Cutaneous basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), collectively called nonmelanoma skin cancer (NMSC), are the most common primary malignant neoplasms, yet recurrence after treatment is unusual.1 With modern antiretroviral therapy, NMSC is also the most frequent cancer in persons infected with human immunodeficiency virus (HIV),2 but recurrence rates in this population are largely unknown.3 We followed a large prospective cohort of patients with NMSC to determine tumor recurrence rates and found unexpectedly high recurrence among the HIV-infected patients.
Methods
This study was approved by the committee on human research, University of California, San Francisco, and details of this study have been described elsewhere.1 Briefly, eligible patients were those with primary NMSC diagnosed in 1999 or 2000 at a university-based practice and its affiliated Veterans Affairs Medical Center. Data were collected via medical records, patient survey, and blinded physical examination, and patients were followed up for a median (interquartile range [IQR]) of 7.3 (3.1–8.7) years after treatment. Given the prevalence of human papilloma virus–associated genital SCCs among HIV-infected patients, tumors located in the genital area were excluded from all analyses.
We compared groups by the χ2 test for categorical characteristics and Wilcoxon rank sum test for continuous characteristics. Cumulative incidence of tumor recurrence over time was displayed using Kaplan-Meier plots. We also constructed a series of Cox proportional hazard models to calculate unadjusted and adjusted 5-year recurrence rates and hazard ratios (HRs). Because of the limited number of recurrences, we forced HIV status and treatment type (categorized as conservative [destruction, topical, or none] or aggressive [excisional or Mohs surgery]) into the adjusted models then applied a forward stepwise selection based on AIC (Akaike Information Criterion) within the entire sample and each histological subtype (BCC and SCC). Potentially selected variables were those significant in bivariate analyses, including age, history of prior NMSC, multiple NMSCs at enrollment, tumor location in the H-zone of the face—a high-risk area for recurrence—histologic type, histologic invasiveness, and number of annual visits to a dermatologist throughout the follow-up period. Unadjusted analyses were repeated in an SCC subset matched at a 1:5 ratio, HIV-infected to HIV-uninfected, on age, sex, and tumor body location. Statistical analyses were performed using R statistical software, version 2.13.
Results
Of the 1534 nongenital NMSCs that occurred among 1202 patients, 50 were primary tumors in 34 HIV-infected patients. Compared with HIV-uninfected patients, HIV-infected patients were younger (median ages, 50.5 vs 70.0 years) (P <.001) and had tumors of similar size and histopathologic characteristics that were, however, less likely to be located in the H-zone (22% [n=11] vs 37% [n=549]) (P =.05). Tumors in HIV-infected patients were more often treated with destructive or topical therapies (54% [n=27] vs 29% [n=430]) than surgical treatments (44% [n = 22] vs 71% [n = 1054]) (P <.001), and HIV-infected patients visited the dermatologist more frequently (median [IQR] number of annual visits, 2.1 [1.0–3.5] vs 1.5 [0.5–3.0]) (P<.001). At enrollment, the median (IQR) duration of HIV infection was 11.0 (9.5–15.0) years. Thirty-one of the 34 HIV-infected patients took approved highly active antiretroviral therapy (91%).
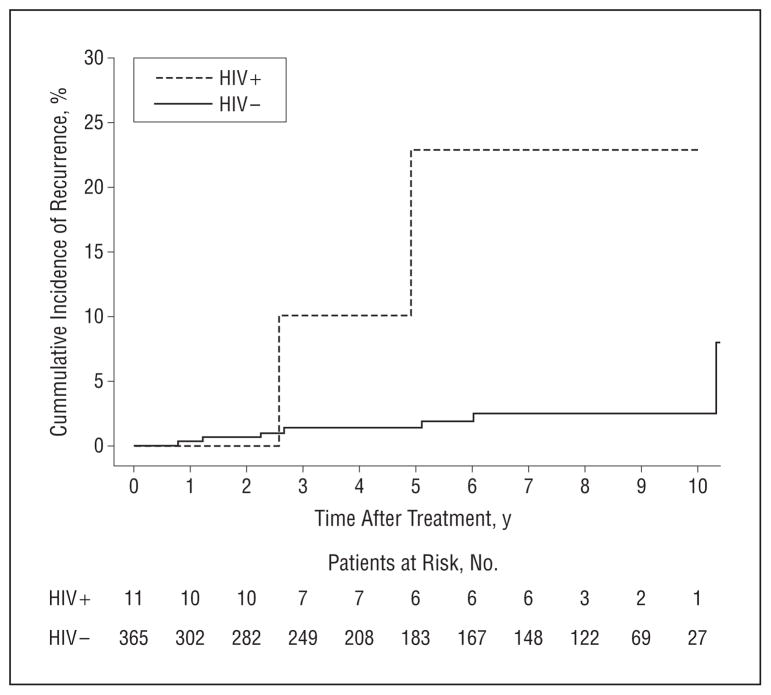
Overall 5-year tumor recurrence rates after treatment were 2.9% in HIV-uninfected patients and 13.8% in HIV-infected patients (HR, 3.1; P=.005) (Table). Rates were statistically significantly higher for the 376 SCCs (Figure). Among SCCs, HIV infection was highly predictive of tumor recurrence in both unadjusted (HR, 9.5; P=.004) and multivariate models (HR, 9.6; P<.001), including tumor location in the H-zone of the face, histologic invasiveness, and treatment type. Unadjusted results were consistent in the matched SCC sample.
Table.
Five-Year Recurrence Rates of Tumors in Patients With and Without HIV
| Characteristics | Patients (N = 1202)a
|
HRb | P Value | |
|---|---|---|---|---|
| With HIV (n = 34 With 50 Tumors) | Without HIV (n = 1168 With 1484 Tumors) | |||
| Unadjusted Findings | ||||
| Overall 5-year recurrence rate | 13.8 | 2.9 | 3.1 | .005 |
| SCC | 21.6 | 1.4 | 9.5 | .004 |
| BCC | 10.4 | 3.3 | 2.2 | .22 |
| Time to detection of recurrence, median (IQR), y | 2.6 (2.0–4.5) | 4.0 (2.1–6.0) | .36 | |
|
| ||||
| Adjusted Findings | ||||
| Overall 5-year recurrence ratec | 11.0 | 2.8 | 2.5 | .07 |
| SCCd | 13.5 | 0.9 | 9.6 | <.001 |
| BCCc | 7.3 | 3.1 | 1.6 | .49 |
Abbreviations: BCC, basal cell carcinoma; HIV, human immunodeficiency virus; HR, hazard ratio; IQR, interquartile range; NMSC, nonmelanoma skin cancer; SCC, squamous cell carcinoma.
Unless otherwise noted, data are reported as percentage recurrence rates.
Hazard ratios for recurrence of tumors in HIV-infected vs HIV-uninfected patients.
Adjusted for HIV status, multiple NMSCs at enrollment, more than 2 annual visits per year to the dermatologist, and treatment type.
Adjusted for HIV status, tumor location in the H-zone of the face, histologic invasiveness, and treatment type.
Figure 1.
Cumulative incidence of recurrence after treatment of 376 squamous cell carcinomas in 337 patients with and without human immunodeficiency virus (HIV).
Median (IQR) CD4 counts and viral loads averaged from time of diagnosis to the end of follow-up were 449/μL (301/mu;L–674/mu;L) and 500/mL (0/mL–27 990/mL), respectively. There was no relationship between recurrence and CD4 count or viral load.
Of note, no recurrences occurred among 51 tumors in patients who had received organ transplants.
Comment
Patients with HIV and nongenital SCCs had higher rates of recurrence after treatment, despite being relatively young with well-controlled HIV. Treatment choices or frequency of follow-up may have contributed to these results, but immune surveillance4 or advanced immunosenescence5 are likely also important. That recurrence did not occur in organ transplant patients or relate to CD4 count suggests that immunosuppression alone is not a sufficient explanation. If replicated, these findings support specific monitoring of HIV-infected individuals with cutaneous NMSC and determination of optimal therapy for primary tumors.
Acknowledgments
Funding/Support: This work was supported by grants R01 AR 054983 and K24 AR052667 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: Dr Chren is a consultant for Genentech Inc.
Author Contributions: All authors had full access to the data in this report and take responsibility for its integrity and the accuracy of the analysis. Study concept and design: Hausauer, Parvataneni, and Chren. Acquisition of data: Hausauer, Parvataneni, Stuart, and Chren. Analysis and interpretation of data: Hausauer, Maurer, Leslie, Parvataneni, and Chren. Drafting of the manuscript: Hausauer, Maurer, Leslie, Parvataneni, and Chren. Critical revision of the manuscript for important intellectual content: Hausauer, Maurer, Leslie, Stuart, and Chren. Statistical analysis: Hausauer, Parvataneni, and Chren. Obtained funding: Chren. Administrative, technical, and material support: Hausauer, Leslie, and Stuart. Study supervision: Hausauer, Maurer, and Chren.
References
- 1.Chren MM, Torres JS, Stuart SE, Bertenthal D, Labrador RJ, Boscardin WJ. Recurrence after treatment of nonmelanoma skin cancer: a prospective cohort study. Arch Dermatol. 2011;147(5):540–546. doi: 10.1001/archdermatol.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crum-Cianflone N, Hullsiek KH, Satter E, et al. Cutaneous malignancies among HIV-infected persons. Arch Intern Med. 2009;169(12):1130–1138. doi: 10.1001/archinternmed.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobo DV, Chu P, Grekin RC, Berger TG. Nonmelanoma skin cancers and infection with the human immunodeficiency virus. Arch Dermatol. 1992;128 (5):623–627. [PubMed] [Google Scholar]
- 4.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23(8):875–885. doi: 10.1097/QAD.0b013e328329216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen ML, Farrell KJ, Gunthel CJ. Non-AIDS-Defining malignancies in patients with HIV in the HAART era. Curr Infect Dis Rep. 2010;12(1):46–55. doi: 10.1007/s11908-009-0075-6. [DOI] [PubMed] [Google Scholar]