Abstract
What can evolutionary biology tell us about male-female differences in preferences concerning family matters? Might mothers be more solicitous toward offspring than fathers, for example? The economics literature has documented gender differences—children benefit more from money put in the hands of mothers rather than fathers, for example—and these differences are thought to be partly due to preferences. Yet for good reason family economics is mostly concerned with how prices and incomes affect behavior against a backdrop of exogenous preferences. Evolutionary biology complements this approach by treating preferences as the outcome of natural selection. We mine the well-developed biological literature to make a prima facie case for evolutionary roots of parental preferences. We consider the most rudimentary of traits—sex differences in gamete size and internal fertilization—and explain how they have been thought to generate male-female differences in altruism toward children and other preferences related to family behavior. The evolutionary approach to the family illuminates connections between issues typically thought distinct in family economics, such as parental care and marriage markets.
Keywords: Altruism, parental care, evolution, reproductive success, paternity, sex ratios
“Sexual gratification, cleaning, feeding, and other services can be purchased, but not own children: both the man and woman are required to produce their own children and perhaps to raise them.”
[Becker (1973), p.818]
“Much as economists postulate that individuals maximize utility, biologists postulate that individuals maximize fitness.”
[Bergstrom (1996), p.1906]
1 Introduction
Mothers tend to spend more time than fathers caring for children. Perhaps this is merely the result of culture and different opportunities for men and women. However, recent neurobiological research suggests that the birth of a child triggers different hormonal patterns in men and in women. While it is too early to say how much these hormonal patterns can explain differences in behavior, it begs the question of whether there is a deeper, evolutionary basis for gender differences in parental preferences. In particular, are there evolutionary reasons to expect mothers to be more altruistic toward children than fathers?
The biological underpinnings of parental preferences can matter for policies to improve the well-being of women and children. Consider a recent example raised by Doepke & Tertilt (2011), concerning policies to empower women. If the maternal-paternal disparity in childcare comes from stronger altruism among mothers, expanding earning opportunities for women can strengthen bargaining power, enabling women to better support their children. But if the disparity emanates from constraints—for example, employment discrimination that confines women to the home—empowerment could hurt children by inducing mothers to spend more time outside of the home.
More generally, mother-father differences in altruistic preferences matter for just about any policy having to do with the well-being of children, including those pertaining to marriage and divorce. For instance, optimal child custody policies (e.g., Rasul 2006) depend, not surprisingly, on parental differences in altruism.
Despite the importance of understanding parental preferences, there is little work in the economics of the family on how or why maternal and paternal altruism might differ.1 Economic models tend to be agnostic about the details of preferences so as to minimize the assumptions required for analyzing the effects of income and prices on household choice. Notwithstanding the virtue of parsimony, this approach can sometimes limit the predictive power of economic models, as the above example illustrates.
Furthermore, recent neuroendocrine evidence (discussed in Section 6) indicates that biological attributes can generate male-female differences in preferences for providing care. For instance, past investments in pregnancy and childbirth can precipitate a cascade of hormonal changes that trigger further maternal care. Indeed, there is mounting neurobiological evidence of such state dependence. For instance, hormonal changes associated with childbirth and lactation have been found to be strongly implicated in mother-child bonding in a way that can conceivably affect the path of maternal care. Distinct hormonal changes in fathers have been documented as well.
Accordingly, rather than take preferences as given, we harness the insights of evolutionary biology to better understand their underpinnings. We ask how biological primitives such as sex differences in gamete size might generate corresponding differences in evolved parental preferences such as altruism towards children.
We make a prima facie case for male-female differences in parental altruism based upon biological fundamentals. The case is not as straightforward as it might seem—there remains much unfinished business in evolutionary biology regarding sex differences in parenting. The broad-brush findings, however, are intuitive. To a first approximation, possession of the larger gamete (i.e., the egg)—plus internal fertilization, pregnancy and lactation—promotes maternal altruism, while having the smaller gamete (i.e., the sperm)—plus paternity uncertainty from internal fertilization—encourages wanderlust, and detracts from paternal altruism.
But this first-pass portrayal needs to be qualified and refined. Investment in the more expensive gamete does not automatically make women more vested in subsequent care. Assuming it does would be to fall prey to the fallacy of sunk cost. In addition, just because fathers produce an abundance of gametes does not necessarily imply that they should be distracted from childcare by the lure of additional mates. Such reasoning ignores the general equilibrium implications of competition for mates, whereby widespread competition drives down expected success from mating effort.
Further, though the daunting odds for finding additional mates might convince the average male to concentrate on caring rather than straying, skewed resources could encourage high status males to do the opposite.
Another potential impediment to paternal care is internal fertilization, which raises the prospect of paternity uncertainty, which in turn could discourage male investment in children.
Finally, an open question in the evolutionary literature concerns the connection between sex ratios and paternal care. A presumption in much of the literature is that male-biased sex ratios promote male-on-male competition for mates, but an alternative perspective is that remote odds of securing extra mates might encourage paternal care as a means of furthering reproductive success.
2 Key Points
Before getting into detail, we list the key points of the arguments connecting evolutionary considerations with maternal versus paternal care.
Fitness The key maximand in evolutionary models is reproductive success, or fitness, rather than utility. Why? Because fitness is the means by which evolution keeps score. Among the variety of traits implicated in the struggle to survive and reproduce, the successful ones are those best represented in nature. Fitness, therefore, is the evolutionary coin of the realm.
Tradeoffs between the intensive and extensive margins The focus on reproductive success highlights the tradeoff between the provision of care versus seeking additional mates—at any given time, these are the two distinct routes to maximizing fitness.
Anisogamy The marked difference between the size and number of eggs versus sperm (so-called anisogamy, or difference between female and male gametes) has traditionally been the first place that biologists have looked for sex differences in parental care (Trivers 1972).
Anisogamy, internal fertilization, and reproductive prospects The simple intuition for parental care is that a father, having the cheap and plentiful gamete, which never cease to be produced, can enhance his reproductive success by pursuing a “go forth and multiply” strategy, seeking additional mates rather than caring. Conversely, the mother, possessor of the scarce and expensive gamete, which ceases to be produced during gestation, does best with a strategy of “go forth and add,” attending to care rather than seeking additional mates.
General Equilibrium Though perhaps superficially appealing, the facile arguments above are at best incomplete and likely misleading. Imagine, for instance, the competition that would ensue if all males seek to expand their progeny by seeking additional mates. Such behavior could drive down the odds of additional matings to a level where provision of care looks good by comparison (Kokko & Jennions 2008).
Sunk Cost Consider too the argument concerning female behavior—that having begun by investing in expensive eggs, she would be bound to follow through continued lopsided investment. To act this way is to commit the “sunk cost” fallacy—a mainstay in economics texts, whereby, having purchased an expensive ticket, a theatergoer feels duty bound to sit through a play till the very end. This problem in Trivers’s (1972) argument was pointed out by Dawkins & Carlisle (1976).
Mammalian Nature Though Trivers’s attempt to connect the dots between anisogamy and parental care proved problematic, the essential concept—that sex differences in reproductive biology matter for parental care—nonetheless remains compelling. The exigencies of lactation and breastfeeding, for example, have obvious implications for the allocation of early parental care. Likewise, internal fertilization sets the backdrop for possible paternity uncertainty.
State Dependent Investment Though anisogamy does not lead inexorably to an advantage in maternal care, if we choose a different starting point—the location of fertilization, say—then it is easy to envision a succession of increasing returns and continued specialization by sex. Whichever parent’s tissue surrounds the zygote is likely to be the one who faces the lower cost of gestation, for instance, with similar logic prevailing for successive stages of care.
Sex Ratios Biology and demographics combine to influence mating and parental care. But much conventional wisdom turns out to be problematic, and biological models can benefit from attention to economics to generate more accurate predictions for humans. For instance, a common premise in the biology literature is that the greater the excess of fertile males over females, the more males concentrate on competing for mates rather than on caring for offspring. Yet the opposite conclusion is at least as compelling: difficulty securing additional mates might steer males away from competing and into caring (Kokko & Jennions 2008). The answer depends on the resolution of opposing forces not unlike income versus substitution effects in the determination of labor supply. A mild shortage of females can discourage male care but a severe shortage can encourage it.
Neurobiology Mothers and fathers have different hormonal responses to the birth of a newborn. Female responses are dominated by hormones associated with bonding; male responses are characterized by hormones associated with vigilance.
3 Darwinian logic
3.1 Reproductive success
Our focus on fitness maximization goes part and parcel with understanding the origin of preferences. Every one of your grandparents—and all their ancestors before them—succeeded at surviving to reproduce and begetting at least one offspring who did likewise. Each one of us, therefore, is the product of a remarkable streak of repeated good fortune, played out innumerable times. The exigencies of scarce resources dictate that not all who are born survive and not all who survive reproduce; countless lineages stopped cold well before the present day. Darwinian logic implies that we are the favored few whose forebears were especially good at surviving and reproducing, traits that have been passed on to us.
These traits come partly in the form of preferences, which encourage us to—for instance—seek out nutritious foods, safe environments and fertile, competent mates. The traits might be biological and unlearned, as with an infant’s fear of abandonment. Or they might be cultural, as in religious restrictions on the consumption of certain foods. Preferences, both biological and cultural, may also be implicated in the provision of parental care. Darwinian success not only demands that we like what is good for us but that we apportion our efforts so as to make useful tradeoffs among competing goals of surviving, securing a mate and rearing offspring. Hence by way of “Darwinian revealed preference,” those who are alive today may be expected to be equipped with preferences that maximize, or at least favor, fitness.2
In the economics of the family, it is common to include children as an argument in the utility functions of adults; see, e.g., Becker (1973, 1974, 1991), Bergstrom (1994a), Siow (1998), Tertilt (2005), and Doepke & Tertilt (2009). Here we rely on evolutionary logic to throw issues of fertility and child care into sharp relief by positing that the sole ultimate objective of individuals is to maximize reproductive success, as is customary in evolutionary biology (Grafen 2000), and in the economics literature on preference evolution.3 This strategy in no way invalidates the common approach in the economics literature, which consists in including own consumption in the utility function, besides children. It merely reflects the idea that own consumption may be viewed as a necessary instrument to reach the ultimate goal of maximizing reproductive success, and that attention to reproductive technology might help economists generate predictions regarding the relative importance of own consumption and children in the utility functions of mothers and fathers.
3.2 Reproductive technology and ensuing trade-offs
How might we broach the idea that parental care emanates from evolved traits? One route is to consider large swaths of the living world, where myriad systems of care evolved. As with natural experiments in economics, focus on exogenous variation—in this context, the most promising variable would be habitat. Consider aquatic versus land-dwelling species. An aqueous medium is needed for egg fertilization. Accordingly, while external fertilization may be adequate for fish, internal fertilization is necessary for, say, red deer. The technology of fertilization makes it easier for the female fish and the male red deer to abandon the fertilized eggs. Hence, we can begin to make connections between the environment, the reproductive technology, and the form of parental care.
The animal kingdom features a rich array of habitats and reproductive technologies. The minimum that is required for an individual to reproduce is the production of a sex cell, or gamete. While some species use parthenogenesis, or asexual reproduction, others use sexual reproduction, which requires the fusion of male and female gametes to beget one new individual. In sexually reproducing species, anisogamy is the norm: one sex produces larger and fewer gametes than the other, and females are defined as the sex whose gametes are the largest. In species with internal fertilization, gestation occurs for some time inside the female’s body. Finally, upon being born, offspring may be precocial and essentially in no need of further help, or altricial, i.e., helpless. Depending upon the species, both paternal and maternal investment runs the gamut from minimal (contributing the gamete and little more) to enormous. Hence, three broad parental care patterns may be distinguished: care from both parents, care from one parent, or no care at all.
Asexually reproducing parents trade off the benefits of caring for existing offspring versus producing more offspring. Sexually reproducing parents face a more complex problem because their decisions are interdependent. A key question for each parent is: “Should I stay (and invest resources in existing children) or should I go (and free-ride on the other mate’s care and seek other mating opportunities elsewhere)?” This question arises because first, each child represents a public good in the fitness of its parents, introducing possible free riding; second, an individual’s fitness may depend on the quantity and quality of mates. In the 1970’s Robert Trivers and John Maynard Smith provided theoretical foundations that still permeate the current thinking on this issue. In the next section we describe the main tenets of their theories.
4 The games parents play
4.1 The parental care game (Maynard Smith, 1977)
Should a mother concentrate resources on caring for existing offspring or on further egg production? Likewise, should a father stay and provide care, or abandon offspring to search for further mating opportunities? Biologist John Maynard Smith posed this question in an early application of evolutionary game theory. Maynard Smith’s (1977) parental care game is general enough to encompass the behavior of several varieties of species, which is instructive for understanding human behavior, since we can examine the theory in light of things we share with other species (as with birds, our offspring are born helpless) and things we do not (unlike for fish, fertilization of human eggs takes place internally).4
Maynard Smith’s model admits a variety of outcomes, depending on background conditions: complete desertion of offspring; care by a single mother or father, and biparental care. What drives the results is a simple tradeoff between enhanced offspring viability from care versus extra reproductive opportunities from desertion. The tradeoffs themselves depend upon, among other things, the importance of care for survival and, for males, on the availability of mates.
Specifically, let Ec denote the number of eggs that a female lays if she provides care and Ed ≥ Ec the (generally greater) number of eggs that a female lays if she does not provide care.5 The number of eggs that a female lays also determines the number of offspring that a male can hope to produce with her. Once produced, the probability that offspring survive depends on parental care; let V0, V1 > V0 and V2 > V1 denote the probability that offspring survive, in the cases where (a) both parents desert, (b) one parent deserts and the other one provides care, and (c) both provide care, respectively. Then, a female’s fitness payoff depends in a straightforward manner on her own behavior and on that of her mate; these payoffs are shown in the upper right corners of the cells of the fitness payoff matrix in Figure 1. For females, then, the trade-off is between providing post-natal care and producing additional eggs.
Figure 1.
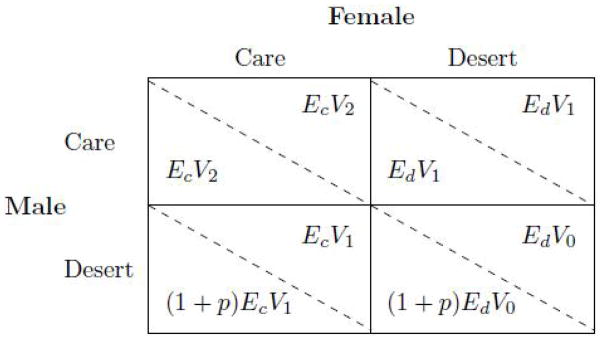
Payoff matrix for the parental care game
The trade-off is different for males. As with females, desertion reduces prospects for offspring survival. But a deserting male can pursue further mating, which has a chance p of succeeding. Such attempts generate p times the payoff that accrues from neither caring for the new offspring nor mating further. These payoffs are shown in the lower left corners of the cells of the matrix in Figure 1. For males, then, the trade-off is between providing post-natal care and fertilizing more eggs.
Before getting to this model’s implications, it is worth noting how games are typically conceptualized in economics versus biology. Economists usually think of individuals choosing strategies; biologists think of individuals as being endowed with a particular strategy, or “type.” The tradition in biology is to conduct analyses at the level of the population, and to focus on type distributions, to either determine stable type distributions, or “population states,” or to study population dynamics. In a stable population state each individual must be endowed with a strategy that is also individually rational given the type distribution in the population (Weibull 1995).
Keeping this in mind, identification of Nash equilibria in the game with the payoff matrix in Figure 1 can be used to understand why evolutionary forces need not necessarily give rise to staying and caring. For instance, suppose conditions are such that it always pays for a female to provide care. Can a male do better by deserting? On the one hand, desertion reduces offspring viability—the probability of survival falls from V2 to V1. On the other hand, there exists a probability (p) that the male might encounter another mate and reproduce with her. If p exceeds the percentage shortfall in viability then males who desert will get a higher fitness payoff than those who stay and care.
Alternatively, suppose that it is always in the male’s interest to provide care. Can a female do better by deserting? As before, desertion reduces offspring viability. But not providing care could free up resources for laying more eggs: Ed ≥ Ec. If the percentage increase in eggs produced, , exceeds the percentage shortfall in viability then females who desert get a higher fitness payoff than those who care.
Finally, if neither parent provides care the probability of survival falls to V0 ≤ V1. If this shortfall is not too large relative to prospective gains from further mating for the male and higher egg production for the female, then it may be expected that both males and females will desert.
In the animal kingdom male-only parental care is not a rarity (Clutton-Brock 1991). For amphibian species, male-only and female-only care are roughly equally common, and male-only parental care occurs in about two thirds of fish species. However, males caring more than females goes against the grain of what we know about mammalian parenting, where the commonest form of care is exclusively maternal (90 percent of species) but never exclusively paternal (Clutton-Brock 1991). Maynard Smith’s (1977) model arguably provides insights into the essential trade-offs that determine these patterns of parental care across species. Consider fish, for which male-only care is common. The external fertilization that characterizes most egg-laying fish gives females a first mover advantage for deserting to lay additional eggs elsewhere, leaving the male to guard the first clutch. In mammals, internal gestation creates a stark difference between the circumstances of males and females: even with multiple mating opportunities, at a given time a male is physically capable of producing many more offspring than a female—conditional on there being females who are willing to mate. Female availability brings us to male competition, an issue at the core of the model proposed by Trivers (1972).
4.2 The parental investment model (Trivers, 1972)
Since Maynard Smith’s (1977) model pays little attention to gamete size, it leaves unexplored the wider implications of anisogamy for parental investment and competition for mates. Trivers (1972) argues that anisogamy is destiny, in the sense that possession of the expensive gamete puts females on a path of investment in offspring while having the inexpensive gamete permits males to seek additional mates.6 The two main ingredients of Trivers’ (1972) original parental investment model are anisogamy and mate competition.
Trivers (1972) argued that since the female invests more heavily than the male in gamete production, she has an incentive to invest more heavily after the offspring’s birth as well. Taken literally, the argument clearly falls prey to the sunk cost fallacy, for which it has been severely, and justifiably, criticized (Dawkins & Carlisle 1976).7 But Trivers’ argument gains momentum if stated as follows: due to anisogamy and internal gestation, relative to a male a female who has just produced a brood faces a significantly higher time cost of producing an additional brood.
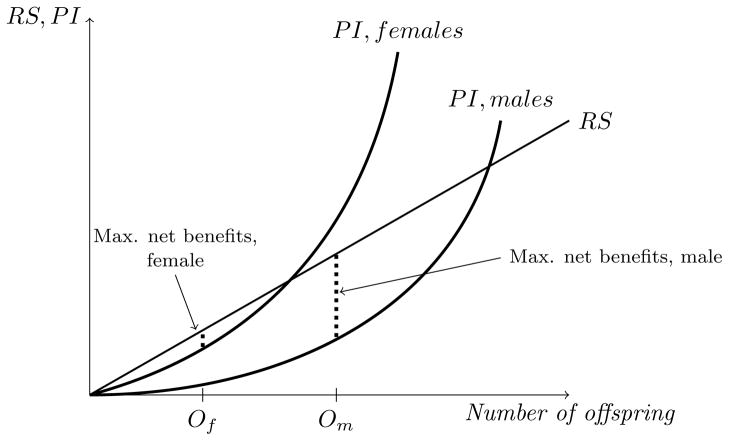
Figure 2 illustrates this: the 45-degree line represents the total benefits of reproductive success (RS) from current offspring, and parental investment (P I) is the foregone reproductive success from producing future offspring. Due to anisogamy and internal gestation, the cost of deserting is lower for males than for females, and therefore the benefit from investing in an existing brood is greater for females than for males. As a result, the optimal number of offspring, which equates marginal cost to marginal benefit, is smaller for females than for males.
Figure 2.
Optimal number of offspring, males versus females
However, whether any given male would be capable of producing his optimal number of off-spring is a different matter, and this leads to Trivers’s (1972) key argument: competition for mates should be more intense among the sex that incurs lower reproductive costs. Since this competition is literally about life and death (or, rather, offspring vs. no offspring), it can be expected to be fierce. Male sage grouse contribute nothing to their offspring beyond their genes, and they compete intensely in high stakes, ritualized displays called leks for mating access to multiple females. In contrast, male pipefishes and seahorses (family Syngnathidae) invest heavily in reproduction undergoing “pregnancy” by gestating embryos in a special broodpouch. For these species, females tend to compete for males, rather than the other way around (Jones & Avise 2001).
4.3 Sexual selection and parental care
The Trivers (1972) model connects two facets of behavior that are typically considered separately in the economics of the family: caring for offspring versus competing for and selecting mates, otherwise known as sexual selection. Sexual selection is surely more nuanced for humans than for sage grouse or seahorses, whose sex differences in reproductive costs lie between these extremes. Our mammalian nature saddles females with a disproportionate share of costs of reproduction, yet we are also among the minority of mammalian species with bi-parental care. Accordingly, one might expect both sexes to be somewhat choosy. Existing evidence suggests this is indeed the case. However, men and women place differing weights on certain qualities in a prospective mate in ways that arguably reflect biological considerations.
Recent evidence is illuminating in this regard. Using data from a French marriage bureau, Vaillant & Wolff (2011) find that men shy away from vulgar partners, whereas women dislike tendencies to be violent and selfish. Based on data from on-line dating sites, Hitsch, Hortaçsu & Ariely (2010) find that, relative to men, women place less emphasis on a prospective partner’s physical appearance compared to their income. In an experimental study of speed dating, Fisman et al. (2006) find similar results. These patterns are consistent with findings from an earlier, cross-cultural study using data from 37 countries (Buss 1989). The disparities are ostensibly consistent with reproductive concerns, whereby men are on the lookout for cues associated with fertility and women are attuned to a prospective partner’s ability to provision offspring. Whether and how much these priorities will change as women’s educational and earnings prospects improve relative to men’s is an open and fascinating question.
There is also evidence that women seek out fewer prospective mates than do men, consistent with the discussion above concerning the propensity for men to compete and for women to choose. In a famous experiment Clark & Hatfield (1989) calculated gender differences in the propensity to accept an offer to have sex with an attractive stranger. The male-female differences were vast—75 percent of male college students expressed willingness to do so; none of the female students did. These unsurprising findings are consistent with Trivers’s (1972) model of sexual selection, which predicts that the sex that incurs the higher cost of reproducing will be less inclined to engage in short-term mating.8 Arcidiocono, Beauchamp & McElroy (2010) study adolescent preferences for being in a sexual relationship using both a structural model of matching and direct subjective reports and their results are consistent with those of Clark & Hatfield (1989): women might reluctantly have sex in order to have a relationship, whereas men might reluctantly abstain from sex for the same reason.
How male competition might play out has been studied by several evolutionary psychologists. Amongst them, Miller (2000) has argued that, like a peacock’s tail, ostentatious displays of wealth, athletic prowess, risk-taking, artistic accomplishments, and the like are used by males to signal their quality as mate. Just one example of behavior consistent with this idea is Barber & Odean’s (2001) finding that male investors trade more frequently, and invest far less successfully, than female investors. There is an emerging economic literature on gender differences in preferences (for a recent survey, see Croson & Gneezy 2009), yet little attention has been given to issues of sexual selection. It is possible that the evolutionary approach described here could help gain a better understanding of gender differences in a variety of economic applications.
Returning to parental care, might sexual selection be expected to affect child well-being? For instance, while among sage grouse the competition between males appears to be a wasteful arms race, in pipefishes and seahorses it seems to have a positive side-effect on parental care. Another colorful example of such a positive side-effect is provided by the purple throat carib, a hummingbird species in which males compete by defending territories; a male’s attractiveness depends on whether his territory is rich in nectar-producing flowers (Temeles & Kress 2003).
In sum, the analyses by Maynard Smith (1977) and Trivers (1972) seem to have provided the first deep insights into the forces that may ultimately be driving differences in maternal and paternal care for offspring. However, they have left a number of questions largely unanswered, in particular the tricky issue of how a new mate is located once an existing brood is deserted. In the recent past quite a few authors have focused on this issue, to which we now turn.
5 What Anisogamists Missed: Sex Ratios and General Equilibrium
The facile intuition of “go forth and multiply” versus “go forth and add” suggests that males should be motivated to seek additional mates while females concentrate on caring for offspring. Recent work by evolutionary biologists, notably Queller (1997) and Kokko & Jennions (2008), shows that this argument turns out to be less compelling than it looks when paired with the self-evident fact that everyone has exactly one father and one mother. This reality—the so-called “Fisher condition” (Fisher 1930)—implies that, in a population with balanced sex ratios, the average reproductive success of one sex cannot exceed that of the other.
Accordingly, a male who seeks to enhance his reproductive success by acquiring a new mate should be mindful of the long odds he faces in a world with similarly ambitious peers. The mating market would be glutted with searching males and hardly any receptive females. A problem with the original anisogamy argument that gamete size is destiny is that it conflates individual potential with aggregate feasibility. Just because a single male can in principle reproduce in abundance with multiple mates doesn’t mean that every male can.
5.1 Supply must meet demand
While the sex ratio derived from counts of adult males and females gives a rough indication of the supply of and the demand for ova, what matters for fitness is the so-called operational sex ratio (OSR), which is determined by focusing on those who are able to reproduce. If females take longer than males to regain reproductive capacity after producing offspring, then the operational sex ratio—the ratio of sexually active males to fertile females—will be male-biased. In turn, the surfeit of males would reduce the chances of finding a fertile mate, thus reducing the opportunity costs of providing care. If males nonetheless persisted in searching for mates, this imbalance would open the door to frequency dependent selection, whereby a mutant male inclined to provide care would enjoy greater reproductive success than his less caring counterparts.
In terms of Maynard Smith’s (1977) model, the key idea here is that p, the probability that a deserting male is able to sire a child with another mate, which in the model is exogenous, in fact depends on the distribution of male types in the population. Clearly, if most males deserted and all females mated with a large number of males, p would be close to zero, and it would be in the interest of males to provide care rather than engage in a fruitless quest for a new mate. On the other hand, if all males provided care, then the net benefit from deserting would be large. It follows that if this large net benefit is positive, the population cannot be monomorphic, but will instead be polymorphic, with some caring and some deserting males.
Wade & Shuster (2002) and Fromhage, McNamara & Houston (2007) provide formal analyses of this idea. They find that precise predictions depend on assumptions regarding whether deserting males who successfully impregnate more than one female, do so at the expense of other deserting males or of caring ones. In general, however, these models predict heterogeneity among males, because some males will be of the caring type whereas others will be of the deserting type.
Besides the competition for the limited supply of females, there is also another reason for why the amount of caring among males may be expected to differ from the amount of caring among females, which was originally suggested by Bateman (1948) and Orians (1969), and exposited more formally by Queller (1997). It has to do with male variance in mating success: more desirable males have more to lose by providing parental care.
5.2 Male variance in mating success
Trivers’s (1972) argument that males invest less in offspring because they produce the less expensive gamete rests on the assumption that an abandoning male has greater mating success than an abandoning female. For this to be true for all males there would have to be more sexually mature females than males in the population. This is clearly not the case for humans, where females are fertile for a much shorter time than males. Nonetheless, it may be true for a fraction of the male population.
Suppose that males vary by some attribute (wealth, say) that contributes to mating success, because it is demanded by females. Now consider the population of individuals, male and female, who have already reproduced—and therefore face a choice between caring for offspring versus seeking other mates. If having reproduced is correlated with future mating success, then the average mating prospects for the males with offspring will exceed those of the average male. If females choose according to quality in a manner that excludes some males from mating, leaving fewer mating males than females, then the average reproductive success of mating males will exceed that of mating females. This advantage raises the benefit from seeking additional matings for males relative to females, since males can reproduce faster than females. Depending on the distribution of the attribute at hand among males, either all males who mate are able to mate with more than one female, or, among those males who mate, some mate with several females while others can secure one mate only (even if they would also mate with several females should the opportunity arise). Furthermore, some males may end up without any mating opportunities at all.
The human species is unique in that the number of socially sanctioned mates is legally circumscribed. Accordingly, in the economics literature polygyny refers to a man marrying more than one woman and polyandry to a woman marrying more than one man.9 While monogamy is the norm in Western countries, polygamous (usually polygynous) unions are socially sanctioned in some cultures; see Bergstrom (1994a), Lagerlöf (2005), and Tertilt (2005), and the references therein, and Zeitzen (2008). Moreover, so-called serial monogamy, whereby a mate leaves one partner to pair with another, has also become increasingly common in Western countries.10 But whether polygamous unions are socially sanctioned or not, multiple matings may arise due to extramarital affairs.11 Perhaps surprisingly, biologists make a similar distinction, but based on behavior rather than on social sanctioning. Thus, “an individual’s pair-bonded mate is the one with whom he or she has a consistent social association” (Westneat, Sherman & Morton 1990, p. 333), and matings with individuals other than the pair-bonded mate are extra-pair matings or copulations.
While social norms and laws clearly affect behavior, anisogamy and internal fertilization imply that males alone are able increase the number of offspring via multiple matings. By contrast, the only benefit that a female can hope to achieve from mating with multiple males is higher quality of offspring.12 Hence, female mate choice typically leads to polygyny (or to monogamy coupled with male unfaithfulness and possibly support to mistresses and concubines who produce offspring) among the “best” males, and to the unavailability of mates for males at the lower end of the distribution; see, e.g., Orians (1969) and Bergstrom (1994a). In fact, taken together, anisogamy and internal gestation may be seen to provide a biological justification for the customary assumption in the economics literature that in societies with polygamy, it is polygyny rather than polyandry that prevails (see Bergstrom 1994a, Lagerlöf 2005).
Male attributes that contribute to mating success vary between species. In humans, as in some bird species with biparental care, a key attribute is wealth (which often comes in the form of a nest for birds), which presumably affects survival of offspring. Insofar as there is female choice, if females are homogeneous and care only about fitness payoffs, the fitness payoff that a female receives from mating with the least wealthy polygynous male cannot be too different from that received from mating with the wealthiest monogamous male (Bergstrom 1994a). In the case where males may accumulate wealth, then, polygyny does not necessarily reduce the amount of care provided by males, since male care comes partly in the form of wealth rather than direct caring effort.13
5.3 Sex ratios and optimal mating effort
Suppose females become relatively scarce. Should a male respond by putting more effort into securing a mate? Or by concentrating on caring for offspring he already has? The question matters for connecting the dots between sex ratios, family life, and the well-being of children.
Despite the importance of the issue, biological discussions are mainly focussed on intuitive, verbal arguments. A prevailing theme in studies such as Trivers (1972) is that the more difficult it is for males to obtain mates, the more they benefit from investments in mating effort relative to parental investment. Recently, however, biologists have made the equally plausible assertion that increased difficulty in locating mates should make the provision of care a relatively more attractive option (Kokko & Jennions 2008). It turns out that each perspective is partially plausible but incomplete. The effect of mate availability on the intensity of mate seeking versus care is similar in form to the standard labor supply problem with its attendant income and substitution effects.
To see this, imagine that reproductive success, or fitness (see Sections 2 and 3.1), F, depends upon mating success, m, and on provision of care to offspring, c, where F is assumed to be strictly increasing in both arguments, and strictly concave.
| (1) |
Suppose that an individual male’s mating success m depends, in turn, on the fraction of fertile females in the population, p, the male’s effort put into mating, e (which can be thought of as quality-adjusted hours), and Ω, which can be thought of as the male’s “mating capital” or attractiveness. Mating success is given by
| (2) |
where e must be within the feasible set, here defined by a time constraint, e + c ≤ T, T being the male’s total available time.
To return to our initial question: What happens to mating effort, e, when the percentage of available mates, p, changes? Mating effort e is analogous to labor supply, and p parallels the wage in that it denotes the expected increase in m from a unit increase in e. So the relationship between e and p is given by the familiar Slutsky equation:14
| (4) |
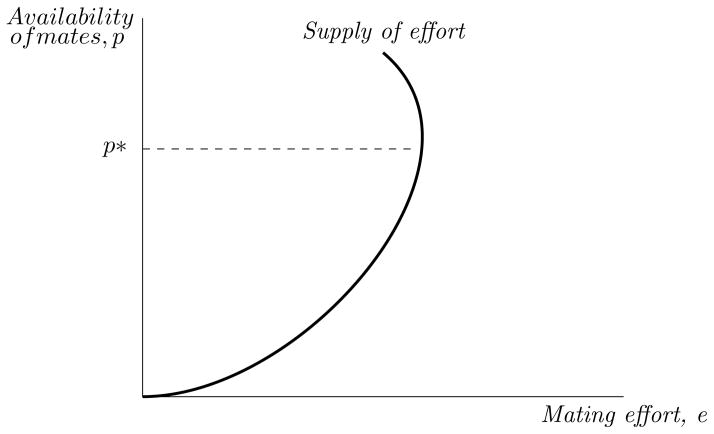
The effect of an increase in mate availability p can be decomposed into a substitution effect and an income effect. As p increases, the benefit of mating effort relative to caring effort (holding reproductive success constant) increases: the substitution effect says that the mating effort will increase. However, the larger the mating capital O, the smaller the marginal effect of mating effort on overall fitness; this “income” effect implies that mating effort will decrease as O increases. The overall effect may be positive or negative. In particular, as in the textbook labor supply example, the end result may be a backward-bending individual mating effort supply, like the one shown in Figure 3.15
Figure 3.
Individual mating effort and the availability of mates
The upward-sloping portion of Figure 3 depicts behavior that is counter to the conventional argument that mate scarcity encourages mating effort. Here, the substitution effect dominates; decreases in p encourage males to devote relatively more effort to parental care.
Considering the aggregate supply of mating effort (roughly, the horizontal sum of individual mating effort supply curves) strengthens the argument that mate scarcity can encourage parental care. Aggregate labor supply curves are generally more elastic than individual curves (e.g., Chang & Kim 2006); by analogy, the same goes for mating effort curves. Accordingly, whatever disincentive mate scarcity poses for individual mating effort would only be stronger in the aggregate.
A key reason has to do with incentives at the extensive margin. Imagine that males vary according to the minimum value of p needed to generate a positive value of e. If this is so, increases in p encourage aggregate mating effort at the extensive margin by drawing more males into the pool of those exerting mating effort. Conversely, unfavorable values of p would reduce aggregate mating effort.
5.4 Competing for a first-time mate
Though the tradeoff between parental care and mating effort is obviously not relevant for the childless, the two can nonetheless be linked, even for those who have yet to find a mate, if today’s mate competition impinges on tomorrow’s parenting. For instance, in the face of imbalanced sex ratios in China it appears that parents attempt to improve their sons’ marriage prospects by increasing their saving (Wei & Zhang 2011).16 The savings, in turn, can arguably affect the well-being of a son’s future offspring.
As Wei and Zhang, as well as Du & Wei (2010) emphasize, the welfare implications of the rise in saving can be complex. Saving for marriage puts families with sons on a positional treadmill that affords no betterment of marriage prospects in the aggregate. And it is not clear how families with daughters might respond to saving fueled by mate competition. They could reduce their saving, substituting wealth accumulated by the groom’s family for their own. Or they might increase saving out of concerns for their daughter’s bargaining power.
In light of crosscurrents like these, it is not clear that male-biased sex ratios in marriage markets need work to the detriment of paternal care and child well-being. For instance, think about the original parents, who save a lot in order to marry their son off. Fast forward to when the son has his own child, a daughter. She might well go into her own marriage wealthier than if savings-related competition had not occurred.
5.5 Paternity uncertainty
Economists who have analyzed marriage market organization (see Footnote 9) have adopted models in which females are faithful.17 However, Hrdy (2000) argues that a woman may have an evolutionary incentive to create confusion about paternity in order to secure resources from more than one man. Thus, if (some) females engage in extra-pair copulations, some males will face paternity uncertainty. By contrast, absent mix-ups in hospital wards, maternal certainty is guaranteed, regardless of any unfaithfulness, thanks to internal fertilization and gestation. Supporting an unrelated child thought to be one’s own (i.e., cuckoldry) can be devastating for a man’s reproductive success, since the benefit in terms of reproductive success associated with this particular child is zero, while the opportunity cost of forgoing other mating opportunities may be positive. Hence, we may expect paternity uncertainty to also matter for maternal versus paternal altruism, in addition to the aforementioned considerations of sexual selection.
Evidence shows that paternity uncertainty is not insignificant in animal species. For example some birds that were once thought to be monogamous, have been found to have cuckoldry rates of 35 percent or more (Birkhead 2000).18 For humans as well, the incidence of misattributed paternity can be non-trivial, depending on the population being sampled. A recent survey by Anderson (2006) finds a median non-paternity rate of 1.9% for a set of studies with presumed high paternity confidence (e.g., serology or genetic studies) conducted in the United States and Canada. Not surprisingly, in these same countries the median non-paternity rate in samples with low paternity confidence (such as paternity testing laboratories) is much higher: 29.4% according to Anderson (2006).
When it comes to parental care paternity uncertainty is a crucial issue, since it determines the expected fitness benefit that a male reaps from giving resources to children born to his mate(s). A crude measure of paternity uncertainty is given by the average degree of female promiscuity. Interestingly, as pointed out by Queller (1997), this average female promiscuity may be expected to entail two distinct effects on paternal care. First, the expected benefit for a male of providing care to his mate’s offspring is smaller the higher female promiscuity. Second, an increase in female promiscuity means that females are on average more willing to engage in multiple matings. As was pointed out above, such an increase has an ambiguous effect on males’ net benefit from exerting mating efforts. Thus, the overall effect of female promiscuity on paternal care is ambiguous (Westneat & Sherman 1993, Houston & McNamara 2002, Houston, Szekely & McNamara 2005).
Some, but not all, genetic studies on the connection between paternity and paternal care in birds have found that care is inversely associated with female extra-pair copulations (Whittingham & Dunn 2001). Some studies suggest that paternity also matters for parental care in some non-human mammals, even though they tend to provide less paternal care than birds (e.g., Buchan et al. 2003). Whether average female promiscuity is expected to have a positive or a negative effect on the amount of paternal care in humans, remains to be determined. However, while average promiscuity may matter for assessing a male’s expected benefit from seeking further mating opportunities, what matters for assessing the benefit from providing care to his mate’s offspring is whether that particular female was faithful or not. Clearly, males may reap huge fitness benefits from gathering better information about a mate’s faithfulness. Two means of obtaining such information that may be at work in humans are mate guarding and the ability to recognize kin.
5.5.1 Mate guarding
Biological thinking has generated a paradoxical concept, namely, that a father’s concerns about paternity might result in his providing more rather than less care to offspring. The idea is that, during ancestral times, paternity concerns and sexual jealousy encouraged males to stick close to their mates in order to prevent them from consorting with other males (Hawkes 2004). According to this hypothesis, paternal care then evolved as a by-product of so-called ‘mate guarding.’ It is thought that hidden ovulation in human females contributes to a male’s incentives to mate guard, since his mate is more or less continuously sexually receptive (Alexander & Noonan 1979).19 Monogamy, too, is thought to have evolved out of a propensity for females to “trade” faithfulness for paternal investment (Fortunato & Archetti 2010).20
In various times and places there have been numerous strategies, running the gamut from intrusive to oppressive, that arguably helped males guard their mates: from stalking to chastity belts, to legal or cultural restrictions on activities that women may engage in. Other examples include chaperoning and sequestration, brought about by, say, foot binding or purdah. Female genital mutilation is thought to be implicated in mate guarding as well. It has also been argued that refundable bride prices is an institution that encourages women’s faithfulness (Bishai & Grossbard 2010).
In addition to the public health and human rights problems created by oppressive mate guarding practices, it might also be at the root of potentially severe misallocations in education and the labor market. If women’s ignorance is perceived as the route to chastity and fidelity, mate guarding could be rationalizing a great deal of discrimination (Posner 1992).
5.5.2 Kin recognition
Because of the fitness cost associated with providing care to an unrelated child, would-be fathers may be expected to have an interest in recognizing their offspring. Consider Anderson’s (1993) ethnography of inner city out-of-wedlock childbearing: “In a number of cases of doubtful paternity, the boy’s mother, sister, aunt, or other female relatives or close family friends may form informal visiting committees, charged with going to see the baby. […] [T]he object is always the same: to see if the baby ‘belongs’ to the boy it is said to.” (pp. 83–84) Intuition further suggests that it may be beneficial for offspring to be recognized by kin so as to trigger parental care, although this intuition is weakened by the observation that an infant whose mother was unfaithful faces a non-zero probability of being born into a family with a man who thinks that he is the biological father but is not (Pagel 1997).
The evidence on parent-child resemblance is mixed (Christenfeld & Hill 1995, Bredart & French 1999, Alvergne et al. 2009). Nonetheless, some studies suggest that parents, especially fathers, are sensitive to resemblance to self (Platek et al. 2002, DeBruine 2004, Platek, Keenan & Mohamed 2005, Volk & Qunisey 2002).
5.6 A seemingly odd exception: genomic imprinting and paternal bias
Mammalian reproduction features a seemingly odd stage in which, counter to the maternal advantages discussed earlier, it is the father who, indirectly at least, can be construed as being more altruistic to the child than the mother. Such paternal bias occurs when the child is in utero, thanks to the placenta, at the behest of the father’s genes. The placenta transfers nutrients from the mother to the fetus. It is better thought of not as an organ of maternal nurturance but a parasite built by the father’s genes for extracting maternal resources. Not that the mother is not altruistic toward the fetus; it’s just that the fetus likes himself even more than his mother likes him. Accordingly, he might want more than she is willing to give.
Haig (1993) discovered that the fetus sometimes expropriates extra glucose by secreting a placental hormone that weakens the mother’s insulin. She responds by increasing her insulin production, but the tug-of-war sometimes goes to the fetus, while the mother incurs heightened risk of gestational diabetes. Further, “sugar grab” has been found to be an expression of paternal, not maternal, genes (Haig 1993). This squares with maternal/paternal differences in costs—he doesn’t bear the hazard of pregnancy.
6 Neurobiology and Parenting
In addition to the ultimate evolutionary forces discussed above are the proximate biological triggers, such as hormones and neurotransmitters, that spur parents to act. Here we consider whether fathers and mothers differ in their hormonal connection to parental care–whether they are primed differently for providing care, and whether care itself, as well as social context, affects hormonal profiles. Because hormones and neurotransmitters are endogenous, interpreting their patterns is difficult. Nonetheless, these proximate variables are arguably indicative of the ultimate evolutionary forces discussed earlier.
Understanding the neurobiology of parental care is important because hormonal patterns are potentially related to preferences and choices. Hormones send signals for controlling bodily processes, including those related to reproduction and social bonding, in ways that differ by sex. Accordingly, hormonal patterns offer possible clues about male-female differences in parental care.
Despite this potential, the economics of the family pays little attention to neurobiology. Conversely, there is little work from the bourgeoning field of neuroeconomics that concerns family behavior. Fortunately, economists can access a wealth of information from the life sciences on the neurobiology of parenting.
We know for example that the hormone oxytocin is associated with several aspects of female reproduction such as the transport of sperm in the genital tract, uterine contractions during childbirth, and milk let down during lactation. Oxytocin has been implicated in maternal bonding: higher first-trimester levels are positively associated with postpartum maternal affection for her newborn (Gordon et al. 2010). Nicknamed the “love hormone” and the “cuddle chemical,” oxytocin has been found to reduce anxiety from social stress and increase trust and cooperation. It also improves the ability to recognize faces and facial expressions.
Though the onset of fatherhood is also marked by increases in oxytocin, the hormonal correlates of parenthood generally differ by gender. One important difference is that fatherhood is marked by an increase in vasopressin (Gray, Parkin & Samms-Vaughan 2007), a hormone whose effects have been found to differ markedly from oxytocin (McCall & Singer 2012). While oxytocin reduces anxiety, vasopressin increases it. Oxytocin acts to promote affiliative bonds with non-kin; vasopressin is associated with male-male competition, courtship, social recognition, and mate and offspring defense. Oxytocin tracks estrogen levels in women; vasopressin is potentiated by testosterone in men (Sanchez et al. 2009).
The onset of fatherhood also coincides with a reduction in testosterone (Storey et al. 2000). In both animal and human studies, testosterone has been found to be positively correlated with mating effort, including, in humans, extramarital sex (Gray et al. 2002).
It could be construed that hormonal gender differences are indicative of evolutionary design. Conceivably, these differences constitute evidence that type of care (nurturance versus defense, say) as well as amount of care that mothers and fathers provide are the outcome of natural selection. For example, it could be argued that hormonal patterns reflect sex-specific evolutionary requirements: oxytocin facilitates the formation of useful social bonds beyond the family, for example, while testosterone reduction influences the male’s tradeoff between mating effort and investment. Indeed, many prominent treatments of sex differences in behavior view these proximate patterns as evidence supporting the evolutionary approach to reproduction and parental care (see, e.g., Geary 2010).
Despite these suggestions, however, it is difficult to craft a fine-grained causal interpretation of hormonal patterns. One reason concerns endogeneity. Oxytocin, for example, can both affect and be affected by the provision of care. The same is true for all of the above mentioned hormones. Indeed, the neuroendocrine system is designed in such a way as to take cues from, as well as to act upon, the social context.
Even aside from issues of causality it can be difficult to make inferences about preferences based upon hormonal processes. In principle, for instance, maternal and paternal altruism could be equivalent even if they are activated by different hormonal triggers. What can be said is that, assuming fathers and mothers do indeed care differently, the hormonal evidence illustrates how gender differences operate within the inner workings of care provision.21
While the study of the neurophysiology of parental investment in humans only has a brief history, research with animal models has a much longer tradition. The latter are also free from some of the constraints that circumscribe research with human subjects. Accordingly, we can get a much more complete picture of parental investment in certain non-human species, including considerations of genomics and habitat in addition to hormonal effects. And it is easier to examine the effects of exogenous changes in hormones in animal models. Of course, such detail comes at the price of external validity since it is difficult to extrapolate from, say, the behavior of rodents or primates to that of humans.22
The premier animal model for studying the neuroendocrinology of reproduction and parenting is the vole. Specifically, two genetically similar but behaviorally distinct species have been compared: the monogamous prairie vole and the polygamous meadow vole (Donaldson & Young 2008). Prairie voles mate for life, and males care for and defend offspring. In contrast, male meadow voles are promiscuous and rarely engage in parenting. The contrasting behavior meshes with differences in habitat: meadow voles are found in moist, dense grasslands while prairie voles tend to occupy the more challenging habitat of drier, patchier grasslands. In the latter environment monogamy would arguably be more urgent for the provisioning of offspring.
Consistent with emerging human evidence, the monogamous behavior of the prairie vole is affected by vasopressin, which stimulates reward pathways in the brain. A preference for a partner is formed when the pleasure derived from sex becomes associated with the partner’s olfactory signature. This occurs with the simultaneous activation of dopamine receptors (rewards) and vasopressin receptors (implicated in social recognition). Vasopressin’s effects carry over to parenting as well, in that they are associated with paternal behavior such as pup licking.
The vasopressin receptor associated with paternal care has a markedly different distribution in prairie versus meadow voles. It is this difference that is thought to account for contrasting paternal behavior. Infusions of vasopressin facilitate pair bonding in monogamous prairie voles, but not in non-monogamous meadow voles (Young et al. 1999).
A candidate gene has been implicated in differences between vasopressin receptors in prairie voles versus meadow voles (Young et al. 1999). Though almost identical for the two species, the prairie vole gene contains one sequence that the meadow vole does not, and the nature of the sequence suggests that it is the product of a sudden mutation. Further, within-species evidence indicates that paternal behavior is sensitive to environment: exogenous treatments of vasopressin were found to have stronger effects on fathering behavior in prairie voles from drier habitats than in prairie voles from less dry habitats (Cushing et al. 2001).
The vole evidence connects genes, environment and function. A difficult habitat favors biparental care; a mutation alters the functioning of the paternal neuroendocrine system so as to facilitate such care.
Of course, it is difficult to know how much of these results will eventually inform studies of human behavior. All animal models are subject to problems of external validity. The behavior of small-brained mammals like voles depends on hormones to a much greater extent than that of humans, who are adapted for more flexible decision making contingent on social and environmental conditions.
Nevertheless, animal models are beginning to prove informative for human behavior. A recent study inspired by vole research honed in on the human analogue of the vasopressin receptor gene in voles and discovered that men (but not women) possessing a particular variant of this gene were more prone to experience marital discord compared to those who did not (Walum et al. 2008).
Despite its early stages then, research on hormonal mechanisms associated with mating and parenting is beginning to reveal a picture that suggests gender differences that are consistent with biological thinking.
7 Conclusion
There appears to be sound evolutionary basis for mother-father differences in altruism toward children. Despite the flaws of earlier, anisogamy-based arguments, later analyses that considered, inter alia, our mammalian nature and internal fertilization, pointed to a maternal edge in altruism toward offspring. The logic associated with disparate parental investment is mirrored in mating, marriage, and sexual selection. Each sex is choosy in long term mating decisions but males and females place different weights on various desirable qualities in a partner.
The gender differences discussed here do not necessarily matter for everyone, however. To cite a maternal edge in altruism toward offspring does not imply the same disparity for every mother-father pair. Nor do the hypotheses advanced here have any normative implications. It would obviously be mistaken to use any of the reasoning above to justify any particular distribution of child care tasks between spouses, for example.
This review suggests that there is considerable overlap between biological and economic approaches to family behavior. There is also ample scope for gains from trade between the two disciplines. Biological problems such as analysis of mating effort are amenable to standard economic analysis, for example, and an evolutionary approach illuminates the workings of parental care in the context of sexual selection. Economists with interest in family behavior can benefit from the synergies between economics and biology.
Acknowledgments
Financial support for this work was provided to Donald Cox by a grant from the National Institute on Child Health and Human Development (R01-HD045637) and to Ingela Alger by the Social Sciences and Humanities Research Council of Canada (SSHRC) as well as the Agence National de la Recherche (ANR). The findings, interpretations and conclusions expressed in this paper are entirely our own and do not necessarily represent the views, opinions, or policy of the National Institutes of Health, the Social Sciences and Humanities Research Council of Canada, the Agence Nationale de la Recherche, or of any other government agency.
Footnotes
An exception is Eswaran & Kotwal (2004).
Since the evolutionary process is not forward-looking, today’s preferences might be better suited to our evolutionary past than for the here and now. However, to the extent that cognitive ability allows humans to adapt to the current environment, it is meaningful to explore the consequences of the assumption that people want to maximize reproductive success.
See, e.g., Frank (1987), Hansson & Stuart (1990), Bergstrom (1995), Robson (2001), Dekel, Ely & Yilankaya (2007), Heifetz, Shannon & Spiegel (2007), Rayo & Becker (2007), Netzer (2009), Robson & Samuelson (2011), Alger (2010), Alger & Weibull (2010, 2012).
Cross-species comparisons are standard among biologists interested in parental care (e.g., Clutton-Brock 1991) since the effects of variations in habitat and reproductive physiology can be examined against a backdrop of the universal Darwinian objective of “survive and reproduce.”
We focus on the version of the model in which each parent faces a discrete choice between caring and deserting. Maynard Smith (1977) also studies a continuous variant; see also Grafen & Sibly (1978).
Due to the (largely verbal) model’s complexity we cannot do full justice to it here. Rather, we will seek to give a simplified account of the model’s main ingredients and predictions.
On the other hand Trivers (1972) can be credited with having introduced the concept of opportunity cost to biology by being the first to define parental investment as “any investment by the parent in an individual offspring that increases the offspring’s chance of surviving (and hence reproductive success) at the cost of the parent’s ability to invest in other offspring” (p. 139; original text in italics).
This is not to say that considerations introduced by Trivers would necessarily be the only determinants of participation in a sexual liaison. There are obviously others to consider, such as the availability of birth control or the chances of contracting a sexually transmitted disease.
See, e.g., Becker (1974), Grossbard (1976, 1980, 1986), Bergstrom (1994a), Bergstrom (1994b), Lagerlöf (2005), and Tertilt (2005).
See, e.g., Becker, Landes & Michael (1977). Divorce rates in the United States remain quite high despite trending downward in recent years (e.g., Stevenson & Wolfers 2007).
Laumann et al.(1994) report that 25 percent of men and 15 percent of women report having at least one extramarital affair during the course of their marriages. (The higher figure for men is due in part to visits to prostitutes.) Smith (2012) further finds that infidelity behaviors are correlated with occupation and education.
This may be, e.g., because she may thus get more wealth, or access to better or more compatible genes (e.g., Neff & Pitcher 2005, Akçay & Roughgarden 2007).
It has even been suggested that monogamy may have arisen because of fierce competition among males in the distant past: monogamous behavior, coupled with an increase in paternal investment, may have spread among males, starting with the low-ranked males (Gavrilets 2012). A different and rather intriguing hypothesis, put forward by Wade (1979), states that male-male competition might in fact have led males to invest less at the gamete stage. This argument puts Trivers’s (1972) theory on its head by suggesting that anisogamy might be the result of male-male competition rather than its cause.
| (3) |
Whether effects similar to these income and substitution effects would remain in a model where the effect of a male’s mating effort also depended on the other males’ mating success, is an open question.
The benchmark sex ratio at birth is 106 boys to 100 girls. But contrast, the corresponding figure in China in 2007 is an estimated 124 boys to 100 girls (Wei & Zhang 2011).
For a model with monogamy and male unfaithfulness, see Bergstrom (1994b). In this model, if some males are unfaithful, depending on the sex ratio some males may end up having no mate at all.
Even maternity uncertainty may arise, due to parasitism; while this can occur among birds (e.g., Friedmann 1928), it is not relevant for humans.
In contrast, male chimpanzees engage in mate guarding only when females are in estrus, and show little interest in guarding during other times.
Mate guarding is not the only evolutionary explanation for paternal care, but it is the leading contender. The alternative hypothesis is household division of labor, whereby male hunters provision their families. A problem with this explanation is that it goes against the grain of evidence for contemporary hunter gatherer societies. Successful hunters direct their largesse toward the community at large and show little favoritism to family members (Balshine 2012).
See Bernheim (2009) for a discussion of these issues in the context of neuroeconomic tests of hypotheses about preferences.
From an endocrinological perspective though, it is striking that the function and workings of hormones tend to be remarkably well conserved through time. Oxytocin- and vasopressin-like neuropeptides have existed and have been implicated in reproductive behavior for at least 700 million years (Donaldson & Young 2008).
Contributor Information
Ingela Alger, Email: ingela.alger@tse-fr.eu, TSE (LERNA, CNRS), IAST, IDEI, Université Toulouse 1 Capitole, F - 31 015 Toulouse Cedex 6.
Donald Cox, Email: donald.cox@bc.edu, Department of Economics, Boston College, Chestnut Hill, MA 02467.
References
- Akçay E, Roughgarden J. Extra-pair paternity in birds: review of the genetic benefits. Evolutionary Ecology Research. 2007;9:855–868. [Google Scholar]
- Alexander RD, Noonan KM. Concealment of ovulation, parental care, and human social evolution. In: Chagnon NA, Irons W, editors. Evolutionary biology and human social behaior: An anthropological perspective. Duxbury; North Scituate, MA: 1979. pp. 402–435. [Google Scholar]
- Alger I. Public goods games, altruism, and evolution. Journal of Public Economic Theory. 2010;12:789–813. [Google Scholar]
- Alger I, Weibull JW. Kinship, incentives, and evolution. American Economic Review. 2010;100(4):1725–58. [Google Scholar]
- Alger I, Weibull JW. A generalization of Hamilton’s rule—Love others how much? Journal of Theoretical Biology. 2012;299:42–54. doi: 10.1016/j.jtbi.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Alvergne A, Oda R, Faurie C, Matsumoto-Oda A, Durand V, Raymond M. Cross-cultural perceptions of facial resemblance between kin. Journal of Vision. 2009;9(6) doi: 10.1167/9.6.23. [DOI] [PubMed] [Google Scholar]
- Anderson E. Sex codes and family life. In: Wilson W, editor. The Ghetto Underclass. Sage Publications; Newbury Park, CA: 1993. pp. 76–95. [Google Scholar]
- Anderson KG. How well does paternity confidence match actual paternity? evidence from worldwide nonpaternity rates. Current Anthropology. 2006;47(3):513–520. [Google Scholar]
- Arcidiocono P, Beauchamp AW, McElroy MB. NBER Working Paper 16517. 2010. Terms of endearment: An equilibrium model of sex and matching. [Google Scholar]
- Balshine S. Patterns of parental care in vertebrates. In: Royale NJ, Smiseth PT, Kölliker M, editors. The evolution of parental care. Oxford University Press; Oxford: 2012. pp. 62–80. [Google Scholar]
- Barber BM, Odean T. Boys will be boys: Gender, overconfidence, and common stock investment. Quarterly Journal of Economics. 2001;116(1):261–292. [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosphilia. Heredity. 1948;2:277–287. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Becker GS. A theory of marriage: Part I. Journal of Political Economy. 1973;81(4):813–846. [Google Scholar]
- Becker GS. A theory of marriage: Part II. Journal of Political Economy. 1974;82(2):S11–S26. [Google Scholar]
- Becker GS. A Treatise on the Family. 2. Harvard University Press; Cambridge, MA: 1991. [Google Scholar]
- Becker GS, Landes EM, Michael RT. An economic analysis of marital instability. Journal of Political Economy. 1977;85(6):1141–1187. [Google Scholar]
- Bergstrom TC. Working paper. University of Michigan; 1994a. On the economics of polygyny. [Google Scholar]
- Bergstrom TC. Working paper. University of Michigan; 1994b. Primogeniture, monogamy, and reproductive success in a stratified society. [Google Scholar]
- Bergstrom TC. On the evolution of altruistic ethical rules for siblings. American Economic Review. 1995;85(1):58–81. [Google Scholar]
- Bergstrom TC. Economics in a family way. Journal of Economic Literature. 1996;34:1903–1934. [Google Scholar]
- Bernheim BD. On the potential of neuroeconomics: A critical (but hopeful) appraisal. American Economic Journal: Microeconomics. 2009;1(2):1–41. [Google Scholar]
- Birkhead T. Promiscuity. Harvard University Press; Cambridge, MA: 2000. [Google Scholar]
- Bishai D, Grossbard S. Far above rubies: Bride price and extramarital sexual relations in uganda. Journal of Population Economics. 2010;23:1177–1187. [Google Scholar]
- Bredart S, French RM. Do babies resemble their fathers more than their mothers? a failure to replicate christenfeld and hill (1995) Evolution and Human Behavior. 1999;20:129–135. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425(6954):179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Buss D. Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures. Behavioral and Brain Sciences. 1989;12:1–49. [Google Scholar]
- Chang Y, Kim S-B. From individual to aggregate labor supply: A quantitative analysis based on a heterogeneous agent macroeconomy. International Economic Review. 2006;47(1):1–27. [Google Scholar]
- Christenfeld NJS, Hill EA. Whose baby are you. Nature. 1995;378:669. doi: 10.1038/378669a0. [DOI] [PubMed] [Google Scholar]
- Clark RD, Hatfield E. Gender differences in receptivity to sexual offers. Journal of Psychology and Human Sexuality. 1989;2(1):39–55. [Google Scholar]
- Clutton-Brock TH. The Evolution of Parental Care. Princeton University Press; Princeton, NJ: 1991. [Google Scholar]
- Croson R, Gneezy U. Gender differences in preferences. Journal of Economic Literature. 2009;47 (2):448–474. [Google Scholar]
- Cushing BS, Martin JO, Young LJ, Carter C. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Hormones and Behavior. 2001;39 (1):48–58. doi: 10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- Dawkins R, Carlisle T. Parental investment, mate desertion and a fallacy. Nature. 1976;262:131–133. [Google Scholar]
- DeBruine LM. Resemblance to self increases the appeal of child faces to both men and women. Evolution and Human Behavior. 2004;25(3):142–154. [Google Scholar]
- Dekel E, Ely JC, Yilankaya O. Evolution of preferences. Review of Economic Studies. 2007;74(3):685–704. [Google Scholar]
- Doepke M, Tertilt M. Women’s liberation: What’s in it for men? Quarterly Journal of Economics. 2009;124(4):1541–1591. [Google Scholar]
- Doepke M, Tertilt M. CEPR Discussion Papers 8441. 2011. Does female empowerment promote economic development? [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Du Q, Wei S-J. NBER Working Paper 16000. 2010. A sexually unbalanced model of current account imbalances. [Google Scholar]
- Eswaran M, Kotwal A. A theory of gender differences in parental altruism. Canadian Journal of Economics. 2004;37(4):918–950. [Google Scholar]
- Fisher RA. The Genetical Theory of Natural Selection. Oxford University Press; Oxford: 1930. [Google Scholar]
- Fisman R, Iyengar SS, Kamenica E, Simonson I. Gender differences in mate selection: Evidence from a speed dating experiment. Quarterly Journal of Economics. 2006;121(2):673–697. [Google Scholar]
- Fortunato L, Archetti M. Evolution of monogamous marriage by maximization of inclusive fitness. Journal of Evolutionary Biology. 2010;23(1):149–156. doi: 10.1111/j.1420-9101.2009.01884.x. [DOI] [PubMed] [Google Scholar]
- Frank RH. If homo economicus could choose his own utility function, would he want one with a conscience? American Economic Review. 1987;77(4):593–604. [Google Scholar]
- Friedmann H. Social parasitism in birds. Quarterly Review of Biology. 1928;3(4):554–569. [Google Scholar]
- Fromhage L, McNamara JM, Houston AI. Stability and value of male care for offspring: is it worth only half the trouble? Biology Letters. 2007;3(3):234–236. doi: 10.1098/rsbl.2006.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S. Human origins and the transition from promiscuity to pair bonding. Proceedings of the National Academy of Sciences. 2012;109:9923–9928. doi: 10.1073/pnas.1200717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary DC. Male, female: The evolution of human sex differences. 2. American Psychological Association; Washington, DC: 2010. [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A. A biological approach to economics through fertility. Economics Letters. 2000;66 (3):241–248. [Google Scholar]
- Grafen A, Sibly R. A model of mate desertion. Animal Behavior. 1978;26:645–652. [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evolution and Human Behavior. 2002;23 (3):193–201. [Google Scholar]
- Gray P, Parkin J, Samms-Vaughan M. Hormonal correlates of human paternal interactions: A hospital-based investigation in urban jamaica. Hormones and Behavior. 2007;52(4):499–507. doi: 10.1016/j.yhbeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Grossbard A. An economic analysis of polygyny: The case of maiduguri. Current Anthropology. 1976;17(4):701–707. [Google Scholar]
- Grossbard-Shechtman A. The economics of polygamy. In: DaVanzo J, Simon J, editors. Research in Population Economics. JAI Press; Boulder, CO: 1980. [Google Scholar]
- Grossbard-Shechtman A. Economic behavior, marriage and fertility: Two lessons from polygyny. Journal of Economic Behavior and Organization. 1986;7(4):415–424. doi: 10.1016/0167-2681(86)90014-4. [DOI] [PubMed] [Google Scholar]
- Haig D. Genetic conflicts in human pregnancy. The Quarterly Review of Biology. 1993;68(4):495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- Hansson I, Stuart C. Malthusian selection of preferences. American Economic Review. 1990;80 (3):529–544. [Google Scholar]
- Hawkes K. Mating, parenting and the evolution of human pair bonds. In: Chapais B, Berman C, editors. Kinship and Behavior in Primates. Oxford University Press; Oxford: 2004. pp. 443–474. [Google Scholar]
- Heifetz A, Shannon C, Spiegel Y. The dynamic evolution of preferences. Economic Theory. 2007;32(2):251–286. [Google Scholar]
- Hitsch GJ, Hortaçsu A, Ariely D. What makes you click? —Mate preferences in online dating. Quantitative Marketing and Economics. 2010;8:393–427. [Google Scholar]
- Houston AI, McNamara JM. A self-consistent approach to paternity and parental effort. Philosophical Transactions: Biological Sciences. 2002;357(1419):351–362. doi: 10.1098/rstb.2001.0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston AI, Szekely T, McNamara JM. Conflict between parents over care. Trends in Ecology & Evolution. 2005;20(1):33–8. doi: 10.1016/j.tree.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. The optimal number of fathers: Evolution, demography, and history in the shaping of female preferences. Annals of New York Academy of Sciences. 2000;907:75–96. doi: 10.1111/j.1749-6632.2000.tb06617.x. [DOI] [PubMed] [Google Scholar]
- Jones AG, Avise JC. Mating systems and sexual selection in male-pregnant pipefishes and seahorses: Insights from microsatellite-based studies of maternity. Journal of Heredity. 2001;92 (2):150–158. doi: 10.1093/jhered/92.2.150. [DOI] [PubMed] [Google Scholar]
- Kokko H, Jennions M. Parental investment, sexual selection and sex ratios. Journal of Evolutionary Biology. 2008;21:919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- Lagerlöf N-P. Sex, equality, and growth. Canadian Journal of Economics. 2005;38(3):807–831. [Google Scholar]
- Laumann EO, Gagnon JH, Michael RT, Michaels S. The Social Organization of Sexuality: Sexual Practices in the United States. The University of Chicago Press; Chicago, IL: 1994. [Google Scholar]
- Maynard Smith J. Parental investment: A prospective analysis. Animal Behavior. 1977;25:1–9. [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nature Neuroscience. 2012;15(5):681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Miller G. The Mating Mind: How Sexual Choice Shaped the Evolution of Human Nature. Doubleday; New York, NY: 2000. [Google Scholar]
- Neff BD, Pitcher TE. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Molecular Ecology. 2005;14(1):19–38. doi: 10.1111/j.1365-294X.2004.02395.x. [DOI] [PubMed] [Google Scholar]
- Netzer N. Evolution of time preferences and attitudes toward risk. American Economic Review. 2009;99(3):937–955. [Google Scholar]
- Orians GH. On the evolution of mating systems in birds and mammals. American Naturalist. 1969;103(934):589–603. [Google Scholar]
- Pagel M. Desperately concealing father: a theory of parent-infant resemblance. Animal Behaviour. 1997;53(5):973–981. [Google Scholar]
- Platek SM, Burch RL, Panyavin IS, Wasserman BH, Gallup GG. Reactions to children’s faces: Resemblance affects males more than females. Evolution and Human Behavior. 2002;23 (3):159–166. [Google Scholar]
- Platek SM, Keenan JP, Mohamed FB. Sex differences in the neural correlates of child facial resemblance: an event-related fmri study. NeuroImage. 2005;25(4):1336–1344. doi: 10.1016/j.neuroimage.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Posner RA. Sex and Reason. Harvard University Press; Cambridge, MA: 1992. [Google Scholar]
- Queller DC. Why do females care more than males? Proceedings: Biological Sciences. 1997;264(1388):1555–1557. [Google Scholar]
- Rasul I. The economics of child custody. Economica. 2006;73(289):1–25. [Google Scholar]
- Rayo L, Becker GS. Evolutionary efficiency and happiness. Journal of Political Economy. 2007;115(2):302–337. [Google Scholar]
- Robson A. Why would nature give individuals utility functions? Journal of Political Economy. 2001;109(4):900–914. [Google Scholar]
- Robson A, Samuelson L. The evolution of decision and experienced utilities. Theoretical Economics. 2011;6(3):311–339. [Google Scholar]
- Sanchez R, Parkin JC, Chen JY, Gray PB. Oxytocin, vasopressin, and human social behavior. In: Gray PB, Ellison PT, editors. Endocrinology of Social Relationships. Harvard University Press; Cambridge, MA: 2009. pp. 317–339. [Google Scholar]
- Siow A. Differential fecundity, markets, and gender roles. Journal of Political Economy. 1998;106(2):334–354. [Google Scholar]
- Smith I. Reinterpreting the economics of extramarital affairs. Review of Economics of the Household. 2012;10(3):319–343. [Google Scholar]
- Stevenson B, Wolfers J. Marriage and divorce: Changes and their driving forces. The Journal of Economic Perspectives. 2007;21(2):27–52. [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evolution and Human Behavior. 2000;21 (2):79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Temeles EJ, Kress WJ. Adaptation in a plant-hummingbird association. Science. 2003;300(5619):630–633. doi: 10.1126/science.1080003. [DOI] [PubMed] [Google Scholar]
- Tertilt M. Polygyny, fertility, and savings. Journal of Political Economy. 2005;113(6):1341–1371. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Vaillant NG, Wolff F-C. Positive and negative preferences in human mate selection. Review of Economics of the Household. 2011;9(2):273–291. [Google Scholar]
- Varian HR. Microeconomic Analysis. 3. W.W. Norton & Company; New York: 1992. [Google Scholar]
- Volk A, Quinsey VL. The influence of infant facial cues on adoption preferences. Human Nature. 2002;13:437–455. doi: 10.1007/s12110-002-1002-9. [DOI] [PubMed] [Google Scholar]
- Wade MJ. Sexual selection and variance in reproductive success. American Naturalist. 1979;114:742–764. doi: 10.1086/424531. [DOI] [PubMed] [Google Scholar]
- Wade MJ, Shuster SM. The evolution of parental care in the context of sexual selection: A critical reassessment of parental investment theory. American Naturalist. 2002;160(3):285–292. doi: 10.1086/341520. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (avpr1a) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences. 2008;105(37):14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-J, Zhang X. The competitive saving motive: Evidence from rising sex ratios and savings rates in china. Journal of Political Economy. 2011;119(3):511–564. [Google Scholar]
- Weibull JW. Evolutionary Game Theory. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Westneat DF, Sherman PW. Parentage and the evolution of parental behavior. Behavioral Ecology. 1993;4(1):66–77. [Google Scholar]
- Westneat D, Sherman P, Morton M. The ecology and evolution of extra-pair copulations in birds. Current Ornithology. 1990;7:331–370. [Google Scholar]
- Whittingham LA, Dunn PO. Male parental care and paternity in birds. Current Ornithology. 2001;16:257–298. [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the v1a receptor from a monogamous vole. Nature. 1999;400(6746):766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Zeitzen MK. Polygamy: A Cross-Cultural Analysis. Berg Publishers; Oxford: 2008. [Google Scholar]