Abstract
Objective
Nicotinic acetylcholine receptors are possible therapeutic targets for schizophrenia, as shown by neurobiological and molecular evidence for deficiencies in expression of α7-nicotinic receptors. Patients’ heavy smoking suggests attempted self-medication through this mechanism. The agent 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A) is a partial α7-nicotinic agonist and can be taken orally. A phase 1 trial showed evidence for cognitive enhancement in schizophrenia.
Method
Thirty-one subjects with schizophrenia received DMXB-A at two different doses and placebo for periods of 4 weeks in a three-arm, two-site, double-blind, crossover phase 2 trial. The MATRICS Consensus Cognitive Battery assessed cognitive effects, and the Scale for the Assessment of Negative Symptoms (SANS) and Brief Psychiatric Rating Scale (BPRS) assessed clinical effects. Subjects continued their current antipsychotic drug during the trial and were nonsmokers.
Results
There were no significant differences in the MATRICS cognitive measures between DMXB-A and placebo over the three treatment arms, but the patients experienced significant improvement at the higher DMXB-A dose on the SANS total score and nearly significant improvement on the BPRS total score. Improvement was most notable on the SANS anhedonia and alogia subscales. Examination of the first treatment arm showed effects of DMXB-A on the attention/vigilance and working memory MATRICS domains, compared to baseline. Five subjects developed mild tremor, and nearly half had mild nausea while taking DMXB-A.
Conclusion
DMXB-A, a nicotinic agonist that activates α7-nicotinic receptors, improved clinical ratings of negative symptoms that are generally resistant to treatment with dopamine antagonist antipsychotic drugs. The clinical utility of this treatment is not yet determined.
Treatment of schizophrenia with dopamine receptor antagonist antipsychotic drugs has significant clinical effects, but patients often have residual cognitive deficits and negative symptoms. The National Institute of Mental Health instituted Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) to find new therapeutic targets for the treatment of cognitive deficits in schizophrenia and to develop the Consensus Cognitive Battery as a common instrument for the evaluation of new drugs (1, 2).
One therapeutic target identified by the MATRICS is the α7-nicotinic acetylcholine receptor. A possible role for the receptor in schizophrenia was first identified in animal models of a sensory gating deficit associated with schizophrenia. Subsequently, evidence for genetic link-age of this sensory gating deficit and for schizophrenia itself was found at the chromosome 15 locus of CHRNA7, the gene for the α7-receptor subunit (3). Molecular analysis indicates that the gene’s coding region is intact. Thus, the receptor has normal structure, but the expression is decreased, possibly because of single nucleotide changes in the promoter of the gene (4). Postmortem studies show decreased expression of the receptor at its two major sites of expression, the inhibitory interneurons of the hippocampus (5) and of the nucleus reticularis thalami (6). These brain regions are thought to play important roles in the regulation of the brain’s sensitivity to sensory stimuli. Inability to filter out unwanted sensory stimuli is one of the mechanisms of poor attention in schizophrenia (7).
Nicotine itself is abused in high doses by many patients with schizophrenia through their heavy cigarette smoking. The α7-nicotinic receptor is an order of magnitude less sensitive to nicotine than other nicotinic receptors, and therefore this heavy smoking may be evidence that patients are trying to activate this receptor, perhaps to compensate for its lower than normal expression (8). Nicotine was initially observed to reverse the adverse neurocognitive effects of haloperidol (9). Positive effects of nicotine on neuropsychological test performance have been observed principally in patients who smoke but have abstained from cigarettes for periods of time ranging from overnight to several weeks. These effects likely represent reversal of withdrawal phenomena (10–13). Transdermal nicotine in nonsmoking patients had no significant effects on the d′ parameter of the Continuous Performance Test, the measure used for the attention/vigilance domain of the MATRICS battery, but effects were found on other test parameters (14). Two studies compared smoking and nonsmoking patients’ responses to nicotine. Among patients who had abstained for only 2 hours, nicotine nasal spay improved delayed recognition but not working memory (15). The effect was not seen in nonsmoking patients. We observed the opposite: negative effects of nicotine gum were found on the attention domain of a neuropsychological battery among patients who smoked up until 2 hours before the test, but positive effects were seen in nonsmokers (16). These last data raise the possibility that patients’ smoking habits cause tachyphylaxis at nicotinic receptors, and therefore we restricted initial experimental trials of nicotinic agonists to nonsmokers.
The nicotinic agonist 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A) is derived from anabaseine, an alkaloid found in nemertine worms (17). The dimethoxybenzylidene derivative is a partial agonist at human α7-nicotinic receptors with a half-life of about 2 hours (18, 19). An initial proof-of-concept trial in schizophrenia involving single-day administration showed positive cognitive effects, particularly on attention (20). The subjects’ antipsychotic drug regimens were maintained, because of the presumption that these drugs are acting through different mechanisms.
On the basis of this initial positive trial, the present phase 2 trial was approved by the U.S. Food and Drug Administration to assess whether cognitive effects would continue during longer-term administration and whether clinical ratings would also change. The doses were those used in the phase 1 trial. The MATRICS battery was chosen because of its recommended use for assessment of drug effects on cognition in schizophrenia (1, 2). The Scale for the Assessment of Negative Symptoms (SANS) (21) and Brief Psychiatric Rating Scale (BPRS) (22) were used to assess symptoms. As in the initial phase 1 trial, nonsmoking patients, almost all of whom were currently taking antipsychotic drugs, were studied.
Method
Subjects
Thirty-four subjects were screened. Two were excluded because of abnormal laboratory values, and one was excluded because of recent hospitalization for an acute psychotic episode (data supplement Figure 1). A total of 31 subjects were enrolled at two sites: the Denver VA Medical Center/University of Colorado (25 subjects) and the Maryland Psychiatric Research Center (six subjects). Twenty-two were male, and nine were female. The age range was 22 to 60 years. All subjects fulfilled the DSM-IV-TR criteria for schizophrenia. As required by the selection criteria, they were clinically stable outpatients with no drug abuse and no tobacco or nicotine use in the past month. Exclusionary criteria included neurological or somatic illness. Because this trial was an initial phase 2 trial, subjects under age 21 or over age 60 and women capable of pregnancy were excluded. Twenty-three were treated with second-generation antipsychotics other than clozapine, two with clozapine, and five with first-generation antipsychotics, and one was receiving no antipsychotic treatment. Antipsychotic treatments were not changed during the trial. All subjects gave informed consent. The trial was approved by the Colorado multi-institutional and University of Maryland institutional review boards and registered on www.clinicaltrials.gov (NCT00100165).
Experimental Drug Protocol
DMXB-A was synthesized and placed into capsules, as previously described (20, 23). Identical-appearing placebo capsules were also prepared. After 1 week of screening, subjects received 1 week of placebo to assess compliance. They then received a baseline assessment consisting of the MATRICS Consensus Cognitive Battery, the BPRS (22), the digit span test (forward subtest only) of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (24), the SANS (21), and the Simpson-Angus Rating Scale for assessment of extrapyramidal symptoms (25). A side effect checklist comprising 37 common side effects was rated on a 1–4 scale for each item. Subjects also received a urinalysis, hematology and serum chemistry tests, vital sign measurements, and an ECG.
The subjects were assigned to 4 weeks of twice-daily placebo, 75 mg b.i.d. of DMXB-A, or 150 mg b.i.d. of DMXB-A. Both patients and investigators, except for the pharmacist and biostatistician, were blind to drug identity. All subjects received each treatment in a balanced crossover design. In addition to the order of treatment, where tests from the MATRICS battery were available in alternate versions, the use of each version was balanced as well (1). The three treatment arms were separated by 1-week washout periods, during which the subjects received placebo. Assessments were repeated at the end of each 4-week treatment arm, immediately after the morning dose of drug. The BPRS and digit span subtest were also administered at the end of each washout period to detect possible carryover effects. Safety assessments were performed every 2 weeks.
One subject was removed from the study after 3 days of treatment because of expressions of suicidality, further described in Results. A second subject completed only one arm and then left Colorado. Data from a third subject, who completed all three arms, were not used except for safety assessments, because inconsistencies in the testing protocols were detected before the order of treatment was unmasked. All other subjects completed all three arms of the trial. Compliance with medication as judged by capsule counts exceeded 90%.
Assessments
The MATRICS Consensus Cognitive Battery is described in data supplement Appendix 1. The social cognition domain was not assessed. The subjects also received the RBANS digit span test (forward subtest only) because this test had been sensitive to DMXB-A in the initial phase 1 trial (20). Modified versions of the BPRS and SANS were used. The BPRS version adds two items to the standard 18-item version: poverty of speech and inappropriate affect. The SANS version uses the four domains most closely associated with core negative symptoms: affective flatness, alogia, anhedonia, and apathy (26).
Plasma Drug Level Assays
Plasma specimens for drug level assays were obtained 2.25 to 2.50 hours after the first morning dose, following MATRICS battery testing, at the Colorado site. Because of an error in the protocol, specimens were obtained 16 hours after the last dose at the Maryland site. The specimens were analyzed by high-performance liquid chromatography as previously described (20, 27). Levels were not detectable in the Maryland samples, which indicates that there was no residual level of drug between doses. In the Colorado samples, DMXB-A and its 4-hydroxy metabolite were detected in the samples, but the metabolite was generally below the level of reliable quantification.
Statistical Analyses
A mixed model was fit to each variable, estimating the effects of encounter number and DMXB-A treatment, as suggested for crossover designs (28). An unstructured multivariate analysis of variance (MANOVA) type covariance matrix was assumed for the three encounter observations common to a subject. The Kenward and Roger method of calculating the denominator degrees of freedom was used (29). Both baseline and site effects were included in the final model. Additional models were computed to assess the effect of DMXB-A plasma level and the type of antipsychotic drug, but these had no effect on the results. For nonparametric tests with nonnormal values, i.e., SANS and BPRS, nonparametric rank tests were used to validate treatment effects, with the observations ranked separately for each encounter number. All tests of significance were two-tailed with an alpha value of 0.05.
Results
Therapeutic Effects
Performance on the six domains of the MATRICS Consensus Cognitive Battery did not differ between either DMXB-A dosage and placebo, which was the primary outcome measurement of the trial (Table 1). Effects of repetition of the tests were observed in several of the domains. For the T score for the speed of processing domain, the effect of encounter number was significant (F=5.96, df=2, 25, p=0.008). The least squares mean difference between week 6, the end of the first arm, and week 16, the end of the third arm, was 4.3 (SD=4.1) (t=3.34, df=26, p=0.002). A nearly significant effect was observed for the T score for the attention/vigilance domain (p=0.08), and changes of similar magnitude, although not significant, were observed for the verbal learning domain T score.
TABLE 1.
Scores on Cognitive Domains for 31 Patients With Schizophrenia During Crossover Treatment With Placebo and Two Doses of DMXB-Aa
| Score |
||||||||
|---|---|---|---|---|---|---|---|---|
| Domain of MATRICS | Baseline |
Placebo |
DMXB-A, 75 mg b.i.d. |
DMXB-A, 150 mg b.i.d. |
||||
| Consensus Cognitive Battery | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Speed of processing | 40.1 | 9.0 | 44.1 | 7.8 | 44.2 | 11.8 | 43.3 | 9.5 |
| Attention/vigilance | 35.8 | 10.3 | 41.6 | 10.3 | 44.3 | 10.2 | 41.3 | 10.6 |
| Working memory | 43.2 | 10.4 | 47.2 | 10.9 | 47.6 | 12.1 | 46.5 | 9.8 |
| Verbal learning | 36.2 | 9.0 | 43.4 | 12.4 | 39.9 | 9.6 | 42.4 | 10.5 |
| Visual learning | 44.1 | 11.2 | 44.4 | 12.6 | 44.9 | 12.2 | 43.2 | 11.6 |
| Reasoning/problem solving | 44.0 | 8.9 | 49.1 | 9.6 | 48.6 | 8.7 | 46.7 | 9.0 |
No domain showed a significant effect of treatment over the three arms of treatment.
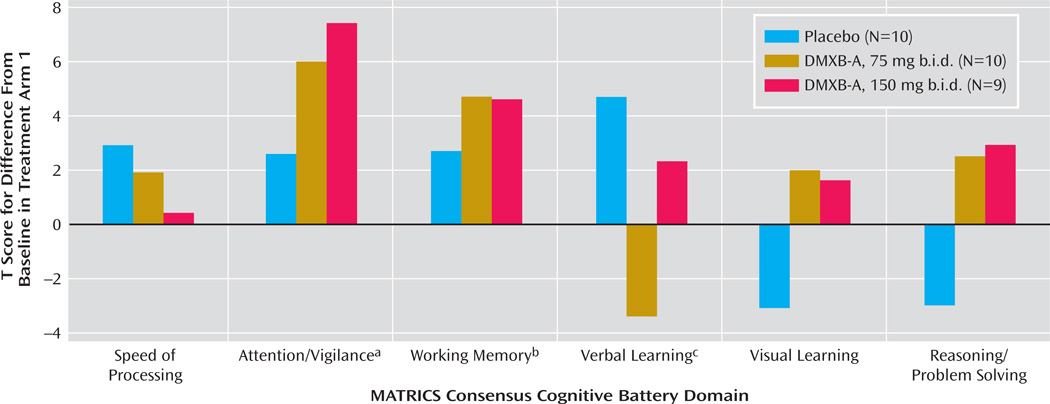
Therefore, we performed a secondary analysis using only the results of the first arm of the study, to minimize the effects of repetition of the tests (Figure 1). This analysis has limited power, because the subjects’ performance could not be compared with their performance in the other two conditions. Ten received placebo, 10 received 75 mg b.i.d. of DMXB-A, and nine received 150 mg b.i.d. of DMXB-A. The verbal learning domain T score significantly increased with placebo, compared to baseline, and nonsignificantly decreased with 75 mg b.i.d. of DMXB-A and increased with 150 mg b.i.d. of DMXB-A. Two domains significantly improved over baseline with DMXB-A treatment in the first arm. The attention/vigilance domain T score did not significantly change over baseline with placebo, but it significantly increased with DMXB-A at both 75 mg b.i.d. (mean=6.1, SD=9.4) and 150 mg b.i.d. (mean=7.6, SD=10.6). The working memory domain T score also did not significantly change over baseline with placebo, but it showed a significant increase with 75 mg b.i.d. of DMXB-A (mean=4.6, SD=6.5) and a nearly significant increase with 150 mg b.i.d. (mean=4.5, SD=7.3).
FIGURE 1.
Scores on Cognitive Domains for Patients With Schizophrenia During the First Arm of Crossover Treatment With Placebo and Two Doses of DMXB-A
a Significant differences from baseline for DMXB-A at both 75 mg b.i.d. (t=2.05, df=25, p=0.05) and 150 mg b.i.d. (t=2.26, df=25, p=0.03).
b Significant difference from baseline for DMXB-A, 75 mg b.i.d. (t=2.25, df=25, p=0.03); nearly significant difference for DMXB-A, 150 mg b.i.d.(t=1.93, df=25, p=0.07).
c Significant difference from baseline for placebo (t=2.18, df=25, p=0.04).
The digit span subtest also did not show significant effects of DMXB-A treatment. For this measure, the effect of encounter number was also significant (F=8.10, df=2, 24, p=0.002). The least squares mean difference in numbers recalled between week 6 and week 16 was also significant, with a mean increase of 1.9 digits (SD=1.9) (t=3.30, df=27, p=0.003). In the first arm, there was improvement during both DMXB-A treatments, but not placebo, compared to baseline, but these effects were not significant.
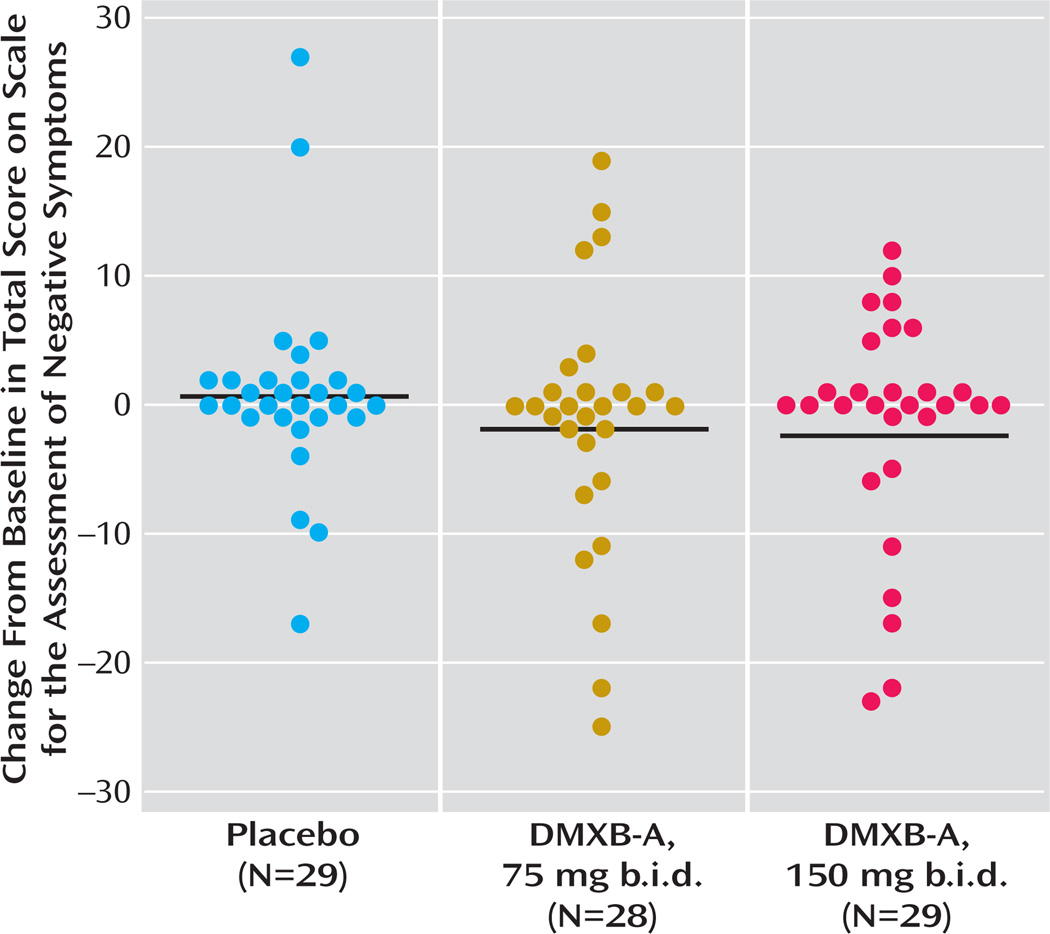
Significant effects of DMXB-A treatment were observed for the SANS total score (Figure 2, data supplement Table 1). For 150 mg b.i.d. of DMXB-A, the mean improvement in ratings compared to placebo was 1.35 (SD=2.80). For 75 mg b.i.d. of DMXB-A, the improvement in ratings compared to placebo fell short of significance (mean=0.96, SD=2.85) (Figure 2). Two of the subscales, alogia and anhedonia, showed significant effects of 150 mg b.i.d. of DMXB-A, compared to placebo (Table 2).
FIGURE 2.
Change in Score for Negative Symptoms for Patients With Schizophrenia During Crossover Treatment With Placebo and Two Doses of DMXB-Aa
a Each symbol is an individual patient’s value, and the horizontal lines are the group means, from the analysis shown in data supplement Table 1. The treatment effect was significant (F=3.62, df=2, 38, p=0.04, nonparametric ranks test), and the ratings during treatment with 150 mg b.i.d. of DMXB-A were significantly lower than during placebo (t=2.61, df=37, p=0.01). The difference with the 75-mg dose fell short of significance (t=1.81, df=37, p=0.08).
TABLE 2.
Scores for Negative Symptoms and Overall Symptoms for 31 Patients With Schizophrenia During Crossover Treatment With Placebo and Two Doses of DMXB-A
| Score |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Placebo |
DMXB-A, 75 mg b.i.d. |
DMXB-A, 150 mg b.i.d. |
150-mg DMXB- A Dose Versus Placebo (p)a |
|||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Scale for the Assessment of Negative Symptoms | |||||||||
| Totalb | 22.0 | 18.3 | 22.3 | 18.8 | 20.4 | 18.2 | 20.6 | 18.3 | 0.01 |
| Affective flattening | 6.9 | 6.8 | 6.7 | 7.4 | 5.7 | 6.8 | 6.4 | 6.9 | n.s. |
| Alogiac | 2.0 | 3.0 | 2.1 | 3.3 | 1.7 | 3.0 | 1.3 | 2.7 | 0.03 |
| Anhedoniad | 6.9 | 7.1 | 7.4 | 6.5 | 6.8 | 6.5 | 6.7 | 6.7 | 0.02 |
| Apathy | 5.9 | 5.6 | 6.1 | 4.9 | 6.1 | 5.5 | 6.3 | 5.5 | n.s. |
| Brief Psychiatric Rating Scalee | 30.0 | 8.8 | 28.7 | 8.9 | 28.0 | 7.7 | 27.4 | 7.0 | 0.06 |
From type III analyses of fixed effects using ranks. Subscale values are not corrected for multiple testing.
Analysis is described in the Figure 2 footnote and in data supplement Table 1.
The overall effect of treatment was nearly significant (F=2.77, df=2, 33, p=0.08). For the 150-mg dose of DMXB-A versus placebo, t=2.31, df= 32, Cohen’s d=0.27.
The overall effect of treatment was significant (F=3.22, df=2, 48, p=0.05). For the 150-mg dose of DMXB-A versus placebo, t=2.40, df=32, Cohen’s d=0.11.
The overall effect of treatment was not significant (F=2.12, df=2, 28, p=0.14).
The BPRS total score showed a nonsignificant effect of DMXB-A treatment with the same analysis used for the SANS. Only the contrast between 150 mg b.i.d. of DMXB-A and placebo approached significance in the nonparametric analysis (Table 2).
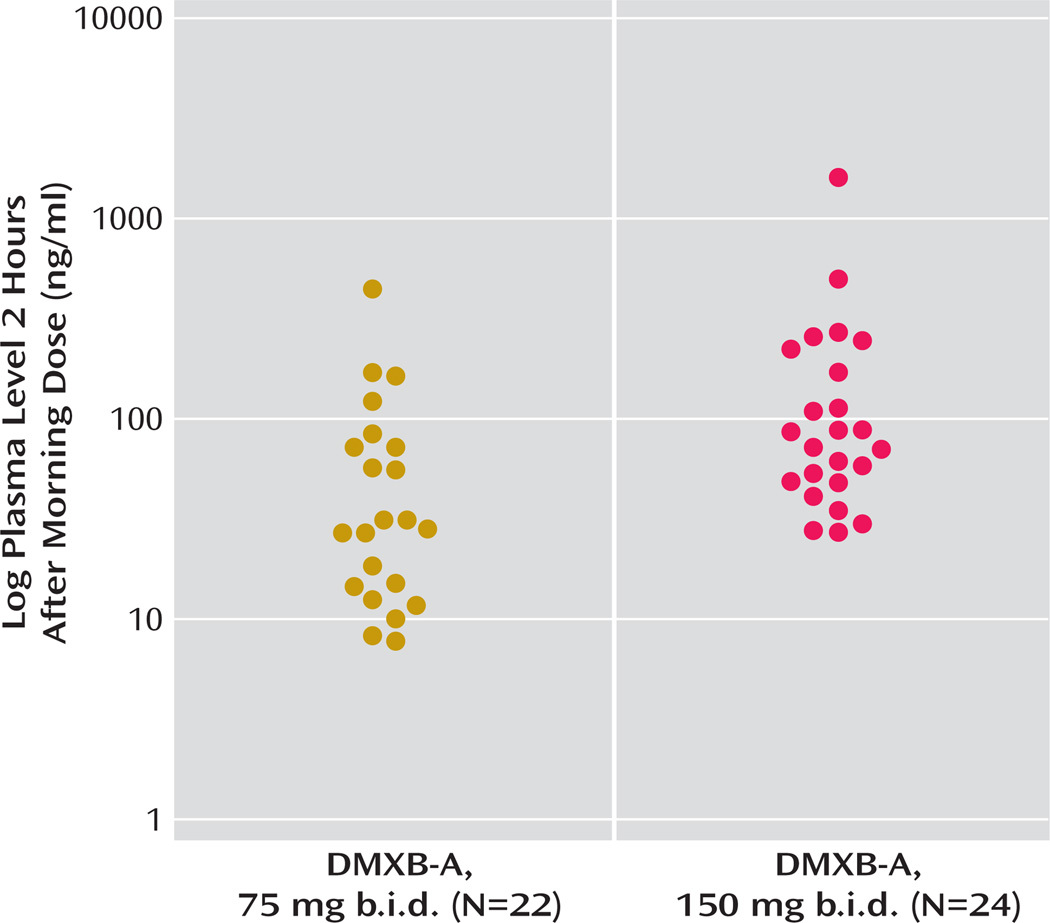
The mean plasma level of DMXB-A obtained after the morning dose on the last day of each 4-week treatment arm with DMXB-A was 50.7 ng/ml (SD=88.7) at the 75-mg dose and 129.9 ng/ml (SD=288.0) at the 150-mg dose. Plasma levels did not influence cognitive effects, symptoms, or any safety measure. One subject’s level was 445 ng/ml at the 75-mg dose and 1570 ng/ml at the 150-mg dose; both values are more than three standard deviations above the mean levels. This subject’s clinical response and serum chemistry results did not differ from the other subjects’ responses. The mean plasma levels were higher than the values previously found in the phase 1 study, 13.2 (SD= 14.4) for the 75-mg dose and 23.2 (SD=16.0) for the 150-mg dose (Figure 3).
FIGURE 3.
DMXB-A Plasma Levels at the End of 4 Weeks for Patients With Schizophrenia Receiving Two Doses of DMXB-A in Crossover Treatment
Type of antipsychotic treatment—no treatment, first-generation antipsychotic, clozapine, or other second-generation antipsychotic—had significant effects only on the speed of processing domain of the MATRICS battery and did not alter the effects of DMXB-A treatment on any measure. There were no effects of site on any of the measures.
Adverse Effects
There was no statistically significant increase in adverse side effects reported by the subjects during DMXB-A treatment, compared with placebo (data supplement Table 2). There were increased reports of nausea and restlessness during DMXB-A treatment, but decreased reports of nervousness. Nausea occurred in 14 patients at the higher dose and is consistent with the known effects of nicotinic agonists on gastrointestinal mobility. None of these effects was severe. One subject became suicidal after 3 days of drug treatment (DMXB-A, 150 mg b.i.d.). He presented himself to an emergency room and was admitted to the hospital, because of a previous severe attempt. He made no attempt during this episode. He said that he had felt well during the treatment, but he became suicidal when his girlfriend told him that she was leaving him. He was removed from the study, but his suicidal ideation was not judged to be related to the study medication.
There were no significant effects of drug treatment on vital signs, ECG, or the results of urinalysis, hematology measurements, or serum chemistry tests (data supplement Table 3). Transient elevations of liver enzymes were observed in different subjects during all three treatment conditions. They had all resolved by the time of repeat testing and did not appear to be related to drug treatment.
Ratings on the Simpson-Angus Scale increased nonsignificantly with the increases in DMXB-A dose, from a mean score of 2.4 (SD=3.2) with placebo to 2.5 (SD=3.3) with 75 mg b.i.d. and 2.8 (SD=3.1) with 150 mg b.i.d. of DMXB-A. Increases were most apparent in head rotation; four of five subjects with ratings of minimal impairment during placebo treatment had increases in ratings to mild or moderate with one or both DMXB-A doses. Two subjects had minimal impairment with DMXB-A that was not observed with placebo. Five subjects had minimal to mild tremors during DMXB-A treatment that did not occur with placebo. Three subjects who had tremors with placebo had increased ratings during DMXB-A treatment, while two had decreases in ratings.
Discussion
The trial did not show significant effects of DMXB-A on cognition over the three treatment arms. What made detection of DMXB-A’s effect difficult may have been the strong effects of test repetition. Subjects improved markedly in their performance over 4 months. We had instituted a baseline test with the MATRICS Consensus Cognitive Battery and digit span subtest, intending to have most of the practice effects occur prior to the three treatment arms, but effects of practice continued throughout the trial. Because the MATRICS tests were chosen for their repeatability, this problem had not been anticipated, but use in a three-arm crossover design was not envisioned in the design of the MATRICS battery (1). Nevertheless, the practice effect would not have obscured a more robust drug effect.
The issue of practice effects has been raised as a possible explanation for the improved performance of patients with first-episode schizophrenia during treatment with second-generation antipsychotic drugs (30). Although none of our patients was in the first episode, a similar phenomenon appeared to occur in this study. We therefore examined performance during the first arm of the protocol only. The effects of DMXB-A after 4 weeks’ treatment on the T score for the attention/vigilance domain, which reflects performance on the Continuous Performance Test—Identical Pairs version, is noteworthy because performance on this test did not change after 6 weeks of treatment with second-generation antipsychotic drugs in first-episode patients (30). Significant effects on the T score for the working memory domain were also observed in the first arm. The decreased performance on the verbal learning domain T score observed in the first arm with 75 mg b.i.d. of DMXB-A reflects significant improvement in the number of items recalled during placebo and a nonsignificant decrease during DMXB-A treatment. The Hopkins Verbal Learning Test, which is used for this domain, asks subjects to learn a list of words. Subjects are given three trials to listen to and then repeat the list; the T score is derived from the sum of items recalled correctly during all three trials. A difference between 75-mg DMXB-A and placebo was observed only in the first learning trial. The test also measures delayed memory, retention, and later recognition of the words. Performance on these measures did not differ between treatments. Thus, viewing the test as a whole, there does not seem to be a strong negative effect of DMXB-A on verbal memory.
The SANS showed no effect of encounter number and did show a significant effect of DMXB-A treatment. The effect on core negative symptoms is also noteworthy, as these symptoms are generally resistant to antipsychotic drugs. Because this study was an initial phase 2 test, we did not establish a priori criteria for clinically significant effects. Many patients expressed that they were substantially more organized in their thoughts and actions, and several spontaneously reported their accomplishment of tasks at home that they had not been previously able to do.
The α7-nicotinic receptors activated by DMXB-A are both presynaptic and postsynaptic. The postsynaptic α7 receptors are predominantly expressed on inhibitory interneurons, particularly in the hippocampus and nucleus reticularis thalami, where they inhibit thalamic input to the cerebral cortex. Activation of these receptors increases inhibitory neuron activity (31). In the phase 1 test, DMXB-A increased inhibition of P50 auditory evoked responses, and this effect is consistent with increased neuronal inhibition (20). It is possible that increased inhibition is the mechanism of improved attention/vigilance and working memory in this study, because inhibition of extraneous activity is necessary for these functions. Improved neurocognition would enable subjects to have better-functioning thought processes, which would be recognized as decreased alogia in the SANS. DMXB-A also increases the release of dopamine through activation of presynaptic receptors, which may account for the decrease in anhedonia observed with the drug (32).
There are other compounds currently in clinical use that have direct or indirect effects on α7-nicotinic receptors. Galantamine, an acetylcholinesterase inhibitor that is also an allosteric modulator of several nicotinic receptors, including the α7-nicotinic receptor, improved several aspects of cognition in schizophrenia and also improved the SANS alogia score (33). In contrast, rivastigmine, which does not have these allosteric properties, had no effect in schizophrenia (34). The most important drug with indirect effects on α7-nicotinic receptors is clozapine. Patients who respond well to clozapine normalize P50 inhibition and decrease their smoking (35–37). Animal model experiments show that clozapine’s neurobiological effects include activation of α7-nicotinic receptors, presumably through the increased release of acetylcholine in the hippocampus (38). The inclusion in this study of two patients taking clozapine may have obscured some of the effects of DMXB-A. However, clozapine, compared to haloperidol, does not improve ratings of alogia (39), which improved with DMXB-A.
Plasma levels of DMXB-A were more variable than observed in the previous phase 1 tests, where DMXB-A was given for 1–5 days (19, 20). While the present study was not designed to be a pharmacokinetic study, changes in metabolism are a possible explanation. The short half-life of DMXB-A could lead to variance in levels if the plasma were sampled at slightly different points in time relative to drug ingestion. Although levels were higher in some patients in this study than previously observed, the finding that levels were undetectable before the first morning dose suggests that there is no accumulation of drug over time due to altered metabolism. The overnight clearing of the drug makes tachyphylaxis from residual drug levels an unlikely explanation for the failure to observe cognitive improvement over all three arms, although longer-term effects mediated by cellular mechanisms cannot be excluded.
Supplementary Material
Acknowledgments
Supported by the VA Biomedical Laboratory and Clinical Science Research and Development Service, by the Mental Illness Research, Education, and Clinical Centers of Veterans Integrated Service Networks 5 and 19, by NIMH grants MH-061412 and MH-068582, by the National Association for Research in Schizophrenia and Affective Disorders, and by the Institute for Children’s Mental Disorders.
The authors thank Drs. Keith Nuechterlein and Barbara Cornblatt from the MATRICS Consensus Cognitive Battery initiative.
ClinicalTrials.gov registry number, NCT00100165 (www.clinicaltrials.gov).
Footnotes
Dr. Freedman has a patent through the Department of Veterans Affairs (VA) on the CHRNA7 gene sequence. The VA has also filed a patent disclosure for the use of nicotinic agonists for schizophrenia. Drs. Kem and Soti have patents through the University of Florida on the manufacture and use of DMXB-A (also known as GTS-21) and have a research grant from CoMentis; Dr. Kem is also a consultant for CoMentis. Dr. Buchanan is on the data safety monitoring boards of Pfizer and Wyeth; is a consultant for Memory Pharmaceuticals, Roche, and Organon; is on advisory boards for AstraZeneca, GlaxoSmithKline, Pfizer, and Solvay; and has received drug samples for research from Ortho-McNeil Neurologics and Janssen. Ms. Ball receives grant support and study medication from Eli Lilly. Dr. Gold has been a consultant for Pfizer and receives royalty payments for the Brief Assessment of Cognition in Schizophrenia. Dr. Stevens receives research support from GlaxoSmithKline and Johnson & Johnson and owns equity in Sierra Puente. Drs. Olincy, Martin, and Johnson receive re-search support from Lundbeck. All other authors report no competing interests.
References
- 1.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, III, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 2.Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RSE, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- 3.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Promoter variants in the α7 nicotinic acetylcholine receptor subunit gene are associated with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1090. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 5.Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 6.Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry E. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–1597. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- 7.Venables PH. In: Input dysfunction in schizophrenia, in Progress in Experimental Personality Research. Maher BA, editor. Orlando, Fla: Academic Press; 1967. pp. 1–64. [PubMed] [Google Scholar]
- 8.Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- 9.Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 10.Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Sacco KA, Termine A, Seyal A, Dudas MM, Vessichio JC, Krishan-Sarin S, Jatlow PI, Wexler PE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- 13.Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhy-anathaswamy S, Khan A. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- 14.Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and non-psychiatric controls. Neuropsychopharmacology. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. Epub 2007, April 18. [DOI] [PubMed] [Google Scholar]
- 15.Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, Thaker GK. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology (Berl) 2004;174:334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- 16.Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- 17.Kem WR, Mahnir VM, Papke RL, Lingle CJ. Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors. J Pharmacol Exp Ther. 1997;283:979–992. [PubMed] [Google Scholar]
- 18.Briggs CA, McKenna DG, Piattoni-Kaplan M. Human alpha-7 nicotinic acetylcholine receptor responses to novel ligands. Neuropharmacology. 1995;34:583–590. doi: 10.1016/0028-3908(95)00028-5. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, Burnett AL. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 20.Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an α7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 21.Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 22.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 23.Kem WR, Mahnir VM, Prokai L, Papke RL, Cao X, LeFrancois S, Wildeboer K, Prokai-Tatrai K, Porter-Papke J, Soti F. Hydroxy metabolites of the Alzheimer’s drug candidate 3-[(2,4-dimethoxy)benzylidene]-anabaseine dihydrochloride (GTS-21): their molecular properties, interactions with brain nicotinic receptors, and brain penetration. Mol Pharmacol. 2004;65:56–67. doi: 10.1124/mol.65.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable Battery for the Assessment of Neuropsychological Status as a screening test in schizophrenia, I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156:1944–1950. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- 25.Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 26.Miller DD, Perry PJ, Cadoret RJ, Andreasen NC. Clozapine’s effect on negative symptoms in treatment-refractory schizophrenics. Compr Psychiatry. 1994;35:8–15. doi: 10.1016/0010-440x(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 27.Mahnir VM, Lin B, Prokai-Tatrai K, Kem WR. Pharmacokinetics and urinary excretion of DMXBA (GTS-21), a compound enhancing cognition. Biopharm Drug Dispos. 1998;19:147–151. doi: 10.1002/(sici)1099-081x(199804)19:3<147::aid-bdd77>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Jones BJ, Kenward MG. Design and Analysis of Cross-Over Trials. 2nd ed. New York: Chapman & Hall/CRC; 2003. [Google Scholar]
- 29.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1999;53:983–997. [PubMed] [Google Scholar]
- 30.Goldberg TE, Goldman RS, Burdick KE, Malhotra A, Lencz T, Patel RC, Woerner MD, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 31.Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective α7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 32.Summers KL, Kem WR, Giacobini E. Nicotinic agonist modulation of neurotransmitter levels in the rat frontoparietal cortex. Jpn J Pharmacol. 1997;74:139–146. doi: 10.1254/jjp.74.139. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, McMahon RP. Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry. 2008;165:82–89. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- 34.Sharma T, Reed C, Aasen I, Kumari V. Cognitive effects of adjunctive 24-weeks rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, doubleblind investigation. Schizophr Res. 2006;85:73–83. doi: 10.1016/j.schres.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, Waldo MC, Hall M-H, Bowles A, Woodward L, Ross RG, Freedman R. Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry. 2004;161:1822–1828. doi: 10.1176/ajp.161.10.1822. [DOI] [PubMed] [Google Scholar]
- 36.George TP, Serynak MJ, Ziedonis DM, Woods SW. Effects of clozapine on smoking in chronic schizophrenic outpatients. J Clin Psychiatry. 1997;56:344–346. [PubMed] [Google Scholar]
- 37.McEvoy JP, Freudenreich O, Wilson W. Smoking and therapeutic response to clozapine in patients with schizophrenia. Biol Psychiatry. 1999;46:125–129. doi: 10.1016/s0006-3223(98)00377-1. [DOI] [PubMed] [Google Scholar]
- 38.Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology. 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- 39.Buchanan RW, Brier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.