Abstract
We reported that both donor CD4+ T and B cells in transplants were required for induction of an autoimmune-like chronic graft versus host disease (cGVHD) in a murine model of DBA/2 donor to BALB/c recipient, but mechanisms whereby donor B cells augment cGVHD pathogenesis remain unknown. Here, we report that, although donor B cells have little impact on acute GVHD (aGVHD) severity, they play an important role in augmenting the persistence of tissue damage in the acute and chronic GVHD overlapping target organs (i.e. skin and lung); they also markedly augment damage in a prototypical cGVHD target organ- the salivary gland. During cGVHD pathogenesis, donor B cells are activated by donor CD4+ T cells to upregulate MHC II and co-stimulatory molecules. Acting as efficient APCs, donor B cells augment donor CD4+ T clonal expansion, autoreactivity, IL-7Rα expression, and survival. These qualitative changes markedly augment donor CD4+ T cells' capacity in mediating autoimmune-like cGVHD, so that they mediate disease in the absence of donor B cells in secondary recipients. Therefore, a major mechanism whereby donor B cells augment cGVHD is through augmenting the clonal expansion, differentiation and survival of pathogenic CD4+ T cells.
Introduction
Graft versus host disease (GVHD) can be divided into acute (a) and chronic (c) GVHD. aGVHD is characterized by T cell infiltration in target organ tissues (i.e. gut, liver, lung, and skin); cGVHD shares characteristics with systemic autoimmune diseases, such as scleroderma and lupus-like syndrome, including elevated serum levels of IgG autoantibodies, sclerodermatous skin tissue damage, and systemic tissue collagen deposition(1-7). The target organ tissues of aGVHD and cGVHD often overlap, such as in the lung and skin, but some target organs (i.e. salivary gland) are mostly unique to cGVHD (1-4). Over the past three decades, there has been little progress in prevention and treatment of cGVHD, due in part to the poor understanding of cGVHD pathogenesis(1). It is clear that aGVHD is mediated by alloreactive donor T cells(8), but it is still unclear whether cGVHD is mediated by the same T cells that mediate aGVHD, although most cGVHD is subsequent to aGVHD(1, 9).
Antigen presentation is known to play a key role in both aGVHD and cGVHD pathogenesis. Host antigen presenting cells (APCs) were reported to initiate acute GVHD, and both donor and host APCs are required for mediating maximal cGVHD(10-14). In autoimmune diseases such as lupus, activated B cells have been shown to be very potent APCs in expanding autoreactive T cells and mediating epitope spreading (15-16). B cells produce autoantibodies in cGVHD patients, leading to the hypothesis that donor B cells play a role in cGVHD pathogenesis (17-18). Indeed, the administration of B cell-depleting anti-CD20 could ameliorate cGVHD in some patients (19-22). In addition, donor B cells were shown to augment priming of T cells that recognize minor antigens (23), and alloantibodies were recently shown to augment cGVHD pathogenesis in an MHC-mismatched murine model(18), but the role of antigen presentation of B cells in cGVHD pathogenesis remains unclear.
In order to clarify the role of donor B cells in GVHD pathogenesis, we utilized a murine cGVHD model of MHC-matched DBA/2 donor to BALB/c recipient (7, 24-25). In this model, although CD8+ T cells have no discernable effect (24), but both donor B and CD4+ T cells are required for disease pathogenesis, offering an opportunity to understand the ways in which donor B cells alter disease progression. We observed that donor B cells in transplants had little impact on aGVHD severity, but did markedly augment cGVHD. Donor B cells in transplants mediated the initial clonal expansion of donor autoreactive CD4+ T cells, augmented their differentiation into the Th2 subset, increased their expression of IL-7Rα, and decreased their apoptosis. Subsequently, these T cells expanded in GVHD target tissues and mediated persistent tissue damage. We also found that after interacting with donor B cells, these donor CD4+ T cells were capable of mediating cGVHD in secondary recipients in the absence of donor B cells. These studies indicate that donor B cells in transplant play a critical APC role in regulating initial expansion, differentiation, and survival of pathogenic CD4+ T cells that mediate cGVHD pathogenesis.
Materials and Methods
Mice
DBA/2 and BALB/c mice were purchased from the National Cancer Institute (NCI) animal production program (Frederick, Maryland). Rag2−/− BALB/c mice were purchased from Taconic Farms, Inc. (Germantown, New York). Luciferase transgenic (Luc+) DBA/2 mice were backcrossed from Luc+ FVB/N mice that was established by C. Contag laboratory (26) for at least 10 generations. Mice were maintained in a pathogen-free room in the City of Hope Animal Resource Center (Duarte, CA). All animal protocols were approved by the City of Hope Institutional Animal Care and Use Committee.
Induction and assessment of GVHD
Mice were irradiated 6-8h prior to HCT using a 137Cs source at a dose of 800 cGy. Recipients were injected with T and B cell-depleted donor BM cells (TBCD-BM) and a dose of splenocytes containing 5×106 CD4+ cells, including CD25− splenocytes (SPL, ∼40×106) and CD25−B220− splenocytes (B220− SPL, ∼20×106). Depletion was achieved using biotin-conjugated antibodies and streptavidin-conjugated magnetic beads, and then passed twice through an AutoMACS cell sorter (Miltenyi Biotec). Purity of depletion was >99%. For experiments using positive selection, CD4-conjugated were used. Purity of the CD4+ fraction exceeded 95%. For adoptive transfer experiments, as CD25 becomes expressed on both Treg and Tcon cells, we used CD103−CD4+ T cells sorted first by depleting CD103 via biotinylated antibody, then enriched CD4+ T cells using anti-CD4 beads. CD4+ T cell purity exceeded 95%. The assessment and scoring of clinical cutaneous GVHD were described in our previous publication (25). In brief, the evaluation was based on the area of alopecia as follows: 0.5, skin ulceration but no hair loss; 1, skin ulcers with alopecia <1 cm2 in area; 2, skin ulcer with alopecia 1–3 cm2; 3, skin ulcer with alopecia >15% body area; and 4, skin ulcer with alopecia >30% body area.
Antibodies, flow cytometry analysis, and cell sorting
FITC-CD5.1 (H11-86.1), APC-CD62L (MEL-14), PE-Cy7-Sca-1 (D7), PE-CD40 (3/23), PE-CD80 (16-10A1), PE-CD86 (GL1), PE-CD40L (MR1), PE-CD28 (37.51), and PE-streptavidin were purchased from BD Pharmingen (San Diego, CA). eFluor450-CD4 (RM4-5), PE-CD44 (IM7), eFluor780-TCRβ (H57-597), APC-IFNγ (XMG1.2), PE-IL-4 (11B11), PE-IL-5 (TRFK5), PE-IL-13 (eBio13A), PE-IL-17A (eBio17B7), APC-Foxp3 (FJK-16S), AlexaFluor780-B220 (RA3-6B2), PE-OX40 (OX-86), and Biotin-CD25 (eBio7D4) were purchased from eBioscience (San Diego, CA). Biotin-PNA was purchased from Vector Laboratories, Inc. (Burlingame, CA). Aqua fluorescent reactive dye for viability analysis was purchased from Invitrogen (Carlsbad, CA). Flow cytometric data were analyzed with FlowJo Software (Treestar, Ashland, OR) as described in our previous publications (24-25, 27).
Proliferation Assays
Proliferating CD4+ T cells were measured as previously reported (25). Briefly, sorted CD4+ T cells (2×105) were incubated with irradiated dendritic cells (1×105) in complete RPMI media containing 10% fetal bovine serum, penicillin/streptomycin, L-glutamine, and 2-mercaptoethanol at the bottom of a 96 U-well plate for 5 days, with tritiated thymidine deoxyribose added to the culture 18h before harvest. Stimulating index was calculated via the formula: ([cpm of culture of responder cells with stimulator] – [cpm of culture of responder alone])/(cpm of culture of responder alone).
Bioluminescent imaging
Mice were injected with luciferase+ CD4+ T cells and monitored for expansion of those cells using bioluminescent imaging every 5 days. Mice were injected with 200 μL of firefly luciferin i.p. (Caliper Life Sciences, Hopkinton, MA), anesthetized, and placed using an IVIS100 charge-coupled device imaging system (Xenogen). Data was analyzed using Igor Pro 4.0 software purchased from WaveMetrics (Lake Oswego, Oregon).
Tissue collection for cellular analysis
Mice were killed using CO2 asphyxiation and their spleens, skin, and lungs were collected for lymphocyte analysis. Spleens were mashed using a 70μm filter. Skin samples were cut into small (<0.5 cm2) pieces, and digested with 5mg/mL collagenase A (Sigma Aldritch, Carlsbad, CA) and 5mg/mL hyaluronidase (Sigma Aldritch, Carlsbad, CA) in complete RPMI media on a shaker at 37°C for 2 hours, then treated with PBS containing 2% BSA and EDTA, mashed through a 70μm filter, and lymphocytes were separated using Lympholyte-M (Burlington, Ontario, Canada). Lung samples were thoroughly flushed of blood by injection of 5-10 mL PBS into the heart and collected in 2% BSA with heparin. Lung samples were then injected collagenase D (Roche, San Francisco, CA) in complete media for 2 hours, then treated with PBS containing 2% BSA and EDTA, mashed through a 70μm filter, and lymphocytes were separated using Lympholyte-M (Burlington, Ontario, Canada).
Histopathology
Tissue specimens were fixed in formalin before embedding in paraffin blocks, cut, and stained with H&E. Slides were examined at 200-400× magnification and visualized with an Olympus and a Pixera (600CL) cooled charge-coupled device camera (Pixera, Los Gatos, CA). Tissue damage was blindly assessed on a scoring system described previously(25). In brief, skin tissue GVHD was scored on the basis of tissue damage in the epidermis, dermis and loss of subcutaneous fat; the maximum score is 9. Lung tissue was blindly evaluated evaluated on a scoring system accounting for perivascular infiltration and inflammation, and peribronchioloar infiltration and inflammation; the maximum score is 15. Salivary gland tissue GVHD was scored on mononuclear cell infiltration and structural disruption, as described previously (25), with a maximum score of 8. Briefly, the degree of inflammatory infiltrates was graded as follows: grade 1: 1–5 foci of mononuclear cells were seen (>20 cells per focus); grade 2: >5 foci of mononuclear cells were seen but without significant parenchymal destruction; grade 3: multiple confluent foci were seen, with moderate degeneration of parenchymal tissue; grade 4: extensive infiltration of the gland with mononuclear cells and extensive parenchymal destruction were seen. Structural and follicular disruption was graded from 0-4, with 0 indicating normal structure, and scores of 1-4 indicating minor, moderate, major, and total disruption of normal structure, respectively. Colon GVHD histopathology was evaluated for increased mononuclear cell infiltration and morphological aberrations (eg hyperplasia and crypt loss), with a maximum score of 10.
TCR Spectratyping
TCR spectratyping was performed as previously reported (25). Briefly, paired groups of experimental mice were sacrificed 15d after HCT, concurrently with healthy DBA/2 control mice. CD4+ T cells were sorted using CD4-beads (Miltenyi Biotec) with purity greater than 98% and lysed in Trizol. RNA was purified, and cDNA was generated and amplified in a series of semi-quantitative PCR assays using a library of murine Vβ primers(28). We then compared the relative area under each peak in recipient mice for each Vβ primer to that of control donor mice using Peak Scanner software (Applied Biosystems, Carslbad, CA). A peak was considered to be significantly skewed if its area was increased by a minimum of 50% relative to the average of the corresponding control mouse peaks. When a majority of samples tested for a given peak registered as skewed, the Vβ was considered to be skewed.
Statistical Analysis
Clinical cutaneous damage scoring and survival in different groups were compared by using the log-rank test (Prism, version 5.0; GraphPad Software, San Diego, CA). Comparison of two means was analyzed using an unpaired two-tail Student t test. Comparison between curves was made by two-way ANOVA. Increases in skewing for TCR spectratyping were compared using a χ2 test from a 2×2 contingency table.
Results
Donor B cells in transplants facilitated the persistence of cGVHD tissue damage in the lung and skin and initiated tissue damage in the salivary gland
We reported that, in an autoimmune-like cGVHD model using DBA/2 donors and BALB/c recipients, donor CD4+ T and B cells were both required to mediate disease pathogenesis(24). It is not yet clear how donor B cells in transplants contribute to cGVHD pathogenesis. We first determined how donor B cells in transplants influenced the course of autoimmune-like cGVHD histopathology development. Since the skin, lung, and salivary gland were reported to be among the most common cGVHD targets(3-4, 29-30), we kinetically compared tissue damage in those organs. As Treg cells can suppress cGVHD induction in this model, especially in the skin (25), sublethally TBI-conditioned BALB/c recipients were injected with CD25+ cell-depleted spleen cells (SPL, ∼40×106) or CD25+ and B220+ cell-depleted spleen cells (B220−-SPL, ∼20×106), which contained an equivalent number of CD4+ T cells (5×106) and other non-B cells. Chimerism and histopathology scores were compared 5, 10, 15, 25, and 40d days post-HCT. Donor B cells had no impact on kinetics of chimerism development, and all recipients became nearly complete chimeras by day 15 after HCT (Sup-Fig.1A).
Skin tissue damage was evaluated by epidermal hyperplasia, dermal infiltration, dermis expansion, hair follicle loss, and dermal fat loss. As shown in Fig. 1A & B, 5-15d post-HCT, severe dermal infiltration was observed; by 15d post-HCT, epidermal hyperplasia occurred. There was no significant difference between recipients given SPL with B cells or B220−-SPL without B cells in this early acute phase of GVHD. Interestingly, thereafter, the inflammation in skin tissue of the recipients without donor B cells rapidly subsided and became nearly normal by 40d post-HCT. In contrast, in the recipients with donor B cells, epidermal hyperplasia, dermal infiltration, dermis expansion, and dermal fat loss persisted, although there was some reduction in dermal infiltration at later time points. Additionally, hair follicles gradually disappeared. Thus, the overall GVHD histopathology score was 6-fold higher than that of recipients without donor B cells (p<0.01), which was comparable to the recipients given T cell-depleted spleen cells by 40d post-HCT (Sup-Fig. 1C). Furthermore, 40 days after HCT, the skin tissues of recipients with donor B cells showed severe collagen deposition as compared with recipients without donor B cells (Sup-Fig. 1B). These results indicate that donor B cells in transplants augment cGVHD skin pathogenesis.
Figure 1. Donor B cells did not augment aGVHD tissue damage early after HCT, but did augment cGVHD tissue damage late after HCT.
BALB/c recipients were irradiated (800cGy) and transplanted with donor B cell-containing SPL or donor B cell-depleted B220−-SPL cells that contained 5×106 CD4+ T cells and were monitored for GVHD development. 5, 10, 15, 25 and 40 days post-HCT, histopathology in skin, lung and salivary grand was evaluated. One representative is shown for 4-6 recipients per group. A. Representative H&E staining of skin tissues at 200× magnification. Arrows indicate hyperplasia, infiltration, subcutaneous fat loss B. Kinetic summary of skin histopathological scores. SPL was more severe than B220−-SPL. (p<0.05 for d25, p<0.01 for d40) C. Representative H&E staining of lung tissues at 200× magnification. Arrows indicate perivascular and peribronchiolar infiltration D. Kinetic summary of lung histopathological scores. SPL was more severe than B220−-SPL. (p=0.05 for d25, p<0.01 for d40) E. Representative H&E staining of salivary tissues at 200× magnification. Arrows indicate infiltration and structural disruptions F. Kinetic summary of salivary histopathological scores. SPL was more severe than B220−-SPL. (p<0.05 for d25, p<0.01 for d40)
Lung tissue was evaluated by examining perivascular and peribronchiolar infiltration. As shown in Fig. 1 C & D, in the recipients given no donor B cells, only mild infiltration in the lung tissue through the whole period from 5-40d post-HCT. In contrast, in the recipients given donor B cells, although infiltration in lung tissue was mild 5-10 days after HCT, the infiltration increased with time. By 40d post-HCT, the overall lung tissue histopathology score was 4-fold higher than that of the recipients given no donor B cells (p<0.01), which was comparable to the recipients given T cell-depleted spleen (Sup-Fig. 1C). These results indicate that donor B cells in transplants augment cGVHD lung tissue damage.
Salivary gland tissue damage was evaluated by examining mononuclear cell infiltration, disruption of normal duct structure, and loss of serous/mucinous fluids. As shown in Fig. 1E & F, in both groups, there was little tissue damage in the salivary gland 5-15d post-HCT. In contrast, the salivary gland tissue damage in the recipients receiving donor B cells gradually increased 15-40d post-HCT and became very severe by day 40, but the salivary gland of the recipients receiving no donor B cells exhibited little damage. The tissue damage score in the former was 5-fold greater than the latter (p<0.01), which was comparable to the recipients given T cell-depleted spleen (Sup-Fig. 1C). These results indicate that donor B cells in transplants significantly augment induction of salivary gland tissue damage.
We also studied whether donor B cells augmented acute GVHD pathogenesis by examining the targets more classically involved in acute GVHD, such as colon. We found that the histopathology of colon of the recipients given SPL or B220−-SPL cells was comparable at 15d and 40d post-HCT (Sup-Fig 1D), suggesting that donor B cells in transplants do not have significant impact on acute GVHD pathogenesis.Taken together, the kinetic study of histopathology shows that donor B cells have little impact on aGVHD pathogenesis but significantly increase persistent tissue damage in overlapping aGVHD and cGVHD target organs such as skin and lung; donor B cells are also critical for damaging cGVHD-specific target tissue salivary gland.
Donor CD4+ T cells in transplants augmented donor B cell activation and upregulation of co-stimulatory molecule expression in cGVHD recipients
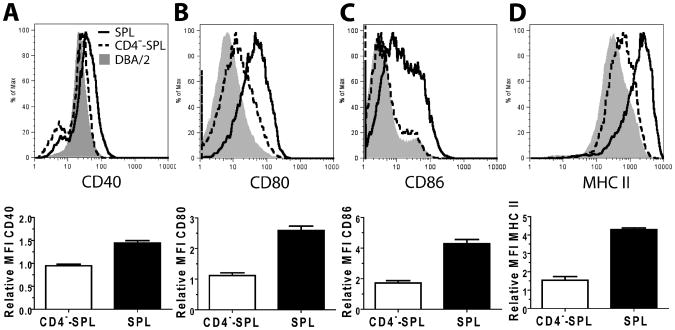
Next, we dissected how donor CD4+ T and B cells in transplants interacted during cGVHD pathogenesis. First, we evaluated the impact of donor CD4+ T cells on donor B cell activation. Since DBA/2 donor cells also induced cGVHD in Rag-2−/− BALB/c recipients, and the severity appeared to be comparable to cGVHD in wild-type (WT) BALB/c (Sup-Fig. 2), we used Rag-2−/− BALB/c recipients for the analysis to avoid any potential contamination by host B cells. Accordingly, sublethally-irradiated Rag-2−/− BALB/c recipients were injected with either DBA/2 donor spleen (SPL) or CD4+ T-depleted spleen (CD4−-SPL) cells. 15d post-HCT, donor B cell expression of costimulatory molecules (i.e. CD40, CD80, and CD86) and MHC II was compared, using healthy DBA/2 donor B cells as controls. At this time point, few de novo-developed B cells were present, so we specifically evaluated the injected B cell population. We found that, although donor B cells showed little upregulation of those markers in recipients given no donor CD4+ T cells as compared to donor B cells before HCT, donor B cells markedly upregulated those markers in the presence of donor CD4+ T cells (p<0.01, Fig. 2). These results indicate that donor CD4+ T cells in transplant play an important role in the initial activation of donor B cells.
Figure 2. Donor B cells upregulated expression of costimulatory molecules upon interaction with donor CD4+T cells in autoimmune-like GVHD recipients.
Rag-2−/− BALB/c recipients were irradiated (800cGy, TBI) and transplanted with SPL or CD4−-SPL cells. 15d post-HCT, mice were sacrificed and donor-type B220+CD19+ splenic B cells were analyzed for expression levels of costimulatory molecules and MHC II, as compared to DBA/2 donor splenic B cells before transplantation (n = 8 from 2 independent experiments). Donor DBA/2 splenic B cells were assigned a relative MFI value of 1 in each experiment and compared to MFI of experimental mice in each experiment. Representative histograms and mean fluorescence intensity comparison summaries are shown. P values indicate an increase in the SPL group compared to the CD4−-SPL group. A. CD40 expression levels were compared, p<0.001. B. CD80 expression levels were compared, p<0.001. C. CD86 expression levels were compared, p<0.001. D. MHC II expression levels were compared, p<0.001.
Donor B cells in transplants mediated initial clonal expansion of the pathogenic autoreactive donor-type CD4+ T cells from the transplant
We recently reported that the alloimmune response was required for initiating clonal expansion of pathogenic donor CD4+ T cells in transplants that possess both donor- and host-reactivity in the autoimmune-like cGVHD recipients (25). Since activated B cells were proposed to be strong APCs in mediating autoreactive CD4+ T clonal expansion and epitope spreading in autoimmune mice (16, 31), and since donor B cells were activated early after HCT and depletion of donor B cells prevented induction of the disease (24), we evaluated the impact of donor B cells in transplants on clonal expansion within donor CD4+ T cell Vβ families, 15d post-HCT, using TCR spectratyping as previously described (25, 32). At this time point, almost no de novo-developed T cells were present, so we could measure the state of the injected CD4+ T cells exclusively. We checked 22 Vβ families. 87/176 total Vβ in 8 recipients given SPL cells but only 57/176 in 8 recipients given B220−-SPL cells exhibited skewing (p<0.01, Fig. 3 A and B). We also found that 13/22 Vβ families in the former and only 8/22 Vβ families in the latter are skewed. These results indicate that B cells can augment clonal expansion of CD4+ T cell Vβ families.
Figure 3. Donor B cells mediate clonal expansion of donor-type CD4+T cells with both donor- and host-reactivity.
BALB/c recipients were irradiated (800cGy) and transplanted with SPL or B220−-SPL cells. A. 15d post-HCT, recipients were sacrificed and the splenic CD4+ T cells were harvested and evaluated for clonal expansion via observations of skewed TCR spectra as compared to donor DBA/2 CD4+ T spectra before HCT . Example unskewed or skewed spectra are shown, with skewed peaks filled with black (n = 8 for experimental samples, n = 4 for controls). B. Summary of TCR spectratype skewing: 22 Vβ in each mouse were analyzed, there were 87 of 176 total Vβ skewed in 8 recipients given SPL cells vs 57 of 176 total Vβ skewed in 8 recipients given B220−-SPL cells, p<0.01. “+” indicates more ≥5/8 recipients exhibited skewing for that Vβ. C. 15d post-HCT, recipients were sacrificed and the splenic donor-type CD4+ T cells were harvested and stimulated with irradiated either DBA/2 or BALB/c dendritic cells and evaluated for their proliferation using 3H-TdR incorporation assay. Stimulating index is shown (n=5, p<0.01 for both).
It was of interest that donor B cells in transplants augmented clonal expansion in donor T cell Vβ families that should have been deleted by Mtv-mediated negative selection in the thymus of DBA/2 mice, including Vβ7, 8.1, 11, and 17 (33), indicating that under GVHD conditions, donor B cells are able to efficiently augment clonal expansion of residual autoreactive donor T cells in the periphery. Consistent with this hypothesis, the donor-type CD4+ T cells from recipients given donor B cells proliferated ∼5-fold more in response to stimulation by syngeneic donor-type DCs and proliferated ∼2-fold more in response to stimulation by allogeneic host-type DCs, as compared with the donor-type CD4+ T cells from recipients given no donor B cells (p<0.01, Fig. 3C). These results indicate that donor B cells in transplants play an important role in augmenting the clonal expansion of cGVHD-pathogenic donor CD4+ T cells with both donor- and host-reactivity.
Donor B cells in transplants augmented donor CD4+ T cell IL-7Rα expression and reduced donor CD4+ T cell apoptosis in GVHD target tissues
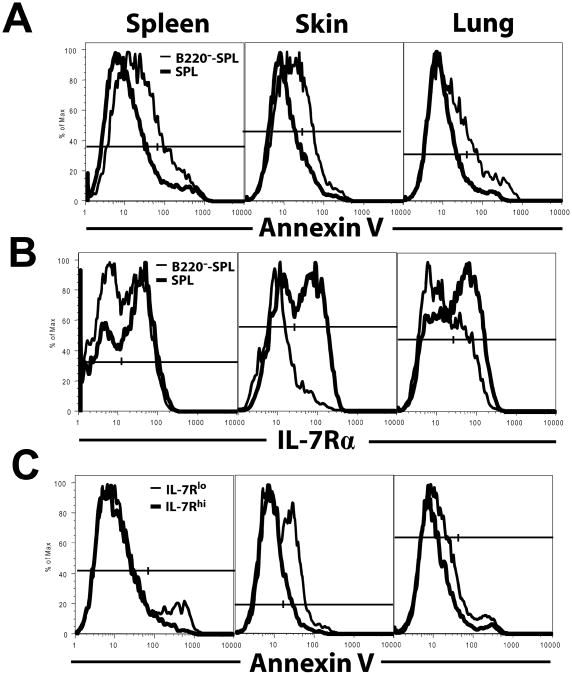
B cells could potentially augment the survival of activated CD4+ T cells (34). We tested whether the persistent GVHD target tissue damage in the presence of donor B cells from the transplant was associated with reduced apoptosis of donor CD4+ T cells. As we had observed other qualitative changes 15d post-HCT, at that same time point, CD4+ T cells in spleen, skin, and lung of recipients given SPL or B220−-SPL cells were measured for apoptosis via Annexin V staining. Indeed, CD4+ T cell apoptosis was significantly decreased in the spleen, skin, and lung of the recipients given donor B cells as compared to that of the recipients given no donor B cells, as judged by a significant reduction of Annexin V staining (p<0.01, Fig. 4A).
Figure 4. Donor B cells increased expression of IL-7Rα but decreased apoptosis on donor CD4+T cells.
BALB/c recipients were irradiated (800 cGy) and transplanted with SPL or B220−-SPL cells. 15d post-HCT, donor CD4+ T cells from the spleen, skin, and lung were evaluated for apoptosis by Annexin V staining and for expression of IL-7Rα, evaluating positive cells by contour or histogram shoulder. One representative is shown from 2-3 replicate experiments, n=8-12. A. Donor CD4+ T cells are shown in histograms of Annexin V staining. A higher percentage of Annexin V+ CD4+ T cells were observed in the B220−-SPL group in all organs (spleen: 23.6±1.9 vs 17.9±0.9, p<0.05; skin: 28.7±3.7 vs 12.9±1.3, p<0.01; lung: 26.82±5.1 vs 14.0±2.2, p<0.05). B. Donor CD4+ T cells are shown in histograms of IL-7Rα expression. A higher percentage of IL-7Rαhi CD4+ T cells were observed in the SPL group in all organs (spleen: 37.2±5.5 vs 21.3±3.7, p<0.05; skin: 61.62±3.8 vs 34.9±3.2, p<0.001; lung: 48.5±2.8 vs 29.4±1.5, p<0.001). C. Spleen-, skin-, and lung-infiltrating donor CD4+ T cells from SPL recipients were gated on IL-7Rαhi or IL-7Rαlo populations and then shown in histograms of Annexin V staining. The percentage of Annexin V+ cells determined with contours/shoulders that show difference between the two comparing groups, IL-7Rαlo group versus IL-7Rαhi group, are spleen: 28.8±3.3 vs 16.5±1.5, p<0.01; skin: 14.1±1.8 vs 8.9±0.6, p<0.05; lung: 14.7±1.0 vs 11.4±0.6, p<0.05.
We then investigated how B cells provided donor CD4+ T cells with a survival advantage. Since PD-1 interaction with its ligand B7-H1 (PD-L1) induces activated T cell apoptosis (35-37), and since IL-7/IL-7Rα signaling augments effector T cell survival (38), besides augmenting naïve T cell expansion, we compared the expression levels of PD-1 and IL-7Rα by donor CD4+ T cells from spleen, skin, and lung. We found almost all infiltrating T cells in the skin and lung were CD44hiCD62Llo effector cells in both recipients with or without donor B cells (Sup-Fig. 3A); however, there was little difference in PD-1 expression by donor CD4+ T cells (Sup-Fig. 3B). B7-H1 expression by infiltrating macrophages and neutrophils was also similar (Sup-Fig. 3C), suggesting that the PD-1/B7-H1 axis was not critical to their survival. Interestingly, donor effector CD4+ T cells from skin and lung tissues, especially the skin, of recipients receiving donor B cells expressed markedly more IL-7Rα, as compared to recipients receiving no donor B cells (p<0.01, Fig. 4B). The IL-7Rαhi donor CD4+ T cells also had less apoptosis, as indicated by reduced Annexin V staining (p<0.01, Fig. 4C). These results indicate that the presence of donor B cells in transplants leads to increased donor CD4+ T effector cell expression of IL-7Rα expression compared to recipients not receiving B cells, subsequently reducing apoptosis of infiltrating activated donor CD4+ T cells in GVHD target tissues.
Donor B cells in transplant augmented donor CD4+ T differentiation into proinflammatory Th2 cells
Proinflammatory TNF-α-producing Th1, Th2, and Th17 have all been shown to mediate GVHD under different circumstances (39-41). Next, we tested whether donor B cells in transplants impact on donor CD4+ T cell differentiation into Th1, Th2, and Th17. Accordingly, 5, 10, 15, 25, and 40d post-HCT, the donor CD4+ T cell cytokine profile in the spleen, skin, and lung of recipients with or without donor B cells was measured by intracellular staining. The IFN-γ-producing Th1 cells were the major Th subset in all tissues, and the percentage of Th1 cells in the recipients with or without donor B cells was not significantly different in all tissues at all time points (p>0.1, Fig. 5A and Sup-Fig. 4A).
Figure 5. Donor B cells increased Th2 among donor CD4+T cells, but not other Th subsets.
BALB/c recipients of SPL or B220−-SPL were sacrificed 5, 10, 15, 25, and 40 days post-HCT, and the donor CD5.1+CD4+ T cells from spleen, skin, and lung were evaluated for intracellular cytokine production. Mean±SE is shown for each time point (n=4-8 from 3-4 replicate experiments). A. Percentage of IFNγ+ cells among donor CD4+ T cells. No significant differences were observed (2-way ANOVA, p>.05). B. Percentage of IL-17+ cells among donor CD4+ T cells Although IL-17 was somewhat higher in the lung of recipients of B220−-SPL, no significant differences were observed (2-way ANOVA, p>.05). C. Percentage of IL-4+ cells among donor CD4+ T cells. A higher percentage of IL-4+ cells were observed in the SPL group compared with the B220−-SPL group (2-way ANOVA, p<0.01 for spleen and lung, p<0.05 for skin)
IL-17-producing Th17 cells were rare in all cases, and largely similar between recipients with or without donor B cells, although somewhat enriched in the lung of recipients without donor B cells as compared to recipients with donor B cells (p>0.1, Fig. 5B and Sup-Fig. 4B). This change was not correlated to lung cGVHD pathogenesis as described in Fig. 1.
In contrast, the percentage of IL-4-producing Th2 cells was significantly increased in recipients with donor B cells as compared to recipients without donor B cells, especially 40d post-HCT (p<0.01, Fig. 5C and Sup-Fig. 4C), which correlated with increased signs of cGVHD as described in Fig. 1. Additionally, the Th2 cells were largely TNF-α producing cells, although the percentage of TNF-α+ cells were similar in recipients with or without donor B cells (p>0.1, Sup-Fig. 4D). We previously demonstrated that TNF-α producing Th2 cells were important mediators of lung GVHD (39). It is interesting that their presence in this model is also correlated with increased skin and salivary gland damage. These results indicate that donor B cells in transplants mainly augment proinflammatory Th2 differentiation in the autoimmune-like cGVHD recipients.
Donor B cells in transplants augmented the expansion of donor-type CD4+ T cells in GVHD target tissues and augmented the development of cGVHD
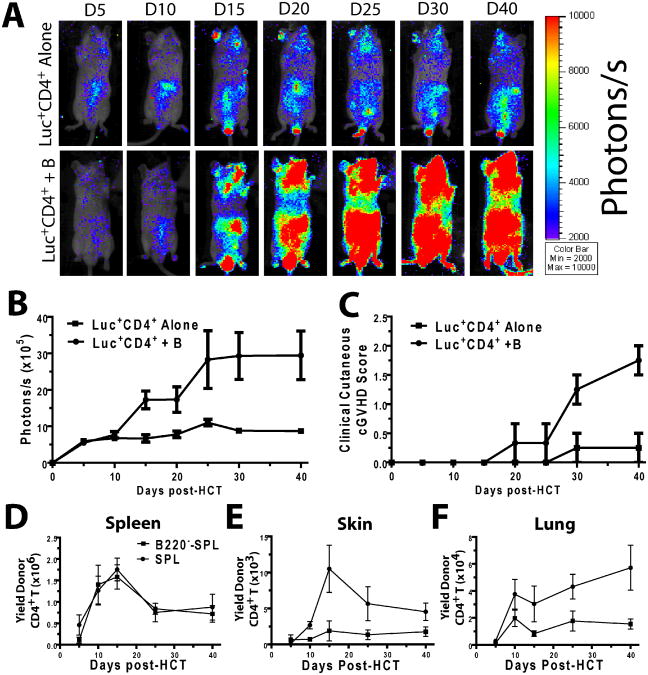
Next, we asked whether these qualitative changes would lead to quantitative changes in the donor CD4+ T cell population during cGVHD pathogenesis. Therefore, we used in vivo bioluminescent imaging (BLI) to visualize the expansion of the injected donor-type CD4+ T cells in recipients given transplants with or without donor B cells. Sorted CD25−CD4+ T cells (5×106) from luciferase-transgenic (luc+) DBA/2 donors were co-injected with T and B cell-depleted (TBCD)-SPL cells (5×106) with or without donor B220+ cells (10×106) into sublethally irradiated BALB/c recipients. Thereafter, in vivo BLI was performed 5, 10, 15, 20, 25, 30, and 40d post-HCT to measure donor CD4+ T expansion. We found that, although there was no significant difference in donor CD4+ T expansion in the presence or absence of donor B cells by 10d post-HCT, donor CD4+ T expansion in the presence of donor B cells was significantly more vigorous by 15-20 days after HCT, as reflected by the in vivo BLI intensity; the donor CD4+ T expansion persisted for more than 40 days in surviving recipients (Fig. 6 A & B). Correspondingly, the cutaneous GVHD score in the latter gradually increased and reached a severity level that was ∼10-fold higher in recipients receiving donor B cells than in recipients receiving no donor B cells by 40d post-HCT (p<0.01, Figure 6B & C).
Figure 6. Donor B cells in transplants augmented donor-type CD4+T cell expansion in GVHD target tissues.
A-C. BALB/c mice were irradiated (800 cGy) and injected with 5×106 CD4+CD25− splenoctyes from luciferase transgenic (luc+) DBA/2 mice and WT DBA/2 TBCD SPL (5×106), with or without WT DBA/2 B220+ B cells (10×106), then monitored for expansion of donor CD4+ T cells and signs of GVHD. A. CD4+ T cell expansion in mice was evaluated with bioluminescent imaging (BLI). One representative BLI pattern is shown per group per time point (n=8 from two replicate experiments). B. BLI intensity in terms of photons/s. A summary curve (mean±SE) is shown. Mice recieiving B cells had increased luminescence (2-way ANOVA, p<0.01). C. Recipieints of luc+CD4+ T cells with or without B cells were evaluated after HCT for GVHD-related skin damage and hair loss. Recipients of B cells had increased clinical cutaneous scores (2-way ANOVA, p<0.01). D-F. BALB/c mice were irradiated (800 cGy) and injected with either SPL or B220−-SPL and sacrificed 5, 10, 15, 25, and 40 days post-HCT, and their tissues were harvested and evaluated for the presence of infiltrating donor CD5.1+CD4+ T cells. Mean±SE is shown at each time point, n=4-8 from 2-3 replicate experiments. D. Donor CD4+ T cell yield in the spleen was evaluated, and was not significantly different between SPL and B220−-SPL groups (2-way ANOVA, p>0.1) E. Donor CD4+ T cell yield in the skin was evaluated. A higher yield was observed in SPL recipients (2-way ANOVA, p<0.01). F. Donor CD4+ T cell yield in the lung was evaluated. A higher yield was observed in SPL recipients (2-way ANOVA, p<0.01).
To further identify the location of donor CD4+ T cell expansion, we kinetically numerated the infiltrating donor CD4+ T cells and total B cells in the spleen, skin, and lung at 5, 10, 15, 25, and 40 days after HCT. There was no difference in the yield of donor CD4+ T cells in the spleen of recipients with or without donor B cells over the course (Fig. 6D). However, the donor CD4+ T cell yield was significantly higher in the skin and lung tissues of the recipients with donor B cells as compared to recipients without donor B cells 15 to 40 days post-HCT (p<0.01, Fig. 6 E&F). This is consistent with stronger BLI and more severe clinical GVHD in the recipients with donor B cells as shown in Fig. 6 B and C.
The changes of B cell percentage and yield were different from that of CD4+ T cells. As shown in Sup-Fig. 4E, there were few (<0.8% or 0.1 ×106) B220+ B cells in the spleen of recipients without injection of donor B cells by 5-15 days after HCT, but donor B cells gradually increased 25 days after HCT; the percentage reached ∼43% and the yield reached ∼3×106 by 40 days after HCT. In contrast, there were significantly more B cells in the spleen of recipients with injection of donor B cells 5-15 days after HCT; the percentage reached ∼18% and the yield reached ∼1.5×106 (p<0.01). However, the percentage and yield significantly declined 25 days after HCT. The percentage was only ∼2.4% and the yield was ∼0.2 ×106 by 40 days after HCT (p<0.01). We also observed little B cell infiltration (<0.5%) in the skin and lung tissues in either group 5-40 days after HCT (data not shown). These results suggest that the interaction between injected donor CD4+ T and B cells mainly takes place early after HCT in the lymphoid tissues.
Additionally, we observed that the persistence of inflammation in the skin tissues of recipients was associated with an increase of donor CD4+ T cell infiltration in the skin tissues. 5-15 days after HCT, skin infiltration consisted of mainly CD11b+/Gr-1+ macrophages and neutrophils, which was markedly reduced in both groups by 25d post-HCT (Sup-Fig. 4F). However, the presence of donor B cells in transplant resulted in a sharp increase of donor CD4+ T cells in the tissue by 15 days after HCT, and the declination of donor CD4+ T cells in the skin tissue was much slower than the decline of macrophages and neutrophils (Fig. 6E and Sup-Fig 4F) and was associated with significantly stronger GVHD. These results indicate that donor B cells in transplants augment donor CD4+ T cell infiltration and expansion in GVHD target tissues (i.e. skin) and support the persistence of tissue inflammation.
Sorted donor-type CD4+ T cells from primary recipients with or without donor B cells in transplant showed a marked difference in cGVHD-inducing capacity
Next, we tested the cGVHD-inducing capacity of donor-type CD4+ T cells with or without prior interaction with donor B cells in transplants in the adoptive recipients. As we observed few donor B cells late after HCT in SPL recipients, but still observed strong GVHD (Fig 1 and Sup-Fig.4E), we hypothesized that the continued presence of B cells may not be necessary after the initial expansion of pathogenic CD4+ T cells. Accordingly, 15d post-HCT, sorted donor-type CD4+ T cells (5×106, purity >98%) from spleens of primary BALB/c recipients with or without donor B cells were injected into sublethally irradiated secondary BALB/c recipients, along with T and B cell-depleted (TBCD) BM cells (2.5×106) from naïve DBA/2 donors. We observed that donor-type CD4+ T cells from primary recipients with donor B cells (B-interacted CD4+ T) induced cutaneous GVHD starting 20d after cell transfer, reaching a plateau 30d after cell transfer. In contrast, donor-type CD4+ T cells from primary recipients without donor B cells (Non-B-interacted CD4+ T) induced little cutaneous GVHD (p<0.01, Fig. 7A).
Figure 7. Donor CD4+T cells from primary recipients receiving donor B cells in transplants mediate cGVHD in secondary recipients.
BALB/c mice were irradiated and given either SPL or B220−-SPL cells. 15d post-HCT, recipients were sacrificed and their spleens and peripheral lymph nodes were harvested, and donor CD4+ T cells were sorted from them. To ensure equal doses of effector CD4+ T cells, CD103+ T cells were depleted. CD103−CD4+ T cells (5×106)from the spleen and LN of primary recipients along with TBCD BM (2.5×106) from healthy DBA/2 donors were injected into sublethally irradiated BALB/c recipients, and the recipients were monitored for GVHD development. A. Secondary recipients were kinetically evaluated for cutaneous cGVHD. Increased cutaneous damage was observed in secondary recipients of CD4+ T cells from primary recipients given donor B cells (B-Int-CD4) as compared with recipients of CD4+ T cells from primary recipients given no donor B cells (B-Non-Int-CD4) recipients. (2-way ANOVA, p<0.01) n=8 from two replicate experiments. B and C. 40d post-HCT, secondary recipients were sacrificed and their lung, skin and salivary glands were evaluated histopathologically. Arrows indicate signs of infiltration or damage. (200×magnification). C. Mean±SE of histopathology scores are shown (n=4-6 from 2 replicate experiments). Increased damage was observed in recipients of B-Int-CD4 as compared with B-Non-Int-CD4 (p<0.05 for lung, p<0.01 for skin and salivary) D-G. 40d post-HCT, recipients were sacrificed and their spleen, skin, and lungs were harvested the yield of infiltrating donor CD4+ T cells from the spleen, skin, and lungs were measured. Cytokine profiles from each organ were measured by intracellular staining. n=8 from two replicate experiments. D. Yield of donor CD4+ T cells. No significant difference was found in the spleen or lung (p>0.05), but significantly more CD4+ T cells infiltrated the skin of recipients of B-Int-CD4 as compared with B-Non-Int-CD4 (p<0.01), E. Percentage of IFNγ+ cells among donor CD4+ T cells. A higher percentage of IFNγ+ cells was observed in recipients of B-Int-CD4 in the spleen and skin (p<0.01), but not the lung (p>0.05). F. Percentage of IL-4+ cells among donor CD4+ T cells. A higher percentage of IL-4+ cells was observed in recipients of B-Int-CD4 in the spleen and skin (p<0.01), as well as the lung (p<0.05). G. Percentage of IL-17+ cells among donor CD4+ T cells. A higher percentage of IL-17+ cells was observed in recipients of B-Int-CD4 in the spleen, lung and skin (p<0.01)
40 days after cell transfer, histopathology of the lung, skin, and salivary gland tissues was evaluated. We found that B-interacted CD4+ T cells induced significantly more tissue damage, especially in the skin and salivary gland, compared to the non-B-interacted CD4+ T cells (Fig. 7B). The histopathology score difference between the two groups varied from ∼2-4 fold (p<0.01, Fig. 7C). While the histopathology score of skin and salivary gland was comparable to that of primary recipients on day 40 after HCT, there was an apparent decrease in the lung in the secondary recipients (Fig. 1 and Fig. 7C). The latter observation is consistent with a recent report that antibodies may be important for lung cGVHD(18), as no B cells or plasma cells were transferred.
We also evaluated the total donor CD4+ T yield and the percentage of Th1, Th2, and Th17 subsets in the spleen, lung, and skin of the recipients. The yield of donor-type CD4+ T cells in the spleen and lung of recipients given B-interacted or non-B-interacted CD4+ T cells was not significantly different (P>0.1). The skin yield was more than 6-fold higher in the B-interacted CD4+ T recipients (p<0.01, Fig. 7D). A plurality of CD4+ T cells were Th1 in both groups. The percentage of Th1 cells in the spleen and skin in recipients given B-interacted CD4+ T cells were 3 to 5-fold higher than that in recipients given non-B-interacted CD4+ T cells (p<0.01, Fig. 7 E), but the difference in the lung was not significant (p>0.1, Fig. 7E). The percentage of Th2 cells and Th17 cells in the spleen, lung, and skin in recipients given B-interacted CD4+ T cells were all significantly higher than in the tissues of recipients given non-B-interacted CD4+ T cells (p<0.01, Fig. 7F-G). These results further support our notion that donor B cells in transplants can augment the generation and expansion of pathogenic donor CD4+ T subsets early after HCT.
Discussion
We have demonstrated that, in a murine autoimmune-like cGVHD model of DBA/2 donor to BALB/c recipient, donor B cells augmented the persistence of GVHD tissue damage in overlapping target organs of aGVHD and cGVHD such as the lung and skin; donor B cells also augmented tissue damage in exclusive cGVHD target organs such as the salivary gland. The mechanisms whereby donor B cells in transplants contribute to pathogenesis of autoimmune-like cGVHD include 1) augmenting the clonal expansion of the residual autoreactive donor CD4+ T cells in transplants; 2) augmenting donor CD4+ T differentiation into proinflammatory Th2 cells; 3) augmenting donor CD4+ T cell expression of IL-7Rα, survival, and expansion in GVHD target tissues.
We previously showed that, in the autoimmune-like cGVHD model of DBA/2 donor and BALB/c recipient, tissue damage occurred in several target organs, including the skin, lung, and salivary gland by late post-HCT (25). However, the kinetics of disease pathogenesis of these target organs remains unknown. The skin and lung are target organs for both acute and chronic GVHD (1-4). We found that, in the skin, donor B cells in transplant had no impact on the severity of dermal infiltration during the early acute phase. The early infiltrating cells were primarily macrophages and neutrophils, and these cells were incapable of persisting as time went on. However, donor B cells had marked impact on the severity of skin tissue damage late after HCT, which manifested with epidermal hyperplasia, loss of subcutaneous fat, loss of hair-follicles, and dermal collagen deposition. This persistent skin tissue damage was strongly associated with the infiltration of donor CD4+ T cells, which was markedly augmented by donor B cells in transplants. Similarly, the increased donor-type CD4+ T cell infiltration in the lung and salivary gland tissues of recipients receiving donor B cells was associated with the GVHD tissue damage in those targets late after HCT. Therefore, donor B cells in transplants augment donor CD4+ T infiltration and cGVHD tissue pathogenesis in the acute and chronic GVHD overlapping target organs skin and lung, as well as in the prototypical cGVHD target organ salivary gland. This augmentation may result from donor B cell expansion of autoreactive T cells that recognize antigens expressed by target tissues.
We have recently reported that donor-type CD4+ T cells that possess both donor- and host-reactivity mediate autoimmune-like cGVHD, and these T cells derived from donor CD4+ T cells in transplants during alloimmune response (25). In the current studies, donor B cells were found to mediate the clonal expansion of these pathogenic CD4+ T cells that possess both donor- and host-reactivity, as judged by TCR spectratyping and MLR. Our finding that additional families of Vβ clones are expanded in SPL recipients as compared to B220−-SPL recipients may also explain why GVHD target organs experience additional CD4+ T cell infiltration in the former group, as they may correspond to clones that mediate organ-specific damage. Unfortunately, we found it too technically challenging to acquire adequate tissue-infiltrating CD4+ T cells to perform TCR spectratyping. Although the mechanisms whereby B cells expand additional CD4+ T cell clones are not yet fully understood, it is known that B cells are much more efficient in mediating CD4+ T expansion when presenting rare antigens (42), possibly allowing for rare autoantigens released by dying cells during the acute GVHD phase to be presented to residual autoreactive CD4+ T cells. The possession of both donor- and host-reactivity by the pathogenic donor CD4+ T cells suggests that those T cell clones recognize non-polymorphic antigens, which have been proposed to be important antigens for mediating cGVHD (9, 43). Our results indicate that donor B cells in transplants play an important role in mediating the clonal expansion of pathogenic CD4+ T cells that mediate autoimmune-like cGVHD.
We found that donor B cells in transplants augmented expansion of TNFα-producing Th2 cells in the lung and skin tissues late after HCT. The increase of Th2 cells is consistent with the fact that B cells can produce IL-4 (44), and IL-4 mediates Th2 differentiation of donor T cells in GVHD recipients (39). Our results show that donor B cells in transplants preferentially augment donor CD4+ T cell differentiation into proinflammatory Th2 cells; the presence of these proinflammatory Th2 cells correlated with the chronic phase of GVHD in this model, suggesting that these cells may be important for cGVHD damage. Proinflammatory Th2 cells have been reported to mediate autoimmune skin tissue damage (45-46) and GVHD lung tissue damage (39). The role of Th subsets in cGVHD pathogenesis is the subject of future studies.
Using in vivo BLI, we found that donor CD4+ T cell expansion in recipients with or without donor B cells appeared to be weak and similar early after HCT. However, donor CD4+ T cells in the recipients with donor B cells showed marked expansion late after HCT. This wave of expansion was associated with increased donor CD4+ T effector cell expansion in GVHD target tissues, as well as associated with increased expression IL-7Rα and reduced apoptosis. Since a plurality of infiltrating donor CD4+ T cells were Th1, this suggests that donor B cells in transplants are able to augment the Th1 cell expression IL-7Rα and increase their survival. Further mechanisms whereby B cells augment T cell expression of IL-7Rα expression need to be studied in the future. Taken together, donor B cells in transplants not only augment the initial clonal expansion of cGVHD-mediating donor-type CD4+ T cells in the lymphoid tissues but also augment their survival and expansion in the GVHD target tissues.
Autoreactive CD4+ T cells in cGVHD recipients can be derived from mature donor CD4+ T cells in transplants (25) and can be derived from donor marrow stem cells in the GVHD-damaged thymus that are defective in negative selection of autoreactive T cells (47-49). In the current study, we mainly address the interactions between donor mature T and B cells in transplants in cGVHD recipients, as de novo-developed cells are not present 15d post-HCT, and the radiation dose used allowed very little mature host T and B cell survival post-HCT. Future studies will address how mature B cells in transplants and de novo-developed donor B cells influence the activation and expansion of de novo-developed autoreactive T cells in cGVHD recipients.
The qualitative impact of donor B cells in transplants on donor CD4+ T cell clonal differentiation, ability of survival and expansion, as well as ability to mediate cGVHD was confirmed by observations in the secondary adoptive recipients. While donor CD4+ T cells from the primary recipients given no donor B cells expanded weakly and induced only a little tissue damage in the adoptive recipients, the donor CD4+ T cells from the primary recipients given donor B cells induced severe tissue damage in the skin and salivary gland in association with expansion of donor CD4+ T subsets in the tissues. Interestingly, increased percentages of Th1, Th2, and Th17 cells were observed in the secondary recipients given CD4+ T cells exposed to B cells in primary recipients, whereas in those primary recipients, only Th2 cells were significantly expanded. This discrepancy is not yet fully understood. Our most recent preliminary data showed that MHC-II KO donor B cells failed to augment chronic GVHD, and IL-4 KO donor B cells still partially augmented chronic GVHD (Young and Zeng, unpublished observation). Furthermore, our data demonstrate that donor B cells augmented donor CD4+ T cell expression of IL-7Rα and increased their survival. These data suggest that donor B cells, acting as APCs, not only augment Th2 differentiation but also improve the survival capacity of other Th subsets. Therefore, besides Th2 cells, Th1 and Th17 cells from primary recipients given donor B cells could also display superior expansion in secondary recipients that have TBI-induced lymphopenia. This observation may be of clinical importance, as it demonstrates that early B cell interaction may provide donor CD4+ T cells with the means to rapidly re-expand from low numbers, as after withdrawal from immunosuppression.
In summary, we propose that donor B cells in transplants augment the pathogenesis of cGVHD in multiple steps: Alloreactive donor CD4+ T cells are first activated by professional host APCs, which then activate autoreactive donor B cells that present alloantigens. Upon activation, these autoreactive B cells then work as potent APCs to mediate initial activation and clonal expansion of the autoreactive CD4+ T cells as well as augment differentiation of Th2 cells. The activated autoreactive B cells also augment the pathogenic CD4+ T cells that possess both allo- and autoreactivity with increased expression of IL-7Rα, leading to their increased survival in GVHD target tissues. Subsequently, the infiltrating pathogenic CD4+ T cells mediate the transition from aGVHD to cGVHD tissue damage in the overlapping target organs such as the skin and lung, as well as initiate tissue damage in cGVHD prototypical organs such as the salivary gland. Additionally, antibodies from donor B cells may also augment some cGVHD tissue damage, especially in the lung, as suggested previously (18).
Our observations provide important insights into human cGVHD pathogenesis. It is known that administration of B cell-depleting anti-CD20 mAb (rituximab) is effective in treating cGVHD in some patients, but others have only a partial response or no response (50-51). The ineffectiveness of anti-CD20 treatment may be due to the fact that cGVHD is mainly mediated by effector T and B cells, and effector lymphocytes are less sensitive to depletion by antibody treatment (50-51). However, we raise another important possibility: once pathogenic CD4+ T cells are generated and expanded by donor B cells, depleting the donor B cells may no longer have a significant impact on the pathogenic activity of the CD4+ T cells, as we observed that purified donor CD4+ T cells from primary recipients with donor B cells were able to induce cGVHD in adoptive recipients, although CD4+ T cells from the recipients without donor B cells could not. Our studies indicate that removal of donor B cells from transplants before HCT or early administration of depleting anti-CD20 prior to pathogenic CD4+ T cell clonal expansion or prior to GVHD onset may be more effective in preventing cGVHD than in treating cGVHD after disease onset.
Supplementary Material
Acknowledgments
We thank Dr. Thea Friedman (Hackensack University Medical Center) for providing the protocol for TCR spectratyping, and Lucy Brown and her staff (City of Hope Flow Cytometry Facility) and Sofia Loera and her staff (City of Hope Anatomic Pathology Laboratory) for excellent technical assistance.
This work was supported by National Institutes of Health Grant R01AI066008 (to D.Z.).
Footnotes
Authorship Contributions: J.S.Y. designed and performed research, and wrote the manuscript; T.W., Y.C., D.Z., H.L, T.Y., H.J., J.R., X.L., and A.W. performed some experiments; I.T. reviewed histopathology slides, and D.Z. designed research, wrote the manuscript, and supervised the research project.
Disclosure of Conflicts of Interest: All authors report no conflicts of interest.
References
- 1.Pavletic SZ, Vogelsang GB. Chronic Graft-versus-Host Disease: Clinical Manifestations and Therapy. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. 4th. Blackwell Publishing; Hoboken, NJ: 2009. pp. 1304–1324. [Google Scholar]
- 2.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, Arora M, Weisdorf DJ, Flowers ME, Martin PJ, Palmer J, Jacobsohn D, Pavletic SZ, Vogelsang GB, Lee SJ. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier JK, Wolff D, Pavletic S, Greinix H, Gosau M, Bertz H, Lee SJ, Lawitschka A, Elad S. Oral chronic graft-versus-host disease: report from the International Consensus Conference on clinical practice in cGVHD. Clin Oral Investig. 2011;15:127–139. doi: 10.1007/s00784-010-0450-6. [DOI] [PubMed] [Google Scholar]
- 5.Parkman R. Is chronic graft versus host disease an autoimmune disease? Curr Opin Immunol. 1993;5:800–803. doi: 10.1016/0952-7915(93)90140-n. [DOI] [PubMed] [Google Scholar]
- 6.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105:4885–4891. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu YW, Gress R, Shlomchik WD. Animal Models of Chronic Graft versus Host Disease. In: Vogelsang GB, Pavletic SZ, editors. Chronic Graft versus Host Disease: Interdisciplinary Management. Cambridge University Press; New York, NY: 2009. pp. 31–45. [Google Scholar]
- 8.Ferrara JLM, Antin JH. The Pathophysiology of Graft-versus-Host Disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic cell transplantation. 4th. Blackwell Publishing Ltd; Hoboken, NJ: 2009. pp. 208–221. [Google Scholar]
- 9.Schultz KR. Pathophysiology of Chronic Graft versus Host Disease. In: Vogelsang GB, Pavletic SZ, editors. Chronic Graft versus Host Disease: Interdisciplinary Management. Cambridge University Press; New York, NY: 2009. pp. 17–30. [Google Scholar]
- 10.Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 11.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 13.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 15.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 16.Singh RR, Hahn BH. Reciprocal T-B determinant spreading develops spontaneously in murine lupus: implications for pathogenesis. Immunol Rev. 1998;164:201–208. doi: 10.1111/j.1600-065x.1998.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 17.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114:4919–4927. doi: 10.1182/blood-2008-10-161638. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, Ritz J, Antin JH, Murphy WJ, Luznik L, Shlomchik MJ, Panoskaltsis-Mortari A, Blazar BR. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2011 doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, Klickstein LB, Levin J, Miller K, Reynolds C, Macdonell R, Pasek M, Lee SJ, Ho V, Soiffer R, Antin JH, Ritz J, Alyea E. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto M, Okano A, Akamatsu S, Ashihara E, Inaba T, Takenaka H, Katoh N, Kishimoto S, Shimazaki C. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20:172–173. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]
- 21.Ratanatharathorn V, Ayash L, Reynolds C, Silver S, Reddy P, Becker M, Ferrara JL, Uberti JP. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9:505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 22.von Bonin M, Oelschlagel U, Radke J, Stewart M, Ehninger G, Bornhauser M, Platzbecker U. Treatment of chronic steroid-refractory graft-versus-host disease with low-dose rituximab. Transplantation. 2008;86:875–879. doi: 10.1097/TP.0b013e318183f662. [DOI] [PubMed] [Google Scholar]
- 23.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16:289–295. [PubMed] [Google Scholar]
- 24.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, Strober S, Zeng D. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 25.Zhao D, Young JS, Chen YH, Shen E, Yi T, Todorov I, Chu PG, Forman SJ, Zeng D. Alloimmune response results in expansion of autoreactive donor CD4+ T cells in transplants that can mediate chronic graft-versus-host disease. J Immunol. 2011;186:856–868. doi: 10.4049/jimmunol.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Currier JR, Robinson MA. Spectratype/immunoscope analysis of the expressed TCR repertoire. Curr Protoc Immunol. 2001;Chapter 10 doi: 10.1002/0471142735.im1028s38. Unit 10 28. [DOI] [PubMed] [Google Scholar]
- 29.Martires KJ, Baird K, Steinberg SM, Grkovic L, Joe GO, Williams KM, Mitchell SA, Datiles M, Hakim FT, Pavletic SZ, Cowen EW. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118:4250–4257. doi: 10.1182/blood-2011-04-350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogelsang GB. Slashing the picture of Dorian Gray. Blood. 2011;117:2990. doi: 10.1182/blood-2011-02-334581. [DOI] [PubMed] [Google Scholar]
- 31.Shlomchik MJ. Activating systemic autoimmunity: B's, T's, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman TM, Goldgirsh K, Berger SA, Zilberberg J, Filicko-O'Hara J, Flomenberg N, Donato M, Rowley SD, Korngold R. Overlap between in vitro donor antihost and in vivo posttransplantation TCR Vbeta use: a new paradigm for designer allogeneic blood and marrow transplantation. Blood. 2008;112:3517–3525. doi: 10.1182/blood-2008-03-145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodes RJ, Abe R. Mouse endogenous superantigens: Ms and Mls-like determinants encoded by mouse retroviruses. Curr Protoc Immunol. 2001;Appendix 1:Appendix 1F. doi: 10.1002/0471142735.ima01fs17. [DOI] [PubMed] [Google Scholar]
- 34.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi T, Li X, Yao S, Wang L, Chen Y, Zhao D, Johnston HF, Young JS, Liu H, Todorov I, Forman SJ, Chen L, Zeng D. Host APCs augment in vivo expansion of donor natural regulatory T cells via B7H1/B7.1 in allogeneic recipients. J Immunol. 2011;186:2739–2749. doi: 10.4049/jimmunol.1002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature Reviews Immunology. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 37.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu D, Chen L, Iwakura Y, Kandee F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft versus host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant. 2008;14:365–378. doi: 10.1016/j.bbmt.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmaltz C, Alpdogan O, Muriglan SJ, Kappel BJ, Rotolo JA, Ricchetti ET, Greenberg AS, Willis LM, Murphy GF, Crawford JM, van den Brink MR. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101:2440–2445. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 42.Malynn BA, Romeo DT, Wortis HH. Antigen-specific B cells efficiently present low doses of antigen for induction of T cell proliferation. J Immunol. 1985;135:980–988. [PubMed] [Google Scholar]
- 43.Shlomchik WD, Lee SJ, Couriel D, Pavletic SZ. Transplantation's greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:2–10. doi: 10.1016/j.bbmt.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Johansson-Lindbom B, Borrebaeck CA. Germinal center B cells constitute a predominant physiological source of IL-4: implication for Th2 development in vivo. J Immunol. 2002;168:3165–3172. doi: 10.4049/jimmunol.168.7.3165. [DOI] [PubMed] [Google Scholar]
- 45.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011;2 doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teshima T, Reddy P, Liu C, Williams D, Cooke KR, Ferrara JL. Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood. 2003;102:429–435. doi: 10.1182/blood-2003-01-0266. [DOI] [PubMed] [Google Scholar]
- 48.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179:3305–3314. doi: 10.4049/jimmunol.179.5.3305. [DOI] [PubMed] [Google Scholar]
- 50.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 51.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.