Abstract
Combinatorial chemistry approaches facilitate drug discovery processes and result in structural modifications of lead compounds that enhance pharmacological activity, improve pharmacokinetic properties, and/or reduce unwanted side effects. Epidemiological and animal model studies have suggested that nonsteroidal anti-inflammatory drugs (NSAIDs) can act as chemopreventive agents. The cyclooxygenase-2 (COX-2) inhibitor nimesulide shows anticancer effect in several cancer cell lines via COX-2 dependent and independent mechanisms. The molecular structure of nimesulide was used as a starting scaffold to design novel sulfonanilide analogs and examine the structural features that contribute to this anticancer effect. A systematic combinatorial chemical approach was used to generate diversely substituted sulfonanilide derivatives that were tested for their effects on the proliferation of human breast cancer cells. Structure–function analysis indicated that the inhibition of cell growth by compounds derived from the novel sulfonanilides required a bulky terminal phenyl ring, a methanesulfonamide, and a hydrophobic carboxamide moiety.
Keywords: breast cancer, cyclooxygenase-2, NSAIDs, nimesulide, sulfonanilide
1. Introduction
The area of drug discovery and drug development has experienced significant advances with the introduction of combinatorial chemistry approaches. This innovative technology of producing libraries of structurally related compounds is particularly beneficial in the step of lead optimization. Lead optimization involves structural modifications of a “lead” compound that has demonstrated desired biological or pharmacological activities, often in an in vitro assay system. Combinatorial chemistry approaches facilitate structural modifications of a lead scaffold to enhance pharmacological activity, improve pharmacokinetic properties, and/or reduce unwanted side effects.
Recent epidemiological and animal model studies have suggested that nonsteroidal anti-inflammatory drugs (NSAIDs) act as chemopreventive agents.1–8 The premise that COX-2 inhibition is integral to this anticarcinogenic effect is based on the assumption that COX-2 generated prostaglandins promote tumor growth in an autocrine and/or paracrine manner.9,10 It is well documented that COX-2 is constitutively overexpressed in many types of human cancers.9 Animal studies have demonstrated that efficient tumor growth requires the presence of COX-2 in the host and that enhanced COX-2 expression in the host was sufficient to induce mammary gland tumorigenesis.11 Furthermore, increased COX-2 expression appears to be involved in the development of cancer by promoting cell division, inhibiting apoptosis, altering cell adhesion and enhancing metastasis, and stimulating neovascularization.12–15 The inhibition of COX-2 activity by traditional NSAIDs blocks these activities and thus may account for the anticarcinogenic activity of these drugs. However, an expanding body of evidence suggests that COX-2-independent mechanism may also be involved in the antitumor effect of COX-2 inhibitors.4,7,8 Each NSAID type appears to have its own non-specific COX-2 independent target. For example, the COX-2 inhibitor celecoxib induces cell apoptosis in prostate cancer cells by inhibiting 3-phosphoinositide-dependent protein kinase via a COX-2 independent mechanism.16
Adenocarcinoma of the breast is the most common cancer in women in the United States and ranks second only to lung cancer as a cause of cancer-related mortality. About 178,500 women in the United States will be found to have invasive breast cancer in 2007. About 40,500 women will die from the disease this year. Currently over 2 million women living in the United States have been treated for breast cancer.17 A growing body of experimental and epidemiological evidence suggests that the use of NSAIDs may decrease the incidence of mammary cancer, tumor burden, and tumor volume.18–20 Although this effect has been studied within the past few decades, the mechanism by which these benefits occur is unclear. Nimesulide [N-(4-nitro-2-phenoxyphenyl) methane sulfonamide] is a nonsteroidal anti-inflammatory drug with a preferential cyclooxygenase-2 inhibitory activity and has been available in some Asia and European countries since 1985. In fact, the anti-inflammatory activity of nimesulide is almost the same as that of indomethacin, but its ulcerogenic potential is much weaker. Nimesulide can induce apoptosis in liver and lung cancer cells; it also suppressed the development of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary gland carcinogenesis in rats.21,22 Aromatase, the key enzyme for estrogen synthesis, is elevated in hormone-dependent breast cancer.23 Research in our laboratory demonstrated that nimesulide also suppressed aromatase activity and expression in several breast cancer cell lines.24
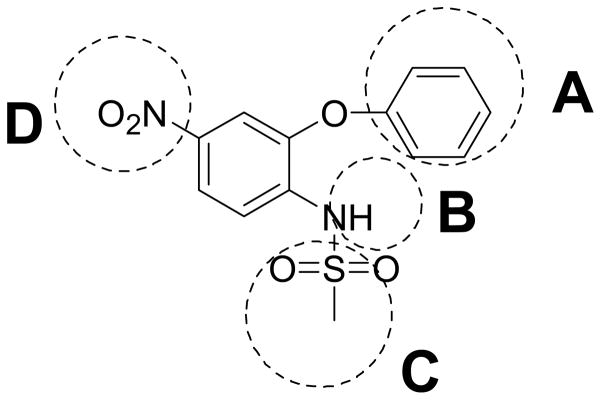
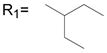
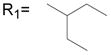
Although nimesulide has been reported as effective in suppressing several types of cancer cell growth, the concentrations used in those studies are much higher than the effective dose for COX-2 inhibition.21,22 This suggests that COX-2 independent mechanisms may be involved. The functionality at the B position is very critical for the COX-2 inhibitory activity (Figure 1).27 Only when the N-H is available in the ionized form, do the compounds inhibit COX-2. Introduction of any group in B position eliminates this ionization process and produce compounds with no COX-2 inhibitory activity.25,27 Previously we tested nimesulide derivatives in SK-BR-3 breast cancer cells at their IC50 for aromatase suppression and also COX-2 inhibition in breast cancer cells. The results demonstrate that the N-methyl substituted compounds at position B did not exhibit COX-2 inhibitory activity.25
Figure 1.
Chemical structure of nimesulide
Two targeted libraries of nimesulide analogs have been synthesized in our laboratory, and their pharmacological effect on aromatase has been extensively studied.25,26 In this publication, their biological effect on breast cancer cell growth was investigated in a panel of breast cancer cell lines. Two analogs significantly inhibited SK-BR-3 breast cancer cell growth, which is a HER2/neu overexpressed breast cancer cell line. Based on the two compounds, a combinatorial approach was used to generate diversely substituted nimesulide derivatives by parallel synthesis. Their preliminary evaluation as antiproliferation agents in breast cancer agents was also further explored.
2. Results and discussion
2.1. Compounds design
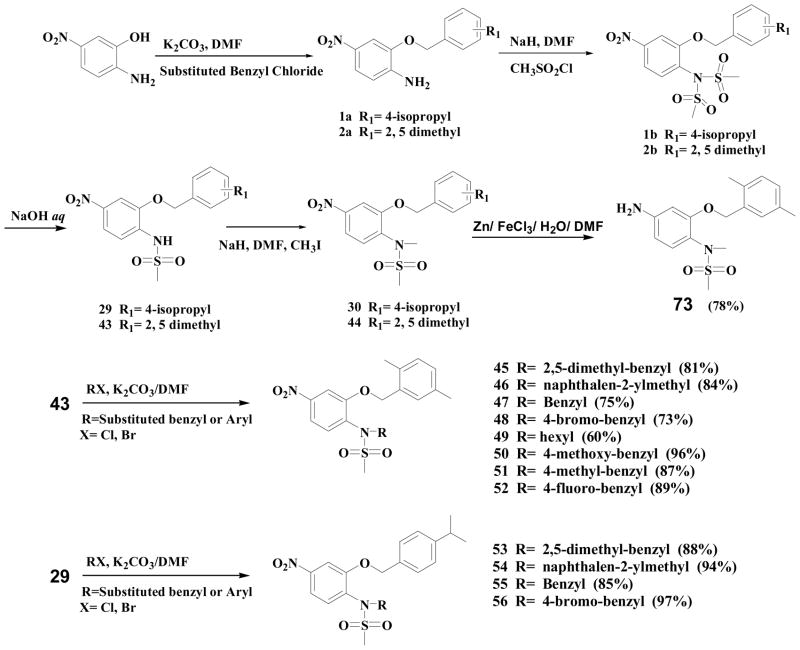
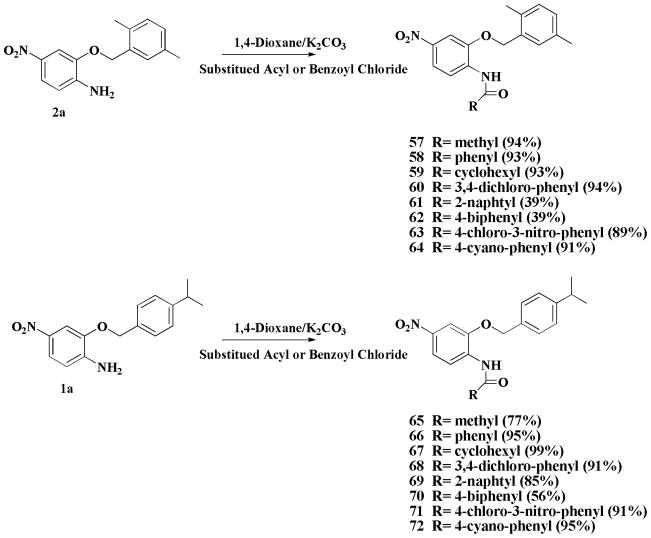
A total of 76 compounds were prepared, which were based on nimesulide structure as a platform (Figure 1). We systematically altered the structure of nimesulide using the combinatorial strategies to modify the four moieties depicted in Figure 1. The A position aromatic ring of nimesulide was modified to either alkyl or substituted aryl groups to generate compounds 1–44 published previously.25,26 Alkyl or substituted benzyl groups were introduced at the B position to produce compounds 45–56. Next, the C position was modified with various substituted benzamides to produce compounds 57–72. Last, the D position nitro group was reduced to the amine moiety to generate compound 73, and then 73 was treated with substituted acyl chloride and K2CO3 to generate the carboxamides 74–76, respectively. The parallel synthesis can generate structure of novel sulfonanilides at the A, B, C and D positions diversity. Further biological investigation of these compounds can reveal the structure requirement for the suppression of breast cancer cell growth. In addition, this combinatory strategy can easily be extended to produce hundreds of new analogs for lead optimization and drug development.
2.2. Parallel synthesis of diverse sulfonanilide derivatives
In the current study, the A moiety of these two compounds was retained and the B, C, or D position was modified to generate several small libraries. The A position modification has been extensively explored previously.25,26 2, 5-Dimethylbenzyl and 4-isopropyl benzyl substituted A position lead to significantly more active compounds.
Modifications at the B position are described in Scheme 1. The starting material 2-amino-5-nitrophenol was refluxed with K2CO3 and 2, 5-dimethylbenzyl chloride or 4-isopropyl benzyl chloride to obtain compounds 1a, 2a, respectively. Sodium hydride and methanesulfonyl chloride were added to compound 1a or 2a in dry dimethylformamide (DMF) at room temperature and the reaction mixture was stirred at room temperature overnight to obtain the N,N-bimethanesulfonamido compound (1b or 2b). Compounds 1b and 2b are hydrolyzed with 10% NaOH solution to generate compounds 29 and 43 as monomethanesulfonamido compounds. Compound 29 or 43 were treated with K2CO3 and substituted benzyl chloride/bromide, alkyl bromide or iodide in DMF at room temperature or at reflux to obtain compounds 45–56, respectively.
Scheme 1.
Modification of B position
Modifications at the C position are described in Scheme 2. Compounds 1a or 2a was treated with different substituted acyl chloride and K2CO3 to generate the carboxamides 57–72.
Scheme 2.
Modification of C position
Modifications at the D position are described in Scheme 3. First, the D position nitro was reduced to the amine group to obtain compound 73 (Figure 1), then compound 73 was treated with different acyl chlorides and K2CO3 to generate the carboxamides 74–76, respectively.
Scheme 3.
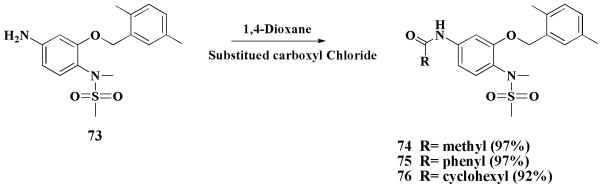
Modification of D position
This parallel combinatory strategy can produce new analogs for lead optimization and drug development. Structures of all the synthesized compounds were determined by 1H-NMR, their purity confirmed by HPLC with two mobile phases, and the structures of significant biologically active compounds also confirmed by HRMS.
2.3. Pharmacological evaluation of sulfonanilide analogs
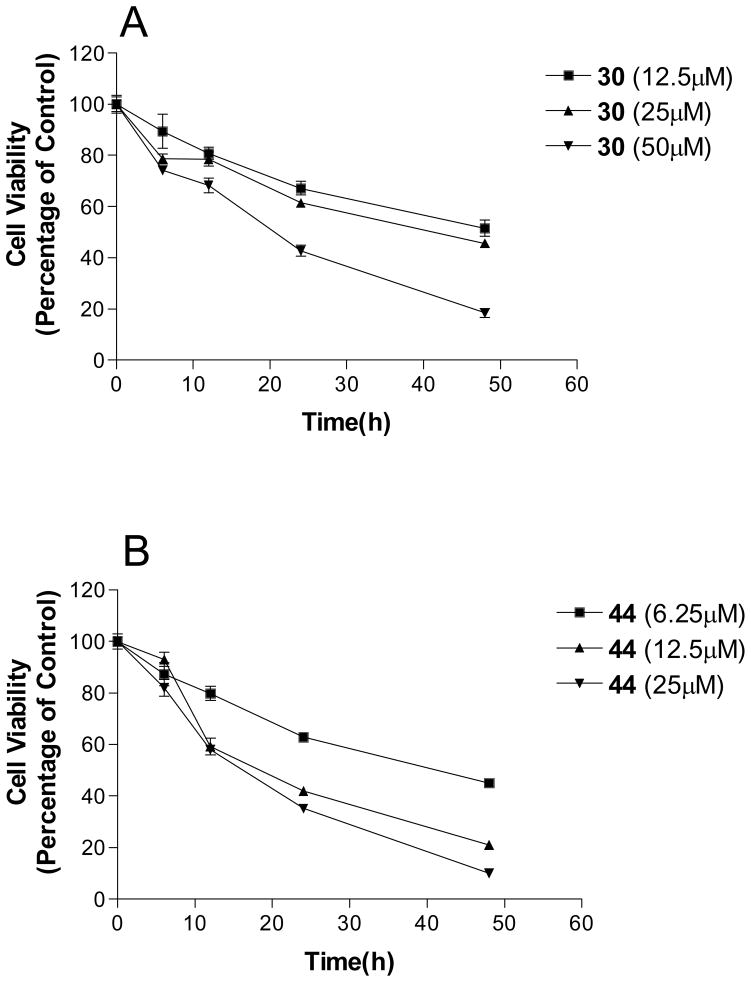
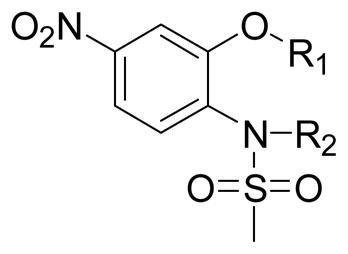
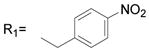
In general, various substitutions at the A position exhibit suppression of the growth of the SK-BR-3 breast cancer cells. The results suggest that the A position bulky group is beneficial for the inhibition of cell growth (Table 1, 2). Compounds 6, 11, 12, 13, 21, 23, 24, 30, 41, 42, 43 and 44 exhibit more than 50% inhibition of SK-BR-3 cell proliferation at 25μM. Particularly, compounds 44 and 30 are more effective than others; showing more than 80% inhibition, with an IC50 of 6.5μM and 20.1 μM respectively. Since these two compounds both are methyl substituted B position nimesulide derivatives, they are not potential COX-2 inhibitors. This suggests that the cell growth suppression effect of these two analogs should be independent of COX-2 inhibition. Further exploration exhibits that the suppression is time and dose dependent (Figure 2, Table 3). (MCF-7, MDA-MB-231, BT-474). Compounds 44 and 30 also significantly suppressed BT474 (estrogen receptor positive and HER2/neu overexpress) breast cancer cell growth with an IC50 of 13.5 μM and 44.7μM, respectively (Table 3). They did not significantly suppress other breast cancer cell growth. These results suggest that compounds 44 and 30 might selectively inhibit cell growth of HER2/neu overexpressing breast cancer cells. Overall, introduction of 2, 5-dimethyl benzyl and 4-isopropyl benzyl groups into the A position of nimesulide generated two compounds that significantly suppressed SK-BR-3 breast cancer cell growth.
Table 1.
A and B moiety alkyl substituted nimesulide and their inhibition of SK-BR-3 cell growth
| Compd |
|
SKBR-3 cell growth inhibition (%) at 25μM |
|---|---|---|
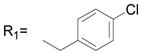
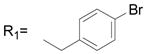
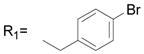
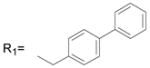
| 1 | R1= CH3 R2= H | 13.9 ± 1.6 |
| 2 | R1= CH3 R2= CH3 | 13.7 ± 3.1 |
| 3 |
|
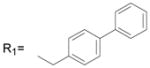
16.6 ± 3.2 |
| 4 |
|
17.1 ± 3.4 |
| 5 |
 R2= H R2= H |
39.3 ± 2.9 |
| 6 |
 R2= CH3 R2= CH3
|
57.5 ± 1.3 |
| 7 |
 R2= H R2= H |
29.1 ± 1.8 |
| 8 |
 R2= CH3 R2= CH3
|
6.1 ± 2.0 |
| 9 |
|
13.8 ± 2.3 |
| 10 |
|
10.6 ± 2.6 |
| 11 |
|
61.6 ± 1.4 |
| 12 |
|
62.8 ± 0.8 |
| 13 |
|
66.5 ± 0.3 |
| 14 |
|
35.2 ± 2.2 |
| 15 |
|
31.3 ± 2.8 |
| 16 |
|
43.0 ± 3.7 |
SK-BR-3 cells were treated with indicated compounds at 25μM for 48 h and cell viability was measured by MTT assay as described in the experimental section, n = 6.
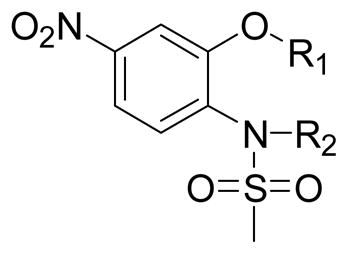
Table 2.
A and B moiety aryl substituted nimesulide and their inhibition of SK-BR-3 cell growth
| Compd |
|
SKBR-3 cell growth inhibition (%) at 25μM |
|---|---|---|
| 17 |
|
34.1 ± 2.9 |
| 18 |
|
38.5 ± 2.2 |
| 19 |
 R2= H R2= H |
53.6 ± 1.6 |
| 20 |
|
31.5 ± 4.9 |
| 21 |
|
55.4 ± 2.4 |
| 22 |
|
41.7 ± 3.0 |
| 23 |
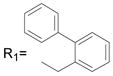 R2= H R2= H |
65.7 ± 1.5 |
| 24 |
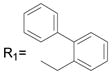 R2= CH3 R2= CH3
|
53.0 ± 2.9 |
| 25 |
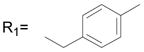 R2= H R2= H |
27.8 ± 2.6 |
| 26 |
|
37.6 ± 3.3 |
| 27 |
|
26.4 ± 1.9 |
| 28 |
|
26.8 ± 3.7 |
| 29 |
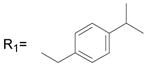 R2= H R2= H |
24.6 ± 3.2 |
| 30* |
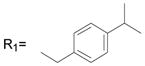 R2= CH3 R2= CH3
|
81.6 ± 1.0 |
| 31 |
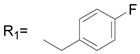 R2= H R2= H |
41.8 ± 1.4 |
| 32 |
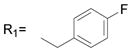 R2= CH3 R2= CH3
|
20.9 ± 5.0 |
| 33 |
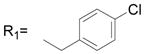 R2= H R2= H |
47.7 ± 3.1 |
| 34 |
 R2= CH3 R2= CH3
|
7.0 ± 1.0 |
| 35 |
 R2= H R2= H |
42.7 ± 1.9 |
| 36 |
 R2= CH3 R2= CH3
|
13.4 ± 2.2 |
| 37 |
 R2= H R2= H |
36.7 ± 1.2 |
| 38 |
 R2= CH3 R2= CH3
|
38.6 ± 3.4 |
| 39 |
|
28.6 ± 2.9 |
| 40 |
|
29.0 ± 4.6 |
| 41 |
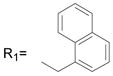 R2= H R2= H |
53.8 ± 2.1 |
| 42 |
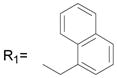 R2= CH3 R2= CH3
|
61.4 ± 1.1 |
| 43 |
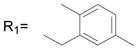 R2= H R2= H |
63.7 ± 1.3 |
| 44* |
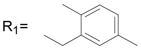 R2= CH3 R2= CH3
|
89.7 ± 0.4 |
SK-BR-3 cells were treated with indicated compounds at 25μM for 48 h and cell viability was measured by MTT assay as described in the experimental section, n = 6.
Significantly active compounds
Figure 2. Time-and dose-dependent effects of compound 30 and 44 on the SK-BR-3 cell growth.
(A) Compound 30, (B) Compound 44. Values obtained from six replicates were plotted for each time point at the indicated concentration of compound 30 or 44. Control SK-BR-3 cells were treated with a dimethyl sulfoxide (DMSO) vehicle.
Table 3.
IC50 of inhibition of breast cancer cells growth by compounds 44, 30 and nimesulide
| Breast cancer cell lines | Comp 44 | Comp 30 | Nimesulide |
|---|---|---|---|
| SK-BR-3 | 6.2 ± 1.8 μM | 20.1 ± 5.5 μM | 111.8 ± 32.3 μM |
| MDA-MB-231 | >50 μM | >50 μM | 123.4 ± 10.8 μM |
| MCF-7 | >50 μM | >50 μM | 120.4 ± 8.6 μM |
| BT-474 | 13.5 ± 2.6 μM | 44.7 ± 16.3 μM | 165.9 ± 22.5 μM |
Cells were treated with indicated compounds at various concentrations for 48h and cell viability was measured by MTT assay as described in the experimental section, n = 6.
The B position alkyl or aryl substituted analogs overall showed weaker cell growth inhibition comparing with proton or methyl group at B position (Table 4). It appears that a smaller group at B position, such as proton or methyl, is better for biological activity. Introduction of carboxamides group to the C position decreases the cell growth suppression activity (Table 5). However, compound 63, which has 4-chloro-3-nitro benzamide group at the C position, showed significant cell growth inhibition with more than 80% of cell growth at 25 μM. Reduction of the D position nitro group to amine decreases the biological activity, with only 63% inhibition compared with compound 44 with 90% inhibition at 25 μM (Table 6). Introduction of acetyl to the D position amine leads to much less active compound 74, with 14.6% cell growth inhibition at 25 μM. However, introduction of benzoyl or cyclohexanacarbonyl group to D amine moiety generates more active compounds 75 and 76, with 73.2% and 75.4% cell growth inhibition activity at 25 μM, respectively. This suggests that a hydrophobic moiety at the D position is better for cell growth inhibition.
Table 4.
Inhibition of SK-BR-3 cells growth by B moiety substituted nimesulide derivatives
| Compd |
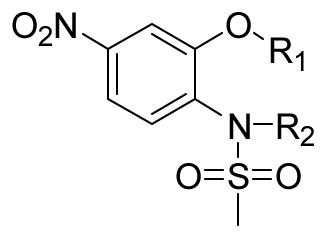
|
SKBR-3 cell growth inhibition (%) at 25μM |
|---|---|---|
| 45 |
|
46.3 ± 4.4 |
| 46 |
|
42.6 ± 2.7 |
| 47 |
|
41.7 ± 2.4 |
| 48 |
|
52.3 ± 3.1 |
| 49 |
|
60.5 ± 2.3 |
| 50 |
|
26.1 ± 0.7 |
| 51 |
|
55.1 ± 2.0 |
| 52 |
|
49.4 ± 0.8 |
| 53 |
|
54.9 ± 1.2 |
| 54 |
|
40.1 ± 1.3 |
| 55 |
|
25.9 ± 0.7 |
| 56 |
|
59.7 ± 0.6 |
SK-BR-3 cells were treated with indicated compounds at 25μM for 48 h and cell viability was measured by MTT assay as described in the experimental section, n = 6.
Table 5.
Inhibition of SK-BR-3 cells growth by C moiety substituted nimesulide derivatives
| Compd |
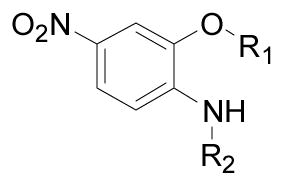
|
SKBR-3 cell growth inhibition (%) at 25μM |
|---|---|---|
| 57 |
|
44.0 ± 1.2 |
| 58 |
|
36.1 ± 1.1 |
| 59 |
|
49.5 ± 1.8 |
| 60 |
|
53.4 ± 1.8 |
| 61 |
|
25.4 ± 1.6 |
| 62 |
|
59.7 ± 0.7 |
| 63* |
|
88.3 ± 1.1 |
| 64 |
|
58.9 ± 1.7 |
| 65 |
|
38.9 ± 2.4 |
| 66 |
|
53.4 ± 1.0 |
| 67 |
|
49.7 ± 1.2 |
| 68 |
|
42.1 ± 2.0 |
| 69 |
|
35.5 ± 1.1 |
| 70 |
|
44.1 ± 1.0 |
| 71 |
|
36.7 ± 0.8 |
| 72 |
|
65.5 ± 1.2 |
SK-BR-3 cells were treated with indicated compounds at 25μM for 48 h and cell viability was measured by MTT assay as described in the experimental section, n = 6.
Significantly active compounds
Table 6.
Inhibition of SK-BR-3 cells growth by D moiety substituted nimesulide derivatives
| Compd |
|
SKBR-3 cell growth inhibition (%) at 25μM |
|---|---|---|
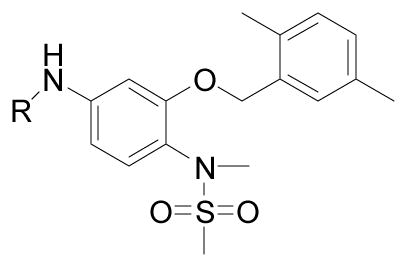
| 73 | R= H | 62.8 ± 1.0 |
| 74 |
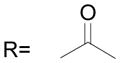
|
14.6 ± 1.9 |
| 75* |
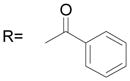
|
73.2 ± 1.2 |
| 76* |
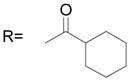
|
75.4 ± 0.4 |
SK-BR-3 cells were treated with indicated compounds at 25μM for 48 h and cell viability was measured by MTT assay as described in the experimental section, n = 6.
Significantly active compounds
3. Conclusion
An efficient method has been developed for the parallel synthesis of diversified novel sulfonanilides via a combinatorial chemistry approach. This parallel is highly efficient and suitable for the synthesis of large libraries of analogs. Through biological activity evaluation of the compound library, we have identified novel compounds 30, 44, 63, 75 and 76 which exhibited potent inhibition against SK-BR-3 breast cancer cell growth at sub-micromolar level. Structure–function analysis indicated that the inhibition of breast cancer cell growth by the synthetic compounds require a hydrophobic group substituted bulky phenyl ring, a methanesulfonamide and a hydrophobic carboxamide moiety (Figure 3). Further research on the mechanisms of these compounds in suppression of SK-BR-3 breast cancer cell growth is ongoing in our laboratory.
Figure 3.
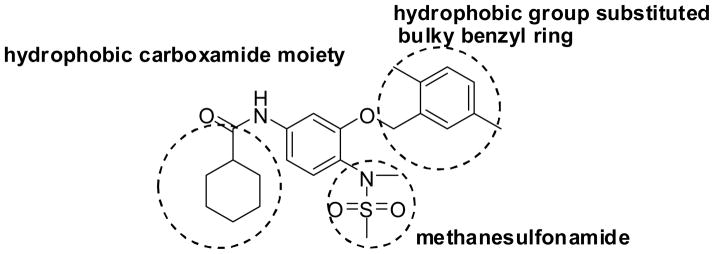
Chemical structure of compound 76
4. Experimental section
4.1. Chemistry
Chemicals were commercially available and used as received without further purification unless otherwise noted. Moisture sensitive reactions were carried out under a dry argon atmosphere in flame-dried glassware. Solvents were distilled before use under argon. Thin-layer chromatography was performed on precoated silica gel F254 plates (Whatman). Silica gel column chromatography was performed using silica gel 60A (Merck, 230–400 Mesh). High-resolution electrospray ionization mass spectra were obtained on the Micromass QTOF Electrospray mass spectrometer at The Ohio State Chemical Instrumentation Center. All the NMR spectra were recorded on a Bruker DPX 250 and DRX 400 MHz in either DMSO-d6 or CDCl3. Chemical shifts (δ) for 1H NMR spectra are reported in parts per million calibrated to residual solvent protons.
For the HPLC analysis, a 1.00 mg/mL stock solution of each standard was prepared in either methanol or acetonitrile. HPLC analysis was performed on a HP1100 system (Hewlett-Packard, Palo Alto, CA), which consists of a binary pump, autosampler, column compartment, and a UV/VIS detector. Reversed phase HPLC was carried out on a C18 column (4.6 × 250 mm, 5μm) from Beckman (Beckman Instruments, Fullenton, CA) at room temperature with a flow rate of 1.0 mL/min. Two mobile phases (mobile phase A: 35%water, 65% acetonitrile; mobile phase B: 25%water, 75% methanol) were employed to run 35 min. An injection volume of 5–15μL was used. The UV detector was set up at 254 and 330nm.
Compounds 1a, 2a, 1b, 2b, 29, 30, 43, 44 were prepared as described by Su et al.26
General procedure for the preparation of B position substituted nimesulide analogs compounds 45–56 from compounds 29 and 43
K2CO3 (5 mmol, 5eq) and aryl or alkyl halide (1 mmol, 1.2 eq) were successively added to a solution of compound 29 or 43 (1.0 mmol, 1.0 eq) in dry DMF and the mixture was stirred at room temperature or 80 °C from 3 h to overnight. After being cooled, 5 mL H2O and 1 mL saturated aqueous Na2CO3 was added to the mixture and it was stirred at room temperature overnight. The precipitated solid was collected by filtration and washed with H2O and cold hexane to afford desired compounds.
(45) N-(2,5-Dimethyl benzyl)-N-[2-(2,5-dimethyl benzyloxy)-4-nitro phenyl]-methanesulfonamide
Compound 43 and 2, 5-dimethyl benzyl chloride were stirred in DMF at 80 °C overnight. Pale yellow solid, yield 81%: 1H-NMR (400 MHz, DMSO-d6) δ 8.00 (1H, d, J = 2.4 Hz), 7.77 (1H, dd, J = 8.7, 2.4 Hz), 7.49 (1H, d, J = 8.6 Hz), 7.34 (1H, s), 7.19 (1H, d, J = 7.7 Hz), 7.13 (1H, d, J = 7.6 Hz), 6.96 (3H, m), 5.27 (2H, s), 4.75 (2H, s), 2.97 (3H, s), 2.37 (3H, s), 2.30 (3H, s), 2.13 (6H, s).
(46) N-[2-(2,5-Dimethyl benzyloxy)-4-nitro phenyl]-N-naphthalen-2-ylmethyl-methanesulfonamide
Compound 43 and 2-(bromomethyl) naphthalene were stirred at room temperature overnight. Yellow solid, yield 84%: 1H-NMR (400 MHz, DMSO-d6) δ 7.99 (1H, d, J = 2.5 Hz), 7.87 (2H, m), 7.77 (2H, m), 7.66 (1H, s), 7.49 (4H, m), 7.35 (1H, s), 7.21 (1H, d, J = 7.7 Hz), 7.15 (1H, d, J = 7.9 Hz), 5.29 (2H, s), 4.95 (2H, s), 3.06 (3H, s), 2.38 (3H, s), 2.31 (3H, s).
(47) N-Benzyl-N-[2-(2, 5-dimethyl benzyloxy)-4-nitro phenyl]-methanesulfonamide
Compound 43 and benzyl bromide were stirred at room temperature overnight. Yellow solid, yield 75%: 1H-NMR (400 MHz, DMSO-d6) δ 8.00 (1H, d, J = 2.4 Hz), 7.76 (1H, dd, J = 2.4, 8.6 Hz), 7.43 (1H, d, J = 8.6 Hz), 7.32 (1H, s), 7.25 (7H, m), 5.28 (2H, s), 4.77 (2H, s), 3.01 (3H, s), 2.36 (3H, s), 2.31 (3H, s).
(48) N-(4-Bromo benzyl)-N-[2-(2,5-dimethyl benzyloxy)-4-nitro phenyl]-methanesulfonamide
Compound 43 and 4- bromobenzyl bromide were stirred at room temperature overnight. Pale yellow solid, yield 73%: 1H-NMR (400 MHz, DMSO-d6) δ 8.00 (1H, d, J = 2.4 Hz), 7.79 (1H, dd, J = 2.4, 8.6 Hz), 7.45 (3H, m), 7.30 (1H, s), 7.18 (4H, m), 5.27 (2H, s), 4.74 (2H, s), 3.02 (3H, s), 2.35 (3H, s), 2.30 (3H, s).
(49) N-[2-(2,5-Dimethyl benzyloxy)-4-nitro phenyl]-N-hexyl-methanesulfonamide
Compound 43 and 1-iodohexane were stirred at 80 °C overnight. Yellow solid, yield 60%: 1H-NMR (400 MHz, DMSO-d6) δ 8.06 (1H, d, J = 2.5 Hz), 7.90 (1H, dd, J = 2.5, 8.6 Hz), 7.60 (1H, d, J = 8.6 Hz), 7.27 (1H, s), 7.16 (1H, d, J = 7.7 Hz), 7.10 (1H, d, J = 801 Hz), 5.27 (2H, s), 3.54 (2H, dd, J = 6.9, 6.9 Hz), 2.90 (3H, s), 2.32 (3H, s), 2.27 (3H, s), 1.32 (9H, m), 0.82 (3H, dd, J = 6.7, 6.7 Hz).
(50) N-[2-(2,5-Dimethyl benzyloxy)-4-nitro phenyl]-N-(4-methoxy benzyl)-methanesulfonamide
Compound 43 and 4-methoxy benzyl chloride were stirred at 80 °C overnight. Yellow solid, yield 96%: 1H-NMR (400 MHz, DMSO-d6) δ 7.99 (1H, d, J = 2.4 Hz), 7.76 (1H, dd, J = 2.4, 8.6 Hz), 7.38 (1H, d, J = 8.8 Hz), 7.31 (1H, s), 7.18 (4H, m), 7.80 (2H, d, J = 8.6 Hz), 5.27 (2H, s), 4.69 (2H, s), 3.69 (3H, s), 2.99 (3H, s), 2.35 (3H, s), 2.30 (3H, s).
(51) N-[2-(2,5-Dimethyl benzyloxy)-4-nitro phenyl]-N-(4-methyl benzyl)-methanesulfonamide
Compound 43 and 4-methyl benzyl chloride were stirred at 80 °C overnight. Pale yellow solid, yield 87%: 1H-NMR (400 MHz, DMSO-d6) δ 7.99 (1H, d, J = 2.5 Hz), 7.76 (1H, dd, J = 2.5, 8.6 Hz), 7.40 (1H, d, J = 8.6 Hz), 7.31 (1H, s), 7.19 (6H, m), 5.27 (2H, s), 4.72 (2H, s), 2.99 (3H, s), 2.35 (3H, s), 2.30 (3H, s), 2.22 (3H, s).
(52) N-[2-(2, 5-Dimethyl benzyloxy)-4-nitro phenyl]-N-(4-fluoro benzyl)-methanesulfonamide
Compound 43 and 4-fluoro benzyl chloride were stirred at 80 °C overnight. Pale yellow solid, yield 89%: 1H-NMR (400 MHz, DMSO-d6) δ 8.00 (1H, d, J = 2.4 Hz), 7.78 (1H, dd, J = 2.5, 8.6 Hz), 7.42 (1H, d, J = 8.6 Hz), 7.30 (1H, s), 7.26(2H, dd, J = 5.6, 8.3 Hz), 7.18(1H, d, J = 7.7 Hz), 7.13(1H, d, J = 7.6 Hz), 7.08 (2H, dd, J = 8.8, 8.8 Hz), 5.27 (2H, s), 4.75 (2H, s), 3.01 (3H, s), 2.35 (3H, s), 2.30 (3H, s).
(53) N-(2,5-Dimethyl benzyl)-N-[2-(4-isopropyl benzyloxy)-4-nitro phenyl]-methanesulfonamide
Compound 29 and 2,5-dimethyl benzyl chloride were stirred at 80 °C overnight. Pale yellow solid, yield 88%: 1H-NMR (400 MHz, DMSO-d6) δ 7.95 (1H, d, J = 1.7 Hz), 7.73 (1H, dd, J = 1.8, 8.6 Hz), 7.50 (2H, d, J = 8.0 Hz), 7.44 (1H, d, J = 8.7 Hz), 7.34 (2H, d, J = 7.9 Hz), 6.94 (3H, m), 5.32 (2H, s), 4.78 (2H, s), 3.04 (3H, s), 2.94 (1H, m), 2.15 (3H, s), 2.11 (3H, s), 1.23 (3H, s), 1.21 (3H, s).
(54) N-[2-(4-Isopropyl benzyloxy)-4-nitro phenyl]-N-naphthalen-2-ylmethyl-methanesulfonamide
Compound 29 and 2-(bromomethyl) naphthalene were stirred at 80 °C overnight. Pale yellow solid, yield 94%: 1H-NMR (400 MHz, DMSO-d6) δ 7.95 (1H, d, J = 1.5 Hz), 7.87 (2H, m), 7.77 (1H, m), 7.71 (2H, m), 7.48 (6H, m), 7.32 (2H, d, J = 7.7 Hz), 5.34 (2H, s), 4.97 (2H, s), 3.12 (3H, s), 2.95 (1H, m), 1.24 (3H, s), 1.22 (3H, s).
(55) N-Benzyl-N-[2-(4-isopropyl benzyloxy)-4-nitro phenyl]-methanesulfonamide
Compound 29 and benzyl bromide were stirred at room temperature overnight. White solid, yield 85%: 1H-NMR (400 MHz, DMSO-d6) δ 7.97 (1H, d, J = 2.5 Hz), 7.74 (1H, dd, J = 2.5, 8.6 Hz), 7.49 (2H, d, J = 8.1 Hz), 7.39 (1H, d, J = 8.6 Hz), 7.34 (2H, d, J = 8.0 Hz), 7.27 (5H, m), 5.32 (2H, s), 4.79 (2H, s), 3.06 (3H, s), 2.95 (1H, m), 1.24 (3H, s), 1.22 (3H, s).
(56) N-(4-Bromo benzyl)-N-[2-(4-isopropyl benzyloxy)-4-nitro phenyl]-methanesulfonamide
Compound 29 and 4-bromobenzyl bromide were stirred at room temperature overnight. White solid, yield 97%: 1H-NMR (400 MHz, DMSO-d6) δ 7.97 (1H, d, J = 2.5 Hz), 7.77 (1H, dd, J = 2.5, 8.6 Hz), 7.46 (5H, m), 7.34 (2H, d, J = 8.1 Hz), 7.20 (2H, d, J = 8.4 Hz), 5.31 (2H, s), 4.77 (2H, s), 3.07 (3H, s), 2.94 (1H, m), 1.24 (3H, s), 1.22 (3H, s).
General procedure for the preparation of C position substituted nimesulide analogs compounds 57–72 from compounds 1a and 2a
K2CO3 (5 mmol, 5eq) and substituted acyl chloride (1.2 mmol, 1.2 eq) were successively added to a solution of compound 1a or 2a (1.0 mmol, 1.0 eq) in dry 1,4 dioxane and the mixture was stirred at room temperature or 80 °C from 3 h to overnight. After being cooled, 10 mL H2O and 3 mL saturated aqueous Na2CO3 was added to the mixture and it was stirred at room temperature overnight. The precipitated solid was collected by filtration and washed with H2O and cold ethyl ether/hexane to afford desired compounds.
(57) N-[2-(2, 5-Dimethyl benzyloxy)-4-nitro phenyl]-acetamide
Compound 2a and acetyl chloride were stirred at room temperature overnight. White solid, yield 94%: 1H-NMR (400 MHz, DMSO-d6) δ 9.54(1H, s), 8.31 (1H, dd, J = 1.7, 8.8 Hz), 7.92 (2H, m), 7.30 (1H, s), 7.15 (1H, d, J = 7.3 Hz), 7.09 (1H, d, J = 7.6 Hz), 5.30 (2H, s), 2.32 (3H, s), 2.27 (3H, s), 2.16 (3H, s).
(58) N-[2-(2,5-Dimethyl benzyloxy)-4-nitro phenyl]-benzamide
Compound 2a and benzoyl chloride were stirred at room temperature overnight. White solid, yield 93%: 1H-NMR (400 MHz, DMSO-d6) δ 9.79(1H, s), 8.23 (1H, d, J = 8.8 Hz), 8.07 (1H, d, J = 2.4 Hz), 7.99 (1H, dd, J = 2.4, 8.8 Hz), 7.95 (2H, d, J = 7.3 Hz), 7.64(1H, m), 7.56 (2H, m), 7.38 (1H, s), 7.12 (1H, d, J = 7.6 Hz), 7.06 (1H, d, J = 7.6 Hz), 5.33 (2H, s), 2.30 (3H, s), 2.22 (3H, s).
(59) Cyclohexanecarboxylic acid [2-(2,5-dimethyl-benzyloxy)-4-nitro phenyl]-amide
Compound 2a and cyclohexanecarbonyl chloride were stirred at room temperature overnight. Pale yellow solid, yield 93%: 1H-NMR (400 MHz, DMSO-d6) δ 9.36(1H, s), 8.25 (1H, d, J = 9.0 Hz), 7.95 (1H, d, J = 2.3 Hz), 7.91 (1H, dd, J = 2.4, 8.9 Hz), 7.34 (1H, s), 7.14 (1H, d, J = 7.7 Hz), 7.08 (1H, d, J = 7.5 Hz), 5.31 (2H, s), 2.60 (1H, m), 2.31 (3H, s), 2.28 (3H, s), 1.83(5H, m), 1.41 (5H, m).
(60) 3,4-Dichloro-N-[2-(2,5-dimethyl benzyloxy)-4-nitro phenyl]-benzamide
Compound 2a and 3, 4 dichlorobenzoyl chloride were stirred at room temperature overnight. White solid, yield 94%: 1H-NMR (400 MHz, DMSO-d6) δ 10.12(1H, s), 8.15 (1H, d, J = 1.9 Hz), 8.07 (3H, m), 7.97 (1H, dd, J = 8.8, 2.4 Hz), 7.92 (1H, dd, J = 2.0, 8.4 Hz), 7.84 (1H, d, J = 8.4 Hz), 7.32 (1H, s), 7.11 (1H, d, J = 7.7 Hz), 7.04 (1H, d, J = 7.7 Hz), 5.30 (2H, s), 2.29 (3H, s), 2.20 (3H, s).
(61) Naphthalene-2-carboxylic acid [2-(2,5-dimethyl benzyloxy)-4-nitro phenyl] amide
Compound 2a and 2-naphthoyl chloride were stirred at 80 °C for three days. Pale yellow solid, yield 39%: 1H-NMR (400 MHz, DMSO-d6) δ 9.98(1H, s), 8.55 (1H, s), 8.27 (1H, d, J = 8.8 Hz), 8.11 (6H, m), 7.68 (2H, m), 7.40 (1H, s), 7.13 (1H, d, J = 7.6 Hz), 7.06 (1H, d, J = 7.7 Hz), 5.36 (2H, s), 2.33 (3H, s), 2.18 (3H, s).
(62) Biphenyl-4-carboxylic acid [2-(2,5-dimethyl benzyloxy)-4-nitro phenyl] amide
Compound 2a and biphenyl-4-carbonyl chloride were stirred at 80 °C for three days. Pale yellow solid, yield 39%: 1H-NMR (400 MHz, DMSO-d6) δ 9.86(1H, s), 8.24 (1H, d, J = 8.8 Hz), 8.07 (3H, m), 8.00 (1H, dd, J = 2.4, 8.8 Hz), 7.85 (2H, d, J = 8.3 Hz), 7.77 (2H, d, J = 7.9 Hz), 7.54 (4H, m), 7.13 (1H, d, J = 7.7 Hz), 7.06 (1H, d, J = 7.8 Hz), 5.34 (2H, s), 2.32 (3H, s), 2.23 (3H, s).
(63) 4-Chloro-N-[2-(2,5-dimethyl benzyloxy)-4-nitro phenyl]-3-nitro benzamide
Compound 2a and 4-chloro-3-nitrobenzoyl chloride were stirred at room temperature overnight. White solid, yield 89%: 1H-NMR (400 MHz, DMSO-d6) δ 10.33(1H, s), 8.58 (1H, d, J = 2.0 Hz), 8.23 (1H, dd, J = 2.1, 8.4 Hz), 8.07 (4H, m), 7.30 (1H, s), 7.08 (1H, d, J = 7.7 Hz), 7.04 (1H, d, J = 7.6 Hz), 5.31 (2H, s), 2.29 (3H, s), 2.18 (3H, s); HRMS calculated for C22H18ClN3NaO6 (M + Na)+ 478.0776, found 478.0774.
(64) 4-Cyano-N-[2-(2,5-dimethyl benzyloxy)-4-nitro phenyl] benzamide
Compound 2a and 4-cyano benzoyl chloride were stirred at room temperature overnight. White solid, yield 91%: 1H-NMR (400 MHz, DMSO-d6) δ 10.17(1H, s), 8.11 (7H, m), 7.32 (1H, s), 7.11 (1H, d, J = 7.6 Hz), 7.05 (1H, d, J = 7.7 Hz), 5.31 (2H, s), 2.29 (3H, s), 2.20 (3H, s).
(65) N-[2-(4-Isopropyl benzyloxy)-4-nitro phenyl] acetamide
Compound 1a and acetyl chloride were stirred at room temperature overnight. Pale yellow solid, yield 77%: 1H-NMR (400 MHz, DMSO-d6) δ 9.57(1H, s), 8.34 (1H, d, J = 8.5 Hz), 7.88 (2H, m), 7.47 (2H, d, J = 8.0 Hz), 7.30 (2H, d, J = 8.1 Hz), 5.35 (2H, s), 2.91 (1H, m), 1.20 (3H, d, J = 1.3 Hz), 1.19 (3H, d, J = 1.3 Hz).
(66) N-[2-(4-Isopropyl benzyloxy)-4-nitro phenyl] benzamide
Compound 1a and benzoyl chloride were stirred at room temperature overnight. White solid, yield 95%: 1H-NMR (400 MHz, DMSO-d6) δ 9.75(1H, s), 8.28 (1H, d, J = 8.7 Hz), 7.98 (4H, m), 7.64 (5H, m), 7.29 (2H, d, J = 7.9 Hz), 5.35 (2H, s), 2.91 (1H, m), 1.20 (3H, s), 1.19 (3H, s).
(67) Cyclohexanecarboxylic acid [2-(4-isopropyl benzyloxy)-4-nitro phenyl] amide
Compound 1a and cyclohexanecarbonyl chloride were stirred at room temperature overnight. White solid, yield 99%: 1H-NMR (400 MHz, DMSO-d6) δ 9.38(1H, s), 8.31 (1H, d, J = 9.1 Hz), 7.88 (2H, m), 7.46 (2H, d, J = 8.0 Hz), 7.29 (2H, d, J = 9.4 Hz), 5.34 (2H, s), 2.91 (1H, m), 2.63 (1H, m), 1.83(5H, m), 1.41 (5H, m), 1.20 (3H, s), 1.19 (3H, s).
(68) 3, 4-Dichloro-N-[2-(4-isopropyl benzyloxy)-4-nitro phenyl] benzamide
Compound 1a and 3, 4-dichlorobenzoyl chloride were stirred at room temperature overnight. White solid, yield 91%: 1H-NMR (400 MHz, DMSO-d6) δ 10.05(1H, s), 8.15 (2H, m), 7.99 (4H, m), 7.49 (2H, d, J = 7.4 Hz), 7.28 (2H, d, J = 7.2 Hz), 5.33 (2H, s), 2.89 (1H, m), 1.20 (3H, s), 1.18 (3H, s).
(69) Naphthalene-2-carboxylic acid [2-(4-isopropyl benzyloxy)-4-nitro phenyl] amide
Compound 1a and 2-naphthoyl chloride were stirred at 80 °C for three days. White solid, yield 85%: 1H-NMR (400 MHz, DMSO-d6) δ 9.92(1H, s), 8.52 (1H, s), 8.33 (1H, d, J = 8.7 Hz), 8.09 (6H, m), 7.68 (2H, m), 7.55 (2H, d, J = 7.8 Hz), 7.31 (2H, d, J = 7.8 Hz), 5.37 (2H, s), 2.92 (1H, m), 1.21 (3H, d, J = 0.5 Hz), 1.18 (3H, d, J = 0.5 Hz).
(70) Biphenyl-4-carboxylic acid [2-(4-isopropyl benzyloxy)-4-nitro phenyl] amide
Compound 1a and biphenyl-4-carbonyl chloride were stirred at 80 °C for three days. Pale yellow solid, yield 56%: 1H-NMR (400 MHz, DMSO-d6) δ 9.81(1H, s), 8.30 (1H, d, J = 8.7 Hz), 8.05 (4H, m), 7.87 (2H, d, J = 8.2 Hz), 7.77 (2H, d, J = 7.5 Hz), 7.55 (5H, m), 7.30 (2H, d, J = 7.9 Hz), 5.37 (2H, s), 2.89 (1H, m), 1.20 (3H, d, J = 1.0 Hz), 1.18 (3H, d, J = 1.0 Hz).
(71) 4-Chloro-N-[2-(4-isopropyl benzyloxy)-4-nitro phenyl]-3-nitro benzamide
Compound 1a and 4-chloro-3-nitrobenzoyl chloride were stirred at room temperature overnight. Pale yellow solid, yield 91%: 1H-NMR (400 MHz, DMSO-d6) δ 10.26(1H, s), 8.58 (1H, d, J = 2.0 Hz), 8.23 (1H, dd, J = 2.0, 8.4 Hz), 8.15 (1H, d, J = 8.8 Hz), 8.00 (3H, m), 7.48 (2H, d, J = 8.0 Hz), 7.27 (2H, d, J = 8.0 Hz), 5.34 (2H, s), 2.90 (1H, m), 1.20 (3H, s), 1.18 (3H, s).
(72) 4-Cyano-N-[2-(4-isopropyl benzyloxy)-4-nitro phenyl] benzamide
Compound 1a and 4-cyanobenzoyl chloride were stirred at room temperature overnight. Pale yellow solid, yield 95%: 1H-NMR (400 MHz, DMSO-d6) δ 10.11(1H, s), 8.18 (1H, d, J = 8.8 Hz), 8.10 (4H, m), 7.99 (2H, m), 7.48 (2H, d, J = 7.8 Hz), 7.28 (2H, d, J = 7.8 Hz), 5.35 (2H, s), 2.88 (1H, m), 1.20 (3H, d, J = 0.7 Hz), 1.18 (3H, d, J = 0.7 Hz).
(73) N-[4-Amino-2-(2,5-dimethyl benzyloxy)-phenyl]-N-methyl-methanesulfonamide
A mixture of ferric chloride (4mmol, 4eq), and compound 44 (1mmol, 1eq) was added to a solvent mixture of dimethylformamide and water (6:1, 7mL). It was stirred for 30min and then zinc dust (10mmol, 10eq) was added slowly. After completion of the reaction (10min, monitored by TLC), the reaction mixture was filtered by passing celite pad. The filtrate was diluted with water and basified by adding saturated aqueous Na2CO3. The precipitated solid was collected by filtration and dried, and then it was dissolved in acetone. After filtration of the insoluble residues, the desired compound was recovered by distillation of the acetone under reduce pressure. White solid, yield 78%: 1H-NMR (400 MHz, DMSO-d6) δ 7.25(1H, s), 7.13 (2H, d, J = 7.6 Hz), 7.07 (2H, d, J = 7.1 Hz), 6.36 (1H, s), 6.13 (1H, d, J = 7.2 Hz), 5.32 (2H, s), 4.99 (2H, s), 3.04 (3H, s), 2.76 (3H, s), 2.28 (3H, s), 2.27 (3H, s).
General procedure for the preparation of the D position substituted nimesulide analogs compounds 74–76 from compound 73
K2CO3 (5 mmol, 5eq) and substituted acyl chloride (1.2 mmol, 1.2 eq) were successively added to a solution of compound 73 (1.0 mmol, 1.0 eq) in dry 1, 4 dioxane and the mixture was stirred at room temperature overnight. After being cooled, 10 mL H2O and 3 mL saturated aqueous Na2CO3 was added to the mixture and it was stirred at room temperature over night. The precipitated solid was collected by filtration and washed with H2O and cold ethyl ether/hexane to afford desired compounds.
(74) N-[3-(2,5-Dimethyl benzyloxy)-4-(methanesulfonyl-methyl-amino)-phenyl] acetamide
Compound 73 and acetyl chloride were stirred at room temperature overnight. White solid, yield 97%: 1H-NMR (400 MHz, DMSO-d6) δ 10.15 (1H, s), 7.54 (1H, d, J = 1.8 Hz), 7.28(1H, s), 7.22(4H, m), 5.06 (2H, s), 3.09 (3H, s), 2.85 (3H, s), 2.30 (3H, s), 2.27 (3H, s), 2.05 (3H, s).
(75) N-[3-(2,5-Dimethyl benzyloxy)-4-(methanesulfonyl-methyl-amino)-phenyl] benzamide
Compound 73 and benzoyl chloride were stirred at room temperature overnight. White solid, yield 97%: 1H-NMR (400 MHz, DMSO-d6) δ 10.38 (1H, s), 7.98 (2H, m), 7.78 (1H, d, J = 2.2 Hz), 7.62(3H, m), 7.43(1H, dd, J = 8.5, 2.2 Hz),7.32(2H, m), 7.13(2H, m), 5.10 (2H, s), 3.11 (3H, s), 2.87 (3H, s), 2.33 (3H, s), 2.28 (3H, s); HRMS calculated for C24H26N2NaO4S (M + Na)+ 461.1511, found 461.1511.
(76) Cyclohexanecarboxylic acid [3-(2,5-dimethyl benzyloxy)-4-(methanesulfonyl-methyl-amino)-phenyl] amide
Compound 73 and cyclohexanecarbonyl chloride were stirred at room temperature overnight. White solid, yield 92%: 1H-NMR (400 MHz, DMSO-d6) δ 9.97 (1H, s), 7.64 (1H, d, J = 2.1 Hz), 7.29(1H, s), 7.21(4H, m), 5.05 (2H, s), 3.08 (3H, s), 2.83 (3H, s), 2.33 (1H, m), 2.32 (3H, s), 2.27 (3H, s), 1.81(5H, m), 1.39 (5H, m); HRMS calculated for C24H32N2NaO4S (M + Na)+ 467.1980, found 467.1977.
4.2. Biological study
4.2.1. Cell culture
SK-BR-3, MDA-MB-231, MCF-7 and BT474 cells were obtained from ATCC (Rockville, MD). All cells were maintained in DMEM/F12 medium supplemented with 5% fetal bovine serum (FBS) and 20 mg/L gentamycin. Fetal bovine serum was heat inactivated for 30 min in a 56 °C water bath before use. Cell cultures were grown at 37 °C, in a humidified atmosphere of 5% CO2 in a Hereaus CO2 incubator.
4.2.2. Cell Viability Analysis
The effect of nimesulides derivatives on breast cancer cell viability was assessed by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay in six replicates. Cells were grown in custom medium in 96-well, flat-bottomed plates for 24 h, and were exposed to various concentrations of nimesulide derivatives dissolved in DMSO (final concentration ≤ 0.1%) in media for different time intervals. Controls received DMSO vehicle at a concentration equal to that in drug-treated cells. The medium was removed, replaced by 200 μl of 0.5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide in fresh media, and cells were incubated in the CO2 incubator at 37°C for 2 h. Supernatants were removed from the wells, and the reduced 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide dye was solubilized in 200 μl/well DMSO. Absorbance at 570 nm was determined on a plate reader.
Supplementary Material
Table 7.
Inhibition of SK-BR-3 cells growth by compound 63, 75 and 76
| Compd | IC50 of inhibiting SK-BR-3 cell growth (μM) |
|---|---|
| 63 | 18.90 ± 4.18 |
| 75 | 3.65 ± 0.19 |
| 76 | 1.38 ± 0.10 |
SK-BR-3 cells were treated with indicated compounds at various concentrations for 48 h and cell viability was measured by MTT assay as described in the experimental section, n = 6.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Grant R01 CA73698 (R.W.B.), and The Ohio State University Comprehensive Cancer Center Breast Cancer Research Fund.
ABBREVIATION LIST
- PGE2
Prostaglandin E2
- COX-2
cyclooxygenase-2
- NSAIDs
nonsteroidal anti-inflammatory drugs
Footnotes
6. Supporting Information Available
HPLC Analysis for compounds 45–76. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Psaty BM, Potter JD. N Engl J Med. 2006;355:950–952. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich CM, Bigler J, Potter JD. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Burford C, Barnes MN, Oelschlager DK, Myers RB, Talley LI, Partridge EE, Grizzle WE. Clin Cancer Res. 2002;8:202–209. [PubMed] [Google Scholar]
- 4.Minter HA, Eveson JW, Huntley S, Elder DJ, Hague A. Clin Cancer Res. 2003;9:1885–1897. [PubMed] [Google Scholar]
- 5.Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. Clin Cancer Res. 2001;7:1410–1418. [PubMed] [Google Scholar]
- 6.Michael MS, Badr MZ, Badawi AF. Int J Mol Med. 2003;11:733–736. [PubMed] [Google Scholar]
- 7.Li M, Wu X, Xu XC. Clin Cancer Res. 2001;7:1010–1016. [PubMed] [Google Scholar]
- 8.Sanchez-Alcazar JA, Bradbury DA, Pang L, Knox AJ. Lung Cancer. 2003;40:33–44. doi: 10.1016/s0169-5002(02)00530-5. [DOI] [PubMed] [Google Scholar]
- 9.Prescott SM, Fitzpatrick FA. Biochim Biophys Acta. 2000;1470:M69–M78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 10.Taketo MM. J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 11.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 12.Fosslien E. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 13.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 14.Tang X, Sun YJ, Half E, Kuo MT, Sinicrope F. Cancer Res. 2002;62:4903–4908. [PubMed] [Google Scholar]
- 15.Li G, Yang T, Yan J. Biochem Biophys Res Commun. 2002;299:886–890. doi: 10.1016/s0006-291x(02)02707-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, Shaw YJ, Kulp SK, Chen CS. Cancer Res. 2004;64:4309–4318. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society. Cancer Facts and Figures. Atlanta: American Cancer Society; 2007. [Google Scholar]
- 18.Harris RE, Namboodiri K, Stellman SD, Wynder EL. Prev Med. 1995;24:119–120. doi: 10.1006/pmed.1995.1022. [DOI] [PubMed] [Google Scholar]
- 19.Harris RE, Namboodiri KK, Farrar WB. Epidemiology. 1996;7:203–205. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Mazhar D, Ang R, Waxman J. Br J Cancer. 2006;94:346–350. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakatsugi S, Ohta T, Kawamori T, Mutoh M, Tanigawa T, Watanabe K, Sugie S, Sugimura T, Wakabayashi K. Jpn J Cancer Res. 2000;91:886–892. doi: 10.1111/j.1349-7006.2000.tb01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaik MS, Chatterjee A, Singh M. Clin Cancer Res. 2004;10:1521–1529. doi: 10.1158/1078-0432.ccr-0902-03. [DOI] [PubMed] [Google Scholar]
- 23.Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Endocr Rev. 2005;26:331–345. doi: 10.1210/er.2004-0015. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. J Clin Endocrinol Metab. 2005;90:2563–2570. doi: 10.1210/jc.2004-2029. [DOI] [PubMed] [Google Scholar]
- 25.Su B, Diaz-Cruz ES, Landini S, Brueggemeier RW. J Med Chem. 2006;49:1413–1419. doi: 10.1021/jm051126f. [DOI] [PubMed] [Google Scholar]
- 26.Su B, Landini S, Davis DD, Brueggemeier RW. J Med Chem. 2007;50:1635–1644. doi: 10.1021/jm061133j. [DOI] [PubMed] [Google Scholar]
- 27.Julemont F, de LX, Michaux C, Damas J, Charlier C, Durant F, Pirotte B, Dogne JM. J Med Chem. 2002;45:5182–5185. doi: 10.1021/jm020920n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.