Abstract
Muscular dystrophy represents a major unmet medical need as only palliative treatments exist for these debilitating diseases. Since multiple forms of muscular dystrophy arise from compromised sarcolemmal membrane integrity a therapeutic approach that can target this loss of membrane barrier function could be applicable to a number of these distinct genetic diseases. One pathway that presents an excellent opportunity to affect compromised membrane integrity is the process that the cell uses to repair injuries to the plasma membrane. Membrane repair is a conserved pathway where disruptions in the plasma membrane of many different cell types are resealed by trafficking of intracellular vesicles to the injury site where these vesicles can fuse and patch the membrane disruption. Recent discoveries of multiple genes associated with this process in skeletal muscle provide opportunities to target this mechanism to increase membrane repair as a therapy to treat muscular dystrophy. One such gene is a member of the tripartite motif (TRIM) family of proteins, mitsugumin 53 (MG53) or TRIM72, that is an essential component of the membrane repair pathway in striated muscles. Recent results indicate that MG53/TRIM72 protein can be directly applied as a therapeutic agent to increase membrane repair capacity of many cell types and treat some aspects of the pathology in mouse models of muscular dystrophy. There is great potential for the use of recombinant human (rhMG53) in the treatment of muscular dystrophy and other diseases where compromised membrane integrity contributes to the progression of the disease. Other TRIM family proteins may provide additional targets for therapeutic intervention in similar disease states.
Membrane repair in physiology and pathophysiology
One fundamental requirement for the maintenance of cellular homeostasis is the presence of an intact plasma membrane bordering the intracellular cytosolic space. Rupture of the plasma membrane allows for free exchange between the extracellular milieu and cytosolic contents so that if the membrane permeability barrier is not rapidly reestablished the cell will die. While a simple lipid bilayer would reseal disruptions by the thermodynamic alignment of the hydrophilic lipid heads, the native plasma membrane is held at tension due to attachment of the membrane to the cytoskeleton and the extracellular matrix. Thus, the plasma membrane has limitations on the extent that it can repair by thermodynamic resealing and an active process must be available to patch membrane disruptions. The membrane repair process is a conserved physiologically relevant cellular response to the disruption of the plasma membrane. In many eukaryotic cell types, disruptions of the plasma membrane trigger an active process where intracellular vesicles or organelles rapidly traffic to the injury site on the plasma membrane. These vesicles provide a source of additional lipids that can fuse with one another, or directly with the plasma membrane, to create a membrane repair patch. This response is Ca2+ dependent and has been compared to the vesicle mediated neurotransmitter release process1.
The membrane repair process was first identified in vivo in the gut2 and skeletal muscles but has since been confirmed to occur in a variety of different tissue types3. While the cellular aspects of membrane repair have been known for some time it is only recently that several genes were determined to mediate membrane repair. One of the first genes to be directly linked to membrane repair was dysferlin4 and, while several other genes have now been linked to membrane repair, there are undoubtedly more to be discovered. Recent findings established that mitsugumin 53 (MG53) is an essential component of the membrane repair mechanism in striated muscles. MG53, also known as TRIM72, is a muscle enriched member of the tripartite motif (TRIM) family of E3 ubiquitin ligase proteins. It was initially isolated from a skeletal muscle immuno-proteomic library in the laboratory of Dr. Hiroshi Takeshima. The structure of the protein consists of a canonical TRIM domain at the N-terminus and a dual-specificity kinase splA (SPRY) domain at the C-terminus, a common configuration of several of the TRIM family of proteins. Initial studies in the laboratory of Dr. Jianjie Ma resolved that MG53/TRIM72 was an important mediator of the cell membrane repair process that nucleates assembly of the membrane repair patch at the membrane injury site5. The loss of MG53/TRIM72 function resulted in muscle fibers that would not properly reseal following membrane damage and a myopathy phenotype could be observed in mg53−/− mice, establishing that MG53/TRIM72 was essential for normal muscle membrane repair. Other recent studies showed that genetic overexpression of MG53/TRIM72 through viral gene delivery could have therapeutic benefit for the treatment of muscular dystrophy and related cardiomyopathy6. Despite this success in this approach the challenges associated with viral gene delivery suggest finding alternative approaches for the treatment of human diseases showing compromised membrane resealing are an attractive target.
In the course of biochemical studies with the recombinant human MG53 (rhMG53) protein a surprising finding revealed that the isolated protein could increase the plasma membrane repair capacity of cells when applied to the extracellular space7. Fluorescently tagged rhMG53 protein provided outside of the cell can specifically localize to membrane disruptions and this interaction allows for more efficient resealing of disruptions in both isolated muscle cells and in non-muscle cell types. The extent of membrane damage that produced by physical, chemical or electrical permeabilization in multiple assays, including quantification of intracellular enzyme leak into the extracellular space or by the entry of indicator dye into the cell. In all tested cases rhMG53 decreased the amount of membrane damage in cultured cells. Functional rhMG53 can be produced in a variety of different host species, including bacteria (Escherichia coli), insect cells via baculovirus methods, and Chinese hamster ovary (CHO) cells. This isolated protein can be used to confirm the protective effects of rhMG53 with in vivo damage assays in which cardiotoxin-mediated myocyte death could be prevented through intramuscular or intravenous injection of rhMG53.
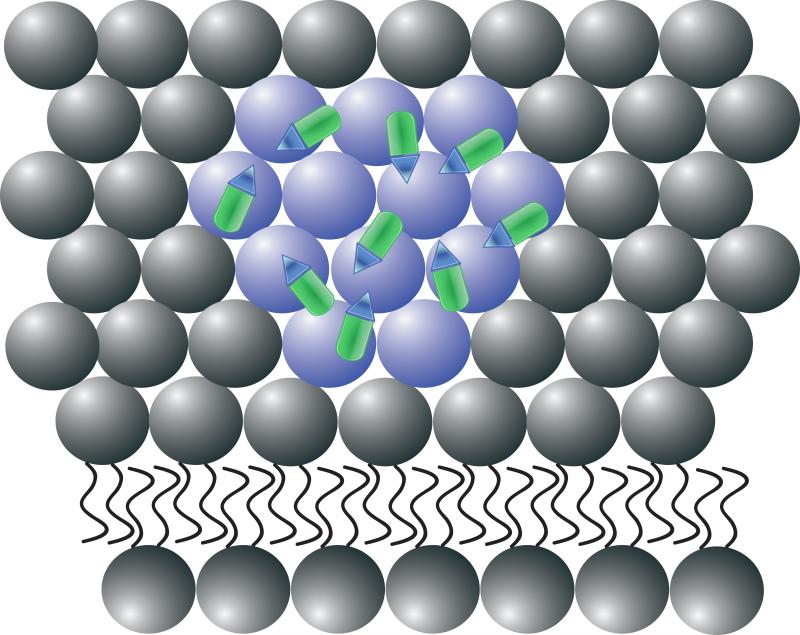
These results indicate that rhMG53 can reduce the extent of membrane injury both in vitro and in vivo however, the mechanisms that allows rhMG53 to function when applied outside of the cell must still be studied. Based on the available data, it appears that expression of neither MG53/TRIM72 nor dysferlin is required for the rhMG53 to increase the membrane repair capacity of a cell. This suggests that externally applied rhMG53 acts by a unique mechanism when compared to endogenously expressed MG53/TRIM72. Based on previous findings that MG53/TRIM72 specifically binds phosphatidylserine (PS)7, it is possible that this activity contributes to rhMG53 action outside of the cell (Fig. 1). In the plasma membrane of a healthy cell, PS is compartmentalized to the inner leaflet. Injury of the cell or the induction of apoptosis results in exposure of PS to the external space. Binding to exposed PS when the membrane is disturbed is one way that externally applied rhMG53 could identify injury sites on the plasma membrane. While this is a likely mechanism for the targeting of rhMG53 to injured cells, the molecular effects that occur at the injury site to increase membrane resealing are not fully established. While future studies will be necessary to fully resolve this mechanism, current studies could directly test if rhMG53 has the capacity to treat disease states where compromised membrane integrity contributes to the progression of the disease, such as muscular dystrophy.
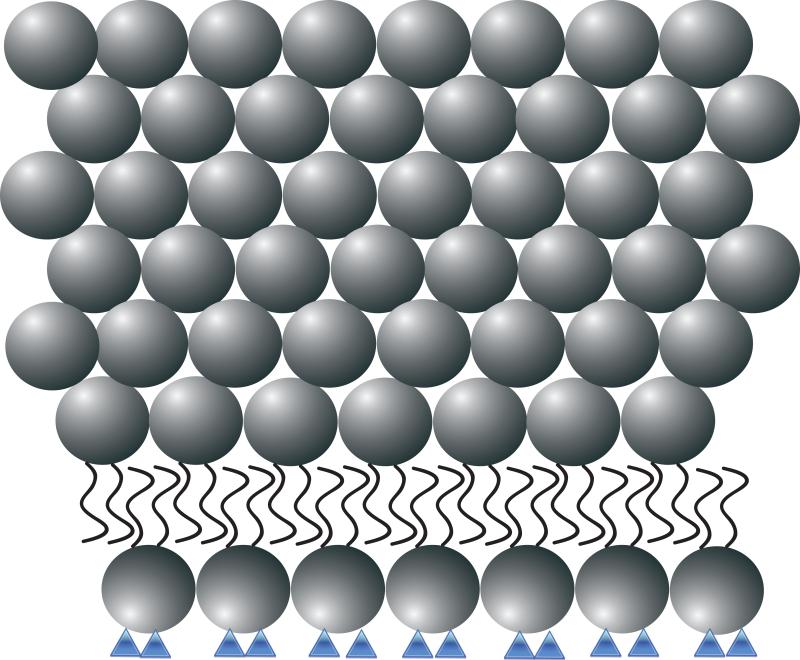
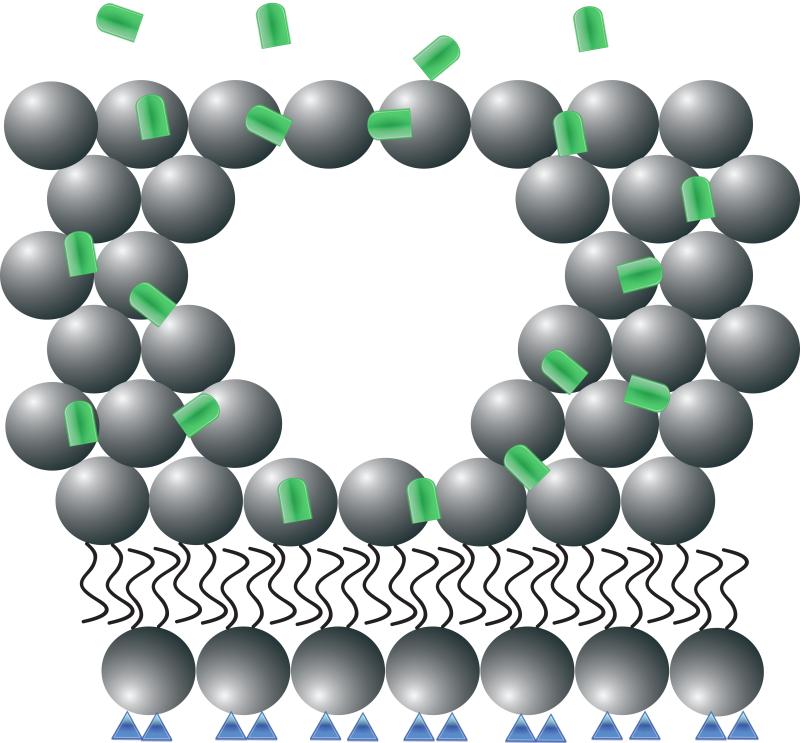
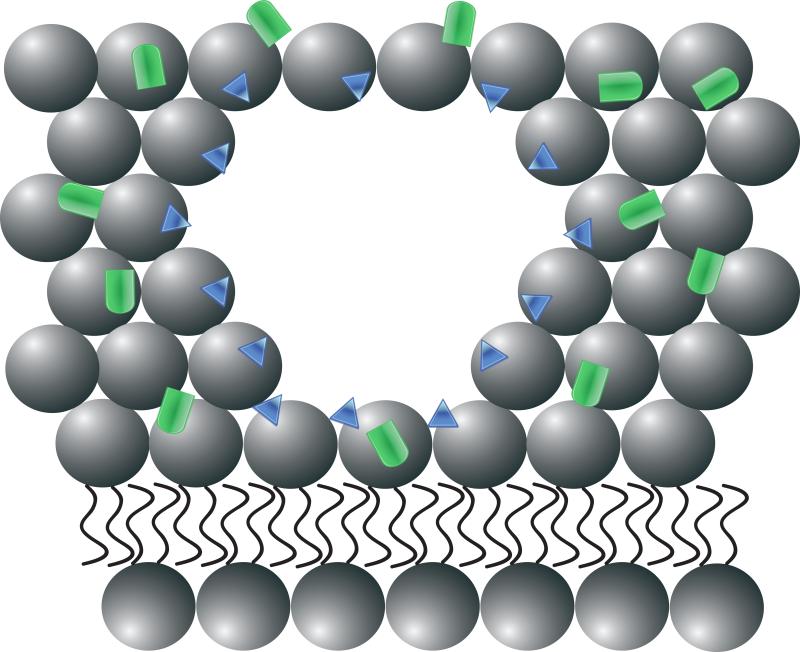
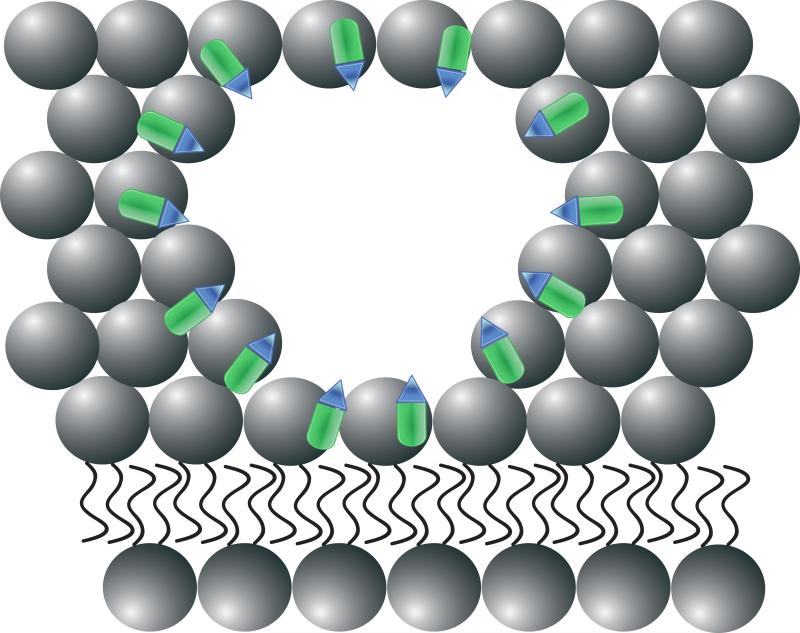
Figure 1. Recombinant human MG53 (rhMG53) associates with phosphatidylserine (PS) during therapetutic membrane repair.
(A) In a healthy cell PS (blue) is generally sequestered on the inner leaflet of the plasma membrane. (B) rhMG53 (green) does not appear to associate with the intact plasma membrane, however disruption of the membrane allows for specific interaction with the injury site. (C) Disruption of the plasma membrane results in the flow of PS from the inner leaflet onto the surface of the cell. (D) The appearance of PS on the surface of the membrane provides a target for rhMG53 binding to the injury site. (E) Association of rhMG53 with the injury site allows for more efficient membrane resealing to patch the disruption in the plasma membrane and allow for survival of the cell.
Potential for rhMG53 in the treatment of muscular dystrophy
Disruption of the plasma membrane as the result of physiological stress is a common occurrence in many of the tissues of the body. Pathophysiological levels of stress can overcome the native membrane repair capacity of the tissues in the body and result in pathology of those tissues. Compromised membrane repair capacity has been linked to a number of different disease states when the reduced membrane repair capacity cannot repair the accumulated damage. The most clearly relevant human disease is muscular dystrophy, where mutations in a number of different genes involved in membrane repair have been linked to the development of limb girdle muscular dystrophy (LGMD). The muscular dystrophies are a diverse group of degenerative muscle diseases with variable symptoms that result from mutations in various genes and are classified based on their clinical phenotype. The more common X-linked dystrophinopathies, such as Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD), produce extensive muscle degeneration early in life that lead to immobility, cardiac complications and, in the case of DMD, death of the patient usually after the second decade of life. LGMD patients generally share the presentation of muscle weakness primarily in the limb musculature with the proximal muscles displaying greater weakness than more distal muscles. The age of onset, extent of muscle wasting and involvement of cardiac pathology vary based on the particular genetic disruption, with mutations in more than 15 genes leading to various types and severity of LGMD. Congenital muscular dystrophies (CMD) are degenerative muscle diseases with genetically heterogeneous origins and variable symptoms where patients generally share the presentation of symptoms near birth or during infancy by displaying muscle weakness and hypotonia with advancing skeletal muscle pathology that can lead to immobilization and respiratory failure. Cardiac and nervous system involvement are sometimes associated with CMD subtypes. Current treatments for muscular dystrophies center on palliative measures that use occupational approaches to allow patients to remain mobile as long as possible. However, none of these treatments have any efficacy on the underlying pathology. While possible future therapies for muscular dystrophies, including gene therapy replacement of affected genes and the use of stem cells, hold promise these treatments are still years away from application in human patients. Genetic replacement approaches are further complicated by the number of different genes mutated in the various muscular dystrophies. As a result, there is a great unmet medical need for intermediate therapies to reduce the pathology associated with muscular dystrophies, particularly if those therapies can treat a number of these different disease states.
One common element in the muscular dystrophies is that many different types display altered membrane integrity. In the case of DMD, the absence of dystrophin increases the fragility of the sarcolemmal membrane of striated muscle cells. Other muscular dystrophies are directly linked to compromised membrane repair capacity, particularly some of the LGMDs that result from certain mutations in dysferlin or caveolin-3. A potential therapeutic agent would be more viable if it could treat multiple forms of LGMD, potentially by targeting a conserved pathologic mechanism shared in several of these distinct diseases. As rhMG53 can boost membrane repair capacity it may be able to address multiple types of muscular dystrophy with a single therapeutic approach. As a first step of testing the efficacy of rhMG53 in the treatment of disease the protein was injected into a DMD mouse model, the mdx dystrophic mice that lack expression of dystrophin7. In these experiments, rhMG53 had protective effects against increased membrane permeability following eccentric running exercise in the mdx mice. Further studies examined the effects of short term injection of rhMG53 during the initial wave of myonecritis that occurs in these mice shortly after they first become ambulatory. This short term treatment with rhMG53 had significant effects in reducing the area of the muscle displaying myonecritis and/or fibrosis in these animals and also had direct effects on the permeability of the dystrophic muscle fibers as the infiltration of Evans blue dye into the muscle fibers was reduced with the injection of rhMG53. These short term trials provide initial evidence that rhMG53 may be an effective treatment for muscular dystrophy arising from fragility of the sarcolemmal membrane. Additional studies will be necessary to establish if chronic application of rhMG53 can provide prolonged relief from the symptoms of the disease. Another useful future trial will be to expand these studies into models of other muscular dystrophies that involve other genetic lesions but share a common pathologic mechanism involving death of myocytes due to breakdown of the sarcolemmal membrane.
There are certain advantages to the use of rhMG53 as a protein therapeutic to treat muscular dystrophy. As native MG53 appears in the blood stream of normal mice and other animals the immune system is exposed to the protein in the course of normal physiological function. This suggests that additional rhMG53 provided as a therapeutic should present minimal risk for developing immunological or toxicological effects. An additional advantage to the use of rhMG53 is that it can be produced effectively in a number of different hosts, including bacteria and mammalian cells, which can simplify production of the protein for preclinical studies and clinical trials for muscular dystrophy. It also appears that rhMG53 can be effectively applied to animal models using subcutaneous injection7 so the protein could potentially be self-administered by patients without the need for additional healthcare visits. Of course, there are still significant questions left to be answered during development of rhMG53 as a treatment for muscular dystrophy. One challenge that remains is to establish if the pharmacokinetics of the unmodified rhMG53 are sufficient to allow for effective application in a muscular dystrophy patient. One would expect that rhMG53 would have to be provided when dystrophic muscle fibers are initially injured and thus treatment would be expected to be required over the course of the life of that patient. Should the pharmacokinetics prove to be insufficient there are well established protein engineering approaches to improve delivery of protein drugs for a variety of human diseases.
Future directions for membrane repair as a therapeutic approach
Given the key contribution of compromised sarcolemmal membrane integrity and repair to the pathophysiology of various muscular dystrophies, these diseases represent a good first target for the treatment of humans diseases by the modulation of membrane repair capacity. However, there are many other disease states in various tissue types where compromised membrane repair capacity contributes to the progression of that disease, such as neurodegeneration and acute lung injury. There are even more diseases where the ultimate endpoint of the pathology leads to the breakdown of the plasma membrane, death of individual cells and progression into eventual organ failure, including heart failure and muscular dystrophy. Application of rhMG53 as a “molecular bandage” to injured cells may increase the membrane integrity of these cells and allow them to recover from damage that would normally result in the death of the cell8. While not all cells would be able to recover, the current results with the mdx mouse model and cell damage assays indicate a sufficient percentage of cells can be spared to improve the structure and function of a target tissue. Boosting membrane repair to prevent cell death could target disease states where traumatic injury leads to a sudden wave of cell death, or chronic diseases where there is continual cell death that contributes to prolonged decreases in the function of the tissue.
Since rhMG53 proved to be effective at increasing membrane repair in a number of different non-muscle cell types7 one future application of rhMG53 may be in treating both acute and chronic diseases outside of the striated muscles. One tissue type with limited regenerative capacity that could benefit from increased membrane repair capacity as a therapeutic approach is the brain. If rhMG53 can effectively diminish neuronal cell death following injury there are multiple potential applications in the neuronal system. Traumatic brain injuries involve mechanical damage to the cell membrane that could be diminished by modulating the membrane repair capacity of these cells. Breakdown of the cell membrane and death of the cell contributes to chronic neurodegenerative diseases, such as Alzheimer's disease, and previous efforts to increase membrane repair show protective effects at the level of the individual neuron. Potential application of rhMG53 in such diseases would require delivery of the protein to the brain, as well as establishing the time window where application of rhMG53 would be necessary to produce effective treatment of the disease.
As rhMG53 appears to be effective in non-muscle cell types, it is also interesting to note that while the expression of native MG53/TRIM72 is primarily restricted to striated muscle cells it is possible to express the cDNA in numerous non-muscle cells types and fully recapitulate the expected membrane repair function. Disruption of the plasma membrane through mechanical or chemical mean results in translocation of exogenous GFP-tagged MG53/TRIM72 to the membrane injury sites in all nearly all cell types that have been tested7. This response is dependent on the expression of nonmuscle myosin type IIA (NM-IIA)9, however it appears to be independent of the expression of known interaction partners of MG53, including dysferlin and caveolin-310. One potential explanation for the capacity of MG53/TRIM72 to recapitulate activity in non-native cell types is that MG53/TRIM72 interacts with components of basal repair machinery present in many different cell types. In this scenario, MG53/TRIM72 would have evolved as a protective mechanism to compensate for the increased damage to the sarcolemmal membrane that occurs in striated muscle due to the contractile nature of the tissue. Another possibility would be that other TRIM family proteins may serve a similar function as MG53/TRIM72 in other cell types. The TRIM family is a large gene family with over 70 members in the human genome, however, very few of these proteins have been extensively studied. Many of the TRIM family proteins show a similar domain structure as MG53/TRIM72 with a C-terminal SPRY domain. It is possible that in other tissue types there are additional TRIM family proteins that may act in a similar fashion as MG53/TRIM72 in the membrane repair process. Determining if this is the case would open a number of different therapeutic possibilities for modulating membrane repair across a wide variety of tissues and disease states and potentially establish new biomarkers for the detection of various pathologies.
Acknowledgements
The authors would like to thank Mr. Eric X. Beck for helpful comments on the manuscript. Grant support is provided to N.W. by the NIH (AR063084) and the Muscular Dystrophy Association. N.W. is a co-Founder and Chief Scientific Officer of TRIM-edicine, Inc., a biotechnology company developing rhMG53 as a therapeutic protein.
References
- 1.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995 Dec;131(6 Pt 2):1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989 May;96(5 Pt 1):1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 4.Bansal D, Miyake K, Vogel SS, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003 May 8;423(6936):168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 5.Cai C, Masumiya H, Weisleder N, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009 Jan;11(1):56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He B, Tang RH, Weisleder N, et al. Enhancing muscle membrane repair by gene delivery of MG53 ameliorates muscular dystrophy and heart failure in δ-Sarcoglycan-deficient hamsters. Mol Ther. 2012 Apr;20(4):727–735. doi: 10.1038/mt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisleder N, Takizawa N, Lin P, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012 Jun 20;4(139):139ra85. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkin DJ, Wuebbles RD. A molecular bandage for diseased muscle. Sci Transl Med. 2012 Jun 20;4(139):139fs19. doi: 10.1126/scitranslmed.3004082. [DOI] [PubMed] [Google Scholar]
- 9.Lin P, Zhu H, Cai C, et al. Nonmuscle myosin IIA facilitates vesicle trafficking for MG53-mediated cell membrane repair. FASEB J. 2012 May;26(5):1875–1883. doi: 10.1096/fj.11-188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai C, Weisleder N, Ko JK, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009 Jan 30;284(5):3314–3322. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]