Abstract
Recent technological advances in photonics are making intravital microscopy (IVM) an increasingly powerful approach for the mechanistic exploration of biological processes in the physiological context of complex native tissue environments. Direct, dynamic and multiparametric visualization of immune cell behavior in living animals at cellular and subcellular resolution has already proved its utility in auditing basic immunological concepts established through conventional approaches and has also generated new hypotheses that can conversely be complemented and refined by traditional experimental methods.
The insight that outgrowing tumors must not necessarily have evaded recognition by the adaptive immune system, but can escape rejection by actively inducing a state of immunological tolerance calls for a detailed investigation of the cellular and molecular mechanisms by which the anti-cancer response is subverted. Along with molecular imaging techniques that provide dynamic information at the population level, IVM can be expected to make a critical contribution to this effort by allowing the observation of immune cell behavior in vivo at single cell-resolution. We review here how IVM-based investigation can help to clarify the role of cytotoxic T lymphocytes (CTL) in the immune response against cancer and identify the ways by which their function might be impaired through tolerogenic mechanisms.
Introduction
The mammalian immune system has, in principle, the ability to reject malignantly transformed cells, which is at least in part based on the recognition of tumor-expressed antigens (1–3). Thus, the clinical manifestation of tumors in cancer patients likely reflects the failure of innate and adaptive immune control mechanisms that may otherwise constantly clear newly transformed cells in healthy individuals. Tumor cells can subvert immune-mediated rejection through loss of antigenicity (4, 5) and through the induction of tumor-specific immune tolerance (6, 7). The latter tenet is the foundation of all forms of immunotherapy, the success of which will ultimately be determined by a detailed understanding of the cellular and molecular processes that regulate immunity and tolerance at the interface with malignancy (8–14).
Our current concepts in this field are for the most part based on the descriptive analysis of blood and tissue samples from human patients as well as on the investigation of various mouse models of transplanted, chemically induced, genetically programmed, or truly spontaneous tumors. Animal models permit flexible perturbation of the experimental system and have allowed us to identify some key molecular and cellular players that determine the outcome of anti-cancer immune responses. The next step will be to define the exact mechanics of these responses, which may differ greatly in various settings. It will be important to elucidate the topography, dynamics, and biological consequences of the various cellular interactions of immune cells with each other and with the tumor cells. However, population level measurements of homogenized tissue samples, static histological snap-shots, and reductionist cellular in vitro systems allow only inferences on what exactly may occur in vivo in the complex microenvironments of secondary lymphoid organs and the tumor tissue, both of which are likely the central battlefields of the tumor-immune system interaction. Various modalities of molecular imaging, such as Positron Emission Tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), or fluorescence molecular tomography (FMT) (reviewed in (15)) add tremendous utility by allowing the non-invasive, longitudinal study of cellular and molecular activity in vivo, for instance by tracking the fate of anti-tumor T cells in adoptive T cell therapy of cancer not only in mice (16), but eventually also in humans. What they will likely not provide, however, is multiplexed information on single cell behavior at high temporal resolution.
Dynamic microscopic imaging in living animals is a rapidly developing methodology that can fill this gap and has already had tremendous impact not only in neuroscience or developmental biology, but more recently also in immunology (17–22). In this review we will briefly describe the features that have made particularly multiphoton intravital microscopy (MP-IVM) such a powerful new tool in immunological research. Then we will discuss some basic features of T cell immune responses, with a focus on CTL and their effector functions and will speculate on how MP-IVM can help to identify the steps at which tumor-induced tolerance mechanisms hinder the effective execution of CTL effector function against tumors.
Mutliphoton Intravital Microscopy in Immunology
The availability of various technologies to study immune phenomena has not only determined the practice of research in different eras of immunology, but has also always defined the perspective from which researchers were at that time able to understand the different features of the immune system. Currently there is some indication that the recent possibility to visualize immune processes in vivo may lead to an enhanced appreciation of the dynamic nature of immune responses at the molecular and cellular level.
IVM is not new, having already been utilized by the natural scientists of the 17th century (23, 24), and has been instrumental in shaping some of the very basic concepts of cellular immunology (25–27). Since the importance of leukocyte trafficking for immune surveillance and response was recognized (28), IVM has been instrumental in elucidating the topography and the molecular mechanisms of directed and regulated cell recruitment from the bloodstream into tissues, a field at the intersection of vascular biology and immunology (29). However, it is only since the development of multiphoton microscopy (30), which has opened up a window to observe what occurs deep in the tissues outside of blood and lymphatic vessels, that IVM-based studies have caught particular attention and began to inspire mainstream immunologists.
The success of multiphoton microscopy (MPM) is based on its potential to overcome several limitations of conventional fluorescence microscopy, namely poor optical penetration into turbid tissues as well as photobleaching and phototoxicity, all of which are based on the efficient interaction of visible light with matter in the form of absorption and scattering. Mitigation of these phenomena is achieved by utilizing the near-simultaneous interaction of fluorochromes with several photons (hence "multi-photon") of low energy, instead of individual, high-energy photons, for fluorescence excitation. Multiphoton excitation events occur only in situations of extremely high photon density, such as in the femtoliter volume around the focal point (the "focal volume") of a high numerical aperture objective lens guiding pulsed infrared light into the sample (30). The instrumentation and theoretical foundation of this technique are well described elsewhere (31–33). Here we will only briefly discuss the chief practical benefits.
Optical penetration
The tissue depths at which one can obtain image information at sufficient signal-to-noise (S/N) ratio and spatial resolution is limited by the scattering and absorption of light. In the case of fluorescence microscopy both the excitation and the emitted light are degraded in this manner. The principle of multiphoton excitation alleviates both limitations to a considerable degree. In MPM light of the infrared spectrum (typically between 700 and 1300 nm wavelength) is used for excitation, which is less efficiently scattered and absorbed by the tissue overlying the structures of interest in the sample compared to light of the visible spectrum. The illumination beam can therefore be focused deeper within turbid tissues. Furthermore, since fluorescence is only generated in the focal volume, the fraction of the emitted light that is scattered on the way out of the tissue does not loose its spatial information as in single-photon microscopy and can be used for the generation of an image (and does not need to be excluded through use of a confocal pinhole aperture). This increases the S/N ratio dramatically in tissue depths at which scattering becomes significant (typically beyond 75–150 µm, depending on the tissue composition). The depth limit of currently available technology lies at around 1 mm in favorable tissues such as the brain (34).
Phototoxicity and photobleaching
High-energy light-illumination of biological tissues affects cellular processes by various mechanisms, such as generation of reactive oxygen species and heat-effects through energy absorption. The biological effects on immune cells, apparent for instance as enhancement of their adhesive interactions with the vascular endothelium, are well documented (35). In MPM, the use of infrared light limits energy absorption of the tissue outside the focal plane, but the harmful side effects of fluorescence excitation still persist. More important in this regard is the limitation of fluorescence excitation to the focal point. In single-photon microscopy, which encompasses both conventional wide-field and laser-scanning confocal microscopy, the tissue is illuminated not only in the focal plane of the objective lens, but also above and below. This means that during imaging of dynamic processes the fluorochromes within the entire light cone entering the tissue are repetitively excited and subjected to phototoxic and photobleaching effects. In MPM this does not occur since fluorescence is excited only in the focal volume. As a consequence, many dynamic biological processes can typically be observed in three dimensions in vivo over the range of many hours at short cycle times of a few seconds without noticeable bleaching or phototoxic effects.
From explanted organs to intravital observations
MPM entered the field of immunology in 2002, when two independent groups published the first observations of immune cell migration in the context of explanted intact or reconstituted organs (36, 37). Although the migration of leukocytes had been studied in culture dishes for a long time, these first observations of cell behavior in a relevant tissue context seemed to make a wider audience of immunologists keenly aware of the relevance of cell motility for immune function. The most surprising aspect of this work was the high degree of motility (average speeds of 10–15 µm/min for T cells) and the apparent lack of directionality of migration, which seemed to contradict the general assumption that chemokines and other chemoattractant cues would guide leukocytes through tissues in a well-coordinated fashion. These observations were later confirmed by studies in living animals, where blood flow, lymph flow, and innervation of the organ under study are generally preserved (38, 39).
The emphasis during the first few years of dynamic in situ visualization of the immune system was on the initiation of immune responses in primary and secondary lymphoid organs (SLO) (37–50). This emphasis could be explained by the availability of model systems for the priming phase of T and B cell responses in the form of mice with transgenically expressed high affinity antigen-receptors specific for well-characterized model antigens. More recently, however, intravital microscopists in immunology have broadened their view to include diverse peripheral tissues, such as the central nervous system (51–53), the liver (54, 55), the intestine (56), or the skin (57) to study effector T cell or innate immune cell behavior in situ. Only three studies so far have investigated CTL in tumor tissue (58–60). In the following paragraphs we will examine how some aspects of CTL biology in the context of tumor disease might benefit from visualization-based studies in vivo in the future.
The CTL response against tumors
Our assumption of a central role of CTL in immunological anti-tumor defense is based on the observations that they are regularly found in the tumor tissue, that their adoptive transfer into tumor-bearing individuals is therapeutically effective in mice (and occasionally in humans), and that deficiency in CTL effector mechanisms confers increased susceptibility to tumor formation and growth (10).
CTL are specialized to execute their function during direct physical encounters with cells presenting the cognate antigens against which they were primed as naïve CD8+ T cells by dendritic cells (DC) in SLO (Fig. 1). Their effector functions include the secretion of cytokines, the ligation of death receptors, and, most prominently, the release of lytic granules (Fig. 2). Linking these functions to the recognition of antigen provides these cells with the discriminatory potential required to avoid excessive collateral damage on healthy cells while focusing the impact on their specific targets, which may be cells infected with intracellular pathogens or transformed cells expressing tumor-associated antigens. Although cancer-related imaging studies addressing the generation of CTL from naïve precursors in SLO are sparse, we will begin this discussion with this topic.
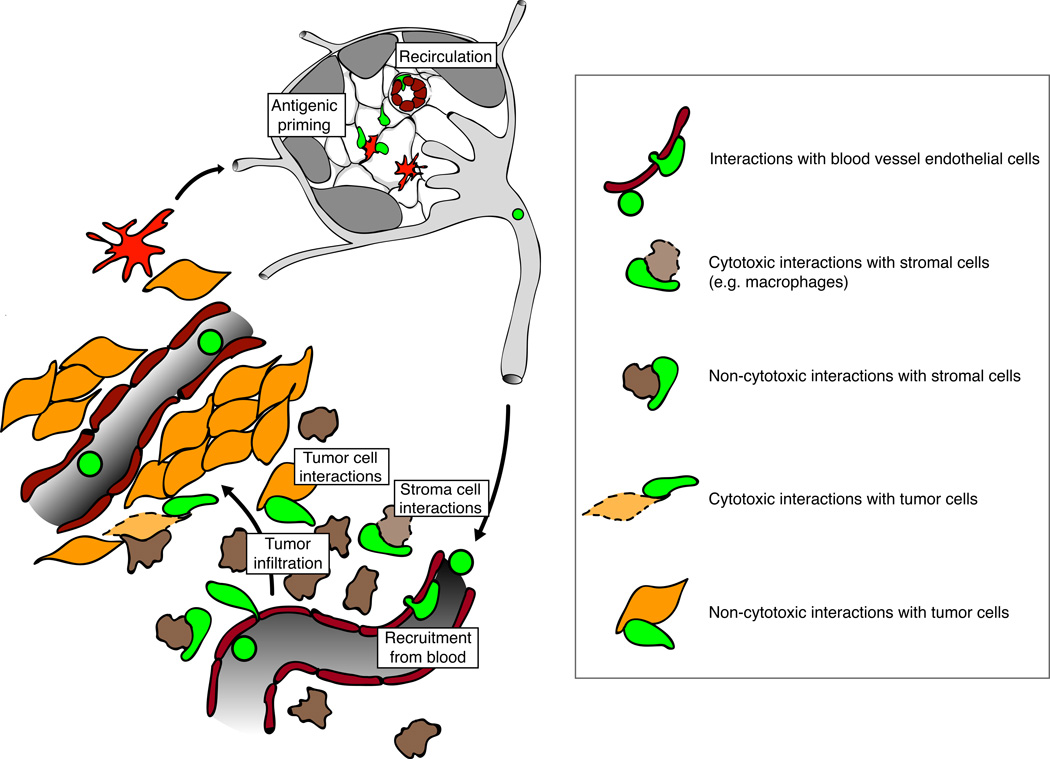
Figure 1.
Antigenic priming and putative cellular interactions of CTL during the effector phase of a successful anti-tumor response. In the tumor-draining LN (top) dendritic cells (red) present tumor-derived antigens to naïve CD8+ T cells, which continuously enter from the bloodstream and scan the T cell area by migration along its network of stromal cells. Cognate encounters with appropriately activated DC lead to proliferation and differentiation into CTL, which eventually leave the LN via afferent lymph vessels to enter the bloodstream. From there CTL are recruited to tumors either directly in the tumor cell islets (left) or in peripheral stromal regions (bottom). In the case of the latter they need to infiltrate the tumor islets in a subsequent migration step in order to engage tumor cells directly. CTL can exert both cytotoxic (lytic granule-mediated) and non-cytotoxic (Cytokines) functions against tumor cells or various cellular components of the tumor stroma, such as macrophages, dendritic cells, neutrophils, fibroblasts, endothelial cells etc. This can occur either through direct cellular interactions the target cell or indirectly through cytokine secretion triggered by cognate cellular interactions with other cells.
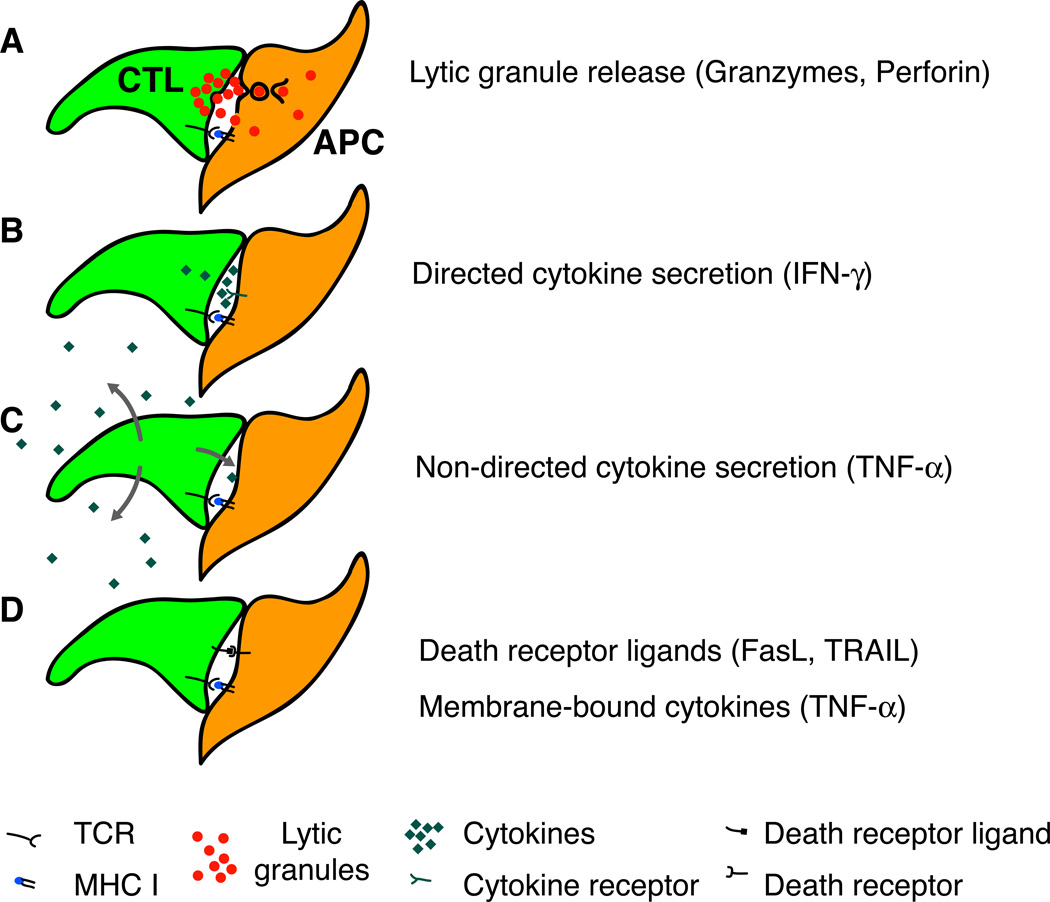
Figure 2.
The execution of CTL effector functions is closely regulated through TCR signals from antigen-presenting cells (APC). A. The contents of lytic granules are released into the CTL interface with the APC and from here reach the APC cytoplasm. B. Some cytokines (e.g. IFN-γ) are secreted in a polarized fashion towards the APC interface. C. Other cytokines (e.g. TNF-α) are secreted multidirectionally and may thus have effects on nearby non-APC. D. Ligands for death receptors are mobilized upon contact with APC similarly to lytic granules.
Antigenic Priming of T cells
As is the case for other effector T cells, the precursors of CTL are generated in the thymus from bone marrow-derived progenitors by a random process of somatic gene rearrangement in their antigen receptor locus. Subsequent selection processes ascertain that the newly obtained, highly diverse receptor repertoire of mature thymocytes is functional to interact with MHC I molecules, but does not recognize self antigen with high affinity (61). After export from the thymus the resulting small clonal pools of naïve CD8+ T cells, lacking effector function, continuously recirculate through SLO, scanning these organs for the cognate antigenic ligands recognized by their various antigen receptors (Fig. 1). Prior to antigenic stimulation the total number of T cells in an individual with specificity for a particular ligand is quite low, between 20 and 200 in mice (62) and several thousands in humans (63, 64). However, encounter with antigenic ligands in the context of appropriate co-stimulation not only triggers cellular differentiation and acquisition of effector functions, but also proliferation and clonal expansion over several orders of magnitude within a few days.
CD8+ T cells "see" antigen only when processed and presented in the context of MHC class I molecules on other cells. This can result from endogenous antigen expression in these cells or from a process called cross-presentation, meaning the presentation of exogenously acquired antigen. It is generally assumed that DC are the only antigen-presenting cells (APC) that are able to fully activate naïve T cells to become effectors. DC acquire antigen directly in peripheral tissues, including solid tumors, and carry it to lymph nodes (LN) by trafficking through afferent lymphatic vessels (65). DC also collect antigen within SLO either from soluble pools of antigen arriving via the lymph (66, 67) or from other DC that have delivered it from the periphery (68, 69). Because of the spatial concentration of antigen-presenting DC in SLO these organs function as "antigen libraries" of the body (70), which allow rare antigen-specific T cells to detect the presence of a foreign antigen in a particular region of the body during short visits to the draining lymph node instead of having to scan the vast volume of peripheral tissues for this purpose.
Essential to the scanning of SLO for "their" cognate Ag is the efficient migration of naïve T cells from the blood into LN via high endothelial venules (HEV). This constitutive process is facilitated by a sequence of molecular interactions mediated by adhesion and signaling molecules expressed by T cells and high endothelial cells (29, 64, 71–73). Interestingly, tumors may possess the ability to interfere with anti-tumor immunity already at this early step in the induction of T cell responses. Carriere et al. observed by conventional video-rate IVM that naïve T cells interacted less avidly with HEV in LN that drained an implanted tumor (74). This correlated with reduced expression of the chemokine CCL21 in stromal cells of the LN. Interaction of CCL21 with its receptor CCR7 on T cells activates the integrin LFA-1, thereby enabling firm adherence to the endothelium during extravasation from the blood. Whether this is a phenomenon specific to tumors, or related to the recent finding that expression of both CCL21 and CXCL13 by stromal cells in the LN is transiently downregulated in an IFN-γ-dependent fashion during the initiation of antiviral or antibacterial immune responses (75), remains an open question.
The most striking initial finding of imaging studies in explanted lymph nodes or in vivo was that once T cells have entered the LN parenchyma, they assume relentless migratory activity along seemingly random paths within the paracortical T cell area (37–40), which is the most likely location to encounter an antigen-presenting DC. Upon closer inspection their migration is however not entirely random, but appears to be guided by contacts with fibroblastic reticular cells (76, 77), which form a dense network in the T cell areas of SLO and may provide haptokinetic or haptotactic cues, or possibly even an adhesive substrate for migrating cells. The fact that stationary DC attach to this stromal cell network (78, 79) and thus position themselves along the T cell "highways", together with the mobility of their long dendritic processes, maximizes the scanning efficiency of naïve T cells to reported values of between 500 and 5000 DC contacts per hour (40, 80). Since CCR7 ligands, such as CCL21, are now known to play a direct pro-migratory role for T cells in LN (45, 81, 82), the aforementioned reduction in CCL21 in tumor-draining LN (74) may also interfere with the efficiency of this scanning process.
A great deal of attention has been devoted to the characterization of the in vivo dynamics of T cell-DC interactions during antigenic priming, because this information is deemed to provide a framework within which to interpret our knowledge of the inter- and intracellular molecular events occurring during this process, including the formation of so-called immunological synapses or supramolecular activation clusters (SMAC) of signaling and adhesion molecules (83–85) between T cells and APC. Although the results from numerous imaging studies in explanted tissues and by IVM are not uniform (37, 39–41, 49, 86–90), some consensus is beginning to emerge on how to unite the differing observations. Initial contacts of T cells with DC presenting cognate antigen can lead to the immediate formation of long-lasting, stable contacts under conditions of a high density of APC presenting high concentrations of high-affinity TCR ligands, (88, 89). We and others have found that under other experimental conditions T cells can also scan the surface of multiple antigen-presenting DC during sequential, short-lasting encounters, and commit to stable encounters only after such hours-long phases of probing (39, 41, 49, 87, 90).
Quite striking is the observation that under some conditions of tolerogenic priming or anergy-induction, T cells are unable to form stable conjugates with DC (41, 91), although this is not the case in all forms of tolerogenic priming (88). Whether absence of stable contact formation is the cause of tolerance or an unrelated event caused by the same factors that lead to tolerance remains to be addressed. In all of these studies the antigen-presenting DC were assumed to be the conveyors of tolerance based on the quality of the peptide they presented on their MHC molecules or on their activation status. Two additional imaging studies in LN also found that the suppressive influence of regulatory T cells (Treg) can prevent stable contact formation of naïve T cells with DC (92, 93). Again, this was interpreted to be the result of the impact of Treg on DC, rather than directly on the effector T cells.
There are multiple reasons why the priming of T cells against tumor antigen could favor the induction of tolerance rather than immunity. For once, DC might acquire antigen from some tumors without receiving concomitant danger signals that would trigger their up-regulation of co-stimulatory molecules, which are required to induce full activation and effector differentation in T cells (94). In addition, tumor-reactive Treg may interfere with the capacity of DC to prime naïve T cells in similar ways as has been reported for autoimmune settings (92, 93, 95, 96). Scholer et al. recently visualized the migratory dynamics of ovalbumin (OVA)-specific T cells in explanted lymph nodes that had drained subcutaneous EG7-OVA tumors, generated by injection of the EL4 thymoma cell line engineered to express the model antigen OVA. (97). When they adoptively transferred naïve OT-I cells into mice bearing established, 10 days old tumors, they found a nearly identical reduction of T cell motility in tumor-draining LN relative to non-draining LN as compared to a highly immunogenic setting generated by targeting the antigen to DC with antibodies against the scavenger receptor DEC-205 and by injection of an activating antibody against CD40. This was interpreted to reflect that T cells established long-lasting interactions with tumor-antigen-presenting DC. If endogenous Treg were induced or activated in this tumor implantation model, as one would expect (98), they apparently did not interfere with the priming of naïve T cells. Not only did naïve T cells show similar motility as under immunogenic conditions, but priming also resulted in comparable production of the effector cytokine IFN-γ (97). Future studies will be required to determine under what condition Treg are expanded or induced in response to tumors and to what extent they impair T cell responses at the level of priming.
Trafficking to effector sites
Poor recruitment of T cells from the blood into tumor tissue and specifically into the tumor cell mass is now recognized as an obstacle to effective immune responses (Fig. 1) (99, 100). Part of the explanation may be that the nature of the tumor environment simply does not support the proper formation of a mature, adhesive microvasculature, but it is equally possible that tumor-secreted factors or tumor-induced cellular mechanisms, which could be therapeutically targeted, actively antagonize cellular recruitment from the blood. A prerequisite to overcoming the limitation of poor recruitment of T cells from the blood into tumor tissue and specifically into the tumor islets themselves will be to identify and dissect the underlying mechanisms.
Differentiation of naïve T cells into effector cells is accompanied by dramatic alterations in their expression of adhesion and signaling molecules. This leads to a change in trafficking behavior from recirculation through SLO via lymph and blood towards a pronounced inclination to enter peripheral tissues, preferentially those in which inflammation has caused up-regulation of the blood vessel endothelial counter-receptors for the newly acquired homing molecules on T cells (reviewed in (101)). Despite the resemblance of tumors to chronically inflamed tissues in many other ways, the tumor vasculature appears to be poorly equipped to support the adhesive interactions leading to the recruitment of immune cells (102, 103). A mostly only rudimentary vascular differentiation into arteriolar, capillary, and venous microvascular beds (104–106) is accompanied by low expression of adhesion molecules, such as intercellular adhesion molecule (ICAM)-1 (107, 108) and vascular cell adhesion molecule (VCAM)-1 (109), and of chemokines, such as the CXCR3 ligands CXCL9 and CXCL10 (110, 111). A particular hindrance in the study of leukocyte recruitment to tumors is also the apparent heterogeneity in vessel function and the fact that preferred sites of recruitment have not yet been identified. Curiously, T cells are often found in greatest density within the stromal components of the tumor mass surrounding the tumor cell islets (100). The variable infiltration of the tumor islets seems to constitute a predictive parameter for a more positive clinical outcome in patients (112). This allows for various interpretations, one of which is that T cell recruitment occurs in the stromal regions of tumors and that subsequent entry into the tumor islets is a limiting step (Fig. 1). It is also possible that direct recruitment from the blood to the tumor islets is what facilitates an effective immune response.
Time-resolved imaging studies of the entire sequence of events leading to trafficking of effector T cells to their final target location is the most promising approach to resolve this issue. In fact, Mrass et al., in the first time-lapse imaging study of T cell migration in explanted tumors, have made the interesting observation that local antigen-recognition through CTL in tumor tissue is required for their sustained motility (58), which may be critical for their ability to enter the tumor islets from the surrounding tumor stroma. When they injected two in vitro-activated populations of TCR transgenic T cells, non-tumor antigen-specific P14 and OVA-specific OT-I CTL, into mice bearing the OVA-expressing EL4-derived thymoma EG7-OVA, both populations entered the tumor. While the P14 cells were initially more motile in the absence of their cognate antigen, the OT-I effectors engaged in partially transient, partially longer-lasting interactions with tumor cells and thus slowed down. The latter finding was also confirmed by the authors using intravital microscopy. Over the next few days, however, the majority of OT-I T cells resumed a motile behavior (and rejected the tumor), while P14 T cells became increasingly immotile. Importantly, such biphasic behavior was also observed for tumor antigen-reactive T cells derived from the endogenous polyclonal repertoire of mice that were implanted with the lung epithelial tumor cell line TC-1 expressing a human papilloma virus antigen. Here, the recovery of motility of tumor-infiltrating T cells was only noted in mice that were in addition vaccinated with an adenoviral vector expressing one of the papilloma virus antigens on the TC-1 tumors, while in absence of vaccination T cells in tumors remained immotile throughout (58).
Similar, but not identical observations were made by Boissonnas et al., who imaged CTL primed in vivo from naïve OT-I T cells in subcutaneous tumors by MP-IVM (59). When mice were implanted in distinct locations with EG7-OVA and EL4 tumors, transferred naïve OT-I cells were primed only in LN draining EG7-OVA, but subsequently migrated to both EG7-OVA and EL4 tumors. Because in this study intravital microscopy was used to visualize CTL migration in tumors, the authors were also able to note that T cell infiltration of tumors seemed to originate from the peritumoral tissue, where CTL migrated preferentially along small blood vessels. OT-I CTL that approached the tumor initially engaged tumor cells most prominently in stable interactions, but at later stages of tumor rejection regained high motility. However, under the experimental conditions used, OT-I CTL were not observed to diminish their motility in EL4 tumors in absence of their cognate antigen as in the study by Mrass et al. (58). The reason for this could be that the continuous supply of newly immigrating fresh CTL generated from naïve cells in the EG7-OVA-draining LN may have obscured the behavior of cells with longer dwell times in EL4 tumors, leading to the conclusion that cells did not decrease their motility in absence of the cognate antigen. On the other hand, non-antigen specific CTL not only remained motile, but were also found in deeper regions of the tumor, when OT-I CTL were simultaneously present in EG7-OVA tumors (59). This argues against a requirement for CTL for direct antigen recognition to migrate from a putative peripheral entry site to central regions of the tumor. Further studies using a larger number of different experimental settings will probably provide a more unified model of CTL recruitment and regional trafficking in tumors.
The cell biology of CTL function
Before we discuss the initial imaging-based observations on CTL-target cell interactions during anti-tumor responses, we will briefly review the cellular functions that CD8+ effector T cells are equipped with to carry out their roles in the immune response (Fig. 2).
Cytokines
Among the cytokines produced by CTL, Interferon (IFN)-γ has been shown to be relevant to immunity against cancer in a number of different settings, including murine models of IFN-γ- or IFN-γ-receptor-deficiency of the host (113, 114) or of IFN-γ-unresponsiveness of the tumor (113, 115). What is less clear is whether it was in every case IFN-γ produced by CTL, and not by NK cells or CD4+ T helper cells, that conferred the observed anti-tumor effects. Also, it is not exactly clear how IFN-γ harms tumors in vivo (116). CTL-derived IFN-γ may activate macrophages to carry out their innate anti-tumor functions (117) or induce anti-angiogenic effects (118–120). IFN-γ may render tumor cells more immunogenic and susceptible to other effector mechanisms (121) by inducing their cellular antigen presentation machinery or via induction of the death-receptor Fas (122) (see below). Secretion of IFN-γ, at least by CD4+ T cells in vitro, occurs in a directional manner towards the interface with antigen-presenting cells (123, 124). Conceivably, CTL also make such economic use of their IFN-γ in vivo, but on which target cells and to what effect remains to be worked out in detail.
Tumor necrosis factor (TNF)-α, on the other hand, is secreted non-directionally by CD4+ T cells upon antigen recognition (124), and may thus, if the same is true for CD8+ effector T cells in vivo, exert rather pleiotropic effects, such as recruitment and activation of innate and adaptive bystander cells, including non-hematopoetic cells. Yet, when mice are co-infected with a mixture of two strains of recombinant vaccinia virus, one of which is engineered to expresses murine TNF-α (and the other not), antiviral CTL-mediated protection is generated only against the TNF-α-expressing strain (125). This suggests that, despite its multidirectional secretion by CTL, TNF-α may act in a highly localized fashion. Generally, a role for TNF-α in anti-tumor immunity is less well substantiated than for IFN-γ, especially when produced by CTL. It may be important when tumors grow in immune-privileged sites (126) or in the elimination of tumor antigen loss variants (127, 128). As for IFN-γ, anti-tumor effects of TNF-α may depend more on effects on the tumor stroma, than on the tumor cells directly (128, 129).
In addition to IFN-γ and TNF-α, IL-4 and IL-10 have also been implicated in anti-tumor effects, although these cytokines are generally associated with immunoregulatory functions. Yet, IL-4 produced by CTL can contribute to tumor rejection, not only during the priming phase (130, 131), but putatively also through anti-angiogenic effects mediated by tumor-associated fibroblasts (132). And finally, since artificial expression of IL-10 by transplanted tumors delays tumor growth, albeit independently of T cells (133), CD8+ T cells, which under some circumstances can be induced to produce this cytokine (134) might also play a role in the anti-tumor response through macrophage-mediated anti-angiogenic effects (133).
Importantly, not only the secretion, but also the production of cytokines is coupled to antigen recognition. IFN-γ and TNF-α are synthesized in CTL partly from preformed, but mostly from newly transcribed mRNA within 30 minutes of binding to Ag-presenting cells (135). This suggests that not only the secretion, but also the expression of cytokines by CTL in tumors is tightly regulated through cellular interactions, but the cellular partners of these interactions and the relevant targets of cytokine effects remain poorly defined in vivo.
Ligands for death-receptors
Tumor cells, like most cells of the body, express, at varying levels, members of the TNF-receptor family, such as Fas, TNF-receptors, and the TRAIL receptors, which, when triggered through their ligands displayed on the surface of activated immune cells, can induce various pathways of apoptosis. The best-studied ligand on CTL is Fas ligand (FasL). FasL has been reported to be stored in the same secretory lysosomes as the granzymes and perforin (discussed below) and, at least in immune cells, to be concomitantly shuttled to the cell surface only upon antigen-triggered degranulation (136). Recently, however, evidence has emerged that FasL may be stored in distinct organelles and the surface mobilization of their content be controlled independently from the classical granule release pathway (137).
The Fas pathway is traditionally believed to be of subordinate or no importance as an effector function in CTL anti-tumor responses compared to its role in immune regulation and homeostasis (138). Yet it may contribute to optimal responses, especially under conditions of large tumor burden (139–141). Its actions, like those of other death receptor ligands, are likely mediated through cellular interactions, but, as is true for the cytokines, the relevant interaction partners in the tumor environment remain to be defined.
Lytic granules
The best-established mechanism used by tumor-reactive CTL is the lytic granule pathway. In addition to above-mentioned effector mechanisms, which they share with CD4+ helper T cells, CTL are also equipped with a specialized form of secretory lysosomes, so-called lytic granules (reviewed in (142)). Naïve CD8+ T cells lack lytic granules, which are only expressed upon effector cell differentiation triggered during antigenic priming. Lytic granules contain multiple members of a family of serine proteases, called granzymes, as well as the membrane pore-forming molecule perforin. Granzymes and perforin most likely act in concert to induce a variety of apoptotic pathways in target cells in a cell contact-dependent fashion.
Upon T cell receptor (TCR)-triggering, CTL polarize their microtubule-organizing center (MTOC) towards the site of contact with antigen-presenting cells. Microtubule-associated lytic granules switch from bidirectional to unidirectional movement along tubules and thus accumulate at the MTOC. Fusion with the cytoplasmic membrane leads to the release of granzymes and perforin through a special secretory domain of the immunological synapse into the intercellular gap (143, 144). It is still a matter of debate whether granzymes then enter the cytoplasm of target cells directly from the synaptic cleft through perforin-mediated pores in the cytoplasmic membranes (142, 145, 146), through perforin-mediated release from endosomes that may form by conventional endocytosis (147) or during membrane repair of the perforin-injured cytoplasmic membrane (148, 149). Within the target cell, granzymes induce a multitude of apoptotic pathways, many, but not all of which depend on the enzymatic activity of caspases (150, 151). In vitro, granule-mediated killing of target cells has been characterized as being significantly faster than apoptosis-induction through death receptors, requiring only a few minutes under optimum conditions (143).
The relevance of the cytotoxic pathway for direct killing of tumor cells versus that of stromal cells cross-presenting tumor-derived antigen could depend on the amount of antigen expressed by the tumor, which may vary in the course of the host-tumor interaction (152), but its general significance is underscored by the enhanced susceptibility to tumor development in perforin-deficient mice (153).
CTL function during interactions with tumor cells in vivo
Based on the efficient perforin-dependent, contact-mediated killing of allogeneic tumor cells in classical in vitro-assays of cytotoxicity (154) one might have assumed that contacts with tumor cells by tumor antigen-specific CTL in vivo would also lead to efficient induction of tumor cell apoptosis, at least under conditions that lead to rejection of tumors in a CTL-dependent fashion. It is therefore surprising that the initial in situ observations of CTL tumor cell interactions yielded no or only anecdotal footage of tumor cell destruction during contacts with tumor-specific CTL (58, 59). Since tumor cell apoptosis under the experimental conditions used was evident, the question arises as to what extent and with what efficiency direct, lytic granule-mediated, contact-dependent cytotoxicity of CTL against tumor cells contributes to tumor rejection, relative to the role of cytotoxic effects on stromal cells and of the other above-mentioned CTL effector mechanisms.
A simple explanation for the paucity of observed killing events could also be that the time it takes to kill a tumor cell in situ is considerably longer than the 30 minute-intervals of continuous observation used for these initial studies. In vitro, signs of structural lysis of target cells can become apparent within a few minutes after engagement with a CTL under optimal conditions (143), but this can also take up to several hours after initial contact formation ((155) and Peter Friedl, personal communication). Many reasons for this heterogeneity are conceivable, including variable degrees of effector differentiation of the CTL, involvement of TCR with different affinities, varying expression of peptide-MHC ligands, or expression of negatively co-stimulating signals by target cells, their induction of anti-apoptotic or down-regulation of pro-apoptotic mechanisms, or differences in the general experimental conditions between studies. This heterogeneity will likely be amplified in vivo, where additional extrinsic factors will contribute to the regulation of killing efficiency.
An additional difficulty of the study of CTL tumor cell interactions in vivo will probably remain that, unless the tumor is continuously observed from the initial infiltration of CTL on, the history of each CTL and tumor cell that interact cannot be known. Even if a tumor cell lysis event is recorded subsequent to a seemingly initial contact by one CTL, it is challenging to exclude with certainty that previous, unobserved interactions with this or other CTL have occurred, have potentially prepared the tumor cell for an easy deathblow. Therefore, to obtain precise information of the killing efficiency of tumor-reactive CTL under conditions of tumor rejection and tumor progression, we recently devised a method where B cells pulsed ex vivo with a tumor-expressed peptide antigen were introduced into tumor-bearing mice where they would be confronted with primed CTL in the tumor-draining LN (156). Since the tumor-reactive CTL expressed EGFP and the surrogate target B cells were labeled with a combination of organic fluorescent dyes that would allow monitoring of cellular viability, we could visualize their encounters in the LN by MP-IVM. At day 5 after implantation of the tumors, CTL had already acquired lytic effector function at the priming site. While CTL contacts with non-antigen pulsed control B cells were, as expected, transient, contacts with antigen pulsed targets very efficiently led to the immediate formation of stable conjugates (Fig. 3). These conjugates subsequently moved jointly through the LN parenchyma at speeds similar to that of unconjugated B cells, with the B cell always leading the way. After an average of 10 minutes the conjugates typically stopped to migrate quite abruptly, which we interpreted, based on the assumption that the B cells were the driving force behind conjugate movement, as a first sign of loss of cellular function in the target cells as a consequence of CTL contact-dependent cytotoxicity. Yet, it was only after a second interval of about 10 minutes that we saw changes in the fluorescent properties of the target cells consistent with their loss of structural integrity. The study of this comparably rapid cytotoxic process with these favorable and fairly standardized targets (high and homogenous levels of surface-peptide/MHC) was hampered by the limited continuous observation time of one hour. But although this timeframe allowed for relatively few observations of the entire sequence of events, it permitted an estimate of the overall efficiency of killing as about 2.5 events per hour of motile CTL-B cell interaction time (156).
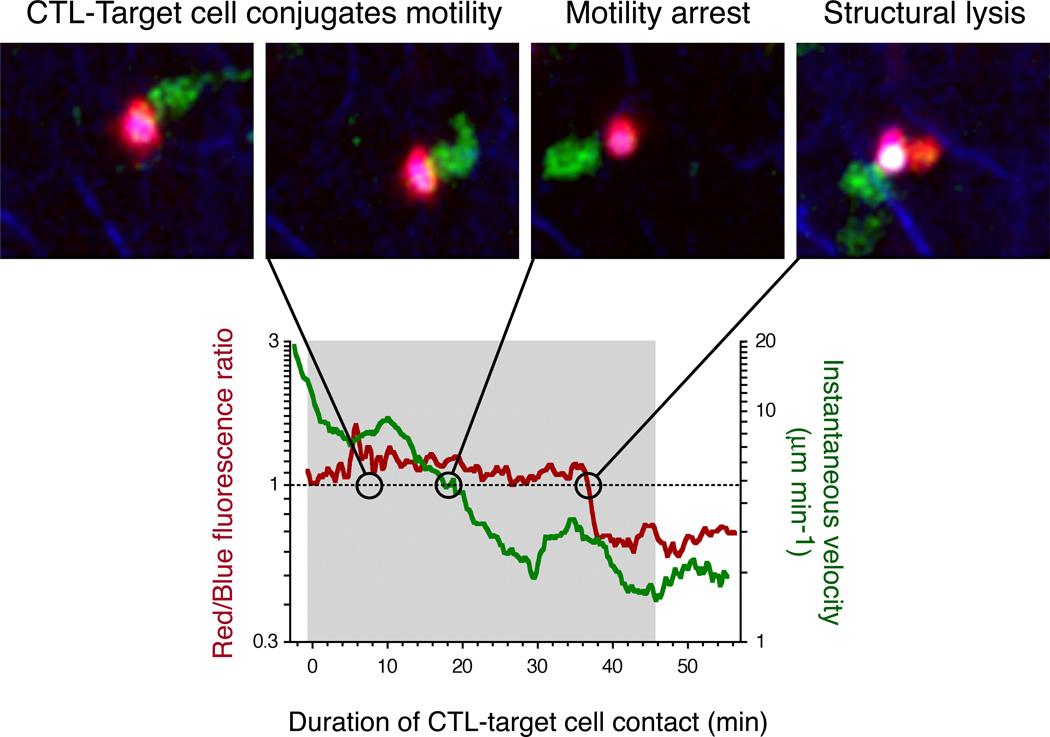
Figure 3.
Dynamic analysis of CTL killing of a motile target in vivo. Engagement of a B cell (purple) presenting cognate antigen by a tumor-reactive CTL (green) leads to formation of a stable conjugate (grey-shaded area in the graph below), which continues to move at the speed of unconjugated B cells (as measured by the instantaneous velocity). After 18 min. the migration speed drops below a threshold value defined by the 95% confidence interval of the migration speed of unconjugated B cells. After another 18 min. the B cell undergoes structural lysis reflected by rapid changes in fluorescent properties (Red/Blue fluorescence ratio). 7 minutes later the CTL disengages from the target cell. Modified from (156).
These observations, although performed on surrogate target cells, highlight some of the challenges to the quantitative characterization of CTL function against tumor cells in vivo. Future studies will hinge on sensitive parameters to monitor the responses of tumor cells to CTL contacts. Signs of cellular disintegration that are detectable by MP-IVM in cells labeled through cytoplasmatic expression of fluorescent proteins may be a late event in apoptosis. More sensitive and easily quantifiable parameters might be obtained by monitoring signaling or enzymatic processes activated earlier in apoptotic cells. Indeed, Philippe Bousso's group recently published the first detailed kinetic in vivo-analysis of tumor cell death resulting from direct cellular interactions with tumor-reactive CTL using caspase activation as a read-out for the induction of cell death (60). For this purpose they transfected the tumor cell line EG7-OVA with a genetically encoded fluorogenic probe that reports caspase activity through spectral changes in fluorescence emission upon cleavage of a caspase-sensitive amino acid sequence used as a linker between two fluorescent proteins. In the uncleaved state the proteins undergo Foerster resonance energy transfer (FRET), which quenches fluorescence of the FRET-donor molecule. When caspases are activated during ongoing apoptosis, they cleave the probe, which can be monitored by microscopy. The authors injected such tumors into mice, followed by adoptive transfer of pre-activated OT-I CTL four days later, which facilitated the rejection of OVA-expressing, but not of non-OVA expressing tumors. When they analyzed the interactions of OT-I cells with tumor cells by MP-IVM they could observe concurrent induction of apoptosis in tumor cells. While these events were infrequent and the duration of continuous observation intervals was limiting, Breart et al. studied a large number of total events and could thus extrapolate the time an individual CTL interacts with one tumor cell before apoptosis is induced. Surprisingly this time was 6 hours in their experimental system and thus much longer than what was observed with peptide-pulsed surrogate target cells in tumor-draining LN (60, 156). Since EG7-OVA cells express considerable amounts of OVA, other reasons apart from limiting presentation of TCR ligands for OT-I cells by the tumor cells, as discussed earlier, should probably be considered to account for this discrepancy.
Complementary to the analysis of the CTLs' effects on their target cells, the ability to monitor the deployment of the cytolytic machinery in CTL during CTL-tumor cell interactions will help to interpret simultaneous observations on tumor cells. This could for instance be achieved through expression of fluorescent fusions to lytic molecules, such as Granzyme B (157) in CTL. What are the interesting questions on CTL-mediated cytotoxicity of tumor cells in vivo, besides the dynamics of the process and its general relevance for tumor elimination? An elegant classic study relying on a simple in vitro cytotoxicity assay initially suggested that CTL possess the ability to lyse several target cells in rapid succession (158). This finding has since been corroborated by several video-microscopic investigations in vitro (159–161), but whether CTL generated in vivo from naïve CD8+ T cells in tumor-draining LN display such serial killing behavior against tumors is not yet known. What is the stoichiometry of tumor cell killing by CTL in vivo? Does the lytic cargo of one CTL suffice to lyse one or several tumor cells or a do several CTL need to engage with one tumor cell simultaneously or in sequence to achieve the kill? The observations from the imaging studies performed so far indicate that CTL-tumor cell interactions are predominantly monogamous, but do not rule out the requirement for sequential engagement of a tumor cell by several CTL to induce cell death (58–60).
A striking observation from imaging studies performed in collagen gels is that CTL remain attached to their targets for significant amounts of time even after the targets show obvious signs of structural disintegration (Peter Friedl, personal communication). We have recorded similar 'necrophilic' interactions in vivo in CTL engaging with peptide-pulsed B cells in tumor-draining LN (156). This seemingly pointless behavior might serve the purpose of sustaining TCR signaling, to induce transcription and recharge the lytic machinery for subsequent encounters, or perhaps to induce the secretion of cytokines that attract phagocytes for clearance of the apoptotic target cells remnants. Finally, once the basic rules of engagement of CTL and tumor cells are characterized in vivo, it will be most rewarding to study the impact of various putative regulatory mechanisms that locally antagonize efficient execution of CTL effector function in the tumor microenvironment.
Indirect anti-tumor functions of CTL
Tumor antigens are not only presented by tumor cells themselves, but also cross-presented by hematopoetic cells and even radio-resistant, possibly non-hematopoetic cells in the tumor stroma and will be encountered in this form by CTL upon entry into the tumor environment. The importance of these encounters was highlighted by a study that showed the relevance of local antigen cross-presentation for the elimination of tumor cells that have downregulated or lost the expression of tumor antigens recognized by tumor-reactive CTL, so-called antigen loss variants (152). The function of these interactions could be to boost CTL function locally and thus enable the direct clearance of tumor cells expressing low amounts of antigen. However, in the study by Spiotto et al. CTL could contain tumors even when they did not recognize their cognate antigen on the tumor cells directly, but only on cross-presenting stromal cells (152). It is therefore also possible that cytotoxic or non-cytotoxic effects on stromal cells is what enables rejection of these tumors. In line with this hypothesis, some studies have found that the capacity of CTL to contribute to tumor rejection is more dependent on cytokines, such as IFN-γ, than on cytotoxicity (162–164). The study by Breart et al., however, provided elegant evidence that, at least under their experimental conditions, indirect effects of CTL on tumors were not dominant in the rejection of tumors. To address this question they injected mice with homogenous mixtures of EG7-OVA and the non OVA-expressing maternal tumor cell line EL4. Three days after adoptive transfer of pre-activated OT-I CTL the OVA-expressing tumor cells had disappeared, but the control tumor cells prevailed in numbers that indicated that they were not affected by the presence of CTL engaging with and killing their direct neighbors (60). This is an important finding since it validates the relevance of direct killing of tumor cells by CTL. Possibly the critical contribution of bystander elimination of tumor cells, as suggested by the study of Spiotto et al. (152) becomes more significant at later stages of tumor development, or the expression of OVA by EG7-OVA is too low to allow for sufficient cross-presentation by stromal cells in the tumor to trigger bystander effects.
Despite these observations, it will be very informative to identify the cellular interactions of CTL with various cells of the tumor stroma. Mrass et al., in their study of tumor explants, found evidence for sustained CTL interactions with an autofluorescent cell population in the stroma, which could be identified as macrophages by correlative histological analysis (58). They did not report macrophage lysis as an outcome of these interactions. Likely, both cell types could influence each other functionally. CTL could activate macrophages to enhance their phagocytic function or secrete cytokines. Conversely, macrophages could modulate CTL function in either immunogenic or tolerogenic ways by regulating their survival, proliferation, and gene expression profile. In light of the recently accumulating evidence for a tumor-promoting function of myeloid-derived suppressor cells (9, 165, 166), the latter may seem more likely under conditions of tumor progression, but macrophage function might be converted by appropriate interventions (167). An examination of the signals that CTL receive during ongoing encounters with macrophages and with other cells of the tumor stroma, using MP-IVM, will provide invaluable insights for the development of effective immunotherapies. This approach will be enabled by the generation of sophisticated fluorescence-based molecular reporters to monitor cellular signaling in vivo at the single cells level.
Extrinsic regulation of CTL function in tumors
The tumor environment is an arena for a multitude of cellular and humoral regulatory networks within the immune system (9, 10, 12–14). It is beyond the scope of this article to review these, but all findings on CTL function in tumors must be interpreted in light of the potential impact of extrinsic regulatory mechanisms.
What orchestrates these regulatory immune mechanisms? It may be helpful to consider that Treg recognizing tumor-expressed or tumor-associated antigens play a central role, by analogy to the diverse functions of CD4+ effector T cells as the conductors of immunogenic responses. It is conceptually attractive to assume that Treg both thrive in and actively maintain environments that antagonize the functions of CTL. We have used our model mouse tumor system to study by MP-IVM the CTL-mediated killing of surrogate target cells in tumor-draining LN to test whether Treg have an impact on CTL cytotoxic function (156). Using the kinetics of killing in the absence of exogenous tumor antigen-specific Treg as baseline value we measured the effect of the presence of tumor-reactive Treg, which globally prevented tumor rejection (168), on CTL function at the single cell level. In the presence of adoptive transferred, clonally expanding Treg, CTL proliferated and migrated normally in the LN, were as efficient in forming stable conjugates with their targets and displayed the same co-migration behavior, but we detected much fewer lytic events during these interactions. Instead we found that prolonged motile interactions frequently ended without visible impact on the targets. The efficiency of killing thus dropped six-fold from 2.5 to less than 0.4 events per hour of motile interaction time. Correlative ex vivo analysis of CTL from LN containing adoptively transferred, tumor-reactive Treg or not, revealed a selective Treg-dependent defect in the CTLs' ability to release their lytic granules toward the target cell interface, suggesting a mechanistic explanation for their inefficient lytic ability in vivo. In these studies we found no convincing evidence that direct Treg-CTL interactions are involved in this process (156). In agreement with imaging studies on Treg-mediated suppression of CD4+ effector T cells in a model of autoimmunity (92), we suggest that Treg may exert their suppressive function through interactions with antigen-presenting cells that subsequently loose their ability to stimulate effector T cells in an immunogenic fashion (19). Importantly we found that the suppressed phenotype of CTL in tumor-draining LN was reversible in absence of Treg. Assuming that the Treg found in tumors operate by similar mechanisms as in the draining LN, it will be interesting to identify tumor-resident partners of antigen-dependent cellular interactions shared by CTL and Treg, which could thus serve as mediators of suppression.
Outlook
Cancer is a complex and multifaceted disease. The at times paradoxical roles of the immune system in disease initiation, progression, and therapy are being increasingly recognized in complementation of the more traditional, 'tumor-centric' perspective (169). Although the limitations of murine tumor implantation models are recognized (7), they still provide useful mechanistic insights into the interaction of the immune system with tumor cells and their surrounding stroma. Due to their experimental flexibility, these models will also, at least initially, be of great value in our visualization-based efforts to understand malignant disease through in vivo-investigation of the cellular interactions that enable and control anti-tumor immune functions. Yet, the predictions from these studies will require validation in tumor models that resemble the sporadic human disease more closely, for instance in the dynamics of disease. Adaptation of existing genetic models of sporadic disease (7) will eventually also allow for imaging-based exploration of immune function during early disease stages and hopefully help address the mechanisms of immunosurveillance (170).
Acknowledgements
We would like to thank Drs. Mikael Pittet and Cathryn Nagler for helpful discussions and critical reading of the manuscript. T.R.M. is supported by NIH grant 4 R00 AI073457 - 02 and C.A.B. is supported by a grant from the Deutsche Forschungsgemeinschaft.
Abbreviations
- APC
Antigen presenting cell(s)
- CTL
Cytotoxic T lymphocyte(s)
- DC
Dendritic cell(s)
- EGFP
Enhanced green fluorescent protein
- FRET
Foerster resonance energy transfer
- HEV
High endothelial venule(s)
- IVM
Intravital microscopy
- LN
Lymph node(s)
- MHC
Major Histocompatibility Complex
- MP-IVM
Multiphoton intravital microscopy
- MPM
Multiphoton microscopy
- MTOC
Microtubule organizing center
- SLO
Secondary lymphoid organ(s)
- SMAC
Supremolecular activation cluster
- TCR
T cell receptor(s)
- Treg
Regulatory T cell(s)
References
- 1.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 3.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 4.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 6.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 7.Willimsky G, Blankenstein T. The adaptive immune response to sporadic cancer. Immunol. Rev. 2007;220:102–112. doi: 10.1111/j.1600-065X.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 8.Stagg J, Johnstone RW, Smyth MJ. From cancer immunosurveillance to cancer immunotherapy. Immunol. Rev. 2007;220:82–101. doi: 10.1111/j.1600-065X.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol. Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 11.Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin. Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 13.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittet MJ, Grimm J, Berger CR, Tamura T, Wojtkiewicz G, Nahrendorf M, Romero P, Swirski FK, Weissleder R. In vivo imaging of T cell delivery to tumors after adoptive transfer therapy. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12457–12461. doi: 10.1073/pnas.0704460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahalan MD, Parker I. Choreography of Cell Motility and Interaction Dynamics Imaged by Two-Photon Microscopy in Lymphoid Organs. Annu. Rev. Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germain RN, Bajenoff M, Castellino F, Chieppa M, Egen JG, Huang AY, Ishii M, Koo LY, Qi H. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunol. Rev. 2008;221:163–181. doi: 10.1111/j.1600-065X.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 19.Pittet MJ, Mempel TR. Regulation of T-cell migration and effector functions: insights from in vivo imaging studies. Immunol. Rev. 2008;221:107–129. doi: 10.1111/j.1600-065X.2008.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Ng LG, Mrass P, Kinjyo I, Reiner SL, Weninger W. Two-photon imaging of effector T-cell behavior: lessons from a tumor model. Immunol. Rev. 2008;221:147–162. doi: 10.1111/j.1600-065X.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- 21.Velazquez P, Waite JC, Dustin ML. Dynamics of host defense: the view at the front lines. Nat Immunol. 2007;8:1153–1157. doi: 10.1038/ni1520. [DOI] [PubMed] [Google Scholar]
- 22.Celli S, Garcia Z, Beuneu H, Bousso P. Decoding the dynamics of T cell-dendritic cell interactions in vivo. Immunol. Rev. 2008;221:182–187. doi: 10.1111/j.1600-065X.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 23.Malpighi M. De Pulmonibus. Bologna: Observationes Anatomicae; 1661. [Google Scholar]
- 24.Leeuwenhoek A. Collected letters. Edited and annotated by a committee of Dutch scientists. Amsterdam: Swets and Zeitlinger; 1939–1999. [Google Scholar]
- 25.Cohnheim J. Lectures on General Pathology: A Handbook for Practitioners and Students. London: The New Sydenham Society; 1889. [Google Scholar]
- 26.Cohnheim J. Über Entzündung und Eiterung. Virchows Arch. 1867;40:1–79. [Google Scholar]
- 27.Metchnikoff E. Untersuchungen über die Mesodermalen Phagozyten einiger Wirbeltiere. Biol. Zentralbl. 1883;3:560–565. [Google Scholar]
- 28.Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in the rat. Proc. R. Soc. Lond. B. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 29.Mempel TR, Scimone ML, Mora JR, von Andrian UH. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr. Opin. Immunol. 2004;16:406–417. doi: 10.1016/j.coi.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 31.Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nature Reviews Immunology. 2002;2:872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 33.Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Theer P, Hasan MT, Denk W. Two-photon imaging to a depth of 1000 microm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 2003;28:1022–1024. doi: 10.1364/ol.28.001022. [DOI] [PubMed] [Google Scholar]
- 35.Saetzler RK, Jallo J, Lehr HA, Philips CM, Vasthare U, Arfors KE, Tuma RF. Intravital fluorescence microscopy: impact of light-induced phototoxicity on adhesion of fluorescently labeled leukocytes. J. Histochem. Cytochem. 1997;45:505–513. doi: 10.1177/002215549704500403. [DOI] [PubMed] [Google Scholar]
- 36.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- 37.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 38.Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 40.Bousso P, Robey E. Dynamics of CD8(+) T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 41.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 42.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 43.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O'Garra A, Cahalan MD, Cyster JG. Antigen-Engaged B Cells Undergo Chemotaxis toward the T Zone and Form Motile Conjugates with Helper T Cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 45.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J. Exp. Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, Leung H, Cherayil BJ, Russell P, von Andrian U, Pillai S. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, von Andrian UH. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Wei SH, Miller MJ, Cahalan MD, Parker I. Two-photon imaging in intact lymphoid tissue. Adv. Exp. Med. Biol. 2002;512:203–208. doi: 10.1007/978-1-4615-0757-4_26. [DOI] [PubMed] [Google Scholar]
- 49.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, Sheetz MP, Littman DR, Dustin ML. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 50.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J. Exp. Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 53.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 54.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinselmeyer BH, Lynch JN, Zhang X, Aoshi T, Miller MJ. Video-rate two-photon imaging of mouse footpad - a promising model for studying leukocyte recruitment dynamics during inflammation. Inflamm. Res. 2008;57:93–96. doi: 10.1007/s00011-007-7195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J. Exp. Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J. Exp. Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8 T cell cytotoxic activity during adoptive T cell therapy in mice. J. Clin. Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kyewski B, Klein L. A central role for central tolerance. Annu. Rev. Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 62.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 64.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 65.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 66.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct Dendritic Cell Populations Sequentially Present a Subcutaneous Antigen to CD4 T Cells and Stimulate Different Aspects of Cell-Mediated Immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 67.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 69.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 70.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nature Reviews Immunology. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 71.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 72.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 73.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 74.Carriere V, Colisson R, Jiguet-Jiglaire C, Bellard E, Bouche G, Al Saati T, Amalric F, Girard JP, M'Rini C. Cancer cells regulate lymphocyte recruitment and leukocyte-endothelium interactions in the tumor-draining lymph node. Cancer Res. 2005;65:11639–11648. doi: 10.1158/0008-5472.CAN-05-1190. [DOI] [PubMed] [Google Scholar]
- 75.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 76.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal Cell Networks Regulate Lymphocyte Entry, Migration, and Territoriality in Lymph Nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mempel TR, Junt T, von Andrian UH. Rulers over randomness: stroma cells guide lymphocyte migration in lymph nodes. Immunity. 2006;25:867–869. doi: 10.1016/j.immuni.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Hayakawa M, Kobayashi M, Hoshino T. Direct contact between reticular fibers and migratory cells in the paracortex of mouse lymph nodes: a morphological and quantitative study. Arch. Histol. Cytol. 1988;51:233–240. doi: 10.1679/aohc.51.233. [DOI] [PubMed] [Google Scholar]
- 79.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 80.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. U. S. A. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okada T, Cyster JG. CC Chemokine Receptor 7 Contributes to Gi-Dependent T Cell Motility in the Lymph Node. J. Immunol. 2007;178:2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- 82.Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J. Exp. Med. 2007;204:1167–1179. doi: 10.1084/jem.20062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;394:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 84.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 85.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol. Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 86.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 87.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–717. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, Nussenzweig MC, Dustin ML. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 92.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J. Immunol. 2004;172:6558–6567. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- 95.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J. Immunol. 2006;176:6202–6210. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 96.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 98.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 99.Mrass P, Weninger W. Immune cell migration as a means to control immune privilege: lessons from the CNS and tumors. Immunol. Rev. 2006;213:195–212. doi: 10.1111/j.1600-065X.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 100.Fisher DT, Chen Q, Appenheimer MM, Skitzki J, Wang WC, Odunsi K, Evans SS. Hurdles to lymphocyte trafficking in the tumor microenvironment: implications for effective immunotherapy. Immunol. Invest. 2006;35:251–277. doi: 10.1080/08820130600745430. [DOI] [PubMed] [Google Scholar]
- 101.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 102.Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52:4265–4268. [PubMed] [Google Scholar]
- 103.Fukumura D, Salehi HA, Witwer B, Tuma RF, Melder RJ, Jain RK. Tumor necrosis factor alpha-induced leukocyte adhesion in normal and tumor vessels: effect of tumor type, transplantation site, and host strain. Cancer Res. 1995;55:4824–4829. [PubMed] [Google Scholar]
- 104.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 105.Munn LL. Aberrant vascular architecture in tumors and its importance in drug-based therapies. Drug Discov Today. 2003;8:396–403. doi: 10.1016/s1359-6446(03)02686-2. [DOI] [PubMed] [Google Scholar]
- 106.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood. 1996;88:667–673. [PubMed] [Google Scholar]
- 108.Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 1996;56:1111–1117. [PubMed] [Google Scholar]
- 109.Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J. Exp. Med. 1995;181:811–816. doi: 10.1084/jem.181.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 111.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-Induced IFN-gamma Production within the Tumor Microenvironment Influences Antitumor Immunity. J. Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 112.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 115.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 116.Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr. Opin. Immunol. 2003;15:148–154. doi: 10.1016/s0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 117.Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–4100. [PubMed] [Google Scholar]
- 119.Kowalczyk DW, Wlazlo AP, Giles-Davis W, Kammer AR, Mukhopadhyay S, Ertl HC. Vaccine-induced CD8+ T cells eliminate tumors by a two-staged attack. Cancer Gene Ther. 2003;10:870–878. doi: 10.1038/sj.cgt.7700653. [DOI] [PubMed] [Google Scholar]
- 120.Girardi M, Oppenheim D, Glusac EJ, Filler R, Balmain A, Tigelaar RE, Hayday AC. Characterizing the protective component of the alphabeta T cell response to transplantable squamous cell carcinoma. J. Invest. Dermatol. 2004;122:699–706. doi: 10.1111/j.0022-202X.2004.22342.x. [DOI] [PubMed] [Google Scholar]
- 121.Weber JS, Rosenberg SA. Modulation of murine tumor major histocompatibility antigens by cytokines in vivo and in vitro. Cancer Res. 1988;48:5818–5824. [PubMed] [Google Scholar]
- 122.Lee JK, Sayers TJ, Brooks AD, Back TC, Young HA, Komschlies KL, Wigginton JM, Wiltrout RH. IFN-gamma-dependent delay of in vivo tumor progression by Fas overexpression on murine renal cancer cells. J. Immunol. 2000;164:231–239. doi: 10.4049/jimmunol.164.1.231. [DOI] [PubMed] [Google Scholar]
- 123.Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc. Natl. Acad. Sci. U. S. A. 1991;88:775–779. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 125.Sambhi SK, Kohonen-Corish MR, Ramshaw IA. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4025–4029. doi: 10.1073/pnas.88.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dace DS, Chen PW, Niederkorn JY. CD8+ T cells circumvent immune privilege in the eye and mediate intraocular tumor rejection by a TNF-alpha-dependent mechanism. J. Immunol. 2007;178:6115–6122. doi: 10.4049/jimmunol.178.10.6115. [DOI] [PubMed] [Google Scholar]
- 127.Prevost-Blondel A, Roth E, Rosenthal FM, Pircher H. Crucial role of TNF-alpha in CD8 T cell-mediated elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J. Immunol. 2000;164:3645–3651. doi: 10.4049/jimmunol.164.7.3645. [DOI] [PubMed] [Google Scholar]
- 128.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J. Clin. Invest. 2008;118:1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stoelcker B, Ruhland B, Hehlgans T, Bluethmann H, Luther T, Mannel DN. Tumor necrosis factor induces tumor necrosis via tumor necrosis factor receptor type 1-expressing endothelial cells of the tumor vasculature. Am. J. Pathol. 2000;156:1171–1176. doi: 10.1016/S0002-9440(10)64986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schuler T, Kammertoens T, Preiss S, Debs P, Noben-Trauth N, Blankenstein T. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8(+) T cells. J. Exp. Med. 2001;194:1767–1775. doi: 10.1084/jem.194.12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schuler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J. Exp. Med. 1999;189:803–810. doi: 10.1084/jem.189.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dobrzanski MJ, Reome JB, Dutton RW. Role of effector cell-derived IL-4, IL-5, and perforin in early and late stages of type 2 CD8 effector cell-mediated tumor rejection. J. Immunol. 2001;167:424–434. doi: 10.4049/jimmunol.167.1.424. [DOI] [PubMed] [Google Scholar]
- 133.Huang S, Xie K, Bucana CD, Ullrich SE, Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin. Cancer Res. 1996;2:1969–1979. [PubMed] [Google Scholar]
- 134.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Slifka MK, Rodriguez F, Whitton JL. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–79. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 136.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 137.He JS, Ostergaard HL. CTLs contain and use intracellular stores of FasL distinct from cytolytic granules. J. Immunol. 2007;179:2339–2348. doi: 10.4049/jimmunol.179.4.2339. [DOI] [PubMed] [Google Scholar]
- 138.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nature Reviews Immunology. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 139.Rosen D, Li JH, Keidar S, Markon I, Orda R, Berke G. Tumor immunity in perforin-deficient mice: a role for CD95 (Fas/APO-1) J. Immunol. 2000;164:3229–3235. doi: 10.4049/jimmunol.164.6.3229. [DOI] [PubMed] [Google Scholar]
- 140.Seki N, Brooks AD, Carter CR, Back TC, Parsoneault EM, Smyth MJ, Wiltrout RH, Sayers TJ. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J. Immunol. 2002;168:3484–3492. doi: 10.4049/jimmunol.168.7.3484. [DOI] [PubMed] [Google Scholar]
- 141.Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J. Immunol. 2003;171:2402–2412. doi: 10.4049/jimmunol.171.5.2402. [DOI] [PubMed] [Google Scholar]
- 142.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu. Rev. Cell Dev. Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 143.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 144.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 145.Millard PJ, Henkart MP, Reynolds CW, Henkart PA. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. J. Immunol. 1984;132:3197–3204. [PubMed] [Google Scholar]
- 146.Tschopp J, Masson D, Stanley KK. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature. 1986;322:831–834. doi: 10.1038/322831a0. [DOI] [PubMed] [Google Scholar]
- 147.Froelich CJ, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, Shah GM, Bleackley RC, Dixit VM, Hanna W. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J. Biol. Chem. 1996;271:29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- 148.Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 149.Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr. Opin. Immunol. 2007;19:301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nature Reviews Immunology. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 151.Martinvalet D, Zhu P, Lieberman J. Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity. 2005;22:355–370. doi: 10.1016/j.immuni.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 152.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 153.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 154.Brunner KT, Mauel J, Cerottini J-C, Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition of isoantibody and by drugs. Immunology. 1968;14:181–196. [PMC free article] [PubMed] [Google Scholar]
- 155.Sanderson CJ. The mechanism of T cell mediated cytotoxicity II. Morphological studies of cell death by time-lapse microcinematography. Proc. R. Soc. Lond. B. 1976;192:241–255. doi: 10.1098/rspb.1976.0011. [DOI] [PubMed] [Google Scholar]
- 156.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 157.Duggan BL, Cabilio NR, Dickie P, Witmer J, Goping IS, Underhill DA, Bleackley RC. A novel lineage-specific hypersensitive site is essential for position independent granzyme B expression in transgenic mice. Biochem. Biophys. Res. Commun. 2008;368:357–363. doi: 10.1016/j.bbrc.2008.01.065. [DOI] [PubMed] [Google Scholar]
- 158.Berke G, Sullivan KA, Amos DB. Tumor immunity in vitro: Destruction of a mouse ascites tumor through a cycling pathway. Science. 1972;177:433–434. doi: 10.1126/science.177.4047.433. [DOI] [PubMed] [Google Scholar]
- 159.Matter A. Microcinematographic and electron microscopic analysis of target cell lysis induced by cytotoxic T lymphocytes. Immunology. 1979;36:179–190. [PMC free article] [PubMed] [Google Scholar]
- 160.Rothstein TL, Mage M, Jones G, McHugh LL. Cytotoxic T lymphocyte sequential killing of immobilized allogeneic tumor target cells measured by time-lapse microcinematography. J. Immunol. 1978;121:1652. [PubMed] [Google Scholar]
- 161.Poenie M, Tsien RY, Schmitt-Verhulst A. Sequential activation and lethal hit measured by [Ca++]i in individual cytolytic T cells and targets. EMBO J. 1987;6:2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barth RJ, Jr, Mule JJ, Spiess PJ, Rosenberg SA. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J. Exp. Med. 1991;173:647–658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Becker C, Pohla H, Frankenberger B, Schuler T, Assenmacher M, Schendel DJ, Blankenstein T. Adoptive tumor therapy with T lymphocytes enriched through an IFN-gamma capture assay. Nat Med. 2001;7:1159–1162. doi: 10.1038/nm1001-1159. [DOI] [PubMed] [Google Scholar]
- 164.Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr. Opin. Immunol. 2005;17:180–186. doi: 10.1016/j.coi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 165.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 167.Ibe S, Qin Z, Schuler T, Preiss S, Blankenstein T. Tumor rejection by disturbing tumor stroma cell interactions. J. Exp. Med. 2001;194:1549–1559. doi: 10.1084/jem.194.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc. Natl. Acad. Sci. U. S. A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 170.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr. Opin. Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]