Abstract
Purpose
Individuals diagnosed with cancer close to death have low access to enrollment in palliative care programs. The purpose of this literature review was to assess the usefulness of pre-diagnostic comorbidity and healthcare utilization as indicators of late-stage colorectal cancer (CRC) diagnosis, to help with early identification of individuals who may benefit from palliative care.
Methods
A literature search was conducted in relevant databases using title/abstract terms which included “cancer,” “stage,” “diagnosis,” “determinants,” “predictors,” and “associated.” Included studies examined whether comorbidity and/or healthcare utilization had an impact on the stage at which CRC was diagnosed. A standardized data abstraction form was used to assess the eligibility of each study. Thirteen articles were included in the literature review. These studies were assessed and synthesized using qualitative methodology.
Results
We found much heterogeneity among study variables. The findings of this literature review point to the presence of comorbidity and non-emergent healthcare utilization as having no association with late-stage diagnosis. Conversely, emergency room presentation (ERP) was associated with late-stage diagnosis.
Conclusions
The results of this literature review did not find strong evidence to suggest that comorbidity and healthcare utilization are potential indicators of late-stage diagnosis. However, ERP may be useful as a flag for consideration of prompt referral to palliative care. Additional research is required to identify potential indicators of late-stage diagnosis that may be available in administrative databases, particularly in the area of healthcare utilization.
Keywords: Colorectal neoplasms, Diagnosis, Neoplasm staging, Epidemiologic factors
Introduction
In Canada, colorectal cancer (CRC) has the second and third highest cancer-related mortality rates for men and women, respectively [1]. Across Canada, the 5-year relative survival of CRC is 63% [1]. The most important prognostic factor in both colon and rectal cancer is stage at diagnosis, with 5-year survival decreasing with increasing stage. While national stage distribution data are limited, data from several provinces indicate that approximately 20% of CRC cases in Canada are diagnosed at Stage IV [1], when treatment with curative intent is no longer a viable option.
Individuals diagnosed with late-stage CRC may benefit from timely enrollment in a palliative care program. By focusing on pain and symptom management, providing psychological and spiritual support, assisting patients in living as actively as possible until death, and offering support to the family, palliative care aims to improve the quality of life for families and patients [2]. The importance of the role of palliative care is reflected in the use of enrollment in a palliative care program as a quality indicator with enrollment near death reflecting poor quality of care [3, 4]. Unfortunately, our research has shown that persons who are diagnosed close to death are at increased risk of not accessing a palliative care program [5, 6].
By identifying factors present prior to diagnosis that are potentially associated with late-stage CRC diagnosis, the individuals affected could be targeted for a palliative care consultation upon diagnosis. Comorbidity and healthcare utilization are two factors that may be useful as potential indicators, or “flags,” due to their availability in administrative data and possible association with the timeliness of a CRC diagnosis.
Two conflicting theories exist as to whether individuals with comorbid conditions are more or less likely to have their cancer detected at an early stage. One theory hypothesizes that those with comorbid conditions may be diagnosed at an earlier stage because of increased contact with the healthcare system due to care related to their comorbidity [7]. Conversely, the competing demands model suggests that comorbid disease may shift healthcare workers’ attention away from early symptoms of CRC, which may result in a later stage at diagnosis [8]. Central to both of these ideas is the assumption that patients with comorbidities have contact with the healthcare system (i.e., access to care). Based on Fienstein’s theory [7], increased contact with the healthcare system would result in diagnosis at an earlier stage, regardless of the presence of comorbidity. As such, a separate exploration of the relationship between healthcare utilization and stage at diagnosis is warranted.
The purpose of this literature review was to assess the usefulness of pre-diagnostic comorbidity and healthcare utilization as indicators of late-stage CRC diagnosis, to help with early identification of individuals who may benefit from palliative care. Thus, we sought to identify and synthesize evidence surrounding whether the presence of comorbid conditions and healthcare utilization prior to diagnosis has an impact on CRC stage at diagnosis, and to identify gaps within the literature that may warrant further investigation.
Methods
The PubMed, EMBASE, and CINAHL electronic databases were searched using title/abstract terms which included “cancer,” “stage,” “diagnosis,” “determinants,” “predictors,” and “associated.” The complete search strategy implemented for each database is available in “Appendix.” The search was current to May 2010. No limitations were placed on publication date or language. Reviews (both systematic and narrative) as well as original studies were eligible for inclusion. Editorials, letters, notes, and similar publication types were excluded. Additional articles were identified through searching reference lists of the retrieved full-text articles.
After deduplication, articles were first screened by title and subsequently by abstract, removing irrelevant entries at each step. Remaining full-text articles were retrieved and assessed for eligibility using several selection criteria. The first selection criterion was whether the study had examined whether comorbidity and/or healthcare utilization had an impact on the stage at which CRC was diagnosed. The second selection criterion was that exposure (i.e., the presence of comorbidity and healthcare utilization) must have been measured prior to diagnosis; studies that determined exposure status after the CRC diagnosis were excluded. Finally, studies must have reported the effect of exposure on the outcome of interest (stage at diagnosis) specifically for CRC patients. If a study’s population contained a heterogeneous mixture of cancer diagnoses, stage at diagnosis must have been reported specifically for CRC patients. If outcomes were only presented for multiple cancer types, the study was excluded.
A standardized data abstraction form was used to assess the eligibility of each study according to the above selection criteria. Data abstracted from each study included location, study design, data source, study population, definition of early/advanced stage, and the effect of each predictor. Included studies were assessed and synthesized using qualitative methodology. Quantitative synthesis was not performed due to excessive heterogeneity present in the classification of both the exposure and outcome variables in all included studies.
Results
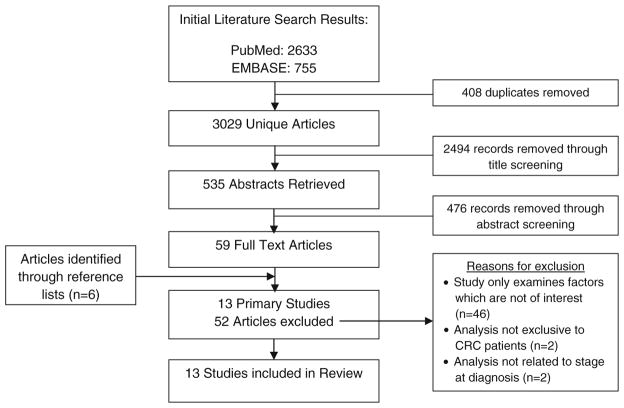
The search strategy retrieved 3,029 unique articles (Fig. 1). Fifty-nine full-text articles were identified as potentially relevant, and 6 additional studies were identified through searching reference lists of full-text articles. Ultimately, 13 studies met the inclusion criteria.
Fig. 1.
Flowchart detailing the selection process for study inclusion and reasons for exclusion from this literature review
Within the 13 identified studies, nine studies reported on the effect of comorbidity on stage at diagnosis [9–17], and five studies reported on healthcare utilization prior to diagnosis and its effect on stage at diagnosis [13, 18–21]. Full details of study selection, including reasons for exclusion of studies, can be seen below in Fig. 1.
Complete details of each included study (study location, study subjects, methods, data source, definition of early/late-stage cancer, and predictors of stage at diagnosis) are available in Table 1. Only one study reported outcomes specifically for colon cancer [13], the other 12 studies grouped colon and rectal cancer. Measurement of both exposure (comorbidity and healthcare utilization) and outcome (CRC stage at diagnosis) varied greatly among the identified literature.
Table 1.
Description of included studies
| First author, date | Country | Sample size | Site | Subject characteristics | Definition of stage at diagnosis | Predictor |
|---|---|---|---|---|---|---|
| Fazio, 2005 [6] | Canada | 816 | CRC | 49.3% men; 98.8% caucasian | Early = Stage I and II Late = Stage III and IV |
Diabetes (self-report) |
| Fisher, 2004 [15] | USA | 547 | CRC | 97.4% men; mean age = 66.4 ± 8.8 years 81% caucasian | Early = Stage I and II Late = Stage III and IV |
Having a usual source of care |
| Fisher, 2010 [7] | USA | 447 | CRC | 98% men; mean age = 67 ± 10.9 years; 66% caucasian | Early = Stage I and II Late = Stage III and IV |
Comorbidity (summary measure) |
| Gonzalez, 2001 [8] | USA | 8,933 | CRC | 51% men; mean age = 71.5 ± 11.6 years; 85.4% caucasian | Early = in situ, local Late = regional, distant |
Comorbidity (summary measure) |
| Gornick, 2004 [9] | USA | 2,946 | CRC | 43% men; ≥67 years; 88.6% caucasian | Early = in situ, local Late = regional, distant |
Comorbidity (summary measure) |
| Gross, 2006 [10] | USA | 44,924 | Colon | 43.7% men; ≥66 years | Early = Stage I Late = Stage II–IV |
Comorbidity Healthcare Utilization |
| Henry, 2009 [11] | USA | 31,603 | CRC | 49.7% men; ≥50 years; 83% caucasian | Early = in situ, local Late = regional, distant |
Being diagnosed with prior cancer (non-CRC) |
| Mitchell, 2007 [16] | Canada | 455 | CRC | 52% men; mean age = 68 years | Individual stages (I, II, III, or IV) | ER presentation of CRC |
| Myers, 1997 [12] | USA | 222 | CRC | 56% men; mean age = 67 years | Early = Duke’s A and B Late = Duke’s C and D |
Being diagnosed with prior cancer |
| Polednak, 2000 [17] | USA | 10,926 | CRC | ED: 46% men; 92.8% caucasian Other: 51.3% men; 94.3% caucasian |
Early = Stage I, II or III Late = Stage IV |
ER presentation of CRC |
| Roetzheim 1999 [18] | USA | 8,933 | CRC | 51% male; mean 51% male; mean age = 71.5 ± 11.6 years; 85.4% Caucasian | Early = in situ, localLate = regional, distant | Physician supply |
| Siddiqui, 2008 [13] | USA | 269 | CRC | T2DM: mean age = 68.3 years Non-T2DM: mean age = 70.5 years |
Early = Stage I, II Late = Stage III, IV |
Type 2 diabetes (T2DM) |
| Zafar, 2008 [14] | USA | 682 | CRC | VA cohort: 98% men; mean age = 67 years; 63% caucasian FFS cohort: 48% men; mean age = 61 years; 73% caucasian |
Early = Stage I, II, and III Late = Stage IV |
Comorbidity (summary measure) |
Comorbidity
There were two methods used to assess the presence of comorbidity in identified literature. Several studies used a summary measure (e.g., Charlson’s Comorbidity Index [22], modified Charlson’s Comorbidity Index, or Adult Comorbidity Evaluation [ACE-27] [23]) to assess comorbidity level. These studies measured comorbidity in terms of presence or absence, or by categorizing the number of comorbid conditions (e.g., 0–1, 2–3, ≥4). Studies also identified patients with a specific comorbid condition (e.g., previous cancer diagnosis, type 2 diabetes mellitus [T2DM]) and compared these patients to controls without the specific comorbid condition. The impact of comorbidity on stage at CRC diagnosis is summarized in Table 2.
Table 2.
Results’ summary of the impact of comorbidity on stage at CRC diagnosis
| Author | Relationship between comorbidity and stage at diagnosis | |
|---|---|---|
| Early stage | Gornick, 2004 [9] | For women, increased comorbidity was associated with earlier stage at diagnosis (OR: 0.69, 95% CI: 0.52–0.91) |
| Gross, 2006 [10] | Increasing number of comorbidities associated with early-stage diagnosis (p <0.001) | |
| Zafar, 2008 [14] | For a cohort of Veteran’s Health Administration patients, an increase in comorbidity was associated with earlier stage at diagnosis (OR: 0.76, 95% CI: 0.58–1.00, p = 0.045) | |
| Fazio, 2005 [6] | Individuals with diabetes were more likely to be diagnosed at an early stage (OR: 0.53, 95% CI: 0.28–0.99) | |
| Henry, 2009 [11] | Individuals with a previous, non-CRC, cancer diagnosis, were less likely to have a late-stage diagnosis (51.2% vs. 55.6%, p <0.001) | |
| No effect | Fisher, 2010 [7] | Comorbidity was not associated with stage at diagnosis for either mild (OR: 0.69, 95% CI 0.38–1.28), moderate (OR: 0.65, 95% CI: 0.34–1.25), or severe comorbidity (OR: 0.60, 95% CI 0.31–1.15) |
| Gornick, 2004 [9] | For men, comorbidity was not associated with stage at diagnosis | |
| Myers, 1997 [12] | Presence of comorbidities (vs. absence) was not associated with early-stage diagnosis (p = 0.33) | |
| Zafar, 2008 [14] | For a cohort of fee-for-service patients, there was no relationship between comorbidity and stage (OR: 1.09, 95% CI: 0.82–1.44) | |
| Siddiqui, 2008 [13] | Well-controlled T2DM was not associated with stage of diagnosis in comparison with controls (OR: 0.9, 95% CI: 0.5–1.6) | |
| Late stage | Gonzalez, 2001 [8] | Presence of comorbidity was associated with late stage at diagnosis (OR: 1.17, 95% CI: 1.06–1.29) |
| Siddiqui, 2008 [13] | Patients with poorly controlled T2DM had a more advanced stage at diagnosis, compared to well-controlled and non-diabetic groups (OR: 2.1, 95% CI: 1.0–4.4) |
Highlighted rows correspond to studies where specific comorbidities were examined
When comorbidity was determined using a summary measure, the presence of comorbidity was associated with a later stage of CRC diagnosis in one study [11] and had no effect on CRC stage at diagnosis in two studies [10, 15] as well as no effect in identified subgroups in two studies: the male cohort in one [12] and a cohort of fee-for-service patients in the other (compared to a cohort from the Veterans Health Administration) [17]. Comorbidity was associated with an earlier stage of CRC at diagnosis in one study [13], and the subgroups in two studies: the female cohort in one study [12] and a cohort of patients from the Veteran’s Health Administration [17].
When individual comorbid diseases were considered, one study found that well-controlled T2DM (HbA1c <7.5%) was not associated with CRC stage at diagnosis; however, poorly controlled T2DM (HbA1c ≥ 7.5%) was associated with a later stage at diagnosis [16]. Other studies found that self-reported diabetes and a prior cancer diagnosis (non-CRC) were associated with an earlier stage of CRC at diagnosis [9, 14].
Healthcare utilization
Five studies were identified surrounding healthcare utilization and its effects on stage at diagnosis. As only one study was identified which contained a direct measure of healthcare utilization (i.e., frequency of visits), we adopted the use of several proxy measures that may be indicative of healthcare utilization (the studies are summarized in Table 3). Specifically, we included measures related to having a usual source of care, physician supply, and emergency room (ER) presentation (ERP). While having a usual source of care does not necessarily correlate with regular interaction with the healthcare system [24], it may be assumed that an individual with a usual healthcare provider can more readily access services when needed. As indicated in the literature [25, 26], we expected increased regional physician supply to improve access to care and subsequently allow increased contact with the healthcare system. In relation, it has been demonstrated that ER services are used by individuals who have difficulty accessing other sources of care, many of whom do not have a primary care physician [27].
Table 3.
Results’ summary of the impact of healthcare utilization on stage at CRC diagnosis
| Author | Measure | Relationship between comorbidity and stage at diagnosis |
|---|---|---|
| Fisher, 2004 [18] | Usual source of care | Individuals with a usual source of care (i.e., clinic or physician’s office) were less likely to be diagnosed at a later stage (OR: 0.4, 95% CI: 0.2–0.6) |
| Gross, 2006 [13] | Number of physician visits | A decreasing number of physician visits, prior to diagnosis, was associated with a later stage at diagnosis (p <0.001) |
| Roetzheim, 1999 [21] | Physician supply | For each 10 percentile increase in primary care physician supply, the odds of late-stage diagnosis decreased by 5% (OR: 0.95, 95% CI: 0.92–0.99) For each 10 percentile increase in specialty physician supply, the odds of late-stage diagnosis increased by 5% (OR: 1.05, 95% CI: 1.02–1.09) |
| Mitchell, 2007 [19] | ERP | Of all individuals who diagnosed with CRC via an emergency room presentation (ERP), a higher percentage of patients presented with Stage IV than Stage I (21.3% vs. 6.5%; p <0.001) |
| Polednak, 2000 [20] | ERP | ERP was associated with “distant” cancer diagnosis compared to all other stages (i.e., in situ, local, regional, unknown) (OR = 2.05, 95% CI: 1.82–2.30) |
In this review, we found one study wherein having a usual source of care (self-reported as having a clinic, physician’s office, etc.) was significantly associated with a decreased likelihood of late-stage diagnosis (OR: 0.4, 95% CI: 0.2–0.6) [18]. Similarly, another study reported that an increasing number of physician visits 2–24 months prior to diagnosis was significantly associated with earlier stage CRC diagnosis (p <0.001) [13].
One study examined the effects of regional physician supply (determined through physician/population ratio of adjacent postal codes) and stage of diagnosis of CRC [21]. It was found that combined physician supply (primary care physicians plus specialists, measured in deciles) did not have an impact on stage at diagnosis; however, when primary care physicians and specialists were analyzed separately, opposing trends were seen. Increasing supply of primary care physicians was associated with a decreased likelihood of late-stage diagnosis (OR: 0.95, 95% CI: 0.92–0.99), while increasing supply of specialists was associated with increased likelihood of late-stage diagnosis (OR: 1.05, 95% CI: 1.02–1.09).
The final two studies examined the effect of ERP on the stage at which CRC was diagnosed, with ERP being associated with late-stage diagnosis [19, 20]. Polednak [20] found that patients with ERP were more likely to be diagnosed with distant stage (OR: 2.05, 95% CI: 1.82–2.30). Similarly, Mitchell et al. [19] found that stage at diagnosis differed significantly between those diagnosed during an emergency resection (i.e., ERP) and those who received an elective resection (p <0.001). Specifically, a higher percentage of patients with ERP received a Stage IV CRC diagnosis (21.3% vs. 10.4%), while a higher percentage of patients who had elective resections were diagnosed with Stage I PC (26.3% vs. 6.5%).
Discussion
This study sought to synthesize evidence on the impact of pre-diagnostic comorbid conditions and healthcare utilization on CRC stage at diagnosis, and to identify gaps in the existing literature. These factors were initially identified as being potential “flags” for late-stage diagnosis that are available from administrative databases. Despite the large number of studies identified in our initial search, few actually assessed the impact of comorbidity or healthcare utilization on stage of diagnosis. Nonetheless, the findings of this review point to the presence of comorbidity as either having no impact on stage of CRC diagnosis or resulting in an earlier stage of CRC diagnosis. We also found several studies indicating that increased (non-emergent) healthcare utilization prior to diagnosis results in an earlier stage at diagnosis. While the impact of comorbidity and healthcare utilization appears to provide some support to the theory presented by Feinstein [7] that earlier stage disease will be found in those with comorbid conditions due to increased contact with healthcare providers, one limitation of the identified literature is that none of the identified studies employed rigorous methodology to examine the potential effect of comorbidity on healthcare utilization, or vice versa. This may warrant investigation given that healthcare utilization varies with number and type of comorbidities [28, 29]. While one study examined the impact of both comorbidity and healthcare utilization on CRC stage at diagnosis, it examined only the univariate impact of each of these factors on stage at diagnosis and did not explore the relationship between comorbidity and healthcare utilization [13]. Furthermore, associations with comorbidity and healthcare utilization may relate to specific components rather summary measures of these concepts.
Siddiqui et al. [16] provide some insight into the interrelation of comorbidity and healthcare utilization through their study of CRC patients with T2DM. Their study examined both the presence and severity of T2DM and found that in those with poorly controlled diabetes (HbA1c ≥ 7.5%), CRC tended to be diagnosed at a later stage in comparison with those with well-managed diabetes (HbA1c <7.5%). It is possible that for those with poorly controlled diabetes, medical care is focused on the management of diabetes and complications related to high blood glucose, which can be quite severe (i.e., neuropathy, infection, nephropathy, eye damage, etc.), rather than on other medical (preventative or symptomatic) concerns. It is also possible that diabetics who do not have the behavioral capability to control their diabetes may not seek screening or visit their physician regarding potential CRC symptoms. In essence, the late-stage diagnosis for individuals with poorly controlled T2DM may rest just as likely with the behavior of the patient as with the provider. But in either case, ensuring the timeliness of the care is important, especially palliative care for late-stage CRC.
Our assessment of the independent effect of healthcare utilization on stage at diagnosis was challenging due to a lack of studies containing direct measures of healthcare utilization, requiring the inclusion of several proxy measures. A lower frequency of physician visits, not having a usual source of care, and decreased primary care physician supply were all associated with later stage at diagnosis. Assuming that increased access to care (having a usual source of care, increased supply of primary care physicians) translates into increased contact with the healthcare system, there are several mechanisms by which earlier diagnosis may occur. Frequent visits to a healthcare provider for persistent issues may prompt referral for definitive diagnostic testing. The literature has also shown that increased contact with the healthcare system (i.e., receiving a regular checkup [30], and increased frequency of physician visits [31]) is associated with increased uptake of CRC screening, suggesting that individuals who have increased contact with healthcare professionals have an increased propensity toward screening (i.e., through increased awareness/opportunity, physician influence, increased awareness, etc.).
In contrast, increased specialist supply was associated with an increased likelihood of late-stage diagnosis. Roetzheim et al. [21] discounted several potential explanations for this finding, including referral patterns (as location of residence, not location of treatment, was used) or any measured confounders (multivariate adjustment was used). We can hypothesize that the difference between primary and specialist care may be a result of the longitudinal relationship between the patient and their primary care provider, as well as the fact that the primary care provider is committed to all of the health risks for a patient, whereas the specialist is focused on fewer aspects of the patient’s health. It is worth noting that an increased supply of primary care physicians has been associated with better health outcomes in other studies of primary care–rich health service environments [32].
The finding that ERP was associated with late-stage diagnosis in two studies [19, 20] lends support to its use as a “flag” for identifying individuals who may benefit from palliative care. These individuals may have been unable to access other health services (i.e., screening or primary care) earlier in their disease trajectory. Han et al. [27] surveyed ER visitors and found that one-fifth of visitors had no primary care physician, and that many had tried to access alternative sources of care prior to visiting the ER. Thus, for some, the ER may be seen as the only option, or last resort, for medical care. Alternatively, ERP may be a consequence of underlying disease symptomatology; acute symptoms, often with large primary tumors, may be more likely to cause a patient to seek urgent care via the emergency department (e.g., profuse rectal bleeding).
Our review has several limitations. First, our study does not present a quantitative estimate of effect. However, not all scientific evidence is necessarily synthesizable in a quantitative manner. We chose to conduct a narrative literature review with a systematic search strategy as opposed to a full systematic review and meta-analysis because much of the identified literature contained extremely heterogeneous definitions of both the exposure and outcome, and because there was an inadequate amount of homogeneous literature in order to calculate a meaningful quantitative estimate. Second, while we placed no limitations on study language, our search strategy was conducted using English terms so only articles with a translated title and/or abstract could have been captured for analysis. Thirdly, we used proxy measures of healthcare utilization; however, this was ultimately conducive to our overall goal of identifying those who may benefit from enrollment in a palliative care program. Finally, as most of the identified studies were conducted in the USA, and only three studies were conducted outside of the USA (two in Canada and one in the Netherlands), the results of this review are not uniformly generalizable.
Strengths of this study include the use of a rigorous, systematic, and well-documented search strategy to identify all relevant literature, and the use of systematic methods to abstract the data from identified studies. This review also summarizes factors related to CRC stage at diagnosis that, as far as we are aware, have not been the subject of prior reviews, systematic or otherwise.
Conclusions/suggestions for future research
The results of this literature review did not find strong evidence to suggest that comorbidity and healthcare utilization are potential indicators of late-stage diagnosis. Whether ERP is a measure of healthcare utilization, or an indicator of access to care, may be debated; nonetheless, it has been shown to be associated with late-stage diagnosis and may be useful as a flag for consideration of prompt referral to palliative care. Determining whether a person is “close to death” is difficult and may not be evident upon diagnosis. However, by identifying individuals who have predictive patterns of health services use, for example, and are diagnosed with Stage IV CRC, here may be the opportunity to improve patient care. Specifically, access to palliative care programs may be improved through targeted and timely palliative care consultations with patients and families.
Future research should include the validation of ERP as an indicator for identifying individuals who are at risk of not accessing or enrolling in a palliative care program. Additional research is required to investigate the relationship between healthcare utilization (i.e., measures of contact with the healthcare system) and stage at diagnosis. Other areas of future investigation include examining the relationship between comorbidity and healthcare utilization in respect to stage at diagnosis, as well as how specific comorbid conditions (e.g., congestive heart disease, renal failure, dementia, etc.), physician supply, and healthcare capacity (i.e., factors relating to access to care) impact stage at diagnosis.
Acknowledgments
This review was funded as part of the Canadian Institutes of Health Research program “Access to Quality Cancer Care,” specifically the CIHR/CCNS Team in Access to CRC Services in Nova Scotia.
Appendix: Details of search strategy
Each term listed below was searched by title and/or abstract. Terms within each section were combined using brackets as well as the Boolean terms “AND” and “OR” where indicated. All three sections were then combined using the Boolean term “AND” to give the final search strategy.
-
Cancer.
AND
-
Advanced OR [(Early OR Late) AND (Stage OR -Stage)] OR [Delay AND (Diagnosed OR Diagnosis OR Diagnostic)].
AND
(Utilization AND Care) OR [(Determinants OR Predictors OR Associated) AND (Stage OR - Stage) AND (Diagnosed OR Diagnosis OR Diagnostic)].
Contributor Information
Mark Corkum, Department of Community Health and Epidemiology, Dalhousie University, Halifax, NS, Canada, Cancer Outcomes Research Program, Cancer Care Nova Scotia, 1276 South Park Street, Room 804, Halifax, NS B3H 2Y9, Canada.
Robin Urquhart, Cancer Outcomes Research Program, Cancer Care Nova Scotia, 1276 South Park Street, Room 804, Halifax, NS B3H 2Y9, Canada.
Cynthia Kendell, Cancer Outcomes Research Program, Cancer Care Nova Scotia, 1276 South Park Street, Room 804, Halifax, NS B3H 2Y9, Canada.
Fred Burge, Department of Community Health and Epidemiology, Dalhousie University, Halifax, NS, Canada, Department of Family Medicine, Dalhousie University, Halifax, NS, Candada, Queen Elizabeth II Health Sciences Centre, Halifax, NS, Canada.
Geoffrey Porter, Department of Community Health and Epidemiology, Dalhousie University, Halifax, NS, Canada, Cancer Outcomes Research Program, Cancer Care Nova Scotia, 1276 South Park Street, Room 804, Halifax, NS B3H 2Y9, Canada, Queen Elizabeth II Health Sciences Centre, Halifax, NS, Canada, Department of Surgery, Dalhousie University, Halifax, NS, Canada.
Grace Johnston, Department of Community Health and Epidemiology, Dalhousie University, Halifax, NS, Canada, School of Health Administration, Dalhousie University, Halifax, NS, Canada, Surveillance and Epidemiology Unit, Cancer Care Nova Scotia, Halifax, NS, Canada.
References
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics. Canadian cancer statistics 2011. Canadian Cancer Society; Toronto, ON: 2011. [Google Scholar]
- 2.World Health Organization Palliative Care. [Accessed 26 Sep 2011]; http://www.who.int/cancer/palliative/en/
- 3.Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld E, Urquhart R, Mykhalovskiy E, et al. Toward population-based indicators of quality end-of-life care: testing stakeholder agreement. Cancer. 2008;112:2301–2308. doi: 10.1002/cncr.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J, Johnston GM, Lavergne MR, McIntyre P. Identifying population groups with low palliative care program enrollment using classification and regression tree analysis. J Palliat Care. 2011;27:98–106. [PMC free article] [PubMed] [Google Scholar]
- 6.Burge FI, Lawson BJ, Johnston GM, Grunfeld E. A population-based study of age inequalities in access to palliative care among cancer patients. Med Care. 2008;46:1203–1211. doi: 10.1097/MLR.0b013e31817d931d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinstein A. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455–468. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 8.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166–174. [PubMed] [Google Scholar]
- 9.Fazio L, Cotterchio M, Manno M, McLaughlin J, Gallinger S. Association between colonic screening, subject characteristics, and stage of colorectal cancer. Am J Gastroenterol. 2005;100:2531–2539. doi: 10.1111/j.1572-0241.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 10.Fisher DA, Zullig LL, Grambow SC, et al. Determinants of medical system delay in the diagnosis of colorectal cancer within the veteran affairs health system. Dig Dis Sci. 2010;55(5):1434–1441. doi: 10.1007/s10620-010-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez EC, Ferrante JM, Van Durme DJ, Pal N, Roetzheim RG. Comorbid illness and the early detection of cancer. South Med J. 2001;94:913–920. [PubMed] [Google Scholar]
- 12.Gornick ME, Eggers PW, Riley GF. Associations of race, education, and patterns of preventive service use with stage of cancer at time of diagnosis. Health Serv Res. 2004;39:1403–1427. doi: 10.1111/j.1475-6773.2004.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296:2815–2822. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 14.Henry KA, Sherman R, Roche LM. Colorectal cancer stage at diagnosis and area socioeconomic characteristics in New Jersey. Health Place. 2009;15:505–513. doi: 10.1016/j.healthplace.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Myers RE, Murray J, Weinberg D, et al. Analysis of colorectal cancer stage among HMO members targeted for screening. Arch Intern Med. 1997;157:2001–2006. [PubMed] [Google Scholar]
- 16.Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case–control study. Dig Dis Sci. 2008;53:2486–2494. doi: 10.1007/s10620-008-0264-4. [DOI] [PubMed] [Google Scholar]
- 17.Zafar SY, Abernethy AP, Abbott DH, et al. Comorbidity, age, race and stage at diagnosis in colorectal cancer: a retrospective, parallel analysis of two health systems. BMC Cancer. 2008;8:345–353. doi: 10.1186/1471-2407-8-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher DA, Martin C, Galanko J, Sandler RS, Noble MD, Provenzale D. Risk factors for advanced disease in colorectal cancer. Am J Gastroenterol. 2004;99:2019–2024. doi: 10.1111/j.1572-0241.2004.40010.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AD, Inglis KM, Murdoch JM, Porter GA. Emergency room presentation of colorectal cancer: a consecutive cohort study. Ann Surg Oncol. 2007;14:1099–1104. doi: 10.1245/s10434-006-9245-z. [DOI] [PubMed] [Google Scholar]
- 20.Polednak AP. Inpatient hospital admission through an emergency department in relation to stage at diagnosis of colorectal cancer. Cancer Detect Prev. 2000;24:283–289. [PubMed] [Google Scholar]
- 21.Roetzheim RG, Pal N, Gonzalez EC, et al. The effects of physician supply on the early detection of colorectal cancer. J Fam Pract. 1999;48:850–858. [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 24.Diamant AL, Brook RH, Fink A, Gelberg L. Assessing use of primary health care services by very low-income adults in a managed care program. Arch Intern Med. 2001;161:1222–1227. doi: 10.1001/archinte.161.9.1222. [DOI] [PubMed] [Google Scholar]
- 25.Wijeysundera HC, Stukel TA, Chong A, Natarajan MK, Alter DA. Impact of clinical urgency, physician supply and procedural capacity on regional variations in wait times for coronary angiography. BMC Health Serv Res. 2010;10:5–13. doi: 10.1186/1472-6963-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guttmann A, Shipman SA, Lam K, Goodman DC, Stukel TA. Primary care physician supply and children’s health care use, access, and outcomes: findings from Canada. Pediatrics. 2010;125:1119–1126. doi: 10.1542/peds.2009-2821. [DOI] [PubMed] [Google Scholar]
- 27.Han A, Ospina M, Blitz SB, Strome T, Rowe BH. Patients presenting to the emergency department: the use of other health care services and reasons for presentation. CJEM. 2007;9:428–434. doi: 10.1017/s1481803500015451. [DOI] [PubMed] [Google Scholar]
- 28.Struijs JN, Baan CA, Schellevis FG, Westert GP, van den Bos GA. Comorbidity in patients with diabetes mellitus: impact on medical health care utilization. BMC Health Serv Res. 2006;6:84–92. doi: 10.1186/1472-6963-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westert GP, Satariano WA, Schellevis FG, van den Bos GA. Patterns of comorbidity and the use of health services in the Dutch population. Eur J Public Health. 2001;11:365–372. doi: 10.1093/eurpub/11.4.365. [DOI] [PubMed] [Google Scholar]
- 30.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23:28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 31.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 32.Macinko J, Starfield B, Shi L. Quantifying the health benefits of primary care physician supply in the United States. Int J Health Serv. 2007;37:111–126. doi: 10.2190/3431-G6T7-37M8-P224. [DOI] [PubMed] [Google Scholar]