 32.Hauri-Hohl MM, Keller MP, Gill J, Hafen K, Pachlatko E, Boulay T, Peter A, Hollander GA, Krenger W. Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation. Blood. 2007;109:4080–4088. doi: 10.1182/blood-2006-07-034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179:3305–3314. doi: 10.4049/jimmunol.179.5.3305. [DOI] [PubMed] [Google Scholar]
- 34.Sakoda Y, Hashimoto D, Asakura S, Takeuchi K, Harada M, Tanimoto M, Teshima T. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109:1756–1764. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 35.Zhao D, Young JS, Chen YH, Shen E, Yi T, Todorov I, Chu PG, Forman SJ, Zeng D. Alloimmune response results in expansion of autoreactive donor CD4+ T cells in transplants that can mediate chronic graft-versus-host disease. J Immunol. 2011;186:856–868. doi: 10.4049/jimmunol.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young JS, Wu T, Chen Y, Zhao D, Liu H, Yi T, Johnston H, Racine J, Li X, Wang A, Todorov I, Zeng D. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J Immunol. 2012;189:222–233. doi: 10.4049/jimmunol.1200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu D, Chen L, Iwakura Y, Kandee F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft versus host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng D, Hoffmann P, Lan F, Huie P, Higgins J, Strober S. Unique patterns of surface receptors, cytokine secretion, and immune functions distinguish T cells in the bone marrow from those in the periphery: impact on allogeneic bone marrow transplantation. Blood. 2002;99:1449–1457. doi: 10.1182/blood.v99.4.1449. [DOI] [PubMed] [Google Scholar]
- 42.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105:4885–4891. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangarajan H, Yassai M, Subramanian H, Komorowski R, Whitaker M, Gorski J, Drobyski WR. Emergence of T cells that recognize nonpolymorphic antigens during graft-versus- host disease. Blood. 2012;119:6354–6364. doi: 10.1182/blood-2012-01-401596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hess AD. Equal opportunity targeting in chronic GVHD. Blood. 2012;119:6183–6184. doi: 10.1182/blood-2012-04-425298. [DOI] [PubMed] [Google Scholar]
- 45.Meier JK, Wolff D, Pavletic S, Greinix H, Gosau M, Bertz H, Lee SJ, Lawitschka A, Elad S. Oral chronic graft-versus-host disease: report from the International Consensus Conference on clinical practice in cGVHD. Clin Oral Investig. 2011;15:127–139. doi: 10.1007/s00784-010-0450-6. [DOI] [PubMed] [Google Scholar]
- 46.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pullen AM, Kappler JW, Marrack P. Tolerance to self antigens shapes the T-cell repertoire. Immunol Rev. 1989;107:125–139. doi: 10.1111/j.1600-065x.1989.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 49.Chao NJ, Sullivan KM. Pharmacologic Prevention of Acute Graft-versus-Host Diease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. Fourth Edition. Wiley-Blackwell; 2009. pp. 1257–1274. [Google Scholar]
- 50.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, Huff CA, Borrello I, Matsui W, Powell JD, Kasamon Y, Goodman SN, Hess A, Levitsky HI, Ambinder RF, Jones RJ, Fuchs EJ. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Na IK, Lu SX, Yim NL, Goldberg GL, Tsai J, Rao U, Smith OM, King CG, Suh D, Hirschhorn-Cymerman D, Palomba L, Penack O, Holland AM, Jenq RR, Ghosh A, Tran H, Merghoub T, Liu C, Sempowski GD, Ventevogel M, Beauchemin N, van den Brink MR. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. The Journal of clinical investigation. 2010;120:343–356. doi: 10.1172/JCI39395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nature reviews Immunology. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Deng R, He W, Liu C, Wang M, Young J, Meng Z, Du C, Huang W, Chen L, Chen Y, Martin P, Forman S, Zeng D. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease. J Immunol. 2012;188:724–734. doi: 10.4049/jimmunol.1102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


