Abstract
Background
Liver gene transfer offers hope for the correction of genetic and acquired disorders. Efficient gene transfer in large animals can be obtained with hydrodynamic gene transfer (HGT), a method that can achieve sufficient levels of gene delivery.
Material/Methods
To test the relative efficiency between plasmid versus foamy virus (FV) vector-based liver gene transfer efficiency, we applied HGT in 4 juvenile pigs, using the same plasmid backbone, either naked or coated as a FV vector particle. Gene transfer efficiency and persistence of expression was assayed by PCR and real-time PCR, respectively, at 1 week and at 1 month after the infusions.
Results
HGT was tolerated well and no adverse reactions were observed. Plasmid injections resulted in no detectable DNA sequences at 1 week. At the 1 month time point, 2/15 liver sections analyzed were positive for the presence of plasmid DNA. When FV vectors were infused under identical conditions, 18/28 (64.3%) of the liver samples were positive for the presence of vector sequences, and the expression levels reached 29.7 and 15.6% of the endogenous GAPDH levels in the injected and the adjacent liver lobes.
Conclusions
Our results indicate that medium-term therapeutic levels of gene expression can be obtained with FV vectors, an effect that can be attributed to the potential of the HGT procedure and to the natural affinity of FV vectors for hepatocytes.
Keywords: liver, swine, foamy virus, hemodynamic, gene therapy
Background
Gene delivery with viral vectors is a relatively novel technology that has the potential to treat both genetic and acquired disorders such as cancer. A major hurdle in all therapeutic gene therapy applications is the delivery method. Most current protocols require the ex vivo manipulation of target cells and reintroduction of the transduced cells in the host [1]. Ideally, gene transfer with minimal manipulation of the target cells would be beneficial in terms of safety, simplicity, logistics, and expenses. Even better, delivery of the vector particles in situ is a highly desired solution because it would result in targeted delivery and minimal off-target transduction events. The liver is an organ that could be targeted for gene transfer applications because its blood-supply can be accessed reliably with current technology. In addition, hepatocytes are long-lived cells that can sustain gene expression from episomal vectors. A number of studies have shown expression of plasmid or naked DNA following hydrodynamic gene delivery to the organ [2].
Hydrodynamic gene transfer (HGT), initially described in mice, is a well-established method for liver-directed gene delivery by means of rapid intravenous injection, via the tail vein, of a large volume of plasmid DNA (pDNA) [3,4]. The rapid and large-volume infusion of solutions containing macromolecules, normally impermeable to the cell membrane, generates high hydrodynamic pressure in the circulation, in effect refluxing to the target organ. The expanded perivenous area, a result of the extravasation of the infused solution, generates high pressure to the exterior of the cells. When this pressure reaches a critical level, breaches (pores) are created on the cell membrane, leading to the intracellular insertion of the macromolecules. After a few seconds, while the pressure declines during the post-injection period, the defects are restored, in effect trapping the infused molecules inside the cytoplasm. Finally, the body adapts to the volume overload over time and homeostasis is restored [5–7]. The relative technique has already been applied to large animal models with promising results [8–11]. In our experiment, the minimally invasive technique that we used involves a selective isolation of liver segments using a custom-made balloon catheter inserted via the external jugular vein under fluoroscopy and the rapid delivery of a large volume (100 ml) of DNA-containing fluids in a short period of time (10 sec) [12]. Overall, the hydrodynamic procedure is considered an effective means for gene transfer with adenoviral, AAV vectors, and integrating retroviral vectors [13].
A number of integrating viral vectors have been used as vehicles for hepatocyte gene transfer. Evidence for efficient gene transfer has been obtained with AAV and lentiviral vectors in rodents and large animals [14–16]. A novel retroviral vector system that has the potential to transduce primary cells has been developed from the Prototype Foamy Virus (PFV) [17]. The virus is non-pathogenic to humans and has a favorable safety profile; zoo-keepers and primate hunters that were infected with the wt virus developed antibodies and no other associated pathology over a long-term follow-up period [18]. FV-derived vectors retain a minimal cis-acting sequence, have a self-inactivating design, and are devoid of the viral transactivator, making them safe alternative vehicles to the current retroviral vectors [19]. In addition, they can transduce non-dividing cells such as HSC, they are not prone to silencing, and they have a relatively safe integration profile, while their LTR seems to isolate the expression cassette from the neighboring genes [20].
Since current gene therapy technology is getting closer to clinical applications, testing in a large animal model such as swine could provide useful information for the optimal design of future human trials. In the current study, we investigated the marking efficiency of liver hydrodynamic injections with plasmid or FV vectors. To our knowledge, liver gene transfer with FV vectors (or other retroviral vectors) in the swine model has not yet been reported. Our results indicate that hydrodynamic injections in the pig liver are safe, can efficiently transfer a reporter gene in the liver tissue, and can deliver FV-derived retroviral vectors with superior long-term expression.
Material and Methods
Animal protocol
All animal manipulation protocols were approved by the Biomedical Research Foundation of the Academy of Athens (BRFAA) Institutional Ethics Committee and the Animal Care Committee of East Attica County, Athens, Greece (Permission Number K2263, 29/3/2006). Four male (Landrace x Large White) swine, 8 months old, weighing on average 82.4±5.8 kg were used for the study. The experiment took place in the Center for Experimental Surgery of the BRFAA, Athens, Greece.
Animal preparation and anesthetic induction
Four pigs underwent 8-day quarantine in the Animal House prior to experimentation. Physical examination and a pre-anesthetic blood work included a complete blood count and serum chemistry profile. Each pig was pre-anesthetized with an IM injection of 10 mg/kg ketamine (Imalgen; Merial), 4 mg/kg azaperone (Suicalm; Jansen-Cilag), and 0.05 mg/kg atropine (Atropine; Demo). Anaesthesia was induced 15 min later with intravenous administration of 2.5–3.5 mg/kg propofol (Diprivan 1% w/v; AstraZeneca) and maintained by closed circuit system inhalation of sevoflurane (Sevorane, Abbott, 2.5–3.5% in 2.5 lt O2). Tidal volume was kept at 10–15 ml/kg and the respiratory rate was 14–16 breaths/min. Lactated Ringers solution was administered intravenously at a rate of 10 ml/kg/h and 4 mg/kg IM of Carprofen (Rimadyl, Pfizer) was used for pre-op analgesia and buprenorphine (5 mg/kg) IM for post-op analgesia every 8 h.
Detailed description of surgical techniques
The animals were placed in a dorsal recumbent position and the skin, at the incision area, was infiltrated with 4–5 ml of lidocaine 2% (Xylocaine, Astra Zeneca). The right external jugular vein was identified and isolated from the surrounding tissues. The vessel was ligated at its distal end and a venotomy was performed to place an introducer sheath (Avanti+, Cordis Europa, NL) proximally. Then, a guide wire (TRIUMPH, Lake Region Mfg. Inc.) was inserted and advanced under radiological control (C-arm, Philips Medical Systems, NL) through the external jugular vein to the right heart into the inferior vena cava (IVC) and then into the right hepatic vein to the periphery of the right lateral liver lobe [2,9,10,12].
Hydrodynamic gene transfer (HGT)
The gene delivery was attempted using a 7Fr rigid-double lumen, custom made, balloon catheter with multiple holes at its end. The catheter was advanced under fluoroscopy over the guide wire in the right lateral liver lobe and inflated with 2–4 ml of saline and contrast medium (Lobiditrol, XENETIX, Guerbet) via the injection channel while the guide wire had been removed. This allowed fluoroscopic confirmation of the correct deployment of the catheter and ensured that there was no venous backflow from the injected liver vein into the IVC before injection of the genetic material. The catheter was then connected to a tomography injector pump (CT 610, Vistron, Medran, U.S.A.) and 100 ml of HBSS with genetic material were injected at a rate of 10ml/sec. The balloon was kept inflated for 10 min after the injection and its placement was rechecked to exclude displacement and backflow. Upon completion, the catheter was removed and the vein was ligated with 3-0 silk suture. The wound was approximated in layers using 2-0 Vicryl suture, and the skin was closed using 0 silk suture [2,9,10,12].
Euthanasia
On days 3, 7, 13, and 22 post-injection, blood samples for standard blood count and liver biochemistry (Glucose, Urea, Creatinine, Total Albumin, SGOT, SGPT, γGT, ALP, CPK, LDH, Amylase, Triglycerides, HDL, LDL, and Uric Acid) were taken along with tru-cut biopsies of the right lateral injected lobe and left lateral untreated lobe of the liver under ultrasound guidance. On day 30, tru-cut biopsy was repeated after laparotomy and the animal was euthanized with a bolus dose of pentobarbital sodium euthanasia solution (Dolethal, Vetoquinol) at a dose of 2ml/kg [12].
Foamy virus (FV) vector production and titration
FV vectors were produced by calcium phosphate transient transfection in 293T cells. Cells were grown in DMEM (GIBCO) supplemented with 10% FCS (GIBCO) and 0.1% penicillin/streptomycin in a fully humidified atmosphere of 5% CO2. For vector production, 3×106 cells were plated in 10-cm dishes and the next day the cells were co-transfected with vector and helper plasmids. The vector plasmid was the expression plasmid used for FV vector production and had the viral Mscv LTR promoter driving expression of an enhanced green fluorescence reporter protein (GFP) [19]. Three different helper plasmids expressing the FV gag, pol, and env proteins were used at 12, 1.5, and 0.8 ug per 10-cm dish, respectively; 12 ug of vector plasmid was added in the mix. Transfection was terminated 16 h later, complete fresh medium was added, and the vector stock was harvested 48 h later. The stock was spun at 200g for 5 min, filtered through a 0.45 um PES filter, and then subjected to ultracentrifugation in Sorvall at 13000g for 4 h at 4°C. The vector was resuspended in complete medium and used either fresh or after storage at −70°C. Vector stocks were titrated in HeLa cells: briefly, 10E5 cells were plated in 6-well dishes and were transduced 24 h later with different volumes of the viral stock. The cells were collected 48 h later, and GFP+ cells were assayed in a flow cytometer (COULTER FC 500). Vector stocks titers are expressed as HeLa transducing particles per ml of vector supernatant.
Plasmid preparation
The plasmid used in the study was the same vector plasmid used for FV vector production. The plasmid grew overnight in 500 ml LB cultures in 2 liter flasks and was isolated using commercially available kits (Quiagen MaxiPrep). A total of 4 mg of vector plasmid expressing GFP was collected, resuspended in sterile Hank’s balanced salt solution (HBSS, GIBCO) at a concentration of 40 μg/ml, and used for hydrodynamic injections.
Foamy virus detection assays
DNA was isolated from liver samples using standard phenol-chloroform extraction techniques. Genomic DNA was subjected to PCR with the following primers; Sense FP3: AATGCTGGCATGGGAATAGT. Antisense FP4: TAACTTCCTTGGGTGGCAAG. The primers hybridize to the cis-acting sequence of the FV provirus and the amplicon is 294 bp long. PCR conditions were 94C for 4 min (1 cycle) followed by 60°C for 1 min, 72°C for 1 min, and 94°C for 1 min for a total of 29 cycles. The products were run on 1% agarose gel.
Relative quantitation assay for GFP expression
For the detection of GFP and pig GAPDH mRNA, RNA was extracted from liver specimens using TriReagent (Molecular Research Center, Cincinnati, Ohio) and reverse-transcribed using MMLV-RT (Promega, Madison, Wisconsin) according to the manufacturer’s recommendations. Using an ABI Prism 7000 instrument (Applied Biosystems, Carlsbad, California), 250 ng of synthesized cDNA was amplified in duplicate 25 ul reactions. The levels of GFP and pig GAPDH mRNA were analyzed using SYBR Green Master mix (Applied Biosystems, Carlsbad, California) and primers: GFP: forward 5’-CAACAGCCACAACGTCTATATCATG-3’, reverse 5’-ATGTTGTGGCGGATCTTGAAG-3’. Pig GAPDH: Forward 5’-CAGCAATGCCTCCTGTACCA-3’, Reverse: 5’-GATGCCGAAGTTGTCATGGA-3’). The relative expression of GFP over endogenous pig GAPDH expression was calculated using the 2Δct method developed as described [21].
Results
Safety of the procedure
The procedure was well tolerated by all animals without any adverse events and no immediate complications were observed. The treated liver was studied ultrasonographically during infusion; dilatation of the liver vein and hyperechoic parenchyma was documented, which returned to normal echogenicity in follow-up ultrasound examinations. In the first animal, on day 3, a hematoma was observed during the ultrasound study, which remained stable in the following days. Liver transaminases were observed to have a mild, not statistically significant, rise on day 3, which returned to normal on day 7. All other measurements remained within normal range.
Plasmid delivery
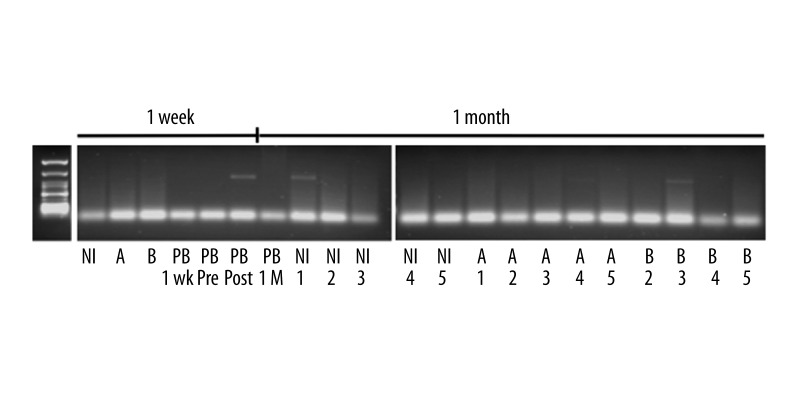
For naked plasmid delivery, 4 mg of plasmid DNA diluted in HBSS to a total volume of 100 ml HBSS were injected within 10 sec according to the protocol. Overall, 2 animals received plasmid infusions; the procedure was well tolerated and no adverse effects were noted during the infusion or the observation period. One of the animals was sacrificed after 1 week and the other after 4 weeks; at this point, the liver was excised and the injected area was sliced and subjected to DNA analysis. In addition, we analyzed an adjacent and a non-injected lobe along with peripheral blood in order to test whether plasmids could be detected in the circulation. At week 1, qualitative PCR analysis showed no plasmid amplicons present in the injected animal (Figure 1(A)). Of note, plasmid sequences could be detected in the peripheral blood obtained at 1 h post-injection, indicating that venous occlusion was not optimal and some leakage of the DNA material occurred during infusion. Alternatively, this could have been the result of plasmid shedding from apoptotic cells or residual non-integrated plasmid still in the circulation.
Figure 1.
Analysis for the presence of plasmid sequences in the non-injected (NI), the injected (A) the adjacent (B) pig liver lobes and in the peripheral blood (PB) after HGT injections. Data at week 1 were obtained from the first animal and at 1 month from the second animal.
The second animal was sacrificed at 1 month post HGT; no plasmid DNA could be detected in the peripheral blood. In the analyzed liver sections, there were overall 2 positive samples from a total of 15 (Figure 1(B)). These 15 samples were derived from the injected lobe (labeled A), the adjacent lobe (labeled B), and from a non-injected site (labeled NI). The presence of vector sequences in the NI and B samples indicates that injections at peripheral branches can clearly result in back-flows and loss of precise tissue targeting. The lack of plasmid amplicons in the peripheral blood at 1 month indicates that whatever plasmid copies had persisted in the animal were confined within the infused tissue and did not circulate. The fact that plasmid DNA persisted in the tissues at 1 month indicates that episomal presence in the long-lived hepatocyte can be obtained in vivo, albeit at relatively low levels.
FV vector delivery
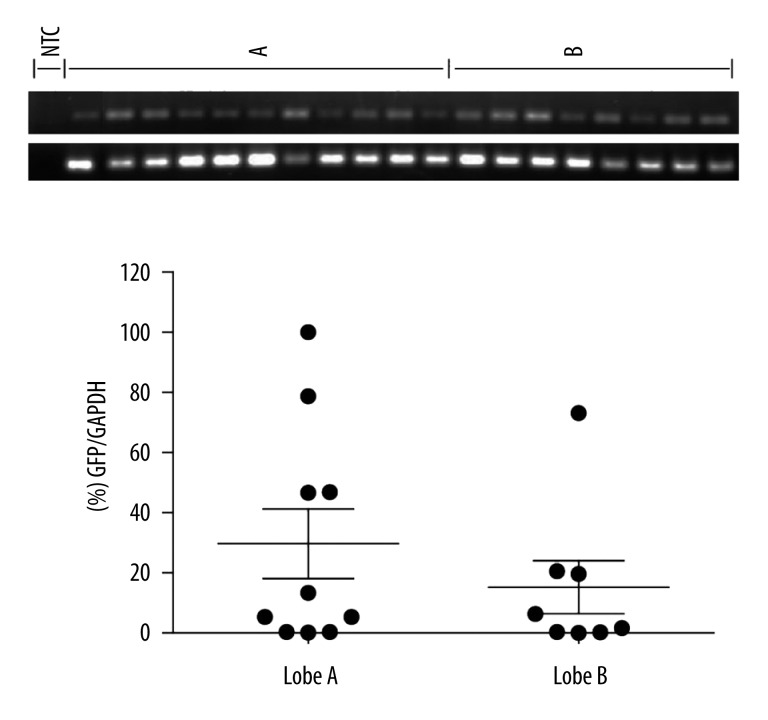
Transduction of pig hepatocytes in situ was attempted with an FV vector that expressed GFP off an Mscv LTR promoter. In the first attempt, a total of 0.74×107 vector particles were administered according to the protocol. At 1 month, the animal was sacrificed and the liver was excised. On macroscopic examination, there was a large hematoma at the site of the injection, presumably a post-catheterization traumatic event. Nevertheless, the lobe was dissected and subjected to DNA isolation. The results showed that the vector could only be detected in the hematoma and in none of the surrounding tissue, indicating that the bulk of the vector preparation was lost in the hematoma (data not shown). In the second experiment, a higher number of vector particles (1.9×107) were administered under identical conditions; the sole modification was that the catheter was not forced to the most peripheral branches in order to avoid the traumatic rupture of the vessels. One month post-injection the animals were sacrificed and the liver was removed; the injected area was excised and further dissected in a total of 28 smaller pieces for DNA analysis. Overall, 18/28 samples (64.3%, data not shown) tested positive for the FV genomes in the liver DNA using a qualitative PCR assay; no positive samples were detected in the non-injected (distal) liver lobes. Subsequently, the positive samples were subjected to realtime PCR to assay expression levels relative to the endogenous GAPDH gene (Figure 2). As shown, GFP mRNA expression in the injected lobe A and in the adjacent lobe B reached 29.7% and 15.6% of the endogenous GAPDH gene, respectively. Our data indicate efficient and persistent expression of the delivered vector in the absence of any adverse effects at 1 month post-liver infusion.
Figure 2.
Relative levels of the foamy virus vector-encoded reporter are presented as percent of the endogenous GAPDH gene in the liver tissue. A and B are the injected and the non-injected (adjacent) lobes. Results were obtained at 1 month post HGT infusion. Dots indicate individual segments of the relative liver lobe. NTC, non-template control.
Discussion
Genetic manipulation of the liver has been explored for the correction of inherited disorders [15,22]. The same technology could be used for the elimination of acquired disorders such as hepatocellular carcinoma [22]. Hydrodynamic gene transfer (HGT) has evolved as the method of choice and has been amply tested in small and large animal models [24,25]. In our study, we used this methodology in order to compare 2 gene delivery methods: naked plasmid and FV vectors. Although HGT allows the introduction of genetic material through transient hepatocyte membrane disruption [26], we reasoned that retroviral vectors would result in superior gene transfer levels because cell entry could also be enhanced by receptor-mediated endocytosis and, persistent expression could be attained through genomic integration. In addition, we hypothesized that cellular entry through a natural receptor would take place in cells not affected by the HGT procedures and would lead to superior vector persistence from naturally transduced, intact cells. We tested this hypothesis using the same plasmid backbone and expression cassette that was used for the generation of vector particles.
At the technical level, we attempted to improve on current HGT techniques by exploring the possibility of infusing peripheral branches, but not end-branches, for the purpose of enhanced targeted delivery without damaging the organ or having backflow of the solution. A major problem of the infusion has been catheter failure due to the high hydrodynamic pressure, causing leakage of the solution or inability to deliver the solution within a short period of time. Solving these problems, we demonstrated that it is feasible to infuse the solution with a single catheter without further disruption of the blood flow such as occlusion of the IVC, the portal vein, or the hepatic artery. Combining these results with the findings of Fabre et al. [24], who proved that minimally invasive HGT is not achieved due to Bernoulli’s principle, we conclude that pressurization is not so critical in this technique; it is rather the prolonged presence of the vector in the organ that makes the difference. For optimal use, the injection-occlusion catheter could be placed in the right hepatic vein and a second occlusion catheter could be percutaneously inserted in the portal vein [27] and guided into the right lobe without obstructing the portal circulation, which in effect would restrict the flow to the left part of liver. Thus, the vector solution would possibly stay in the organ for a prolonged period of time with minimal effect on blood circulation.
In the case of plasmid infusions, we analyzed the animals at 1 week and 1 month post-infusion. At the 1-week time point we could not detect any plasmid sequences. Since HGT-mediated plasmid delivery has been tested successfully in earlier experiments [10,24], we reasoned that the lack of plasmid DNA in the analyzed samples was due to loss of hepatocytes from the high pressure applied in the peripheral branches. This explanation is further supported by the results obtained in the second animal, where plasmid DNA could be traced in the adjacent and non-injected lobes; these findings indicate that the relative mild pressure obtained by the back-flow of the infusion material and the flooding of the liver was adequate for the entry of plasmids in the hepatocytes. However, the overall efficiency of the procedure was suboptimal; in the non-injected and adjacent lobes, a similar degree of PCR-positive material was detected (13.3%). Our findings are consistent with those reported from similar experiments [28–30] in which a picture of lower gene expression in pigs compared to rodents and a rapid reduction in gene expression that subsided in the first 3 days accompanies the HGT of plasmids in pig livers.
The efficiency, however was significantly improved when foamy virus vectors were used for delivering the DNA. Overall, we observed that 64.3% of the tissue sections were PCR-positive for vector genomes, a nearly 5-fold improvement relative to the values observed with the plasmid method. In addition, expression relative to the endogenous GAPDH gene was at levels that could be considered as therapeutic for genetic disorders such as hemophilia A or B [31]. Specifically, foamy virus mRNA levels reached 29.7% in the injected lobe and 15.6% in the distant lobe. Since vector performance requires the use of tissue-specific promoters [32], our data underestimate the potential of FV vectors for liver gene expression. The presence of significant transduced liver tissue in the distant non-injected lobe can be attributed to the natural suitability of hepatocytes for FV transduction. Although no specific reference in the literature has reported primary hepatocyte transduction with FV vectors, the fact that no FV-restricted cell line has been identified and the report of the HepG2 cell line transduction by FV vectors [33] suggests that hepatocytes could be a valid target for FV vectors. In addition, our data show that persistent expression can be obtained with the FV vectors. Expression was analyzed at 1 month post-HGT, a short time-frame for the long-lived hepatocytes, which have an average life span of 5 months [34]. For such slow-dividing cells, FV vectors may be well suited as gene transfer vehicles because they can survive in quiescent cells for up to 10 days [35] and enter cells upon nuclear membrane breakdown [36]. However, gene addition in non-dividing cells would be a highly desirable property of any gene transfer vehicle; lentiviral vectors have a clear advantage over the rest of the retroviral vectors in transducing cells in G0 [37]. In the case of liver gene transfer, the advantage of lentiviral vectors would be critical only if modifications of the methodology would allow a mild treatment of the organ with minor damage and tissue regeneration. Until then, the potential of FV vectors to survive as DNA particles in the host cell [35] could outweigh their inability to access the nucleus of the cell in the absence of nuclear membrane breakdown.
Conclusions
Our data show that medium-term therapeutic levels of gene expression can be obtained with FV vectors, an effect that can be attributed both to the potential of the HGT procedure and to the natural affinity of FV vectors for hepatocytes.
Footnotes
Source of support: Departmental sources
Statement
All authors disclose that they had no sponsorship or funding arrangements relating to the research protocol and no conflict of interest.
References
- 1.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–58. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 2.Kamimura K, Liu D. Physical approaches for nucleic acid delivery to liver. AAPS J. 2008;10(4):589–95. doi: 10.1208/s12248-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6(7):1258–66. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 4.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10(10):1735–37. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 5.Al-Dosari MS, Knapp JE, Liu D. Hydrodynamic delivery. Adv Genet. 2005;54:65–82. doi: 10.1016/S0065-2660(05)54004-5. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi N, Kuramoto T, Yamaoka K, et al. Hepatic uptake and gene expression mechanisms following intravenous administration of plasmid DNA by conventional and hydrodynamics-based procedures. J Pharmacol Exp Ther. 2001;297(3):853–60. [PubMed] [Google Scholar]
- 7.Lecocq M, Andrianaivo F, Warnier MT, et al. Uptake by mouse liver and intracellular fate of plasmid DNA after a rapid tail vein injection of a small or a large volume. J Gene Med. 2003;5(2):142–56. doi: 10.1002/jgm.328. [DOI] [PubMed] [Google Scholar]
- 8.Eastman SJ, Baskin KM, Hodges BL, et al. Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum Gene Ther. 2002;13(17):2065–77. doi: 10.1089/10430340260395910. [DOI] [PubMed] [Google Scholar]
- 9.Yoshino H, Hashizume K, Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13(24):1696–702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- 10.Kamimura K, Suda T, Xu W, et al. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17(3):491–99. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunetti-Pierri N, Stapleton GE, Law M, et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17(2):327–33. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsimpoulas M, Zacharoulis D, Rountas C, et al. Minimal invasive technique for gene delivery in porcine liver lobe segment. J Invest Surg. 2011;24(1):13–17. doi: 10.3109/08941939.2010.519815. [DOI] [PubMed] [Google Scholar]
- 13.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15:2063–69. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 14.Follenzi A, Sabatino G, Lombardo A, et al. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum Gene Ther. 2002;13(2):243–60. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- 15.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–47. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 16.Patijn GA, Lieber A, Meuse L, et al. High-efficiency retrovirus-mediated gene transfer into the livers of mice. Hum Gene Ther. 1998;9(10):1449–56. doi: 10.1089/hum.1998.9.10-1449. [DOI] [PubMed] [Google Scholar]
- 17.Lindemann D, Rethwilm A. Foamy virus biology and its application for vector development. Viruses. 2011;3(5):561–85. doi: 10.3390/v3050561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gessain A, Calattini S. Emergence of simian foamy viruses in humans: facts and unanswered questions. Future Virol. 2008;3:71–81. [Google Scholar]
- 19.Trobridge G, Josephson N, Vassilopoulos G, et al. Improved foamy virus vectors with minimal viral sequences. Mol Ther. 2002;6(3):321–28. doi: 10.1006/mthe.2002.0672. [DOI] [PubMed] [Google Scholar]
- 20.Hendrie PC, Huo Y, Stolitenko RB, Russell DW. A rapid and quantitative assay for measuring neighboring gene activation by vector proviruses. Mol Ther. 2008;16(3):534–40. doi: 10.1038/sj.mt.6300398. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Paulk NK, Wursthorn K, Wang Z, et al. Adeno-associated virus gene repair corrects a mouse model of hereditary tyrosinemia in vivo. Hepatology. 2010;51(4):1200–8. doi: 10.1002/hep.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smerdou C, Menne S, Hernandez-Alcoceba R, Gonzalez-Aseguinolaza G. Gene therapy for HCV/HBV-induced hepatocellular carcinoma. Curr Opin Investig Drugs. 2010;11(12):1368–77. [PubMed] [Google Scholar]
- 24.Fabre JW, Whitehorne M, Grehan A, et al. Critical physiological and surgical considerations for hydrodynamic pressurization of individual segments of the pig liver. Hum Gene Ther. 2011;22(7):879–87. doi: 10.1089/hum.2010.144. [DOI] [PubMed] [Google Scholar]
- 25.Aliño SF, Herrero MJ, Noguera I, et al. Pig liver gene therapy by noninvasive interventionist catheterism. Gene Ther. 2007;14(4):334–43. doi: 10.1038/sj.gt.3302873. [DOI] [PubMed] [Google Scholar]
- 26.Zhang G, Gao X, Song YK, et al. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11(8):675–82. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastman SJ, Baskin KM, Hodges BL, et al. Development of catheter- based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum Gene Ther. 2002;13:2065–77. doi: 10.1089/10430340260395910. [DOI] [PubMed] [Google Scholar]
- 28.Kamimura K, Suda T, Xu W, et al. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17(3):491–99. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Song YK, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy. 1999;6:1258–66. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 30.Fabre JW, Grehan A, Whitehorne M, et al. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther. 2008;15(6):452–62. doi: 10.1038/sj.gt.3303079. [DOI] [PubMed] [Google Scholar]
- 31.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frauli M, Ribault S, Neuville P, et al. Adenoviral-mediated skeletal muscle transcriptional targeting using chimeric tissue-specific promoters. Med Sci Monit. 2003;9(2):BR78–84. [PubMed] [Google Scholar]
- 33.Moore MD, McGarvey MJ, Russell RA, et al. Stable inhibition of hepatitis B virus proteins by small interfering RNA expressed from viral vectors. J Gene Med. 2005;7(7):918–25. doi: 10.1002/jgm.739. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald RA. “Lifespan” of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats”. Arch Intern Med. 1961;107:335–43. doi: 10.1001/archinte.1961.03620030023003. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann-Che J, Renault N, Giron ML, et al. Centrosomal latency of incoming foamy viruses in resting cells. PLoS Pathog. 2007;3(5):e74. doi: 10.1371/journal.ppat.0030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trobridge G, Russell DW. Cell cycle requirements for transduction by foamy virus vectors compared to those of oncovirus and lentivirus vectors. J Virol. 2004;78(5):2327–35. doi: 10.1128/JVI.78.5.2327-2335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naldini L, Blömer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–67. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]